Palatability and Acceptability of Flaxseed-Supplemented Foods in Children with Sickle Cell Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Design/Participants
2.2. Materials
2.3. Data Collection
2.4. Statistical Considerations
3. Results
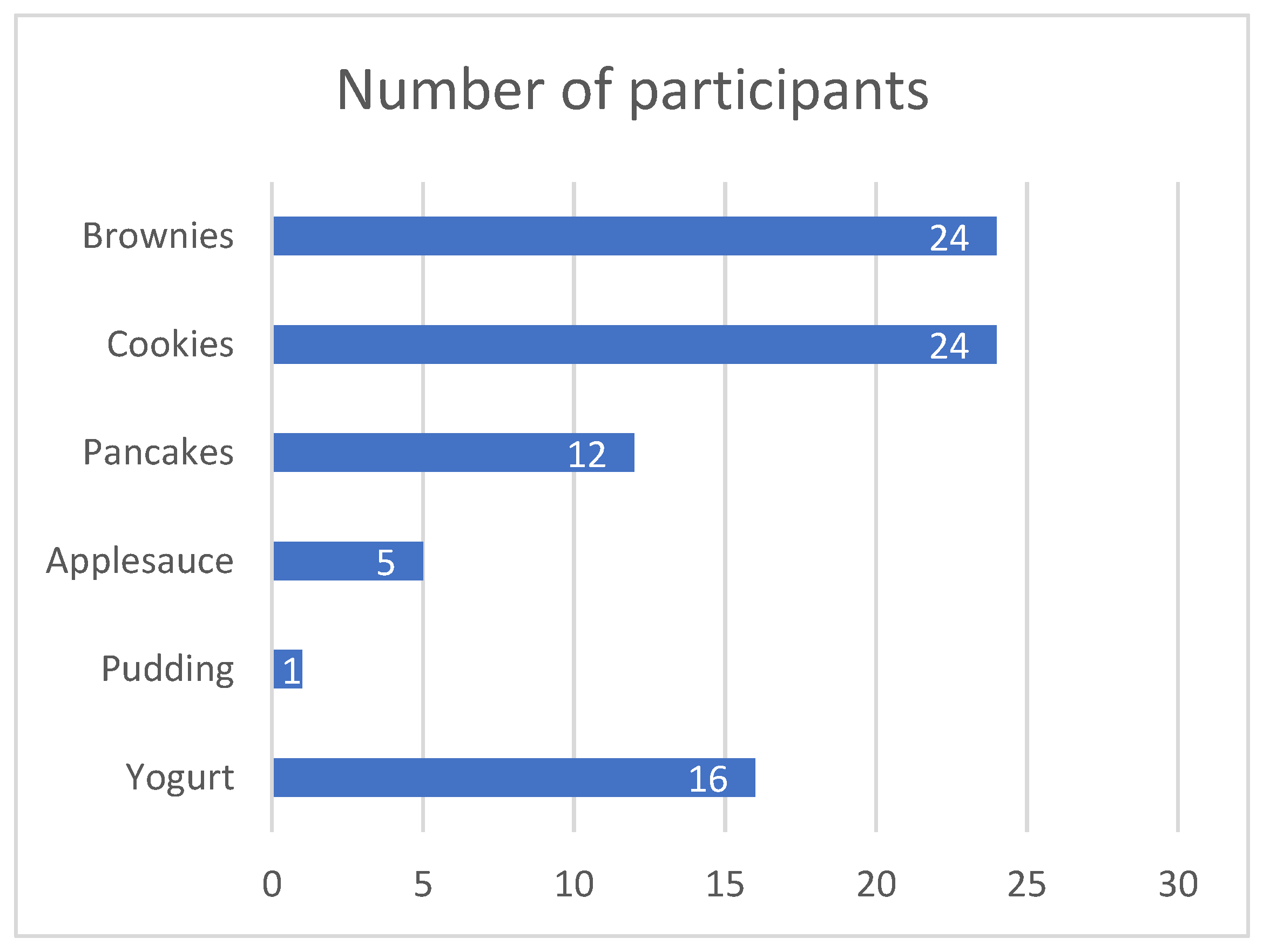
3.1. Cohort Characteristics
3.2. Outcomes
Product Preference Scores
- Appearance
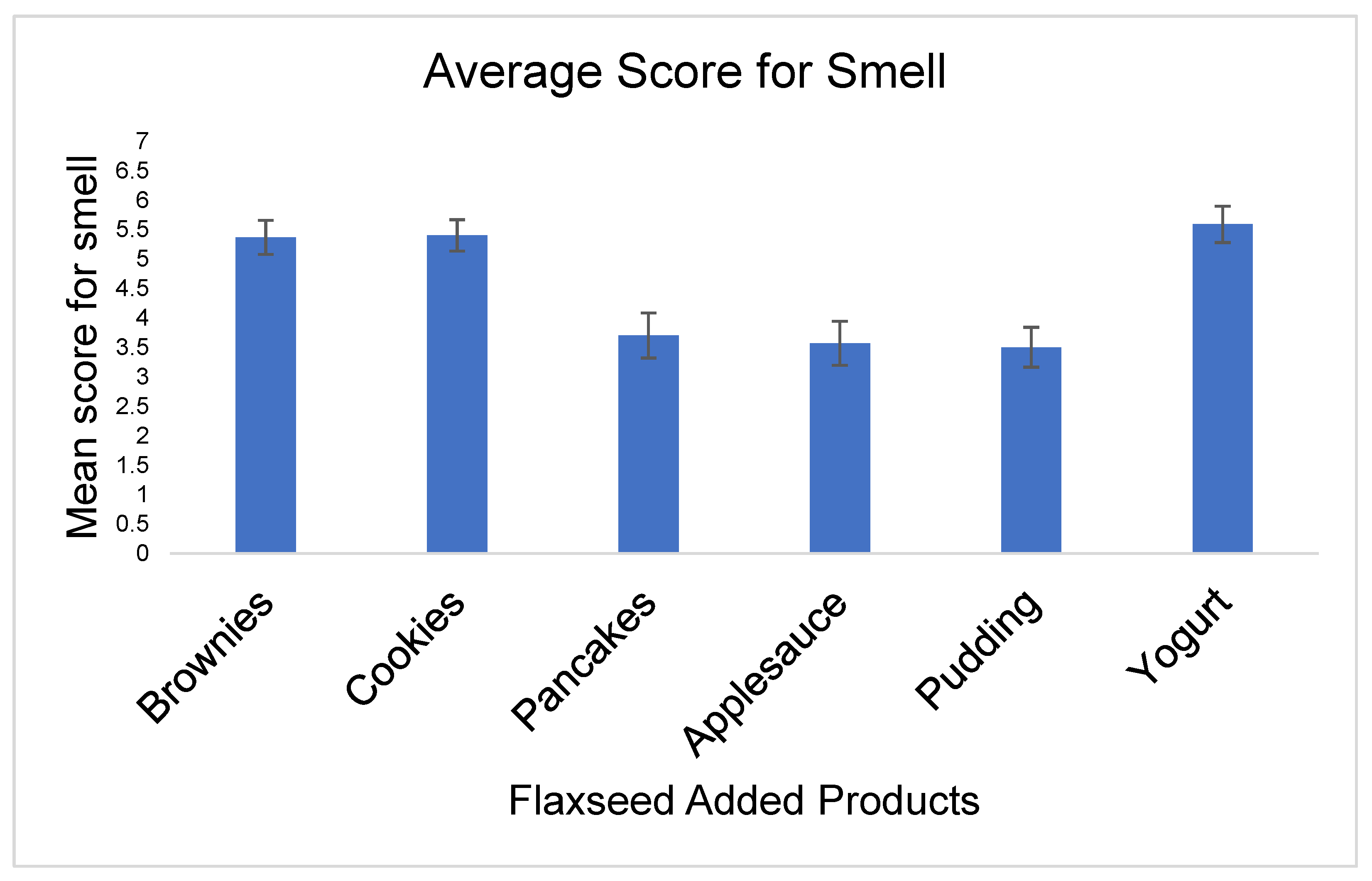
- Smell
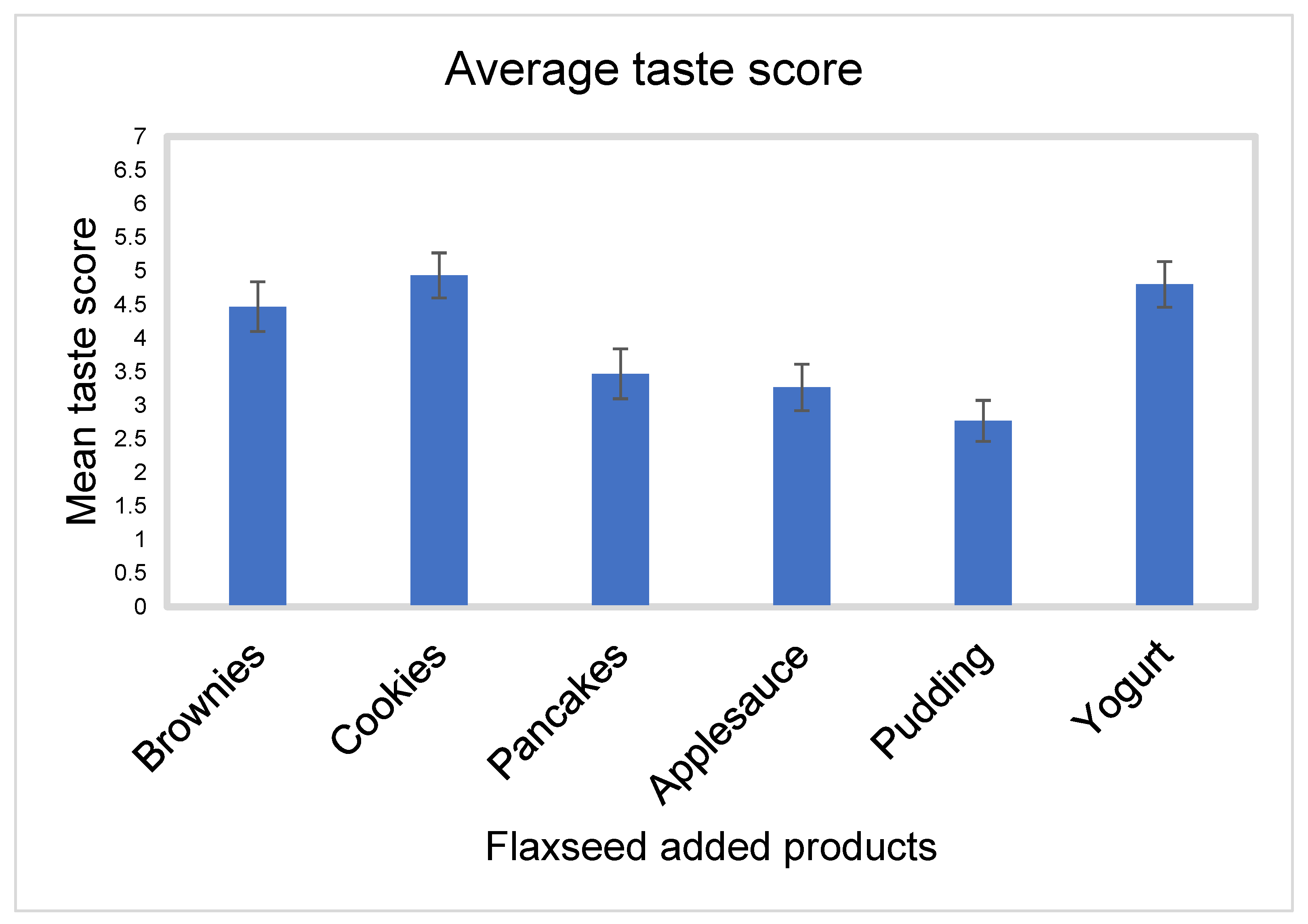
- Taste
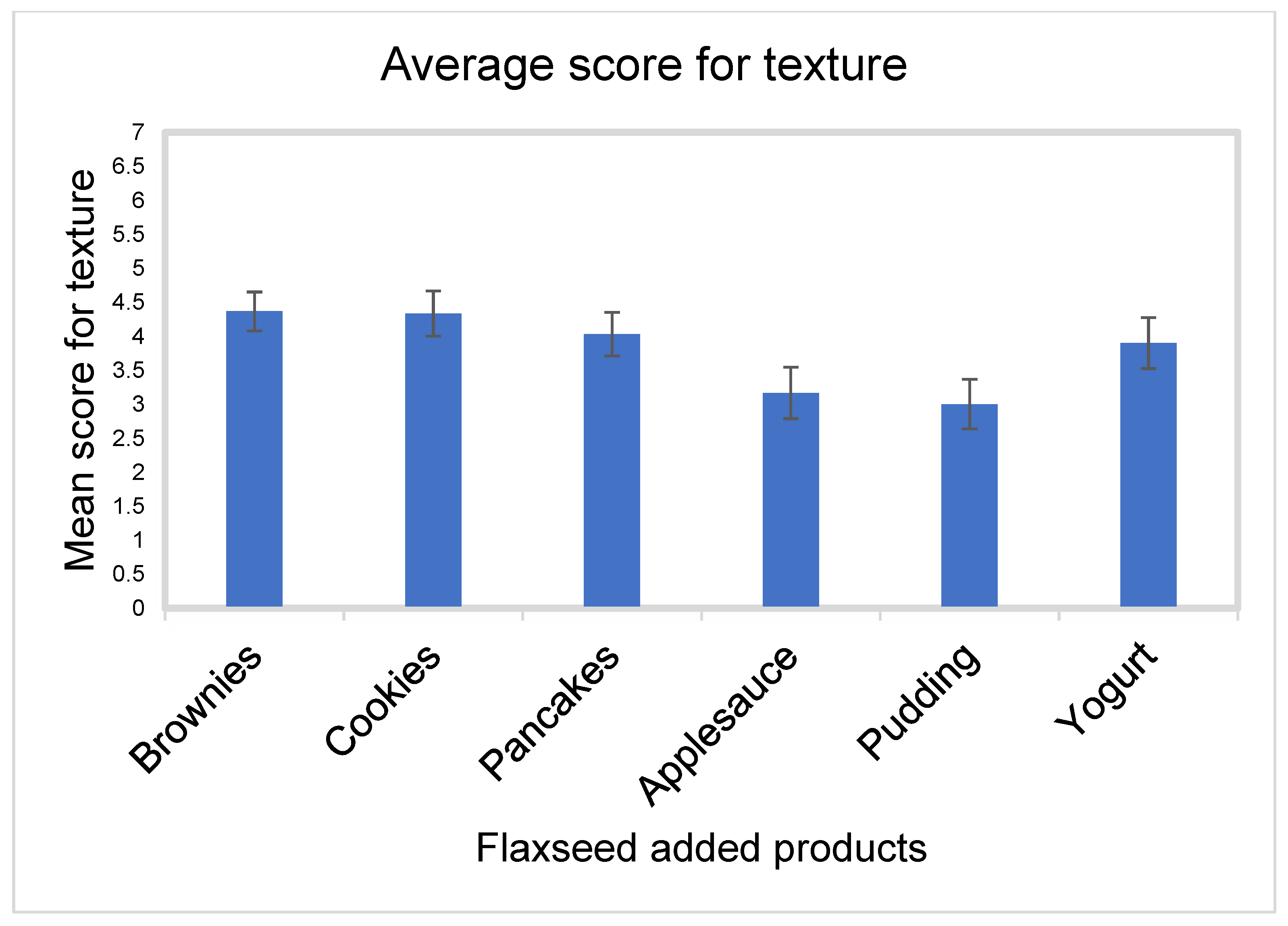
- Texture
3.3. Other Outcomes
- Overall score
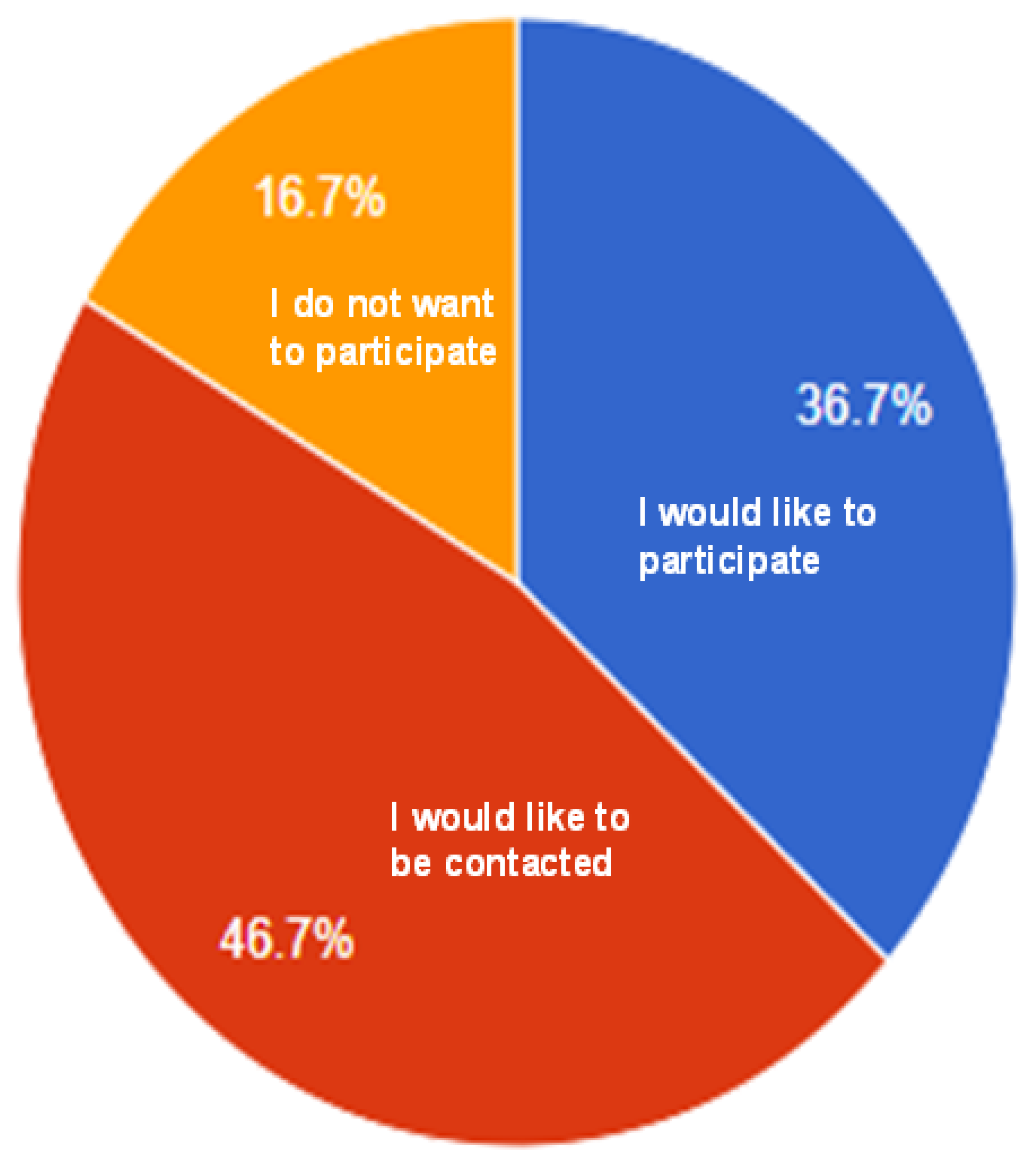
- Willingness to participate in future studies.
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rees, D.C.; Williams, T.; Gladwin, M. Sickle-cell disease. Lancet 2010, 376, 2018–2031. [Google Scholar] [CrossRef] [PubMed]
- Kavanagh, P.L.; Fasipe, T.A.; Wun, T. Sickle Cell Disease: A Review. JAMA 2022, 328, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Peterson, E.E.; Salemi, J.L.; Dongarwar, D.; Salihu, H.M. Acute care utilization in pediatric sickle cell disease and sickle cell trait in the USA: Prevalence, temporal trends, and cost. Eur. J. Pediatr. 2020, 179, 1701–1710. [Google Scholar] [CrossRef] [PubMed]
- Lanzkron, S.; Carroll, C.P.; Haywood, C., Jr. The burden of emergency department use for sickle-cell disease: An analysis of the national emergency department sample database. Am. J. Hematol. 2010, 85, 797–799. [Google Scholar] [CrossRef] [PubMed]
- Delesderrier, E.; Curioni, C.; Omena, J.; Macedo, C.R.; Cople-Rodrigues, C.; Citelli, M. Antioxidant nutrients and hemolysis in sickle cell disease. Clin. Chim. Acta 2020, 510, 381–390. [Google Scholar] [CrossRef]
- Sundd, P.; Gladwin, M.T.; Novelli, E.M. Pathophysiology of Sickle Cell Disease. Annu. Rev. Pathol. Mech. Dis. 2019, 14, 263–292. [Google Scholar] [CrossRef] [PubMed]
- Pace, B.S.; Shartava, A.; Pack-Mabien, A.; Mulekar, M.; Ardia, A.; Goodman, S.R. Effects of N-acetylcysteine on dense cell formation in sickle cell disease. Am. J. Hematol. 2003, 73, 26–32. [Google Scholar] [CrossRef]
- Morris, C.R.; Kuypers, F.A.; Lavrisha, L.; Ansari, M.; Sweeters, N.; Stewart, M.; Gildengorin, G.; Neumayr, L.; Vichinsky, E.P. A randomized, placebo-controlled trial of arginine therapy for the treatment of children with sickle cell disease hospitalized with vaso-occlusive pain episodes. Haematologica 2013, 98, 1375–1382. [Google Scholar] [CrossRef]
- Niihara, Y.; Miller, S.T.; Kanter, J.; Lanzkron, S.; Smith, W.R.; Hsu, L.L.; Gordeuk, V.R.; Viswanathan, K.; Sarnaik, S.; Osunkwo, I.; et al. A Phase 3 Trial of l-Glutamine in Sickle Cell Disease. N. Engl. J. Med. 2018, 379, 226–235. [Google Scholar] [CrossRef]
- Bao, B.; Prasad, A.S.; Beck, F.W.; Snell, D.; Suneja, A.; Sarkar, F.H.; Doshi, N.; Fitzgerald, J.T.; Swerdlow, P. Zinc supplementation decreases oxidative stress, incidence of infection, and generation of inflammatory cytokines in sickle cell disease patients. Transl. Res. 2008, 152, 67–80. [Google Scholar] [CrossRef]
- Brownell, J.N.; Schall, J.I.; Mcanlis, C.R.; Smith-Whitley, K.; Norris, C.F.; Stallings, V.A. Effect of High-dose Vitamin A Supplementation in Children With Sickle Cell Disease: A Randomized, Double-blind, Dose-finding Pilot Study. J. Pediatr. Hematol. 2019, 42, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Dougherty, K.A.; Bertolaso, C.; Schall, J.I.; Smith-Whitley, K.; Stallings, V.A. Safety and Efficacy of High-dose Daily Vitamin D3 Supplementation in Children and Young Adults with Sickle Cell Disease. J. Pediatr. Hematol. 2015, 37, e308–e315. [Google Scholar] [CrossRef]
- Daak, A.A.; Ghebremeskel, K.; Hassan, Z.; Attallah, B.; Azan, H.H.; Elbashir, M.I.; Crawford, M. Effect of omega-3 (n-3) fatty acid supplementation in patients with sickle cell anemia: Randomized, double-blind, placebo-controlled trial. Am. J. Clin. Nutr. 2013, 97, 37–44. [Google Scholar] [CrossRef]
- Sins, J.W.R.; Mager, D.J.; Davis, S.C.A.T.; Biemond, B.J.; Fijnvandraat, K. Pharmacotherapeutical strategies in the prevention of acute, vaso-occlusive pain in sickle cell disease: A systematic review. Blood Adv. 2017, 1, 1598–1616. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.A.; Damanhouri, G.A.; Ahmed, T.J.; Halawani, S.H.; Ali, A.; Makki, A.; Khan, S.A. Omega 3 fatty acids—Potential modulators for oxidative stress and inflammation in the management of sickle cell disease. J. Pediatr. 2022, 98, 513–518. [Google Scholar] [CrossRef]
- Tomer, A.; Kasey, S.; Connor, W.E.; Clark, S.; Harker, L.A.; Eckman, J.R. Reduction of pain episodes and prothrombotic activity in sickle cell disease by dietary n-3 fatty acids. Thromb. Haemost. 2001, 85, 966–974. [Google Scholar] [CrossRef]
- Ugwu, A.; Iloanusi, N.; Ugwu, N.; Chukwu, B.; Ezenwosu, O.; Modebe, E.; Duru, A.; Madu, A.; Chibueze, E.; Igboke, M.; et al. Pilot assessment of omega-3 fatty acids and potassium thiocyanate in sickle cell anemia patients with conditional peak systolic cerebral artery blood velocity. Blood Cells Mol. Dis. 2021, 89, 102564. [Google Scholar] [CrossRef]
- Cameron-Smith, D.; Albert, B.; Cutfield, W. Fishing for answers: Is oxidation of fish oil supplements a problem? J. Nutr. Sci. 2015, 4, e36. [Google Scholar] [CrossRef]
- Sullivan, J.C.; Budge, S.M.; St-Onge, M. Modeling the Primary Oxidation in Commercial Fish Oil Preparations. Lipids 2010, 46, 87–93. [Google Scholar] [CrossRef]
- Kolanowski, W.; Berger, S. Possibilities of fish oil application for food products enrichment with omega-3 PUFA. Int. J. Food Sci. Nutr. 1999, 50, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Jackowski, S.A.; Alvi, A.Z.; Mirajkar, A.; Imani, Z.; Gamalevych, Y.; Shaikh, N.A.; Jackowski, G. Oxidation levels of North American over-the-counter n-3 (omega-3) supplements and the influence of supplement formulation and delivery form on evaluating oxidative safety. J. Nutr. Sci. 2015, 4, e30. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Resurreccion, A.; Paguio, L. Age appropriate hedonic scales to measure food preferences of young children. J. Sens. Stud. 1996, 11, 141–163. [Google Scholar] [CrossRef]
- Lebensburger, J.D.; Hilliard, L.M.; Pair, L.E.; Oster, R.; Howard, T.H.; Cutter, G.R. Systematic review of interventional sickle cell trials registered in ClinicalTrials.gov. Clin. Trials 2015, 12, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Wandersee, N.J.; Maciaszek, J.L.; Giger, K.M.; Hanson, M.S.; Zheng, S.; Guo, Y.; Mickelson, B.; Hillery, C.A.; Lykotrafitis, G.; Low, P.S.; et al. Dietary supplementation with docosahexanoic acid (DHA) increases red blood cell membrane flexibility in mice with sickle cell disease. Blood Cells Mol. Dis. 2015, 54, 183–188. [Google Scholar] [CrossRef]
- Demark-Wahnefried, W.; Polascik, T.J.; George, S.L.; Switzer, B.R.; Madden, J.F.; Ruffin, M.T.; Snyder, D.C.; Owzar, K.; Hars, V.; Albala, D.M.; et al. Flaxseed Supplementation (Not Dietary Fat Restriction) Reduces Prostate Cancer Proliferation Rates in Men Presurgery. Cancer Epidemiol. Biomark. Prev. 2008, 17, 3577–3587. [Google Scholar] [CrossRef] [PubMed]
- Parikh, M.; Maddaford, T.G.; Austria, J.A.; Aliani, M.; Netticadan, T.; Pierce, G.N. Dietary Flaxseed as a Strategy for Improving Human Health. Nutrients 2019, 11, 1171. [Google Scholar] [CrossRef] [PubMed]
- Ghaseminasab-Parizi, M.; Nazarinia, M.-A.; Akhlaghi, M. The effect of flaxseed with or without anti-inflammatory diet in patients with rheumatoid arthritis, a randomized controlled trial. Eur. J. Nutr. 2021, 61, 1377–1389. [Google Scholar] [CrossRef]
- Zhu, L.; Sha, L.; Li, K.; Wang, Z.; Wang, T.; Li, Y.; Liu, P.; Dong, X.; Dong, Y.; Zhang, X.; et al. Dietary flaxseed oil rich in omega-3 suppresses severity of type 2 diabetes mellitus via anti-inflammation and modulating gut microbiota in rats. Lipids Health Dis. 2020, 19, 20. [Google Scholar] [CrossRef]
- Wong, H.; Chahal, N.; Manlhiot, C.; Niedra, E.; McCrindle, B.W. Flaxseed in Pediatric Hyperlipidemia: A Placebo-Controlled, Blinded, Randomized Clinical Trial of Dietary Flaxseed Supplementation for Children and Adolescents with Hypercholesterolemia. JAMA Pediatr. 2013, 167, 708–713. [Google Scholar] [CrossRef] [PubMed]
- Gholami, Z.; Akhlaghi, M. The effect of flaxseed on physical and mental fatigue in children and adolescents with overweight/obesity: A randomised controlled trial. Br. J. Nutr. 2021, 126, 151–159. [Google Scholar] [CrossRef]
- Kazem, Y.; Zarouk, W.A.; Hamed, K.; Tosson, A.M.; Essa, H.A.; El-Bassyouni, H.T. The Effect of Anti-inflammatory Diet and Vitamin D Supplementation on the Amelioration of the Clinical Status and Cognitive functions of Familial Mediterranean Fever Patients. Kobe J. Med. Sci. 2021, 66, E159–E165. [Google Scholar] [PubMed]
- Gneezy, A.; Imas, A.; Jaroszewicz, A. The impact of agency on time and risk preferences. Nat. Commun. 2020, 11, 2665. [Google Scholar] [CrossRef] [PubMed]
- Baccarani, A.; Donnadieu, S.; Pellissier, S.; Brochard, R. Relaxing effects of music and odors on physiological recovery after cognitive stress and unexpected absence of multisensory benefit. Psychophysiology 2023, e14251. [Google Scholar] [CrossRef] [PubMed]
- Tonacci, A.; Billeci, L.; Di Mambro, I.; Marangoni, R.; Sanmartin, C.; Venturi, F. Wearable Sensors for Assessing the Role of Olfactory Training on the Autonomic Response to Olfactory Stimulation. Sensors 2021, 21, 770. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dike, C.R.; Lebensburger, J.; Mitchell, C.; Darnell, B.; Morrow, C.D.; Demark-Wahnefried, W. Palatability and Acceptability of Flaxseed-Supplemented Foods in Children with Sickle Cell Disease. Nutrients 2023, 15, 1245. https://doi.org/10.3390/nu15051245
Dike CR, Lebensburger J, Mitchell C, Darnell B, Morrow CD, Demark-Wahnefried W. Palatability and Acceptability of Flaxseed-Supplemented Foods in Children with Sickle Cell Disease. Nutrients. 2023; 15(5):1245. https://doi.org/10.3390/nu15051245
Chicago/Turabian StyleDike, Chinenye R., Jeffrey Lebensburger, Ciara Mitchell, Betty Darnell, Casey D. Morrow, and Wendy Demark-Wahnefried. 2023. "Palatability and Acceptability of Flaxseed-Supplemented Foods in Children with Sickle Cell Disease" Nutrients 15, no. 5: 1245. https://doi.org/10.3390/nu15051245
APA StyleDike, C. R., Lebensburger, J., Mitchell, C., Darnell, B., Morrow, C. D., & Demark-Wahnefried, W. (2023). Palatability and Acceptability of Flaxseed-Supplemented Foods in Children with Sickle Cell Disease. Nutrients, 15(5), 1245. https://doi.org/10.3390/nu15051245




