The Impact of Sarcopenia Onset Prior to Cancer Diagnosis on Cancer Survival: A National Population-Based Cohort Study Using Propensity Score Matching
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Cohort
2.2. Patients Selection
2.3. Covariates and Propensity Score Matching
2.4. Sensitivity Analysis
2.5. Statistical Analysis
3. Results
3.1. Study Cohort
3.2. Multivariate Cox Regression Analysis
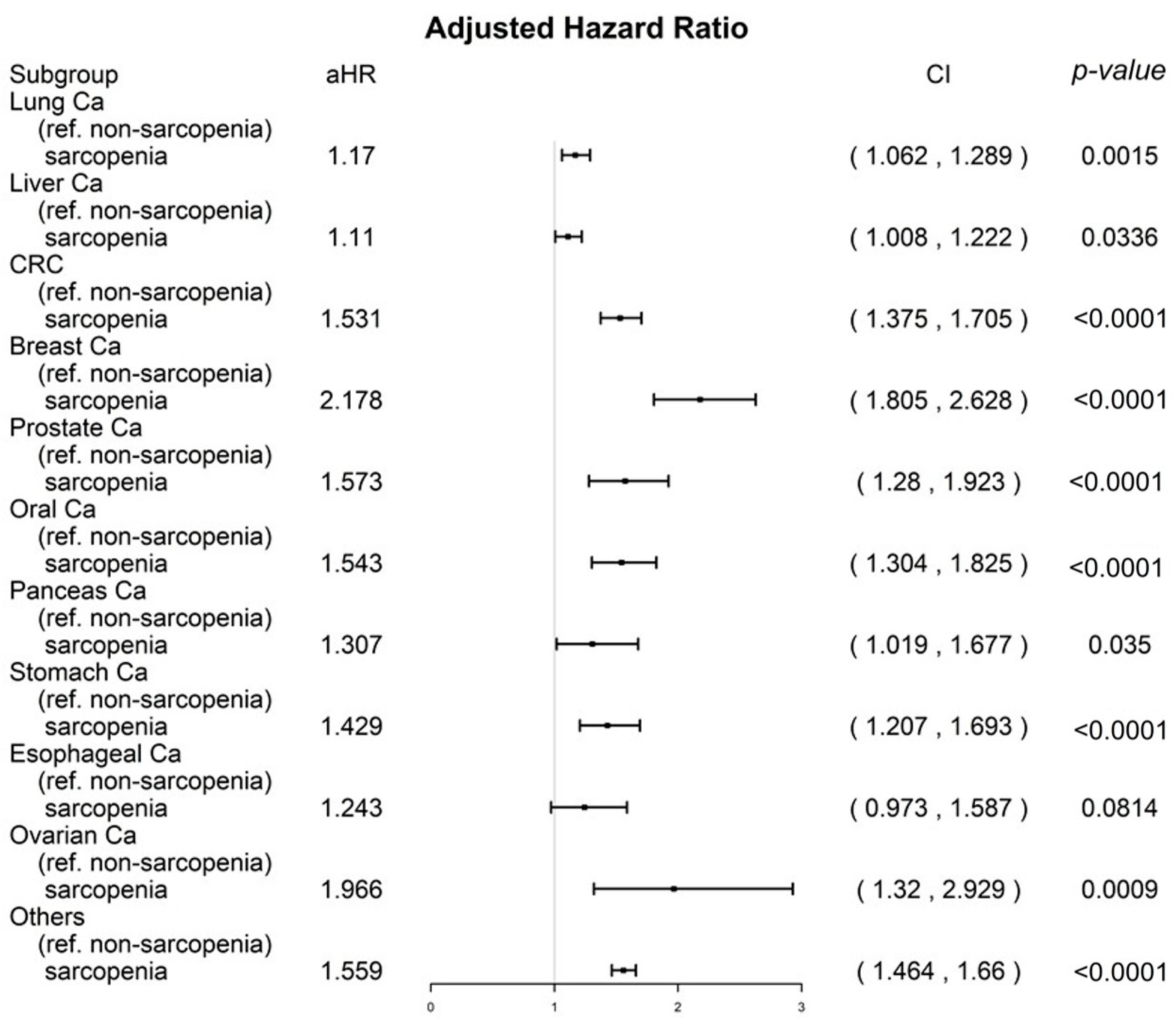
3.3. Sensitivity Analysis for Cancer Types
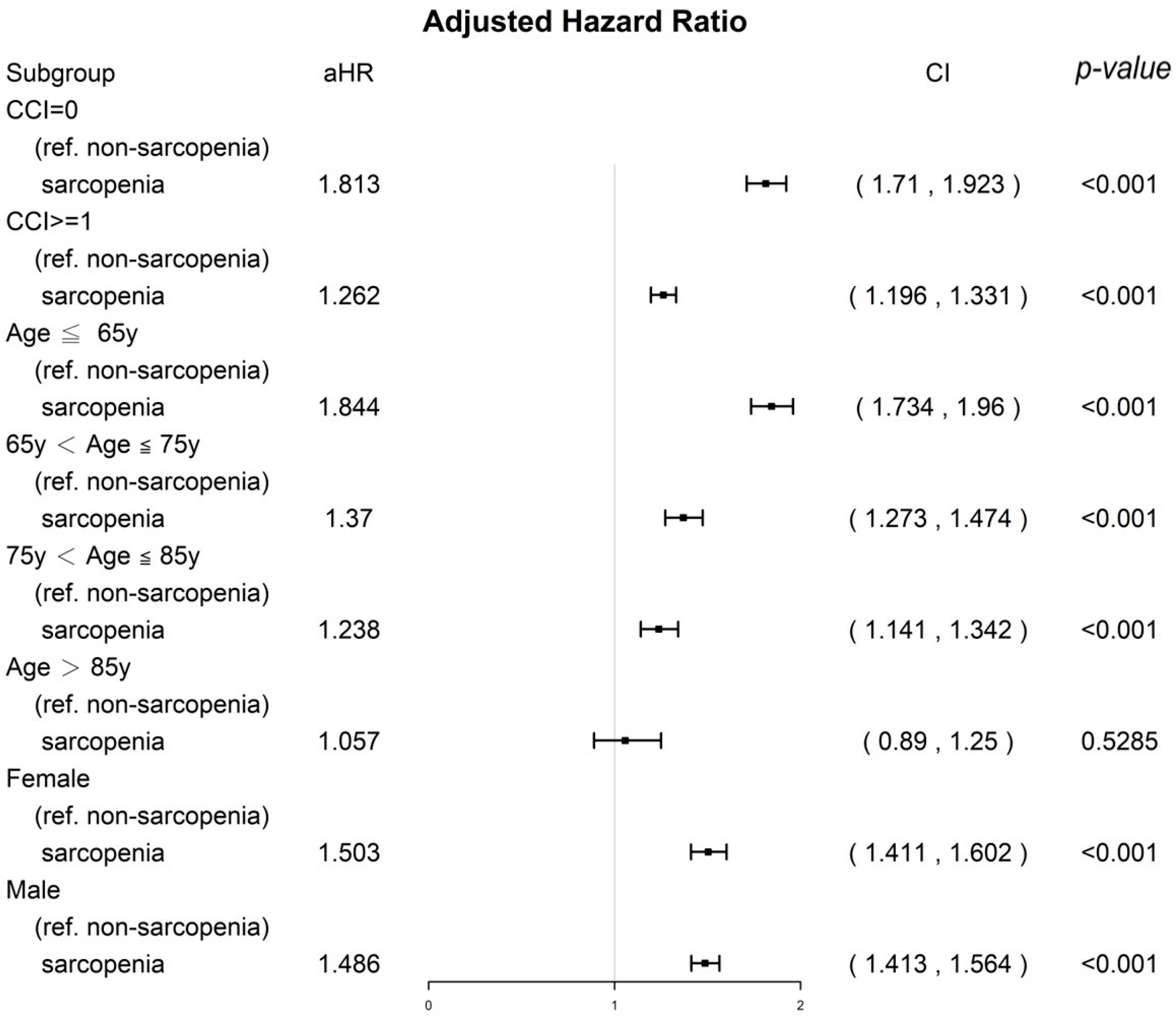
3.4. Sensitivity Analysis of CCI Score, Age Groups, and Sex
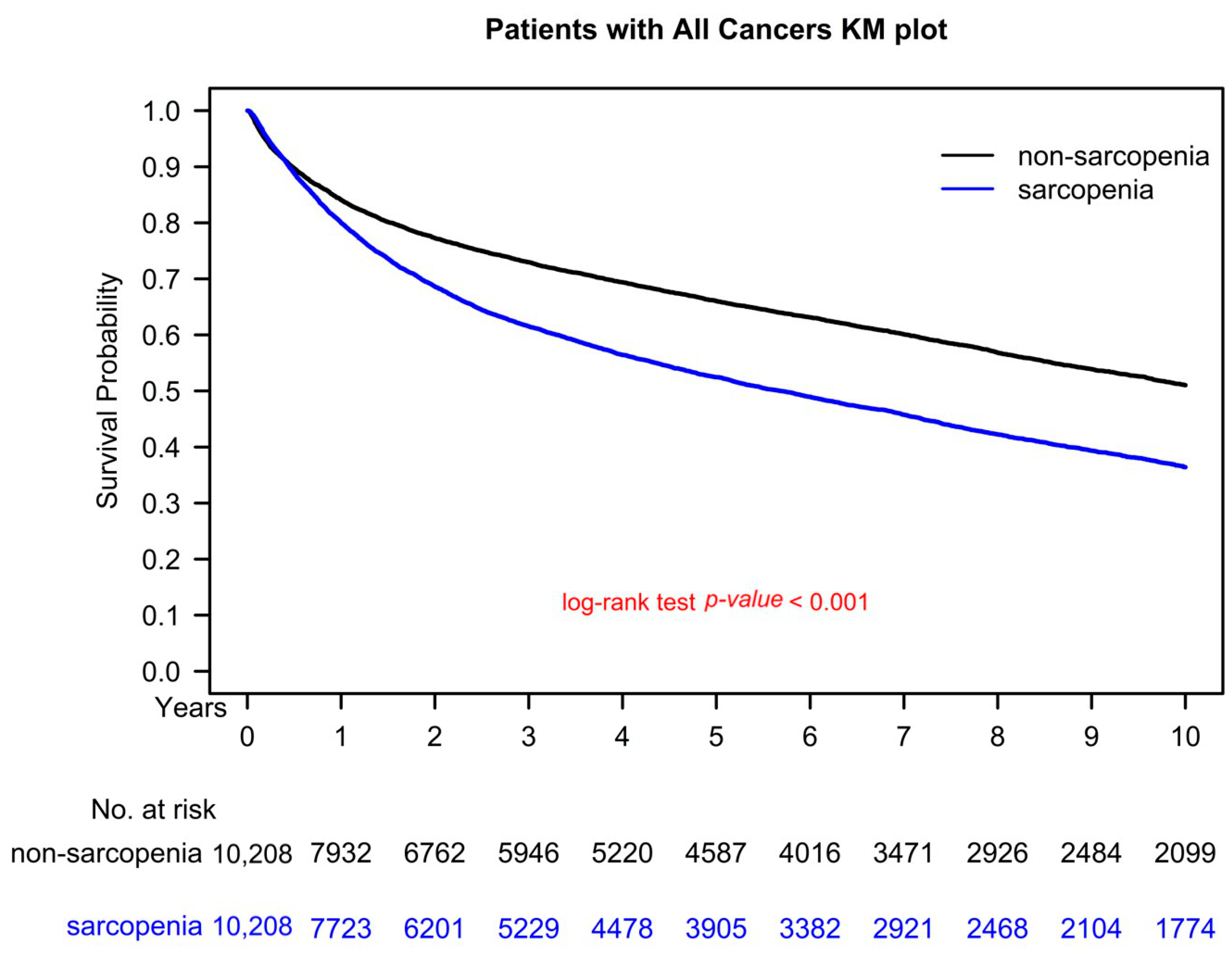
3.5. Kaplan–Meier Survival Curves
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Janssen, I. The epidemiology of sarcopenia. Clin. Geriatr. Med. 2011, 27, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, R.N.; Waters, D.L.; Gallagher, D.; Morley, J.E.; Garry, P.J. Predictors of skeletal muscle mass in elderly men and women. Mech. Ageing Dev. 1999, 107, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Muscaritoli, M.; Anker, S.D.; Argiles, J.; Aversa, Z.; Bauer, J.M.; Biolo, G.; Boirie, Y.; Bosaeus, I.; Cederholm, T.; Costelli, P.; et al. Consensus definition of sarcopenia, cachexia and pre-cachexia: Joint document elaborated by Special Interest Groups (SIG) “cachexia-anorexia in chronic wasting diseases” and “nutrition in geriatrics”. Clin. Nutr. 2010, 29, 154–159. [Google Scholar] [CrossRef]
- Janssen, I. Influence of sarcopenia on the development of physical disability: The Cardiovascular Health Study. J. Am. Geriatr. Soc. 2006, 54, 56–62. [Google Scholar] [CrossRef]
- Houston, D.K.; Tooze, J.A.; Garcia, K.; Visser, M.; Rubin, S.; Harris, T.B.; Newman, A.B.; Kritchevsky, S.B.; Health, A.B.C.S. Protein Intake and Mobility Limitation in Community-Dwelling Older Adults: The Health ABC Study. J. Am. Geriatr. Soc. 2017, 65, 1705–1711. [Google Scholar] [CrossRef]
- Kyle, U.G.; Morabia, A.; Schutz, Y.; Pichard, C. Sedentarism affects body fat mass index and fat-free mass index in adults aged 18 to 98 years. Nutrition 2004, 20, 255–260. [Google Scholar] [CrossRef]
- Rasmussen, B.B.; Fujita, S.; Wolfe, R.R.; Mittendorfer, B.; Roy, M.; Rowe, V.L.; Volpi, E. Insulin resistance of muscle protein metabolism in aging. FASEB J. 2006, 20, 768–769. [Google Scholar] [CrossRef]
- Joseph, C.; Kenny, A.M.; Taxel, P.; Lorenzo, J.A.; Duque, G.; Kuchel, G.A. Role of endocrine-immune dysregulation in osteoporosis, sarcopenia, frailty and fracture risk. Mol. Aspects Med. 2005, 26, 181–201. [Google Scholar] [CrossRef]
- Roberts, S.B. Regulation of energy intake in relation to metabolic state and nutritional status. Eur. J. Clin. Nutr. 2000, 54 (Suppl. S3), S64–S69. [Google Scholar] [CrossRef]
- Szulc, P.; Duboeuf, F.; Marchand, F.; Delmas, P.D. Hormonal and lifestyle determinants of appendicular skeletal muscle mass in men: The MINOS study. Am. J. Clin. Nutr. 2004, 80, 496–503. [Google Scholar] [CrossRef]
- Li, H.L.; Au, P.C.; Lee, G.K.; Li, G.H.; Chan, M.; Cheung, B.M.; Wong, I.C.; Lee, V.H.; Mok, J.; Yip, B.H.; et al. Different definition of sarcopenia and mortality in cancer: A meta-analysis. Osteoporos. Sarcopenia 2021, 7, S34–S38. [Google Scholar] [CrossRef]
- Au, P.C.; Li, H.L.; Lee, G.K.; Li, G.H.; Chan, M.; Cheung, B.M.; Wong, I.C.; Lee, V.H.; Mok, J.; Yip, B.H.; et al. Sarcopenia and mortality in cancer: A meta-analysis. Osteoporos. Sarcopenia 2021, 7, S28–S33. [Google Scholar] [CrossRef]
- Ibilibor, C.; Psutka, S.P.; Herrera, J.; Rivero, J.R.; Wang, H.; Farrell, A.M.; Liss, M.A.; Pruthi, D.; Mansour, A.M.; Svatek, R.; et al. The association between sarcopenia and bladder cancer-specific mortality and all-cause mortality after radical cystectomy: A systematic review and meta-analysis. Arab. J. Urol. 2021, 19, 98–103. [Google Scholar] [CrossRef]
- Zhang, X.M.; Dou, Q.L.; Zeng, Y.; Yang, Y.; Cheng, A.S.K.; Zhang, W.W. Sarcopenia as a predictor of mortality in women with breast cancer: A meta-analysis and systematic review. BMC Cancer 2020, 20, 172. [Google Scholar] [CrossRef]
- Joglekar, S.; Nau, P.N.; Mezhir, J.J. The impact of sarcopenia on survival and complications in surgical oncology: A review of the current literature. J. Surg. Oncol. 2015, 112, 503–509. [Google Scholar] [CrossRef]
- Zhang, J.; Lu, C.Y.; Chen, H.M.; Wu, S.Y. Neoadjuvant Chemotherapy or Endocrine Therapy for Invasive Ductal Carcinoma of the Breast with High Hormone Receptor Positivity and Human Epidermal Growth Factor Receptor 2 Negativity. JAMA Netw. Open 2021, 4, e211785. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyere, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Sun, M.Y.; Chang, C.L.; Lu, C.Y.; Wu, S.Y.; Zhang, J.Q. Sarcopenia as an Independent Risk Factor for Specific Cancers: A Propensity Score-Matched Asian Population-Based Cohort Study. Nutrients 2022, 14, 1910. [Google Scholar] [CrossRef]
- Bijlsma, A.Y.; Meskers, C.G.; Ling, C.H.; Narici, M.; Kurrle, S.E.; Cameron, I.D.; Westendorp, R.G.; Maier, A.B. Defining sarcopenia: The impact of different diagnostic criteria on the prevalence of sarcopenia in a large middle aged cohort. Age 2013, 35, 871–881. [Google Scholar] [CrossRef]
- Anker, S.D.; Morley, J.E.; von Haehling, S. Welcome to the ICD-10 code for sarcopenia. J. Cachexia Sarcopenia Muscle 2016, 7, 512–514. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.H.; Chiu, S.Y.; Chang, P.H.; Lai, Y.L.; Chen, P.C.; Ho, W.C. Hyperlipidemia and Statins Use for the Risk of New Diagnosed Sarcopenia in Patients with Chronic Kidney: A Population-Based Study. Int. J. Environ. Res. Public Health 2020, 17, 1494. [Google Scholar] [CrossRef] [PubMed]
- Austin, P.C. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm. Stat. 2011, 10, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Austin, P.C. The performance of different propensity score methods for estimating marginal hazard ratios. Stat. Med. 2013, 32, 2837–2849. [Google Scholar] [CrossRef]
- Yuan, Y.; Yung, Y.-F.; Stokes, M. Propensity Score Methods for Causal Inference with the PSMATCH Procedure. In Proceedings of the SAS Global Forum 2017 Conference, Orlando, FL, USA, 2–5 April 2017. [Google Scholar]
- Austin, P.C. The use of propensity score methods with survival or time-to-event outcomes: Reporting measures of effect similar to those used in randomized experiments. Stat. Med. 2014, 33, 1242–1258. [Google Scholar] [CrossRef]
- Baumgartner, R.N.; Koehler, K.M.; Gallagher, D.; Romero, L.; Heymsfield, S.B.; Ross, R.R.; Garry, P.J.; Lindeman, R.D. Epidemiology of sarcopenia among the elderly in New Mexico. Am. J. Epidemiol. 1998, 147, 755–763. [Google Scholar] [CrossRef]
- Kim, T.N.; Yang, S.J.; Yoo, H.J.; Lim, K.I.; Kang, H.J.; Song, W.; Seo, J.A.; Kim, S.G.; Kim, N.H.; Baik, S.H.; et al. Prevalence of sarcopenia and sarcopenic obesity in Korean adults: The Korean sarcopenic obesity study. Int. J. Obes. 2009, 33, 885–892. [Google Scholar] [CrossRef]
- Rolland, Y.; Lauwers-Cances, V.; Cristini, C.; Abellan van Kan, G.; Janssen, I.; Morley, J.E.; Vellas, B. Difficulties with physical function associated with obesity, sarcopenia, and sarcopenic-obesity in community-dwelling elderly women: The EPIDOS (EPIDemiologie de l’OSteoporose) Study. Am. J. Clin. Nutr. 2009, 89, 1895–1900. [Google Scholar] [CrossRef]
- Prado, C.M.; Lieffers, J.R.; McCargar, L.J.; Reiman, T.; Sawyer, M.B.; Martin, L.; Baracos, V.E. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: A population-based study. Lancet Oncol. 2008, 9, 629–635. [Google Scholar] [CrossRef]
- Antoun, S.; Baracos, V.E.; Birdsell, L.; Escudier, B.; Sawyer, M.B. Low body mass index and sarcopenia associated with dose-limiting toxicity of sorafenib in patients with renal cell carcinoma. Ann. Oncol. 2010, 21, 1594–1598. [Google Scholar] [CrossRef]
- Alibhai, S.M.; Breunis, H.; Timilshina, N.; Johnston, C.; Tomlinson, G.; Tannock, I.; Krahn, M.; Fleshner, N.E.; Warde, P.; Canning, S.D.; et al. Impact of androgen-deprivation therapy on physical function and quality of life in men with nonmetastatic prostate cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2010, 28, 5038–5045. [Google Scholar] [CrossRef]
- Smith, M.R.; Saad, F.; Egerdie, B.; Sieber, P.R.; Tammela, T.L.; Ke, C.; Leder, B.Z.; Goessl, C. Sarcopenia during androgen-deprivation therapy for prostate cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2012, 30, 3271–3276. [Google Scholar] [CrossRef]
- Van Vugt, J.L.A.; Buettner, S.; Levolger, S.; Coebergh van den Braak, R.R.J.; Suker, M.; Gaspersz, M.P.; de Bruin, R.W.F.; Verhoef, C.; van Eijck, C.H.C.; Bossche, N.; et al. Low skeletal muscle mass is associated with increased hospital expenditure in patients undergoing cancer surgery of the alimentary tract. PLoS ONE 2017, 12, e0186547. [Google Scholar] [CrossRef]
- Hilmi, M.; Jouinot, A.; Burns, R.; Pigneur, F.; Mounier, R.; Gondin, J.; Neuzillet, C.; Goldwasser, F. Body composition and sarcopenia: The next-generation of personalized oncology and pharmacology? Pharmacol. Ther. 2019, 196, 135–159. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, J.L.; Lu, J.; Song, Y.; Kwak, K.S.; Jiao, Q.; Rosenfeld, R.; Chen, Q.; Boone, T.; Simonet, W.S.; et al. Reversal of cancer cachexia and muscle wasting by ActRIIB antagonism leads to prolonged survival. Cell 2010, 142, 531–543. [Google Scholar] [CrossRef]
- Advani, S.M.; Advani, P.G.; VonVille, H.M.; Jafri, S.H. Pharmacological management of cachexia in adult cancer patients: A systematic review of clinical trials. BMC Cancer 2018, 18, 1174. [Google Scholar] [CrossRef]
- Shachar, S.S.; Williams, G.R.; Muss, H.B.; Nishijima, T.F. Prognostic value of sarcopenia in adults with solid tumours: A meta-analysis and systematic review. Eur. J. Cancer 2016, 57, 58–67. [Google Scholar] [CrossRef]
- Buentzel, J.; Heinz, J.; Bleckmann, A.; Bauer, C.; Rover, C.; Bohnenberger, H.; Saha, S.; Hinterthaner, M.; Baraki, H.; Kutschka, I.; et al. Sarcopenia as Prognostic Factor in Lung Cancer Patients: A Systematic Review and Meta-analysis. Anticancer Res. 2019, 39, 4603–4612. [Google Scholar] [CrossRef]
- Hua, X.; Liu, S.; Liao, J.F.; Wen, W.; Long, Z.Q.; Lu, Z.J.; Guo, L.; Lin, H.X. When the loss costs too much: A systematic review and meta-analysis of sarcopenia in head and neck cancer. Front. Oncol. 2020, 9, 1561. [Google Scholar] [CrossRef]
- Austin, P.C. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivar. Behav. Res. 2011, 46, 399–424. [Google Scholar] [CrossRef]
- Wu, S.Y.; Fang, S.C.; Shih, H.J.; Wen, Y.C.; Shao, Y.H.J. Mortality associated with statins in men with advanced prostate cancer treated with androgen deprivation therapy. Eur. J. Cancer 2019, 112, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.K.; Hsieh, M.C.; Wang, W.W.; Lin, Y.C.; Chang, W.W.; Chang, C.L.; Cheng, Y.F.; Wu, S.Y. Outcomes of adjuvant treatments for resectable intrahepatic cholangiocarcinoma: Chemotherapy alone, sequential chemoradiotherapy, or concurrent chemoradiotherapy. Radiother. Oncol. 2018, 128, 575–583. [Google Scholar] [CrossRef] [PubMed]
| Nonsarcopenia | Sarcopenia | SMD | |||
|---|---|---|---|---|---|
| N = 10,208 | N = 10,208 | ||||
| N | % | N | % | ||
| Age (mean ± SD) | 61.05 ± 15.78 | 62.17 ± 14.28 | 0.075 | ||
| Age (years) | 0.031 | ||||
| Age ≤ 65 | 5584 | 54.70 | 5620 | 55.05 | |
| 65 < Age ≤ 75 | 2573 | 25.21 | 2496 | 24.45 | |
| 75 < Age ≤ 85 | 1729 | 16.94 | 1719 | 16.84 | |
| Age > 85 | 322 | 3.15 | 373 | 3.65 | |
| Sex | 0.008 | ||||
| Female | 4843 | 47.44 | 4883 | 47.84 | |
| Male | 5365 | 52.56 | 5325 | 52.16 | |
| Diabetes | 2085 | 20.43 | 2100 | 20.57 | 0.004 |
| Hypertension | 4388 | 42.99 | 4345 | 42.56 | 0.009 |
| Hyperlipidemia | 2212 | 21.67 | 2254 | 22.08 | 0.010 |
| ESRD | 133 | 1.30 | 127 | 1.24 | 0.005 |
| Liver cirrhosis | 2370 | 23.22 | 2433 | 23.83 | 0.015 |
| AMI | 180 | 1.76 | 209 | 2.05 | 0.021 |
| Coronary arterial disease | 2114 | 20.71 | 2178 | 21.34 | 0.015 |
| Stroke | 582 | 5.70 | 652 | 6.39 | 0.029 |
| Hepatitis C | 317 | 3.11 | 329 | 3.22 | 0.002 |
| Hepatitis B | 581 | 5.69 | 574 | 5.62 | 0.003 |
| CCI score (mean ± SD) | 0.91 ± 1.24 | 0.93 ± 1.27 | 0.018 | ||
| CCI score | 0.005 | ||||
| =0 | 5613 | 54.99 | 5589 | 54.75 | |
| ≥1 | 4595 | 45.01 | 4619 | 45.25 | |
| CCI | |||||
| Congestive heart failure | 549 | 5.38 | 552 | 5.41 | 0.001 |
| Dementia | 208 | 2.04 | 229 | 2.24 | 0.014 |
| Chronic pulmonary disease | 1889 | 18.51 | 2019 | 19.78 | 0.032 |
| Rheumatic disease | 133 | 1.30 | 154 | 1.51 | 0.017 |
| Liver disease | 2173 | 21.29 | 2228 | 21.83 | 0.013 |
| Diabetes mellitus with complications | 479 | 4.69 | 465 | 4.56 | 0.007 |
| Hemiplegia and paraplegia | 0 | 0 | - | ||
| Renal disease | 590 | 5.78 | 593 | 5.81 | 0.001 |
| AIDS | 5 | 0.05 | 4 | 0.04 | 0.005 |
| Income levels | 0.009 | ||||
| Low income | 96 | 0.94 | 104 | 1.02 | 0.001 |
| Income ≤20,000 NTD/month | 6305 | 61.77 | 6284 | 61.56 | |
| 20,000 < income ≤ 30,000 NTD/month | 2370 | 23.22 | 2377 | 23.29 | |
| Income > 30,000 NTD/month | 1437 | 14.08 | 1443 | 14.14 | |
| Urbanization | 0.001 | ||||
| Rural | 3209 | 31.44 | 3204 | 31.39 | |
| Urban | 6999 | 68.56 | 7004 | 68.61 | |
| Cancer types | |||||
| Lung cancer | 1474 | 14.44 | 1474 | 14.44 | 0.000 |
| Early stages | 678 | 6.64 | 678 | 6.64 | |
| Advanced stages | 796 | 7.80 | 796 | 7.80 | |
| Hepatocellular carcinoma | 1174 | 11.50 | 1174 | 11.50 | 0.000 |
| Early stages | 698 | 6.84 | 698 | 6.84 | |
| Advanced stages | 476 | 4.66 | 476 | 4.66 | |
| Colorectal cancer | 1501 | 14.70 | 1501 | 14.70 | 0.000 |
| Early stages | 911 | 8.92 | 911 | 8.92 | |
| Advanced stages | 590 | 5.78 | 590 | 5.78 | |
| Breast cancer | 1004 | 9.84 | 1004 | 9.84 | 0.000 |
| Early stages | 502 | 4.92 | 502 | 4.92 | |
| Advanced stages | 502 | 4.92 | 502 | 4.92 | |
| Prostate cancer | 396 | 3.88 | 396 | 3.88 | 0.000 |
| Early stages | 132 | 1.29 | 132 | 1.29 | |
| Advanced stages | 264 | 2.59 | 264 | 2.59 | |
| Head and neck cancer | 592 | 5.80 | 592 | 5.80 | 0.000 |
| Early stages | 269 | 2.64 | 269 | 2.64 | |
| Advanced stages | 323 | 3.16 | 323 | 3.16 | |
| Pancreatic cancer | 232 | 2.27 | 232 | 2.27 | 0.000 |
| Early stages | 106 | 1.04 | 106 | 1.04 | |
| Advanced stages | 126 | 1.23 | 126 | 1.23 | |
| Gastric cancer | 503 | 4.93 | 503 | 4.93 | 0.000 |
| Early stages | 240 | 2.35 | 240 | 2.35 | |
| Advanced stages | 263 | 2.58 | 263 | 2.58 | |
| Esophagus cancer | 252 | 2.47 | 252 | 2.47 | 0.000 |
| Early stages | 42 | 0.41 | 42 | 0.41 | |
| Advanced stages | 210 | 2.06 | 210 | 2.06 | |
| Ovarian cancer | 164 | 1.61 | 164 | 1.61 | 0.000 |
| Early stages | 66 | 0.65 | 66 | 0.65 | |
| Advanced stages | 98 | 0.96 | 98 | 0.96 | |
| Other cancers | 4115 | 40.31 | 4115 | 40.31 | 0.000 |
| Early stages | 2244 | 21.98 | 2244 | 21.98 | |
| Advanced stages | 1871 | 18.33 | 1871 | 18.33 | |
| p value | |||||
| Follow-up, years (mean ± SD) | 6.49 ± 4.82 | 5.88 ± 4.63 | <0.0001 | ||
| Follow-up, years; median (IQR, Q1,Q3) | 5.17 (1.19, 8.82) | 4.16 (1.04, 7.80) | <0.0001 | ||
| All-cause death | <0.0001 | ||||
| No | 5920 | 57.99 | 4306 | 42.18 | |
| Yes | 4288 | 42.01 | 5902 | 57.82 | |
| Crude HR (95% CI) | Adjusted HR * (95% CI) | p Value | |||
|---|---|---|---|---|---|
| Sarcopenia (ref.: Nonsarcopenia) | |||||
| Yes | 1.5 | (1.44, 1.56) | 1.49 | (1.43, 1.55) | <0.001 |
| Sex (ref.: Female) | |||||
| Male | 1.74 | (1.67, 1.81) | 1.56 | (1.50, 1.62) | <0.001 |
| Age (years; ref.: Age ≤ 65) | |||||
| 65 < Age ≤ 75 | 1.64 | (1.57, 1.72) | 1.29 | (1.23, 1.36) | <0.001 |
| 75 < Age ≤ 85 | 2.75 | (2.61, 2.89) | 2.00 | (1.89, 2.12) | <0.001 |
| Age > 85 | 4.64 | (4.24, 5.08) | 3.26 | (2.97, 3.59) | <0.001 |
| CCI score (ref. = 0) | |||||
| ≥1 | 1.82 | (1.75, 1.89) | 1.34 | (1.28, 1.40) | <0.001 |
| Diabetes (ref.: No) | |||||
| Yes | 1.17 | (1.02, 1.78) | 1.06 | (0.89, 1.32) | 0.357 |
| Hyperlipidemia (ref.: No) | |||||
| Yes | 1.14 | (1.08, 1.41) | 1.08 | (0.93, 1.03) | 0.446 |
| Liver cirrhosis (ref.: No) | |||||
| Yes | 1.11 | (1.05, 1.38) | 1.02 | (0.91, 1.22) | 0.275 |
| Hypertension (ref.: No) | |||||
| Yes | 1.25 | (1.18, 1.82) | 1.04 | (0.79, 1.20) | 0.428 |
| ESRD (ref.: No) | |||||
| Yes | 1.19 | (1.05, 1.96) | 1.07 | (0.88, 1.60) | 0.131 |
| AMI (ref.: No) | |||||
| Yes | 1.47 | (0.83, 2.34) | 1.12 | (0.85, 1.36) | 0.226 |
| Coronary arterial disease (ref.: No) | |||||
| Yes | 1.66 | (1.58, 1.74) | 1.09 | (0.74, 1.50) | 0.390 |
| Stroke (ref.: No) | |||||
| Yes | 1.23 | (1.07, 2.39) | 1.17 | (0.98, 1.37) | 0.071 |
| Hepatitis C (ref.: No) | |||||
| Yes | 1.88 | (1.17, 2.08) | 1.06 | (0.95, 1.18) | 0.284 |
| Hepatitis B (ref.: No) | |||||
| Yes | 1.67 | (0.93, 1.68) | 1.11 | (0.88, 1.81) | 0.393 |
| Urbanization (ref.: Rural) | |||||
| Urban | 0.87 | (0.83, 0.9) | 0.94 | (0.90, 1.02) | 0.196 |
| Income (ref.: Low income) | |||||
| Income ≤ 20,000 NTD/month | 0.78 | (0.65, 0.94) | 0.88 | (0.73, 1.06) | 0.179 |
| 20,000 < Income ≤ 30,000 NTD/month | 0.68 | (0.56, 0.82) | 0.82 | (0.68, 1.09) | 0.155 |
| Income > 30,000 NTD/month | 0.39 | (0.32, 0.48) | 0.61 | (0.50, 1.14) | 0.101 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, C.-H.; Chen, W.-M.; Chen, M.-C.; Shia, B.-C.; Wu, S.-Y. The Impact of Sarcopenia Onset Prior to Cancer Diagnosis on Cancer Survival: A National Population-Based Cohort Study Using Propensity Score Matching. Nutrients 2023, 15, 1247. https://doi.org/10.3390/nu15051247
Su C-H, Chen W-M, Chen M-C, Shia B-C, Wu S-Y. The Impact of Sarcopenia Onset Prior to Cancer Diagnosis on Cancer Survival: A National Population-Based Cohort Study Using Propensity Score Matching. Nutrients. 2023; 15(5):1247. https://doi.org/10.3390/nu15051247
Chicago/Turabian StyleSu, Chih-Hsiung, Wan-Ming Chen, Ming-Chih Chen, Ben-Chang Shia, and Szu-Yuan Wu. 2023. "The Impact of Sarcopenia Onset Prior to Cancer Diagnosis on Cancer Survival: A National Population-Based Cohort Study Using Propensity Score Matching" Nutrients 15, no. 5: 1247. https://doi.org/10.3390/nu15051247
APA StyleSu, C.-H., Chen, W.-M., Chen, M.-C., Shia, B.-C., & Wu, S.-Y. (2023). The Impact of Sarcopenia Onset Prior to Cancer Diagnosis on Cancer Survival: A National Population-Based Cohort Study Using Propensity Score Matching. Nutrients, 15(5), 1247. https://doi.org/10.3390/nu15051247







