Composition and Biological Properties of Blanched Skin and Blanch Water Belonging to Three Sicilian Almond Cultivars
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Sample Collection and Preparation
2.3. Nutritional Profile and Fatty Acids Composition of Blanched Skin
2.4. Phytochemical Screening
2.4.1. Total Phenols
2.4.2. Total Flavonoids
2.5. Qualitative and Quantitative Analysis of Polyphenols by LC-DAD-ESI-MS
2.6. Antioxidant Activity
2.6.1. 2,2-Diphenyl-1-picrylhydrazyl (DPPH) Assay
2.6.2. Trolox Equivalent Antioxidant Capacity (TEAC) Assay
2.6.3. Ferric-Reducing Antioxidant Power (FRAP) Assay
2.6.4. Oxygen Radical Absorbance Capacity (ORAC) Assay
2.7. Antimicrobial Activity
2.8. Antiviral Potential
2.8.1. Cells Culture and Virus
2.8.2. Cell Proliferation Assay
2.8.3. Plaque Reduction Assay
2.9. Prebiotic Properties
2.10. Statistical Analysis
3. Results
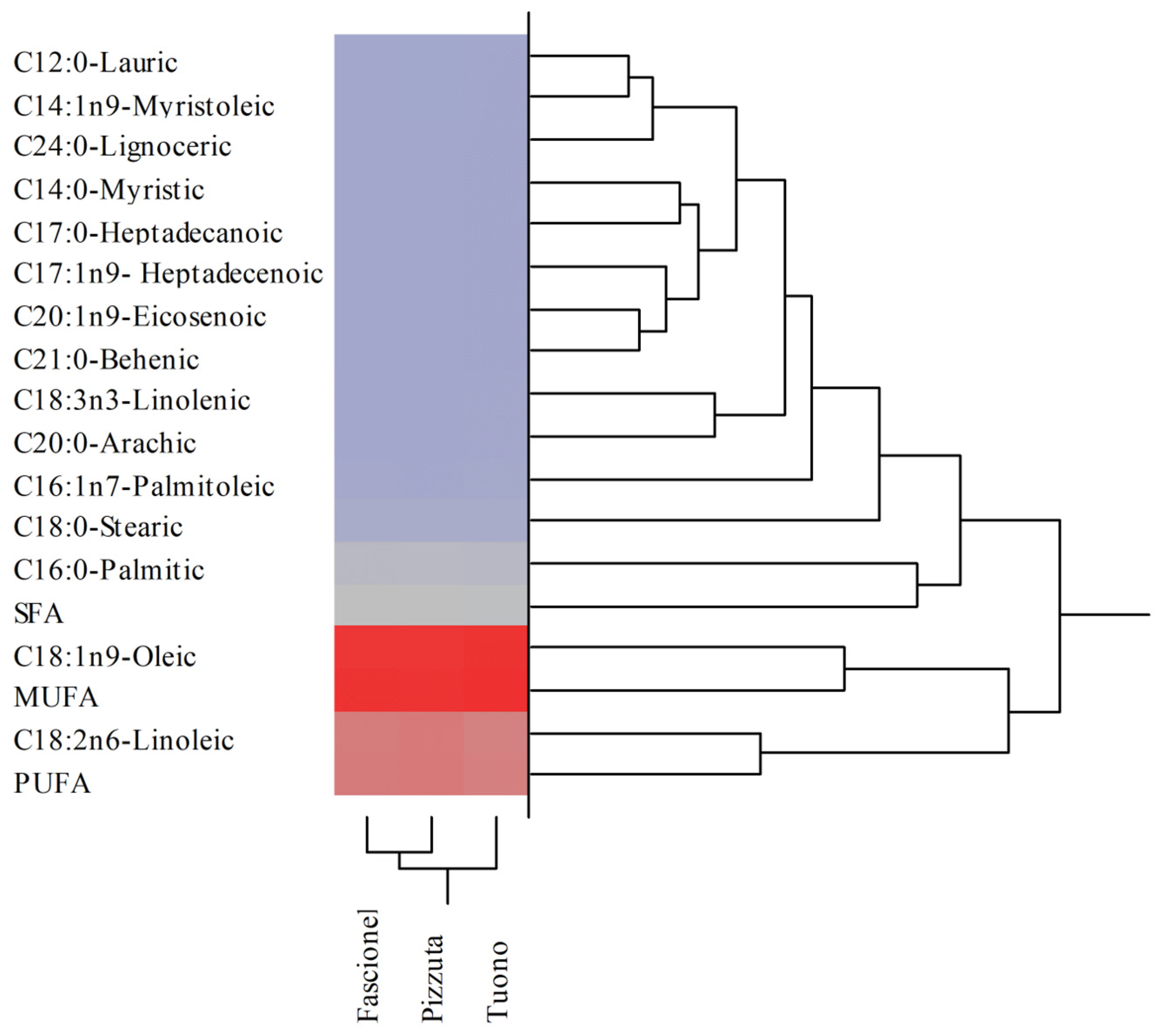
3.1. Nutritional Properties and Fatty Acids Profiles of BS Samples
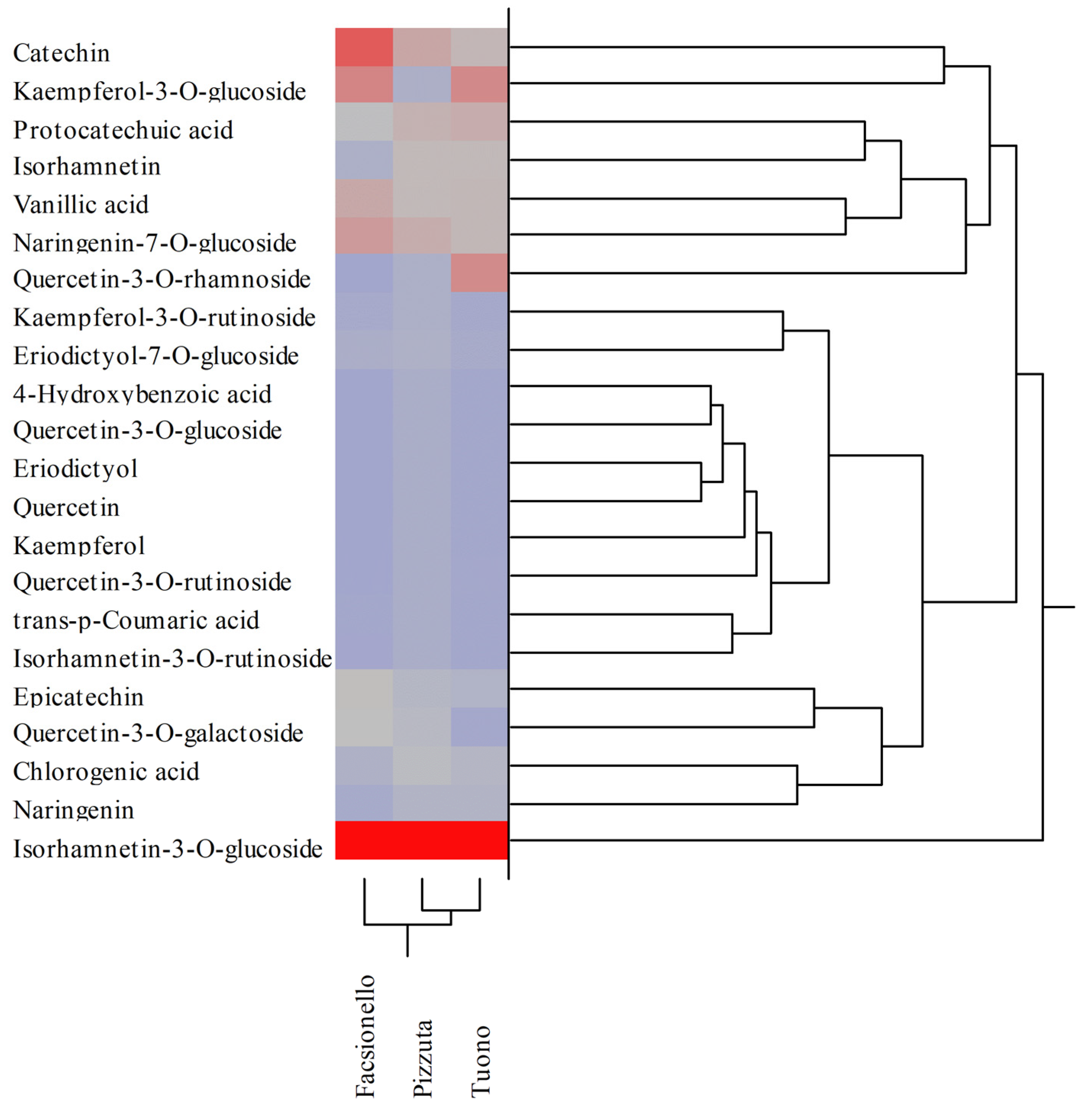
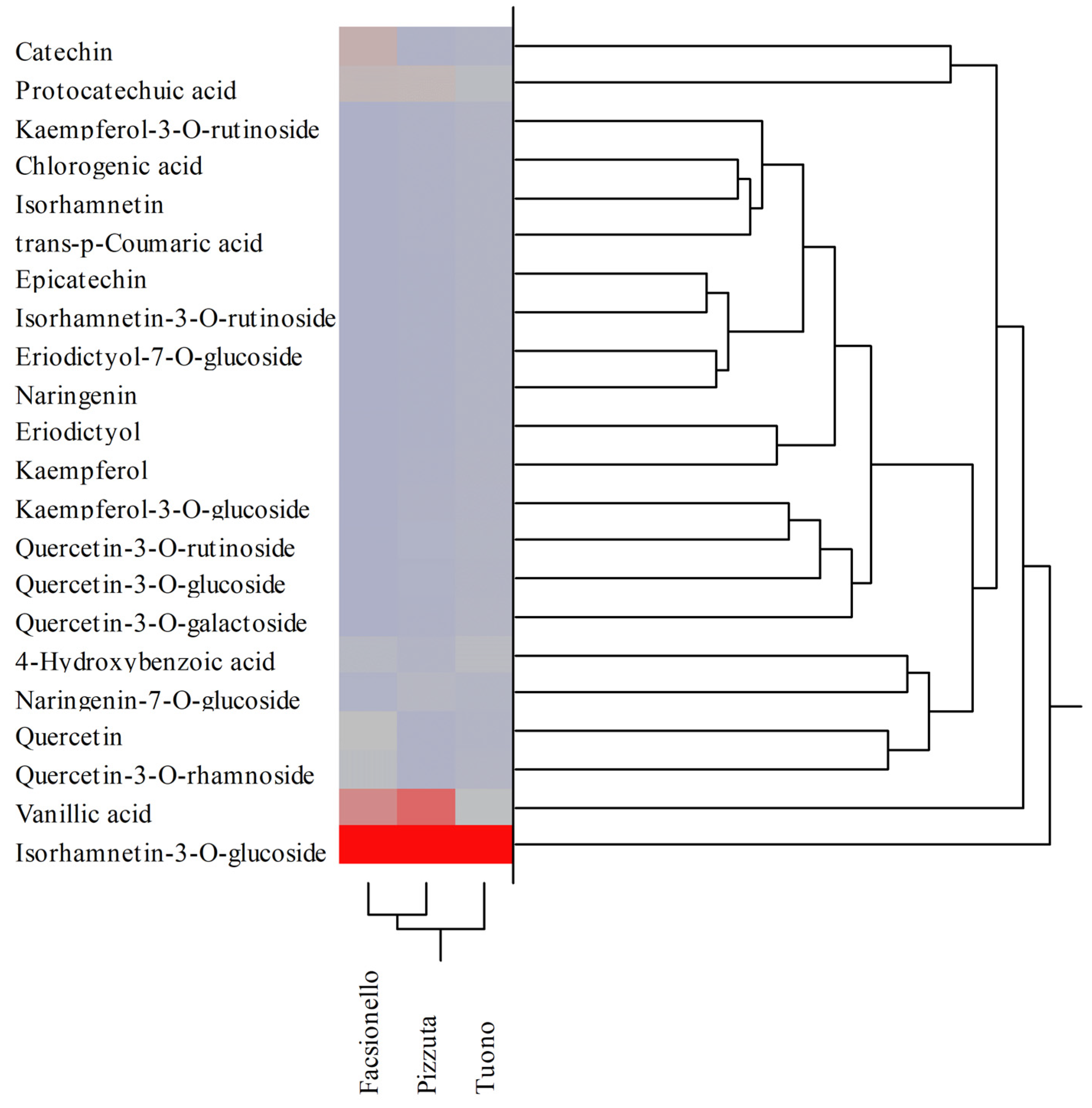
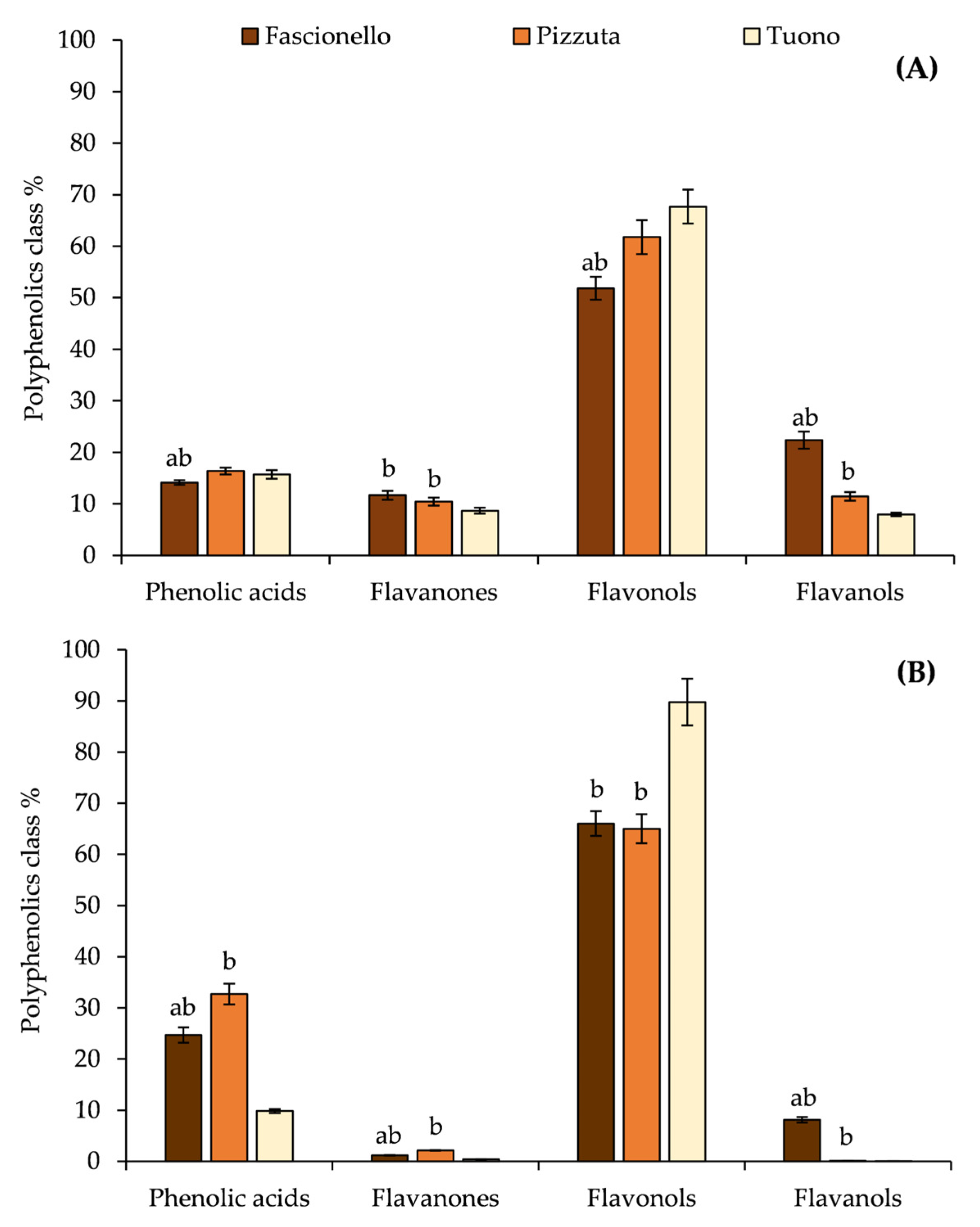
3.2. Phytochemical Analyses of BSE and BWE
3.3. Antioxidant and Free-Radical Scavenging Activity of BSE and BWE
3.4. Antimicrobial and Antiviral Activity of BSE
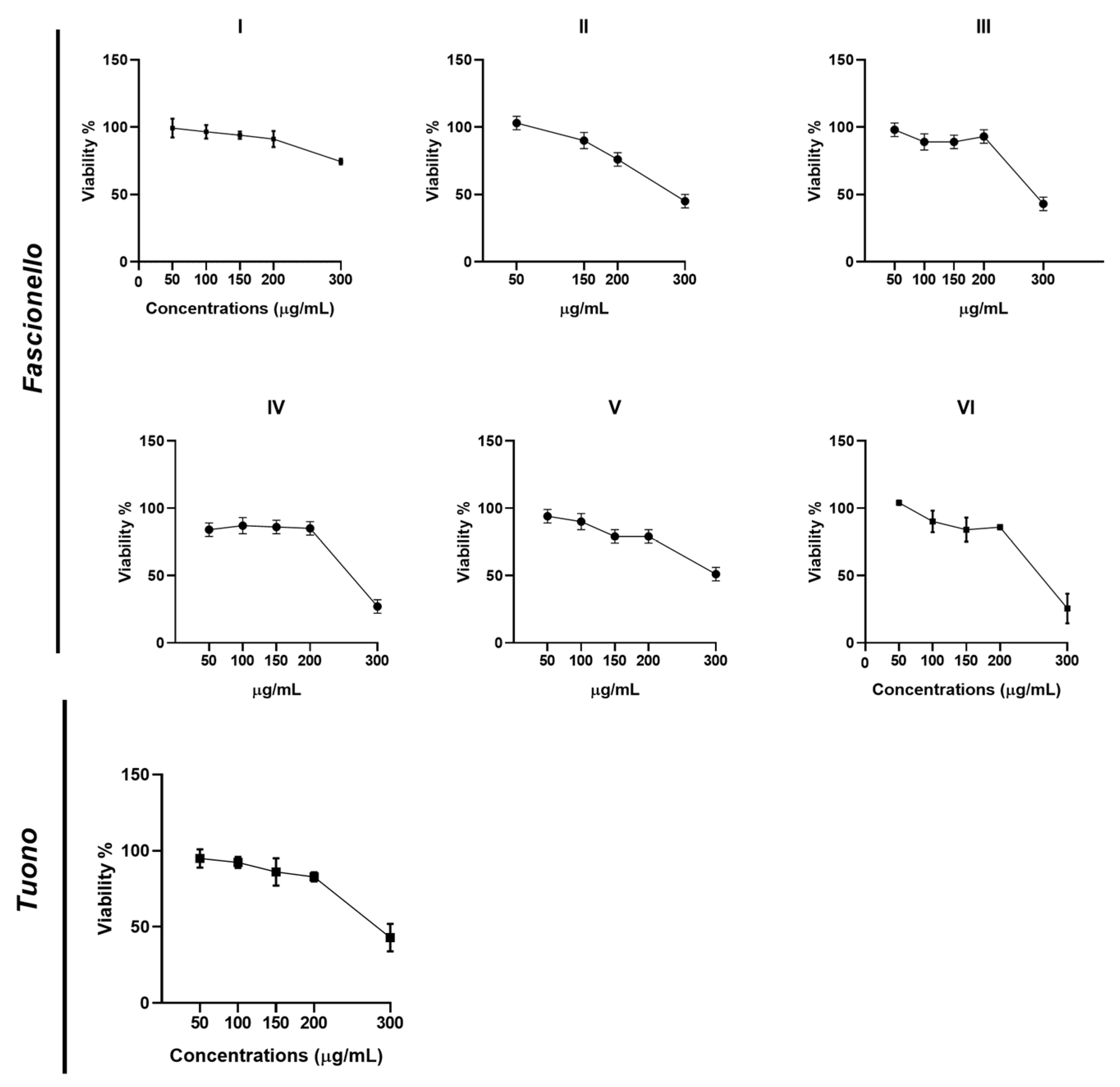
3.4.1. Cell Viability Assay
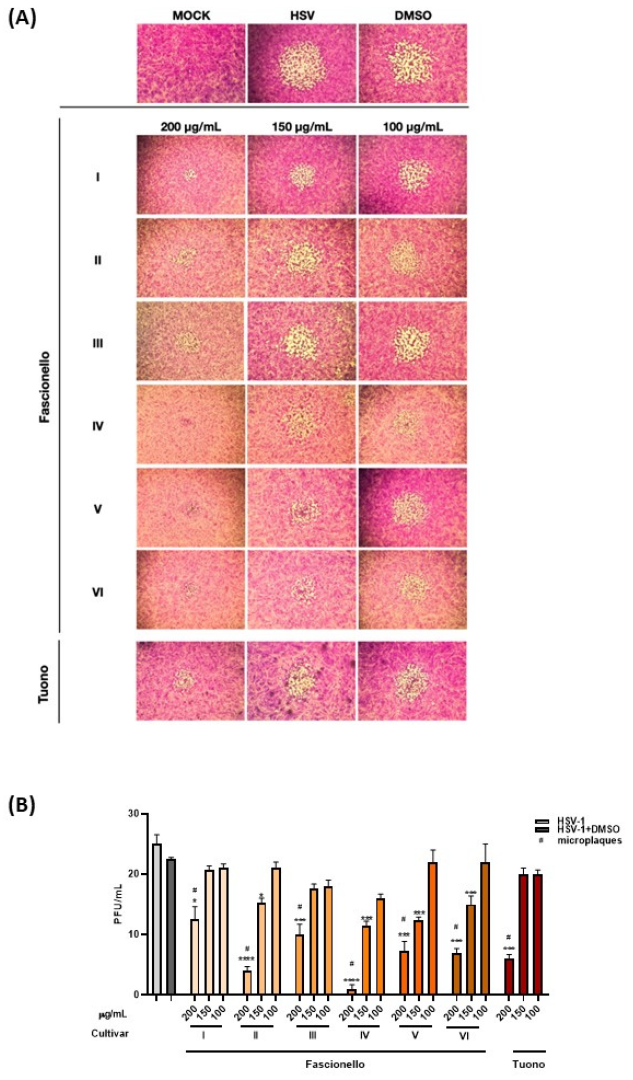
3.4.2. Antiviral Activity
3.5. Prebiotic Effects of BS Samples
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prgomet, I.; Gonçalves, B.; Domínguez-Perles, R.; Pascual-Seva, N.; Barros, A.I. Valorization Challenges to Almond Residues: Phytochemical Composition and Functional Application. Molecules 2017, 22, 1774. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Perez, P.; Xiao, J.; Munekata, P.E.S.; Lorenzo, J.M.; Barba, F.J.; Rajoka, M.S.R.; Barros, L.; Mascoloti Sprea, R.; Amaral, J.S.; Prieto, M.A.; et al. Revalorization of Almond By-Products for the Design of Novel Functional Foods: An Updated Review. Foods 2021, 10, 1823. [Google Scholar] [CrossRef] [PubMed]
- Caltagirone, C.; Peano, C.; Sottile, F. Post-harvest Industrial Processes of Almond (Prunus dulcis L. Mill) in Sicily Influence the Nutraceutical Properties of By-Products at Harvest and During Storage. Front. Nutr. 2021, 8, 659378. [Google Scholar] [CrossRef]
- Barreca, D.; Nabavi, S.M.; Sureda, A.; Rasekhian, M.; Raciti, R.; Silva, A.S.; Annunziata, G.; Arnone, A.; Tenore, G.C.; Süntar, İ.; et al. Almonds (Prunus Dulcis Mill. D. A. Webb): A Source of Nutrients and Health-Promoting Compounds. Nutrients 2020, 12, 672. [Google Scholar] [CrossRef] [PubMed]
- Smeriglio, A.; Mandalari, G.; Bisignano, C.; Filocamo, A.; Barreca, D.; Bellocco, E.; Trombetta, D. Polyphenolic content and biological properties of Avola almond (Prunus dulcis Mill. D.A. Webb) skin and its industrial byproducts. Ind. Crops Prod. 2016, 83, 283–293. [Google Scholar] [CrossRef]
- Sottile, F.; Massaglia, S.; Peano, C. Ecological and Economic Indicators for the Evaluation of Almond (Prunus dulcis L.) Orchard Renewal in Sicily. Agriculture 2020, 10, 301. [Google Scholar] [CrossRef]
- Barral-Martinez, M.; Fraga-Corral, M.; Garcia-Perez, P.; Simal-Gandara, J.; Prieto, M.A. Almond By-Products: Valorization for Sustainability and Competitiveness of the Industry. Foods 2021, 10, 1793. [Google Scholar] [CrossRef]
- Mandalari, G.; Bisignano, C.; D’Arrigo, M.; Ginestra, G.; Arena, A.; Tomaino, A.; Wickham, M.S. Antimicrobial potential of polyphenols extracted from almond skins. Lett. Appl. Microbiol. 2010, 51, 83–89. [Google Scholar] [CrossRef]
- Bottone, A.; Montoro, P.; Masullo, M.; Pizza, C.; Piacente, S. Metabolite profiling and antioxidant activity of the polar fraction of Italian almonds (Toritto and Avola): Analysis of seeds, skins, and blanching water. J. Pharm. Biomed. Anal. 2020, 190, 113518. [Google Scholar] [CrossRef]
- Bolling, B.W. Almond Polyphenols: Methods of Analysis, Contribution to Food Quality, and Health Promotion. Compr. Rev. Food Sci. Food Saf. 2017, 16, 346–368. [Google Scholar] [CrossRef]
- Savoia, M.A.; Del Faro, L.; Venerito, P.; Gaeta, L.; Palasciano, M.; Montemurro, C.; Sabetta, W. The Relevance of Discovering and Recovering the Biodiversity of Apulian Almond Germplasm by Means of Molecular and Phenotypic Markers. Plants 2022, 11, 574. [Google Scholar] [CrossRef]
- Consorzio Mandorla di Avola. Available online: https://consorziomandorlaavola.it/ (accessed on 13 December 2022).
- Mandalari, G.; Arcoraci, T.; Martorana, M.; Bisignano, C.; Rizza, L.; Bonina, F.P.; Trombetta, D.; Tomaino, A. Antioxidant and photoprotective effects of blanch water, a byproduct of the almond processing industry. Molecules 2013, 18, 12426–12440. [Google Scholar] [CrossRef]
- Mandalari, G.; Faulks, R.M.; Bisignano, C.; Waldron, K.W.; Narbad, A.; Wickham, M.S. In vitro evaluation of the prebiotic properties of almond skins (Amygdalus communis L.). FEMS Microbiol. Lett. 2010, 304, 116–122. [Google Scholar] [CrossRef]
- Mandalari, G.; Tomaino, A.; Arcoraci, T.; Martorana, M.; Lo Turco, V.; Cacciola, F.; Rich, G.T.; Bisignano, C.; Saija, A.; Dugo, P.; et al. Characterization of polyphenols, lipids and dietary fibre from almond skins (Amygdalus communis L.). J. Food Comp. Anal. 2010, 23, 166–174. [Google Scholar] [CrossRef]
- Mandalari, G.; Vardakou, M.; Faulks, R.; Bisignano, C.; Martorana, M.; Smeriglio, A.; Trombetta, D. Food Matrix Effects of Polyphenol Bioaccessibility from Almond Skin during Simulated Human Digestion. Nutrients 2016, 8, 568. [Google Scholar] [CrossRef]
- Bisignano, C.; Mandalari, G.; Smeriglio, A.; Trombetta, D.; Pizzo, M.M.; Pennisi, R.; Sciortino, M.T. Almond Skin Extracts Abrogate HSV-1 Replication by Blocking Virus Binding to the Cell. Viruses 2017, 9, 178. [Google Scholar] [CrossRef] [PubMed]
- Musarra-Pizzo, M.; Ginestra, G.; Smeriglio, A.; Pennisi, R.; Sciortino, M.T.; Mandalari, G. The Antimicrobial and Antiviral Activity of Polyphenols from Almond (Prunus dulcis L.) Skin. Nutrients 2019, 11, 2355. [Google Scholar] [CrossRef]
- ISTISAN 1996/34. Available online: https://www.iss.it/Rapp_ISTISAN_96_34_def.pdf (accessed on 7 January 2022).
- Occhiuto, C.; Aliberto, G.; Ingegneri, M.; Trombetta, D.; Circosta, C.; Smeriglio, A. Comparative Evaluation of the Nutrients, Phytochemicals, and Antioxidant Activity of Two Hempseed Oils and Their Byproducts after Cold Pressing. Molecules 2022, 27, 3431. [Google Scholar] [CrossRef]
- Trombetta, D.; Cimino, F.; Cristani, M.; Mandalari, G.; Saija, A.; Ginestra, G.; Speciale, A.; Chirafisi, J.; Bisignano, G.; Waldron, K.; et al. In vitro protective effects of two extracts from bergamot peels on human endothelial cells exposed to tumor necrosis factor-alpha (TNF-alpha). J. Agric. Food Chem. 2010, 58, 8430–8436. [Google Scholar] [CrossRef]
- Lenucci, M.S.; Cadinu, D.; Taurino, M.; Piro, G.; Dalessandro, G. Antioxidant composition in cherry and high-pigment tomato cultivars. J. Agric. Food Chem. 2006, 54, 2606–2613. [Google Scholar] [CrossRef]
- Smeriglio, A.; Denaro, M.; D’Angelo, V.; Germanò, M.P.; Trombetta, D. Antioxidant, Anti-Inflammatory and Anti-Angiogenic Properties of Citrus lumia Juice. Front. Pharmacol. 2020, 11, 593506. [Google Scholar] [CrossRef] [PubMed]
- Smeriglio, A.; Bonasera, S.; Germanò, M.P.; D’Angelo, V.; Barreca, D.; Denaro, M.; Monforte, M.T.; Galati, E.M.; Trombetta, D. Opuntia ficus-indica (L.) Mill. fruit as source of betalains with antioxidant, cytoprotective, and anti-angiogenic properties. Phytother. Res. 2019, 33, 1526–1537. [Google Scholar] [CrossRef]
- Bazzicalupo, M.; Burlando, B.; Denaro, M.; Barreca, D.; Trombetta, D.; Smeriglio, A.; Cornara, L. Polyphenol Characterization and Skin-Preserving Properties of Hydroalcoholic Flower Extract from Himantoglossum robertianum (Orchidaceae). Plants 2019, 8, 502. [Google Scholar] [CrossRef] [PubMed]
- Cornara, L.; Sgrò, F.; Raimondo, F.M.; Ingegneri, M.; Mastracci, L.; D’Angelo, V.; Germanò, M.P.; Trombetta, D.; Smeriglio, A. Pedoclimatic Conditions Influence the Morphological, Phytochemical and Biological Features of Mentha pulegium L. Plants 2023, 12, 24. [Google Scholar] [CrossRef] [PubMed]
- CLSI M100-S22; Institute Performance Standards for Antimicrobial Susceptibility Testing. Twentieth Informational Supplement. Clinical and Laboratory Standards Institute (CLSI): Wayne, PA, USA, 2012.
- CLSI M27-A3; Clinical and Laboratory Standards Institute Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts, Approved Standard. Clinical and laboratory Standards Institute (CLSI): Wayne, PA, USA, 2008.
- Herigstad, B.; Hamilton, M.; Heersink, J. How to optimize the drop plate method for enumerating bacteria. J. Microbiol. Methods 2001, 44, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Swanson, K.L.; Bill, H.M.; Asmus, J.; Heguy, J.M.; DePeters, E.J. Feeding high amounts of almond hulls to lactating cows. J. Dairy Sci. 2021, 104, 8846–8856. [Google Scholar] [CrossRef] [PubMed]
- Kaur, M.; Kumar, M.; Sachdeva, S.; Puri, S.K. An efficient multiphase bioprocess for enhancing the renewable energy production from almond shells. Energy Convers. Manag. 2020, 203, 112235. [Google Scholar] [CrossRef]
- Rahimian, R.; Zarinabadi, S. A review of Studies on the Removal of Methylene Blue Dye from Industrial Wastewater UsingActivated Carbon Adsorbents Made from Almond Bark. Prog. Chem. Biochem. Res. J. 2020, 3, 251–268. [Google Scholar]
- Varzakas, T.; Zakynthinos, G.; Verpoort, F. Plant food residues as a source of nutraceuticals and functional foods. Foods 2016, 5, 88. [Google Scholar] [CrossRef]
- Wijeratne, S.S.K.; Abou-Zaid, M.M.; Shahidi, F. Antioxidants polyphenols in almond and its coproducts. J. Agric. Food Chem. 2006, 54, 312–318. [Google Scholar] [CrossRef]
- Sang, S.; Lapsley, K.; Jeong, W.S.; Lachance, P.A.; Ho, C.T.; Rosen, R.T. Antioxidant phenolic compounds isolated from almond skins (Prunus amygdalus Batsch). J. Agric. Food Chem. 2002, 50, 2459–2463. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Milbury, P.E.; Lapsley, K.; Blumberg, J.B. Flavonoids from almond skins are bioavailable and act synergistically with vitamins C and E to enhance hamster and human LDL resistance to oxidation. J. Nutr. 2005, 135, 1366–1373. [Google Scholar] [CrossRef] [PubMed]
- Monagas, M.; Garrido, I.; Lebron-Aguilar, R.; Bartolome, B.; Gomez-Cordoves, C. Almond (Prunus dulcis [Mill.] D.A. Webb) skins as a potential source of bioactive polyphenols. J. Agric. Food Chem. 2007, 55, 8498–8507. [Google Scholar] [CrossRef] [PubMed]
- Bolling, B.W.; McKay, D.; Blumberg, J. The phytochemical composition and antioxidant actions of tree nuts. Asia Pac. J. Clin. Nutr. 2010, 19, 117. [Google Scholar]
- Chen, C.Y.O.; Holbrook, M.; Duess, M.A.; Dohadwala, M.M.; Hamburg, N.M.; Asztalos, B.F.; Milbury, P.E.; Blumberg, J.B.; Vita, J.A. Effect of almond consumption on vascular function in patients with coronary artery disease: A randomized, controlled, cross-over trial. Nutr. J. 2015, 14, 61. [Google Scholar] [CrossRef]
- Li, Z.; Bhagavathula, A.; Batavia, M.; Clark, C.; Abdulazeem, H.M.; Rahmani, J.; Yin, F. The effect of almonds consumption on blood pressure: A systematic review and dose-response meta-analysis of randomized control trials. J. King Saud. Univ. Sci. 2020, 2, 1757–1763. [Google Scholar] [CrossRef]
- Hughey, C.A.; Janusziewicz, R.; Minardi, C.S.; Phung, J.; Huffman, B.A.; Reyes, L.; Wilcox, B.E.; Prakash, A. Distribution of almond polyphenols in blanch water and skins as a function of blanching time and temperature. Food Chem. 2012, 131, 1165–1173. [Google Scholar] [CrossRef]
- Garrido, I.; Monagas, M.; Gómez-Cordovés, C.; Bartolomé, B. Polyphenols and antioxidant properties of almond skins: Influence of industrial processing. J. Food Sci. 2008, 73, C106–C115. [Google Scholar] [CrossRef]
- Bartolomé, B.; Monagas, M.; Garrido, I.; Gómez-Cordovés, C.; Martín-Alvarez, P.J.; Lebrón-Aguilar, R.; Urpí-Sardà, M.; Llorach, R.; Andrés-Lacueva, C. Almond (Prunus dulcis (Mill.) D.A. Webb) polyphenols: From chemical characterization to targeted analysis of phenolic metabolites in humans. Arch Biochem. Biophys. 2010, 501, 124–133. [Google Scholar] [CrossRef]
- Yu, J.; Ahmedna, M.; Goktepe, I.; Dai, J. Peanut skin procyanidins: Composition and antioxidant activities as affected by processing. J. Food Comp. Anal. 2006, 19, 364–371. [Google Scholar] [CrossRef]
- Rohn, S.; Buchner, N.; Driemel, G.; Rauser, M.; Kroh, L.W. Thermal degradation of onion quercetin glucosides under roasting conditions. J. Agric. Food Chem. 2007, 55, 1568–1573. [Google Scholar] [CrossRef]
- Buchner, N.; Krumbein, A.; Rohn, S.; Kroh, L.W. Effect of thermal processing on the flavonols rutin and quercetin. Rapid Commun. Mass Spectrom 2006, 20, 3229–3235. [Google Scholar] [CrossRef]
- Milbury, P.E.; Chen, C.Y.; Dolnikowski, G.G.; Blumberg, J.B. Determination of flavonoids and phenolics and their distribution in almonds. J. Agric. Food Chem. 2006, 54, 5027–5033. [Google Scholar] [CrossRef]
- Harrison, K.; Were, L.M. Effect of gamma irradiation on total phenolic content yield and antioxidant capacity of almond skin extracts. Food Chem. 2007, 102, 932–937. [Google Scholar] [CrossRef]
- Chen, C.O.; Milbury, P.E.; Blumberg, J.B. Polyphenols in Almond Skins after Blanching Modulate Plasma Biomarkers of Oxidative Stress in Healthy Humans. Antioxidants 2019, 8, 95. [Google Scholar] [CrossRef]
- Liu, Z.; Lin, X.; Huang, G.; Zhang, W.; Rao, P.; Ni, L. Prebiotic effects of almonds and almond skins on intestinal microbiota in healthy adult humans. Anaerobe 2014, 26, 1–6. [Google Scholar] [CrossRef]
- Parkar, S.G.; Stevenson, D.E.; Skinner, M.A. The potential influence of fruit polyphenols on colonic microbiota and human gut health. Int. J. Food Microbiol. 2008, 124, 295–298. [Google Scholar] [CrossRef]
- Brigidi, P.; Vitali, B.; Swennen, E.; Bazzocchi, G.; Matteuzzi, D. Effects of probiotic administration upon the composition and enzymatic activity of human fecal microbiota in patients with irritable bowel syndrome or functional diarrhea. Res. Microbiol. 2001, 152, 735–741. [Google Scholar] [CrossRef]
- El-Aguel, A.; Pennisi, R.; Smeriglio, A.; Kallel, I.; Tamburello, M.P.; D’Arrigo, M.; Barreca, D.; Gargouri, A.; Trombetta, D.; Mandalari, G.; et al. Punica granatum Peel and Leaf Extracts as Promising Strategies for HSV-1 Treatment. Viruses 2022, 14, 2639. [Google Scholar] [CrossRef]
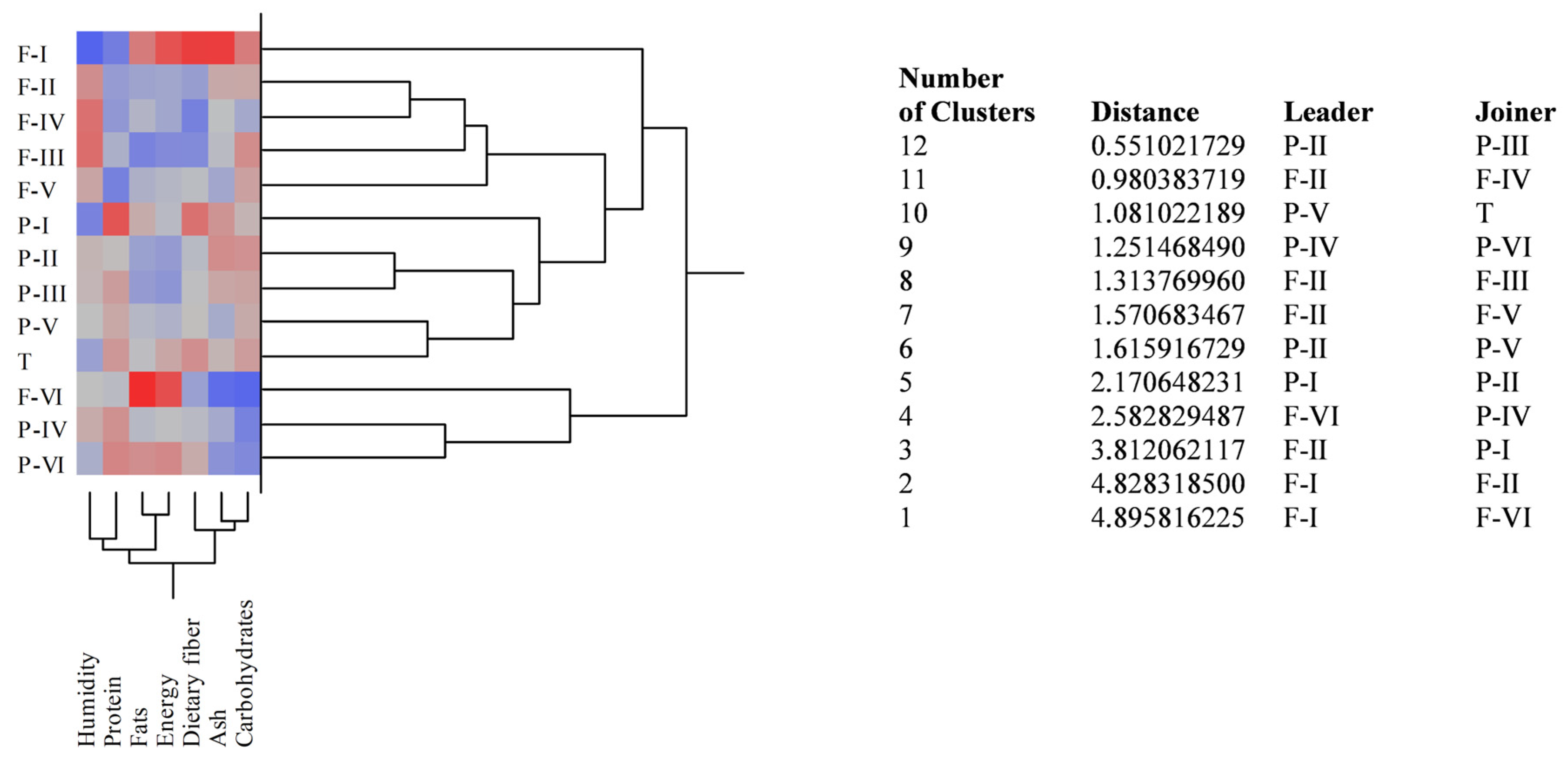
| Cultivar | Moisture | Ash | Fats | Proteins | Fibres | Sugars | Energy | |
|---|---|---|---|---|---|---|---|---|
| g/100 g | Kcal | KJ | ||||||
| Fascionello | ||||||||
| I | 6.43 ± 0.12 aA | 3.41 ± 0.06 aA | 13.37 ± 0.67 aA | 10.99 ± 0.42 aD | 60.25 ± 1.22 A | 5.55 ± 0.08 aJ | 307 ± 6.52 aL | 1258 ± 12.44 aL |
| II | 16.55 ± 0.85 aB | 2.64 ± 0.02 aF | 10.63 ± 0.28 G | 11.37 ± 0.21 | 53.65 ± 1.27 | 5.16 ± 0.12 B | 269 ± 2.88 M | 1104 ± 10.67 G |
| III | 18.39 ± 0.34 aC | 2.42 ± 0.03 aC | 9.5 ± 0.12 aE | 11.61 ± 0.19 I | 52.67 ± 1.28 aI | 5.41 ± 0.06 aK | 259 ± 1.95 E | 1062 ± 8.67 E |
| IV | 18.22 ± 0.58 aC | 2.46 ± 0.02 aC | 11.22 ± 0.41 D | 11.31 ± 0.26 a | 52.15 ± 1.35 aI | 4.64 ± 0.04 aC | 269 ± 2.43 aC | 1103 ± 12.66 aD |
| V | 15.24 ± 0.67 aD | 2.21 ± 0.03 D | 11.07 ± 0.23 D | 11.03 ± 0.22 aD | 55.23 ± 1.17 | 5.22 ± 0.14 D | 275 ± 2.66 D | 1128 ± 14.62 D |
| VI | 13.67 ± 0.24 a | 1.63 ± 0.01 a | 15.35 ± 0.37 a | 11.76 ± 0.18 | 53.77 ± 2.05 | 3.82 ± 0.03 a | 308 ± 3.54 a | 1263 ± 12.08 a |
| Pizzuta | ||||||||
| I | 8.88 ± 0.06 A | 2.78 ± 0.04 E | 12.17 ± 0.42 H | 12.78 ± 0.25 J | 58.34 ± 1.42 H | 5.05 ± 0.22 K | 276 ± 2.67 N | 1132 ± 13.65 N |
| II | 14.33 ± 0.22 E | 2.84 ± 0.05 F | 10.55 ± 0.25 E | 11.85 ± 0.27 D | 55.07 ± 1.08 | 5.36 ± 0.14 K | 265 ± 2.38 E | 1087 ± 7.56 E |
| III | 14.28 ± 0.12 E | 2.65 ± 0.04 E | 10.35 ± 0.46 E | 12.14 ± 0.32 | 55.37 ± 1.32 | 5.21 ± 0.07 K | 263 ± 4.42 E | 1078 ± 6.88 E |
| IV | 14.93 ± 0.08 C | 2.24 ± 0.07 C | 11.37 ± 0.38 D | 12.24 ± 0.11 | 55.08 ± 1.88 | 4.14 ± 0.04 | 278 ± 3.62 D | 1140 ± 10.05 D |
| V | 13.69 ± 0.04 D | 2.26 ± 0.04 D | 11.35 ± 0.18 D | 12.04 ± 0.38 | 55.53 ± 1.57 | 5.13 ± 0.18 K | 273 ± 4.08 D | 1120 ± 12.22 D |
| VI | 12.27 ± 0.33 | 2.01 ± 0.08 | 12.88 ± 0.24 | 12.35 ± 0.08 | 56.25 ± 1.62 | 4.24 ± 0.21 | 293 ± 2.44 | 1201 ± 8.48 |
| Tuono | 11.16 ± 0.21 | 2.55 ± 0.06 | 11.56 ± 0.36 | 12.18 ± 0.10 | 57.28 ± 1.29 | 5.27 ± 0.08 | 285 ± 1.82 | 1169 ± 6.54 |
| Fatty Acids | Fascionello | Pizzuta | Tuono |
|---|---|---|---|
| C12:0-Lauric | 0.06 ± 0.00 a | 0.04 ± 0.00 | 0.06 ± 0.00 |
| C14:0-Myristic | 0.09 ± 0.00 b | 0.10 ± 0.01 | 0.03 ± 0.00 |
| C14:1n9-Myristoleic | 0.05 ± 0.00 | 0.05 ± 0.00 | 0.05 ± 0.00 |
| C16:0-Palmitic | 8.38 ± 0.18 | 8.33 ± 0.42 | 7.92 ± 0.24 |
| C16:1n7-Palmitoleic | 0.77 ± 0.02 b | 0.81 ± 0.03 b | 0.68 ± 0.03 |
| C17:0-Heptadecanoic | 0.11 ± 0.00 ab | 0.15 ± 0.01 b | 0.07 ± 0.00 |
| C17:1n9- Heptadecenoic | 0.10 ± 0.00 a | 0.13 ± 0.01 | 0.12 ± 0.01 |
| C18:0-Stearic | 2.38 ± 0.12 | 2.39 ± 0.12 | 2.18 ± 0.12 |
| C18:1n9-Oleic | 54.79 ± 1.24 | 54.14 ± 2.78 | 56.69 ± 3.08 |
| C18:2n6-Linoleic | 32.56 ± 2.08 | 33.05 ± 1.49 | 31.36 ± 1.52 |
| C18:3n3-Linolenic | 0.31 ± 0.02 | 0.33 ± 0.02 b | 0.27 ± 0.02 |
| C20:0-Arachic | 0.22 ± 0.01 | 0.24 ± 0.01 | 0.25 ± 0.01 |
| C20:1n9-Eicosenoic | 0.07 ± 0.00 ab | 0.11 ± 0.00 | 0.12 ± 0.01 |
| C21:0-Behenic | 0.08 ± 0.00 b | 0.09 ± 0.00 b | 0.12 ± 0.01 |
| C24:0-Lignoceric | 0.06 ± 0.00 b | 0.07 ± 0.00 | 0.08 ± 0.00 |
| SFA | 11.36 | 11.39 | 10.71 |
| MUFA | 55.77 | 55.24 | 57.66 |
| PUFA | 32.87 | 33.38 | 31.63 |
| Cultivar | Blanched Skin | Blanch Water | ||
|---|---|---|---|---|
| TPC (g GAE/100 g DE) | TFC (g RE/100 g DE) | TPC (g GAE/100 g DE) | TFC (g RE/100 g DE) | |
| Fascionello | ||||
| I | 5.07 ± 0.36 aA | 1.20 ± 0.03 aN | 2.08 ± 0.68 aG | 0.77 ± 0.03 aA |
| II | 3.97 ± 0.09 aE | 0.95 ± 0.01 aO | 2.20 ± 0.13 aO | 0.40 ± 0.01 O |
| III | 3.88 ± 0.29 aE | 0.84 ± 0.01 K | 1.18 ± 0.03 aK | 0.23 ± 0.01 aQ |
| IV | 2.93 ± 0.02 aC | 0.89 ± 0.02 D | 1.69 ± 0.08 aC | 0.56 ± 0.03 aC |
| V | 2.48 ± 0.20 aD | 0.86 ± 0.02 aD | 1.30 ± 0.10 aD | 0.29 ± 0.01 aD |
| VI | 2.24 ± 0.07 a | 0.70 ± 0.01 a | 0.56 ± 0.01 a | 0.22 ± 0.02 a |
| Pizzuta | ||||
| I | 4.39 ± 0.18 A | 0.88 ± 0.03 P | 3.36 ± 0.04 O | 0.60 ± 0.01 A |
| II | 3.65 ± 0.13 O | 0.78 ± 0.03 D | 3.26 ± 0.10 O | 0.35 ± 0.03 Q |
| III | 7.07 ± 0.05 E | 0.81 ± 0.04 C | 2.28 ± 0.08 E | 0.37 ± 0.01 E |
| IV | 3.85 ± 0.01 C | 0.88 ± 0.07 C | 1.37 ± 0.05 C | 0.23 ± 0.02 D |
| V | 3.25 ± 0.09 D | 0.70 ± 0.06 D | 1.70 ± 0.06 D | 0.20 ± 0.01 D |
| VI | 1.72 ± 0.04 | 0.52 ± 0.02 | 1.55 ± 0.06 | 0.31 ± 0.01 |
| Tuono | 2.76 ± 0.08 | 0.91 ± 0.01 | 1.00 ± 0.09 | 0.18 ± 0.00 |
| Polyphenols | RT (min) | λmax (nm) | [M-H]− | Blanched Skin | Blanch Water | ||||
|---|---|---|---|---|---|---|---|---|---|
| Fascionello | Pizzuta | Tuono | Fascionello | Pizzuta | Tuono | ||||
| Hydroxybenzoic acids | |||||||||
| Protocatechuic acid | 7.04 | 258;293 | 137 | 9.37 ± 0.12 ab | 16.69 ± 0.72 b | 11.98 ± 0.58 | 3.03 ± 0.17 b | 2.91 ± 0.10 b | 1.38 ± 0.08 |
| 4-Hydroxybenzoic acid | 12.00 | 253 | 153 | 0.04 ± 0.00 ab | 0.09 ± 0.00 b | 0.01 ± 0.00 | 1.23 ± 0.03 b | 0.44 ± 0.02 b | 1.31 ± 0.06 |
| Vanillic acid | 16.00 | 262;291 | 167 | 16.50 ± 0.28 ab | 13.46 ± 0.65 b | 9.58 ± 0.48 | 7.70 ± 0.31 ab | 11.67 ± 0.72 b | 1.98 ± 0.05 |
| Hydroxycinnamic acids | |||||||||
| Chlorogenic acid | 20.50 | 291;319 | 353 | 4.38 ± 0.08 a | 8.66 ± 0.44 b | 4.53 ± 0.21 | 0.03 ± 0.00 ab | 0.03 ± 0.00 b | 0.01 ± 0.00 |
| trans-p-Cumaric acid | 22.80 | 309 | 163 | 0.55 ± 0.01 ab | n.d. | 0.01 ± 0.00 | n.d. | 0.07 ± 0.00 | n.d. |
| Flavanones | |||||||||
| Eriodictyol-7-O-glucoside | 29.71 | 283 | 449 | 3.20 ± 0.10 ab | 2.03 ± 0.05 b | 1.08 ± 0.02 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 |
| Naringenin-7-O-glucoside | 32.43 | 282 | 433 | 20.59 ± 0.34 ab | 18.88 ± 0.67 b | 9.41 ± 0.23 | 0.46 ± 0.02 ab | 0.97 ± 0.03 b | 0.11 ± 0.01 |
| Eriodictyol | 35.66 | 287 | 287 | 0.01 ± 0.00 | 0.01 ± 0.00 | n.d. | 0.09 ± 0.00 ab | 0.01 ± 0.00 | 0.02 ± 0.00 |
| Naringenin | 40.26 | 289 | 271 | 1.66 ± 0.02 ab | 3.91 ± 0.17 | 3.94 ± 0.11 | 0.01 ± 0.00 b | 0.01 ± 0.00 b | 0.04 ± 0.00 |
| Flavonols | |||||||||
| Quercetin-3-O-galactoside | 32.36 | 253;354 | 463 | 10.10 ± 0.35 ab | 6.98 ± 0.24 b | 0.07 ± 0.00 | 0.02 ± 0.00 ab | 0.07 ± 0.00 b | 0.28 ± 0.02 |
| Quercetin-3-O-rutinoside | 32.41 | 254;354 | 609 | 0.11 ± 0.01 a | 0.04 ± 0.00 b | 0.12 ± 0.00 | 0.05 ± 0.00 ab | 0.19 ± 0.01 b | 0.13 ± 0.01 |
| Quercetin-3-O-glucoside | 32.64 | 254;354 | 463 | 0.02 ± 0.00 a | 0.06 ± 0.00 b | 0.02 ± 0.00 | 0.06 ± 0.00 a | 0.13 ± 0.01 b | 0.07 ± 0.00 |
| Kaempferol-3-O-rutinoside | 33.96 | 265;348 | 593 | 1.50 ± 0.02 ab | 1.18 ± 0.03 b | 0.40 ± 0.02 | 0.02 ± 0.00 ab | 0.07 ± 0.00 | 0.07 ± 0.00 |
| Kaempferol-3-O-glucoside | 34.34 | 264;347 | 447 | 26.58 ± 0.37 ab | 1.00 ± 0.02 b | 20.13 ± 0.74 | 0.03 ± 0.00 ab | 0.26 ± 0.02 b | 0.06 ± 0.00 |
| Quercetin-3-O-rhamnoside | 34.36 | 257;358 | 447 | n.d. | 1.22 ± 0.02 b | 19.63 ± 0.86 | 1.82 ± 0.02 ab | 0.12 ± 0.01 b | 0.31 ± 0.02 |
| Isorhamnetin-3-O-rutinoside | 34.63 | 254;354 | 624 | 0.44 ± 0.01 | n.d. | n.d. | n.d. | n.d. | n.d. |
| Isorhamnetin-3-O-glucoside | 34.88 | 254;353 | 477 | 70.60 ± 1.88 ab | 122.94 ± 4.67 b | 63.07 ± 2.36 | 27.91 ± 1.06 b | 29.09 ± 0.55 b | 41.71 ± 2.65 |
| Quercetin | 39.39 | 255;370 | 301 | 0.01 ± 0.00 | 0.01 ± 0.00 | n.d. | 2.10 ± 0.03 ab | 0.02 ± 0.00 | 0.01 ± 0.00 |
| Kaempferol | 43.66 | 264;365 | 285 | 0.11 ± 0.00 | n.d. | n.d. | 0.08 ± 0.00 a | 0.05 ± 0.00 b | 0.07 ± 0.00 |
| Isorhamnetin | 44.47 | 253;368 | 315 | 3.70 ± 0.18 ab | 13.44 ± 0.56 b | 9.14 ± 0.43 | 0.01 ± 0.00 ab | 0.04 ± 0.00 | 0.04 ± 0.00 |
| Flavanols | |||||||||
| Catechin | 18.65 | 279 | 289 | 38.26 ± 1.24 ab | 21.73 ± 0.88 b | 9.69 ± 0.27 | 3.95 ± 0.06 ab | 0.05 ± 0.00 b | 0.01 ± 0.00 |
| Epicatechin | 23.64 | 279 | 289 | 10.60 ± 0.27 ab | 5.46 ± 0.14 b | 3.53 ± 0.12 | n.d. | 0.01 ± 0.00 | n.d. |
| Total polyphenols (mg/100 g DE) | 218.35 | 237.80 | 166.33 | 48.62 | 46.21 | 47.63 | |||
| Cultivar | Blanched Skin | Blanching Water | ||||||
|---|---|---|---|---|---|---|---|---|
| DPPH | FRAP | TEAC | ORAC | DPPH | FRAP | TEAC | ORAC | |
| g TE/100 g DE | g TE/100 g DE | |||||||
| Fascionello | ||||||||
| I | 11.79 ± 0.16 aA | 5.43 ± 0.30 aJ | 15.25 ± 1.11 aA | 34.21 ± 1.48 aA | 7.44 ± 0.68 aR | 3.32 ± 0.07 aJ | 5.57 ± 0.62 aS | 20.81 ± 0.14 aA |
| II | 10.43 ± 0.25 aM | 3.93 ± 0.22 O | 8.45 ± 0.19 G | 28.23 ± 0.69 aJ | 4.33 ± 0.12 aM | 2.38 ± 0.11 aO | 2.23 ± 0.10 aG | 10.48 ± 0.09 aE |
| III | 8.92 ± 0.59 aE | 5.12 ± 0.50 aE | 12.81 ± 1.09 aJ | 27.22 ± 1.15 aJ | 2.65 ± 0.18 aJ | 0.89 ± 0.03 aE | 1.49 ± 0.01 aE | 3.87 ± 0.13 aE |
| IV | 10.55 ± 0.08 aC | 6.01 ± 0.26 aC | 8.93 ± 0.70 D | 22.85 ± 1.40 aC | 7.92 ± 0.36 aC | 3.09 ± 0.16 C | 6.63 ± 0.27 aD | 10.54 ± 0.50 aC |
| V | 7.61 ± 0.17 aD | 3.31 ± 0.32 aD | 7.99 ± 0.52 D | 20.34 ± 0.77 aD | 3.99 ± 0.23 aD | 3.08 ± 0.42 D | 6.05 ± 0.46 aD | 16.22 ± 0.32 aD |
| VI | 3.84 ± 0.02 a | 2.61 ± 0.16 a | 6.65 ± 0.53 a | 18.50 ± 1.02 a | 0.83 ± 0.01 a | 0.92 ± 0.03 a | 1.34 ± 0.13 a | 3.83 ± 0.08 a |
| Pizzuta | ||||||||
| I | 7.48 ± 0.15 O | 6.49 ± 0.57 A | 8.38 ± 0.54 D | 48.16 ± 2.46 O | 5.30 ± 0.30 M | 4.36 ± 0.27 E | 7.94 ± 0.72 D | 28.30 ± 0.93 O |
| II | 7.64 ± 0.36 M | 4.47 ± 0.41 G | 8.51 ± 0.76 D | 49.00 ± 2.61 O | 5.89 ± 0.33 M | 4.05 ± 0.09 G | 7.79 ± 0.96 D | 26.47 ± 2.20 O |
| III | 6.79 ± 0.36 C | 8.43 ± 0.68 E | 8.10 ± 0.22 D | 68.41 ± 4.28 C | 4.84 ± 0.06 C | 4.70 ± 0.33 E | 6.49 ± 0.36 D | 22.65 ± 0.93 C |
| IV | 7.31 ± 0.29 C | 5.10 ± 0.36 C | 8.43 ± 0.39 D | 63.58 ± 2.17 C | 4.61 ± 0.31 C | 3.28 ± 0.33 C | 3.82 ± 0.30 D | 14.75 ± 1.17 C |
| V | 5.75 ± 0.17 D | 4.17 ± 0.12 D | 8.30 ± 0.18 D | 30.79 ± 2.21 | 2.62 ± 0.06 D | 2.68 ± 0.27 D | 3.80 ± 0.40 D | 22.90 ± 1.25 D |
| VI | 3.53 ± 0.06 | 3.56 ± 0.31 | 5.38 ± 0.02 | 26.60 ± 2.61 | 1.30 ± 0.03 | 2.19 ± 0.20 | 1.80 ± 0.10 | 24.19 ± 0.95 |
| Tuono | 6.69 ± 0.34 | 3.07 ± 0.14 | 5.72 ± 0.20 | 67.69 ± 5.83 | 1.26 ± 0.01 | 1.96 ± 0.18 | 1.83 ± 0.12 | 9.55 ± 0.31 |
| Cultivar | CC50 (μg/mL) | EC50 (μg/mL) | SI |
|---|---|---|---|
| Fascionello | |||
| I | 778.57 | 168.54 | 4.6 |
| II | 289.23 | 154.75 | 1.8 |
| III | 290.54 | 165.94 | 1.7 |
| IV | 290.22 | 155.03 | 1.8 |
| V | 290.19 | 144.90 | 2.0 |
| VI | 260.25 | 151.03 | 1.7 |
| Tuono | 290.91 | 186.52 | 1.6 |
| Cultivar | 24 h | 48 h | 72 h |
|---|---|---|---|
| Fascionello | |||
| I | 7.95 ± 0.15 *a | 6.40 ± 0.16 aR | 6.18 ± 0.36 *S |
| II | 7.70 ± 0.21 * | 7.54 ± 0.18 *Q | 5.70 ± 0.12 *T |
| III | 7.67 ± 0.25 * | 7.30 ± 0.16 *Q | 6.24 ± 0.25 *S |
| IV | 7.47 ± 0.20 | 6.00 ± 0.42 aD | 5.40 ± 0.16 I |
| V | 7.82 ± 0.12 * | 6.00 ± 0.12 *aD | 6.10 ± 0.14 * |
| VI | 7.63 ± 0.14 * | 7.18 ± 0.28 * | 5.70 ± 0.32 |
| Pizzuta | |||
| I | 7.64 ± 0.08 * | 7.44 ± 0.10 * | 6.18 ± 0.25 *U |
| II | 7.81 ± 0.18 * | 7.10 ± 0.24 * | 5.70 ± 0.12 *V |
| III | 7.89 ± 0.12 * | 7.30 ± 0.34 * | 6.24 ± 0.22 *K |
| IV | 7.87 ± 0.29 * | 7.40 ± 0.18 * | 5.40 ± 0.15 I |
| V | 7.88 ± 0.16 * | 7.18 ± 0.12 * | 6.10 ± 0.24 |
| VI | 7.89 ± 0.15 * | 7.18 ± 0.14 * | 5.70 ± 0.25 |
| Tuono | 7.95 ± 0.22 * | 7.40 ± 0.28 * | 6.00 ± 0.18 * |
| Control | 7.40 ± 0.08 | 6.40 ± 0.05 | 5.40 ± 0.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ingegneri, M.; Smeriglio, A.; Rando, R.; Gervasi, T.; Tamburello, M.P.; Ginestra, G.; La Camera, E.; Pennisi, R.; Sciortino, M.T.; Mandalari, G.; et al. Composition and Biological Properties of Blanched Skin and Blanch Water Belonging to Three Sicilian Almond Cultivars. Nutrients 2023, 15, 1545. https://doi.org/10.3390/nu15061545
Ingegneri M, Smeriglio A, Rando R, Gervasi T, Tamburello MP, Ginestra G, La Camera E, Pennisi R, Sciortino MT, Mandalari G, et al. Composition and Biological Properties of Blanched Skin and Blanch Water Belonging to Three Sicilian Almond Cultivars. Nutrients. 2023; 15(6):1545. https://doi.org/10.3390/nu15061545
Chicago/Turabian StyleIngegneri, Mariarosaria, Antonella Smeriglio, Rossana Rando, Teresa Gervasi, Maria Pia Tamburello, Giovanna Ginestra, Erminia La Camera, Rosamaria Pennisi, Maria Teresa Sciortino, Giuseppina Mandalari, and et al. 2023. "Composition and Biological Properties of Blanched Skin and Blanch Water Belonging to Three Sicilian Almond Cultivars" Nutrients 15, no. 6: 1545. https://doi.org/10.3390/nu15061545
APA StyleIngegneri, M., Smeriglio, A., Rando, R., Gervasi, T., Tamburello, M. P., Ginestra, G., La Camera, E., Pennisi, R., Sciortino, M. T., Mandalari, G., & Trombetta, D. (2023). Composition and Biological Properties of Blanched Skin and Blanch Water Belonging to Three Sicilian Almond Cultivars. Nutrients, 15(6), 1545. https://doi.org/10.3390/nu15061545