Abstract
Sleeve gastrectomy (SG) induces weight loss but its effects on body composition (BC) are less well known. The aims of this longitudinal study were to analyse the BC changes from the acute phase up to weight stabilization following SG. Variations in the biological parameters related to glucose, lipids, inflammation, and resting energy expenditure (REE) were concomitantly analysed. Fat mass (FM), lean tissue mass (LTM), and visceral adipose tissue (VAT) were determined by dual-energy X-ray absorptiometry in 83 obese patients (75.9% women) before SG and 1, 12 and 24 months later. After 1 month, LTM and FM losses were comparable, whereas at 12 months the loss of FM exceeded that of LTM. Over this period, VAT also decreased significantly, biological parameters became normalized, and REE was reduced. For most of the BC, biological and metabolic parameters, no substantial variation was demonstrated beyond 12 months. In summary, SG induced a modification in BC changes during the first 12 months following SG. Although the significant LTM loss was not associated with an increase in sarcopenia prevalence, the preservation of LTM might have limited the reduction in REE, which is a longer-term weight-regain criterion.
1. Introduction
Bariatric surgery (BS) is an effective method for both acute and long-term weight loss in obese patients when other treatments have failed and when the body mass index (BMI) is greater than 40 kg/m2 (severe obesity) or greater than 35 kg/m2 with obesity-related comorbidities [1]. The loss of excess body weight is generally a criterion for successful surgery. However, while the change in absolute weight loss provides an indication of progress [2] and is easy to use in clinical practice, it cannot adequately reflect changes in fat mass (FM) and lean tissue mass (LTM), the two compartments that constitute body composition. These two indicators are more closely related to all-causes and cardiovascular mortality than to weight [3].
After BS, the greatest weight loss occurs in the first year and weight stabilization is achieved in the second [4,5]. Weight regain may start after the second or third year [4,5]. Conversely, the long-time course of body composition change is unclear, probably because the techniques of investigation—including bioelectrical impedance analysis (BIA) and dual-energy X-ray absorptiomety (DXA)—may introduce measurement bias [6]. Moreover, although BIA is less expensive than DXA, it may underestimate FM and overestimate LTM in patients with obesity [7]. The body hydration disturbance present in this population may lead to measurement errors [8]. When the reference technique is used (i.e., DXA) [9], a biphasic variation is observed. This has been characterized by an acute and concomitant reduction in FM and LTM in the first few months after BS, followed by a sustained reduction in FM during the subsequent prolonged weight-loss period [10,11,12]. Generally, the time of follow-up in these studies has been limited to the first 12 months [11,12,13], and very few longitudinal studies have been performed between 24 and 36 months [14,15]. Yet monitoring body composition change over a longer postsurgery period may provide clinically relevant information to better identify and treat patients according to lifestyle and medical care [13,16]. Moreover, although it is not clear whether body composition changes vary with the surgical procedure, studies have preferentially focused on the Roux-en-Y gastric bypass (RYGB) technique rather than sleeve gastrectomy (SG) [13], despite SG recently becoming the most common bariatric approach [17].
It was also reported that patients with weight regain presented higher %FM and lower %LTM than patients maintaining stable weight [18]. Moreover, a lower weight-adjusted resting energy expenditure (REE) was observed in the weight regain group [18], suggesting that this clinical parameter should also be routinely evaluated. These studies, thus, seem to indicate that an excessive reduction in LTM during weight loss programs may have some deleterious effects on metabolism, thermoregulation, functional capacity and weight regain [19].
While body composition analysis appears to be an improvement over simple body weight assessment, the measurement of visceral adipose tissue (VAT) may be even more relevant in patients with obesity. For example, VAT excess induces and maintains lipotoxicity and insulin resistance [20,21], playing a central role in cardiac dysfunction [22]. VAT is also an important endocrine organ that secretes pro-inflammatory factors, resulting in chronic low-grade systemic inflammation that may be involved in the pathogenesis of metabolic abnormalities [23]. However, to the best of our knowledge, few studies have investigated the VAT change after BS [24,25].
The aims of this study were to analyse the FM, LTM and VAT changes from the acute phase of body weight loss (1 month) until a recognized phase of body weight stabilization (12 and 24 months) following SG. We also analysed the variation in the biological parameters related to glucose, lipids and inflammation, as well as the resting energy expenditure.
2. Materials and Methods
2.1. Subjects and Method
The study was approved by the local research ethics committee (ID RCB: 2015-A01047-42). All patients signed a consent form before entering the study. The clinical trial number is NCT02712086.
2.1.1. Subjects
From November 2016 to July 2020, 83 Caucasian patients (women n = 63; 75.9%) from 18.4 to 60.0 years old were recruited from candidates for BS in the obesity management centre, University Hospital of Montpellier (Montpellier, France). The inclusion criteria were: inaction of other weight loss treatments, BMI > 40 or BMI ≥ 35 kg/m2 with the presence of comorbidities [type 2 diabetes (T2D), sleep apnoea syndrome or arterial hypertension (HTA)] and more than 4 years of obesity [1]. The exclusion criteria were: previous BS, pregnancy, medical treatment or physical handicap that might affect body composition evaluation, and body weight >190 kg or height >192.5 cm (limitations of the densitometry device). Physical activity levels were not specifically determined, but none of the participants was participating in a training program in the period before inclusion. Medical history was obtained by questionnaires. All the BS procedures were sleeve gastrectomy (SG), which consists of resecting most of the greater curvature to reduce gastric size and leaving a narrow stomach tube. The SGs were performed in a single institution and in only one surgical department.
2.1.2. Methods
This study followed a longitudinal design; its methodology was previously described in detail [10,11]. However, the data of the patients included in this study have never been published. Briefly, all the patients were evaluated the day before the operation (baseline) and 1, 12 and 24 months after SG. After SG, patients were encouraged to increase their physical activity, improve protein intake, and reduce fat intake in order to lose weight, while avoiding side effects, such as muscle mass loss or steatorrhea. For each visit, standing height and weight were measured with a stadiometer and a weight scale with a precision of 0.1 kg. BMI was determined as weight (kg)/height2 (m). The ideal body weight (IBW in kg) was obtained from the Lorentz equations to calculate ideal body weight (IBW): (height [cm]—100)—((height [cm]—150)/4) for men and (height [cm]—100)—((height [cm]—150)/2.5) for women.
2.1.3. Comorbidity Definitions
- T2D was defined as glycated haemoglobin (HbA1c) ≥6.5% and/or fasting glycaemia ≥7 mmol/L and/or antidiabetic treatment [26].
- Arterial hypertension (HTA) was defined as systolic blood pressure >140 mmHg and/or diastolic blood pressure >90 mmHg and/or use of antihypertensive medications [27].
2.1.4. Regional and Whole Body FM and LTM
FM (kg, %) and LTM (kg) were measured using DXA (Horizon A, Hologic, Inc., Waltham, MA, USA) at whole body and regional sites (upper limbs, trunk, and lower limbs). The same operator performed all scanning and analyses to ensure consistency after following standard quality control procedures. For LTM and FM, the coefficients of variation (CVs) given by the manufacturer were <1%. In the abdominal region, total adipose tissue (TAT), VAT and superficial adipose tissue (SAT) were measured according to a previously validated method [28].
To define sarcopenia in terms of low LTM, we chose the most current cut-offs used for the Caucasian population. The sum of the LTM of the arms and legs defined the appendicular lean mass (ALM; kg). ALM/height2 index [ALMI(h2); kg/m2] or ALMI/body mass index [ALMI(BMI)] defined the ALM index. ALM < 20 kg and ALMI(h2) < 7 kg/m2 in men and ALM < 15 kg and ALMI(h2) < 5.5 kg/m2 in women [29] defined sarcopenia according to The European Working Group on Sarcopenia in Older People (EWGSOP2). ALM < 19.75 kg and ALMI(BMI) < 0.789 in men and ALM < 15.02 kg and ALMI(BMI) <0.512 in women defined the cut-points for low LTM for sarcopenia according to the Foundation for the National Institutes of Health (FNIH) [30]. Finally, ALMI(h2) ≤ 7.23 kg/m2 in men and ≤5.67 kg/m2 in women [31] defined sarcopenia according to the International Working Group on Sarcopenia (IWGS, Albuquerque, NM, USA).
2.1.5. Assays
Blood samples (35 mL) were collected in fasting conditions in the morning (8:30–9:00 am). The samples were centrifuged at 2500× g for 10 min at 4 °C. Serum samples were stored at −80 °C and most analyses were performed at the end of the study to reduce interassay variation. In premenopausal women, the blood samples were obtained at an unsynchronized menstrual stage.
Albumin, HbA1c, cholesterol, HDL, triglyceride, glucose, insulin and CRP were routinely analysed by Cobas 101, 501, 602 or 701 (Roche Diagnostic, Mannheim, Germany). The interassay and intraassay coefficients of CVs for the majority of these parameters were lower than 5%. The IGF-1 (Reference IS-3900) and IGFBP-3 (Reference IS-4400) assays are based on chemiluminescence technology and were analysed with IDS-iSYS (Immunodiagnostic Systems, Tyne & Ware, Boldon, UK). The intraassay and interassay CVs were 1.9 and 3.9% for IGF-1 and 1.8 and 6.3% for IGFBP-3. For all the biological parameters, the CVs for the intraassay and interassay variations were given by the manufacturer.
Insulin resistance was estimated using the homeostasis model assessment of insulin resistance (HOMA-IR) according to the following formula: fasting serum insulin (mIU/mL)/fasting plasma glucose (mmol/L)/22.5 [26].
2.1.6. Resting Energy Expenditure (REE)
Measured REE (REEm) was performed in all patients between 8:00 and 8:30 h after overnight fasting and without the practice of physical exercise for 48 h. REEm was assessed by indirect calorimetry (Quark RMR, Cosmed, Rome, Italy), which analysed oxygen, carbon dioxide and ventilation in a mixing chamber over a period of at least 30 min. Predicted REE values (%; REEp) were calculated from the equation of Harris and Benedict modified by Roza and Shizgal [32] as follows: REEp = 667.051 + 9.74 × (weight) + 1.729 × (height) − 4.737 × (age).
2.1.7. Statistical Analysis
Quantitative variables are described with means and standard deviations (SD) after normality testing of the continuous variables with Shapiro–Wilk’s test. For categorical variables, the numbers and associated percentages are presented.
Paired Wilcoxon or paired Student’s tests were used, depending on the normality of the distribution, to compare the relative variations (100 × (measure 2 − measure 1)/measure 1) between baseline and 1, 12 and 24 months for the patients’ clinical characteristics; whole-body composition; android, gynoid, and abdominal adipose tissue; and biological parameters.
Relationships between preoperative characteristics (age, BMI, whole-body LTM, FM, TAT, VAT, SAT and biological parameters) and baseline or relative variations in body composition parameters at 1, 12 or 24 months were assessed using Spearman or Pearson correlation coefficients, depending on the normality of the distributions.
All analyses were two-tailed, with a p-value of < 0.05 considered statistically significant. SAS® Enterprise Guide software (version 8.2, SAS Institute, Cary, NC, USA) was used to perform the analyses and graphs were generated using R statistical software (www.r-project.org, version 4.1.3) with ggplot2 package (version 3.3.3).
3. Results
3.1. Anthropometric Parameters
A total of 83 patients with obesity underwent preoperative and at least one postoperative assessment of body composition (n = 83 with 1-month data; n = 76 with 12-month data. and n = 60 with 24-month data). The major reasons why 23 of the 83 subjects did not complete the 24-month follow-up were the following: they chose not to repeat the measurements (n = 15), were pregnant (n = 3), were relocated for work (n = 3), could not be located for follow-up (n = 2), or died (n = 1) (Table 1).

Table 1.
Clinical characteristics of the patients at baseline and 1, 12 and 24 months after sleeve gastrectomy.
All the baseline and variations in anthropometric characteristics and comorbidity prevalence are presented in Table 1 and Figure 1. At inclusion, the mean age was 40.9 ± 12.3 years, and the mean BMI was 40.7 ± 4.2 kg/m2. The relative mean weight loss was −9.1 ± 2.1% after 1 month, −29.3 ± 8.4% after 12 months, and −27.5 ± 9.6% after 24 months. As expected, all the anthropometric parameters were lower at all times compared to baseline, but no additive loss was observed between 12 and 24 months. All the comorbidities decreased with time. Specifically, T2D was only present in six patients (<10%) at 24 months. Among the 17 patients who presented T2D at baseline, only two were lost to follow-up (one had died).
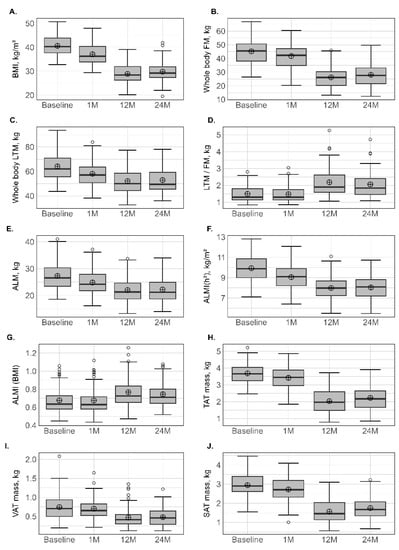
Figure 1.
Variation in anthopometric and body composition parameters at different times. BMI: body mass index (A); FM: fat mass (B); LTM: lean tissue mass (C); LTM/FM ratio (D); ALM: appendicular lean mass (E); ALMI(h2): appendicular lean mass index (height2) (F); ALM/height2, ALMI(BMI) (G): appendicular lean mass index (BMI); TAT: total adipose tissue (H); VAT: visceral adipose tissue (I); SAT: subcutaneous adipose tissue (J). Lower whisker represents the smallest observation greater than or equal to lower hinge −1.5 × IQR. Upper whisker represents the largest observation less than or equal to upper hinge +1.5 × IQR. Lower and upper hinges represent, respectively, the 25 and the 75% quartile values. The open circles represent the mean value in each time. Data beyond the end of the whiskers are called outliers points and are plotted individually. IQR, interquartile range.
3.2. Body Composition
Initial values and changes in FM and LTM are presented in Table 2 and Figure 1. At 1 month, the reduction in % relative variation at the different sites ranged between −5.3% (±7.7%) at the upper limbs and −9.0% (±4.9%) at the trunk for FM (kg), and between −8.9% (±5.1%) at the lower limbs and −10.4% (±4.8 %) at the trunk for LTM (kg). At 12 months, all the parameters related to FM and LTM had significantly decreased, but the % relative variation in LTM was lower than in FM, which was also supported by an increase in the LTM/FM ratio. At 12 months, the reduction in % relative variation at the different sites ranged between −39.0% (±12.1%) at the lower limbs and −47.9 % (±14.1%) at the trunk for FM (kg), while the loss for LTM was about 20% and homogeneous between sites. No subsequent loss in FM or LTM was observed at 24 months. The prevalence of T2D, HTA and obstructive sleep apnoea decreased following surgery.

Table 2.
Whole body composition of the patients at baseline and 1, 12 and 24 months after sleeve gastrectomy.
3.3. Sarcopenia Prevalence
ALM and ALM(h2) were significantly reduced, with a comparable percentage relative variation between presurgery and 1 month, and between 1 month and 12 months, around 10%. Conversely, ALMI(BMI) increased at 12 and 24 months in comparison with baseline values. Sarcopenia was diagnosed in 10 patients (4 men and 6 women) at baseline and in eight patients (4 men and 4 women) at 1 month only when the FNIH (ALMI(BMI)) criteria were used. Four patients (all women) and two patients (1 man and 1 woman) presented sarcopenia at 12 and 24 months, defined mainly by FNIH and EWGSOP (Table 2 and Figure 1).
3.4. Android, Gynoid and Abdominal Body Composition
All parameters (total mass, LTM and FM) measured at android or gynoid regions decreased significantly at 1 and 12 months and this was accentuated with the time from surgery, following the same pattern as observed for the whole body (Table 3 and Figure 1). This was characterized by an accentuated LTM at 1 month, while, for FM, the decrease was maintained over the first 12 months. At abdominal regions, a progressive FM loss (p < 0.001) for TAT (−7.69% at 1 month, −45.75% at 12 months), VAT (−7.16% at 1 month, −34.8% at 12 months) and SAT (−7.98% at 1 month, −46.89% at 12 months) was also observed. At the android, gynoid and abdominal regions, no subsequent loss was observed between 12 and 24 months.

Table 3.
Android, gynoid and abdominal adipose tissue at baseline and 1, 12 and 24 months after sleeve gastrectomy.
3.5. Biological Parameters
The variation in biological parameters is presented in Table 4 and Figure 2. For glucose homeostasis, a significant decrease was observed in the levels of fasting glucose, HbA1c, insulin and HOMA-IR, suggesting improved insulin sensitivity at all postsurgical time points. No subsequent loss was observed for these biological parameters after 12 months. IGF-1 and IGF-BP3 decreased simultaneously after 1 month, whereas IGF-1 had higher values and IGFBP-3 had lower values at 12 and 24 months in comparison with presurgical values. The concentration of albumin did not change significantly with time, whereas a significant decrease in CRP at 1 month was observed and accentuated at 12 and 24 months.

Table 4.
Biological parameters at baseline and 1, 12 and 24 months after sleeve gastrectomy.
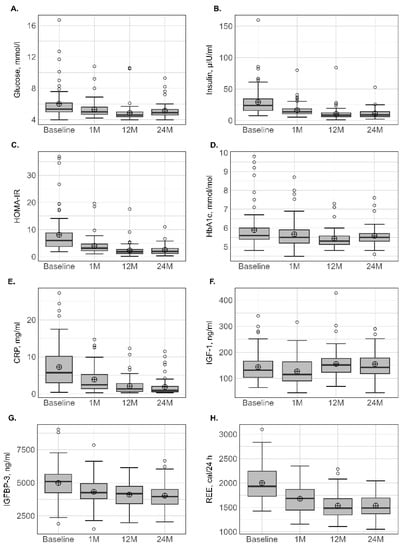
Figure 2.
Variation in biological parameters at different times. BMI: body mass index (A); FM: fat mass (B); LTM: lean tissue mass (C); LTM/FM ratio (D); ALM: appendicular lean mass (E); ALMI(h2): appendicular lean mass index (height2) (F); ALM/height2, ALMI(BMI) (G): appendicular lean mass index (BMI); TAT: total adipose tissue (H); VAT: visceral adipose tissue (I); SAT: subcutaneous adipose tissue (J). Lower whisker represents the smallest observation greater than or equal to lower hinge −1.5 × IQR. Upper whisker represents the largest observation less than or equal to upper hinge + 1.5 × IQR. Lower and upper hinges represent, respectively, the 25 and the 75% quartile values. The open circles represent the mean value in each time. Data beyond the end of the whiskers are called outlier points and are plotted individually. IQR, interquartile range.
Compared to baseline, absolute REEm significantly decreased by −15.7% at 1 month and continued to decrease at 12 months, which totalled a reduction of −23.2%. No subsequent decrease was observed between 12 and 24 months.
3.6. Correlations between Basal Parameters and Body Composition Change
The correlation analysis between the basal parameters and % relative body composition changes at the various time points is presented in Table 5. Few significant correlations were observed and only age seemed clearly associated with LTM and FM losses at 12 and 24 months.

Table 5.
Correlation between preoperative characteristics and basal or variations in body composition parameters.
4. Discussion
This study aimed to characterize the changes in body composition and biological and metabolic parameters in patients with obesity over a 24-month period following SG. The study demonstrated that a significant modification in body composition was associated with the body weight loss and was characterized by an acute reduction in LTM and a continuous loss of FM and VAT over the first 12 months. Concomitantly, an improvement in the lipid, glycaemic, inflammatory and somatotropic profiles was also demonstrated.
This longitudinal study, based on a cohort composed mostly of women, showed that SG induced a loss of approximately 29% of the presurgical body weight and 20% of the anthropometric parameters, including waist and hip circumferences, after 12 and 24 months. The positive effect of weight loss on glycaemic, lipid, inflammatory and somatotropic profiles was clearly confirmed even though some of the patients continued to be overweight or obese at 24-months postsurgery. Moreover, the LTM and FM losses were significant from the first month and maintained over the 2 years. However, the kinetics of loss seemed to be specific to the components. Thus, although the magnitude of variation was relatively comparable between FM and LTM in the first month—i.e., around 8–10%—almost half of the total loss in LTM observed in the first 12 months occurred during this acute phase. The loss in FM appeared more progressive and sustained during the subsequent prolonged weight-loss period, and at 12 months it largely exceeded that of LTM (−38 vs. −20%). It is interesting to note that the % relative variation in LTM and FM appeared relatively comparable between sites, including limbs, trunk and whole body throughout the study, suggesting a uniform body loss. These results are completely superimposable in terms of kinetics and intensity with those of two previously published studies by our group performed in a limited group of patients (n = 30) after the same follow-up periods [10,11]. The findings from our previous and current studies suggest that, for the same surgical procedure (i.e., SG) and patient characteristics (sex, age and BMI), the expected body composition changes are very reproducible. Comparison with other works must be made with caution because the type of surgical procedure (SG vs. RYGB vs. one-anastomosis gastric bypass) and the device used to measure body composition (BIA vs. DXA) may interfere with the results [6,13,14,33]. In a group of mainly female patients who had undergone predominantly SG, Sivakumar et al. [12] also reported that LTM depletion evaluated with DXA occurred predominantly in the initial month, whereas FM declined more progressively over the 12-month follow-up. A recent meta-analysis of 122 studies [13] highlighted that all types of BS cause LTM decline and, although the rate of LTM decrement decreases over time, it follows a significant downward trend over the first 12 months. It should be noted that no subgroup analysis according to the surgical procedure was performed in this meta-analysis [13]. Last, using BIA, two longitudinal studies [14,34] demonstrated that the greatest LTM loss (~7–10%) occurred between 1 and 3 months and that the loss slowed down at 12 months, with values of approximately 80−85% of the presurgical values.
The highest rate of LTM loss in the first months following BS may be explained by the reduction in physical activity, inadequate protein intake, and restricted global food intake during this period of about 700 kcal/day, which can promote proteolysis to meet the metabolic demands [35,36]. The initial decrease in IGF-1, an anabolic hormone that is sensitive to nutritional intake and even more so to protein intake, might confirm this hypothesis. In humans, weight decrease is associated with IGF-1 reduction only when energy intake is below 50% of the daily ration. [37,38]. In our study, we reported, for the first time, that, after 12 months, the IGF-1 values increased and exceeded the presurgical values. Functional hyposomatotropism is a situation frequently observed in obesity [39], and normalization of nutritional intake alone cannot explain this overcompensation. Obesity is also a disease characterized by the presence of low-grade chronic inflammation [40], and the recovery of normal IGF-1 synthesis over time after BS was reported to be independently related with CRP [39]. Consequently, the decrease in CRP found in our study might have led to an increase in IGF-1, which, in turn, might have reduced the rate of LTM loss after 1 month [41]. The favourable effect of IGF-1 in our study might even have been more effective because its free form was increased due to the reduction in its main binding protein (IGFBP-3) throughout the study. This finding has never before been reported. Ohira et al. [42] reported an important effect of IGF-1 on LTM because they found that preoperative values were related to maintaining skeletal muscle mass and decreasing body FM in a sex-dependent manner. Unfortunately, in this study [42], no information concerning the variation in IGF-1 after BS was reported. In line with our results, Juiz-Valina et al. [39] investigated 116 patients who had undergone RYGB or SG, reporting an initial decrease in IGF-1 after 1 month followed by a progressive increase in growth hormone and IGF-1 up to 12 months. Conversely, De Marinis et al. [43] studied 15 obese female patients 16–24 months after biliopancreatic diversion (BPD) and reported that, although the GH response to GH-releasing hormone markedly increased, the initially low IGF-1 and IGFBP-3 concentrations remained unchanged.
Very few studies have analysed body composition changes after weight stabilization over the 12 months following BS, and specifically SG [14,15,25,33,34]. Moreover, the data are generally drawn from cross-sectional analyses, which limits their scope [25,33]. Our results offer new findings by demonstrating that no substantial loss was observed for LTM and FM between 12 and 24 months, which suggests that a new steady state was established 12 months following BS. Two longitudinal studies of 24 and 36 months, using BIA, also observed minor body composition changes after 12 months following BS [14,34].
The reduction in body weight and FM after BS is a desired result, while the LTM loss may have deleterious functional and metabolic consequences. However, we observed, for the first time, that 24 months post-SG, the prevalence of low muscle mass—a criterion involved in the definition of sarcopenia evaluated by DXA—did not increase. This observation was made regardless of the threshold [ALM, ALMI(h2) or ALMI(BMI)]. The presurgical high LTM values in patients with obesity [44] and the maintenance of relative subnormal mean BMI (~28–29 kg/m2) after BS could be responsible. In line with our results, previous studies using DXA have reported that none of the patients presented pathologically low LTM from one to several years (2–18) after RYGB, thus ruling out the diagnosis of sarcopenia [25,45]. We note that the prevalence of sarcopenia may vary with the technique. For example, Vassilev et al. [46] used magnetic resonance imaging (RMI) and reported that 57% of the patients were sarcopenic after 24 weeks following RYGB, whereas Voican et al. [47] used computed tomography and observed a sarcopenia prevalence of 32% in patients 1 year after SG.
Although the LTM loss seems to have no or only a limited effect on the sarcopenia prevalence up to 24 months, it may alter the metabolic status because—as observed in previous studies as well as this one—muscle mass is the primary determinant of REE [19,34,48] rather than other parameters such as FM [34]. We observed that the main reductions in REE and LTM were concomitant at 1 month, with values continuing to decrease at 12 months and stabilized at 24 months. Two studies performed in patients with extreme obesity (BMI > 50 kg/m2) who underwent different surgical procedures [34,49] also reported this initial REE loss at 1 month that continued up to 24 months. However, it should be noted that body composition was not concomitantly evaluated [49] and that REE was indirectly evaluated by BIA [34], limiting the scope of these works. Our results clearly underlined that the first months, and particularly the first month, are crucial for LTM loss. Consequently, the implementation of programs in this period based on a nutritional approach, such as protein supplementation [50], or exercise interventions, particularly resistance training [51], should help preserve LTM and thus REE [52,53]. The maintenance of REE after BS is crucial and this problem needs to be resolved because low values are associated with weight regain [49,54]. The crucial role of LTM in weight homeostasis and metabolism point to the importance of evaluating body composition in each patient to improve weight loss [34]. However, as underlined by Martinez et al. [34], LTM is rarely evaluated in daily clinical practice.
In parallel to the conventional parameters of body composition obtained with DXA, we also evaluated the variation in abdominal adiposity. This approach may be more pertinent in this population because most of the obesity-related comorbidities are more related to VAT than whole body FM. For example, obesity, especially when linked to increases in visceral fat, has more deleterious effects on the cardiovascular system than subcutaneous compartments [22]. We demonstrated herein that abdominal FM parameters, including TAT, VAT and SAT, followed a profile similar to that of whole-body FM in terms of kinetics and intensity. Despite their pertinence, these parameters are largely under-analysed [10,24,55,56,57,58,59] and, to our knowledge, only two studies have evaluated them with comparable techniques (i.e., DXA) and surgical procedures [10,57], but with a limited follow-up duration. For example, Zang et al. [57] reported a single measurement point after only 3 months, with VAT decreased by approximately 22.5%. In our study, the relative variation in VAT was −7.8 at 1, −34.8 at 12, and −38.4 % at 24 months, and these results are totally in line with those previously published by our group at 1 and 12 months [10]. It is well established that diabetic patients have a greater amount of VAT before surgery compared to normoglycaemic subjects [24], and this observation persisted even later after surgery [25].
The identification of the basal parameters potentially associated with postsurgical body composition change is an attractive approach to implementing corrective measures. In this study, only age was positively but weakly correlated with LTM and FM loss at 12 and 24 months. Conversely, a recent study reported that the loss of LTM measured with BIA at 24 months was only associated with presurgical values of LTM and HOMA-IR independent of age, gender and BS techniques [34]. Other studies have reported that higher BMI and LTM before surgery, male gender and older age were associated with greater LTM loss [60]. Finally, higher preoperative BMI, female gender and patients undergoing SG were also found to be determinants of greater LTM [14,61]. Many factors, including gender and the age distribution of the studied group, may explain the discrepancies in the results.
5. Limitations and Strength
The strength of this longitudinal study is the long follow-up period after surgery (24 months) with four repeated measures from the acute loss of body weight up to body weight stabilization. Moreover, as recommended by the International Society for Clinical Densitometry (ISCD) guidelines [9], the body composition change after BS was evaluated by the gold standard technique (DXA). In addition, the concomitant evaluation of biological parameters reflecting glucose homeostasis, inflammation and metabolism may improve our knowledge on the potential link between body composition change and these parameters. We are aware that our study presents some limitations, including a limited number of patients at inclusion and after 24 months, due to the difficulties in maintaining patient compliance and the COVID-19 pandemic. However, the study group reflects the population managed in our surgical department, and the number of included patients is in line with previous studies [13], reflecting the difficulties of including and following these patients after BS. In addition, a longer study until weight regain occurs might provide more clues with factors that are closely associated with weight regain after SG. Moreover, our cohort was composed of men and women, and it cannot be excluded that a sex-specific body composition change before surgery occurred, particularly for VAT [59]. Our population was mainly women, and, thus, no subgroup analysis was performed. Last, specific data on food intake and physical activity levels were not collected. However, all patients had received the same hygienic and dietetic advice from a specialized team before and during the follow-up.
6. Conclusions
Our longitudinal study provides crucial findings on the kinetics and specificity of the two components of body composition change over a 2-year period after SG. FM tended to progressively decrease up to 12 months, whereas LTM loss was predominant in the first month. Taking into account the crucial role of LTM on REE regulation and potential weight regain, multidisciplinary (surgeons, dietitians, physiotherapists, …) reflection and dialogue should be encouraged to develop strategies for limiting LTM loss, particularly in the early postoperative period.
Author Contributions
L.M., M.D., M.-C.P. and D.N. conceptualized the original research study; L.M., P.L., M.P., L.M., E.R., J.-P.C., J.M., D.M.-G. and D.N. provided input into this analysis; L.M. wrote the initial manuscript and its subsequent drafts; L.M. and M.P. coordinated data collection; L.M., S.A. and M.-C.P. conducted the data analysis; L.M. and M.P. provided support for the data analyses; and P.d.S.B., L.M., P.L., M.P., L.M., E.R., J.-P.C., J.M., D.M.-G. and D.N. provided inputs, edits, and revisions for the subsequent drafts of the manuscript and contributed to substantial revisions of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
Support for this research was provided by the AOI of CHU Montpellier ID: 9590. The opinions expressed herein are solely those of the authors.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the COMITE DE PROTECTION DES PERSONNES SUD MÉDITERRANÉE I (ID RCB: 2015-A01047-42). The Clinical Trial Number is: NCT02712086.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data used in the present analysis can be obtained through request to the corresponding author.
Acknowledgments
We express special gratitude to the CHU Montpellier, France, for funding this study. We are thankful to the technicians and nurses from the Departments of Clinical Physiology, Endocrinology and Nuclear Medicine for their support of the research study. Last, we are thankful to the study participants without whom this research endeavour would not have been possible.
Conflicts of Interest
I certify that neither I nor my co-authors have a conflict of interest as described above that is relevant to the subject matter or materials included in this work.
References
- Jensen, M.D.; Ryan, D.H.; Apovian, C.M.; Ard, J.D.; Comuzzie, A.G.; Donato, K.A.; Hu, F.B.; Hubbard, V.S.; Jakicic, J.M.; Kushner, R.F.; et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Obesity Society. J. Am. Coll. Cardiol. 2013, 63, 2985–3023. [Google Scholar] [CrossRef] [PubMed]
- Albanopoulos, K.; Tsamis, D.; Natoudi, M.; Alevizos, L.; Zografos, G.; Leandros, E. The impact of laparoscopic sleeve gastrectomy on weight loss and obesity-associated comorbidities: The results of 3 years of follow-up. Surg. Endosc. 2016, 30, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Beamish, A.J.; Olbers, T.; Kelly, A.S.; Inge, T.H. Cardiovascular effects of bariatric surgery. Nat. Rev. Cardiol. 2016, 13, 730–743. [Google Scholar] [CrossRef] [PubMed]
- Kennedy-Dalby, A.; Adam, S.; Ammori, B.J.; Syed, A.A. Weight loss and metabolic outcomes of bariatric surgery in men versus women—A matched comparative observational cohort study. Eur. J. Intern. Med. 2014, 25, 922–925. [Google Scholar] [CrossRef]
- Voorwinde, V.; Steenhuis, I.H.M.; Janssen, I.M.C.; Monpellier, V.M.; van Stralen, M.M. Definitions of Long-Term Weight Regain and Their Associations with Clinical Outcomes. Obes. Surg. 2020, 30, 527–536. [Google Scholar] [CrossRef]
- Frisard, M.I.; Greenway, F.L.; Delany, J.P. Comparison of methods to assess body composition changes during a period of weight loss. Obes. Res. 2005, 13, 845–854. [Google Scholar] [CrossRef]
- Schiavo, L.; Pilone, V.; Tramontano, S.; Rossetti, G.; Iannelli, A. May Bioelectrical Impedance Analysis Method Be Used in Alternative to the Dual-Energy X-Ray Absorptiometry in the Assessment of Fat Mass and Fat-Free Mass in Patients with Obesity? Pros, Cons, and Perspectives. Obes. Surg. 2020, 30, 3212–3215. [Google Scholar] [CrossRef]
- Johnson Stoklossa, C.A.; Forhan, M.; Padwal, R.S.; Gonzalez, M.C.; Prado, C.M. Practical Considerations for Body Composition Assessment of Adults with Class II/III Obesity Using Bioelectrical Impedance Analysis or Dual-Energy X-Ray Absorptiometry. Curr. Obes. Rep. 2016, 5, 389–396. [Google Scholar] [CrossRef]
- Shepherd, J.A.; Baim, S.; Bilezikian, J.P.; Schousboe, J.T. Executive summary of the 2013 International Society for Clinical Densitometry Position Development Conference on Body Composition. J. Clin. Densitom. 2013, 16, 489–495. [Google Scholar] [CrossRef]
- Maimoun, L.; Lefebvre, P.; Aouinti, S.; Picot, M.C.; Mariano-Goulart, D.; Nocca, D.; Montpellier Study Group of Bariatric, S. Acute and longer-term body composition changes after bariatric surgery. Surg. Obes. Relat. Dis. 2019, 15, 1965–1973. [Google Scholar] [CrossRef]
- Maimoun, L.; Lefebvre, P.; Jaussent, A.; Fouillade, C.; Mariano-Goulart, D.; Nocca, D. Body composition changes in the first month after sleeve gastrectomy based on gender and anatomic site. Surg. Obes. Relat. Dis. 2017, 13, 780–787. [Google Scholar] [CrossRef]
- Sivakumar, J.; Chen, Q.; Sutherland, T.R.; Read, M.; Ward, S.; Chong, L.; Hii, M.W. Body Composition Differences Between Excess Weight Loss ≥ 50% and < 50% at 12 Months Following Bariatric Surgery. Obes. Surg. 2022, 32, 2556–2566. [Google Scholar] [CrossRef]
- Haghighat, N.; Ashtary-Larky, D.; Bagheri, R.; Aghakhani, L.; Asbaghi, O.; Amini, M.; Moeinvaziri, N.; Hosseini, B.; Wong, A.; Shamekhi, Z.; et al. Preservation of fat-free mass in the first year after bariatric surgery: A systematic review and meta-analysis of 122 studies and 10,758 participants. Surg. Obes. Relat. Dis. 2022, 18, 964–982. [Google Scholar] [CrossRef]
- Barzin, M.; Heidari Almasi, M.; Mahdavi, M.; Khalaj, A.; Valizadeh, M.; Hosseinpanah, F. Body Composition Changes Following Sleeve Gastrectomy Vs. One-Anastomosis Gastric Bypass: Tehran Obesity Treatment Study (TOTS). Obes. Surg. 2021, 31, 5286–5294. [Google Scholar] [CrossRef]
- Sherf-Dagan, S.; Zelber-Sagi, S.; Buch, A.; Bar, N.; Webb, M.; Sakran, N.; Raziel, A.; Goitein, D.; Keidar, A.; Shibolet, O. Prospective Longitudinal Trends in Body Composition and Clinical Outcomes 3 Years Following Sleeve Gastrectomy. Obes. Surg. 2019, 29, 3833–3841. [Google Scholar] [CrossRef]
- Gomez-Ambrosi, J.; Andrada, P.; Valenti, V.; Rotellar, F.; Silva, C.; Catalan, V.; Rodriguez, A.; Ramirez, B.; Moncada, R.; Escalada, J.; et al. Dissociation of body mass index, excess weight loss and body fat percentage trajectories after 3 years of gastric bypass: Relationship with metabolic outcomes. Int. J. Obes. 2017, 41, 1379–1387. [Google Scholar] [CrossRef]
- Angrisani, L.; Santonicola, A.; Iovino, P.; Formisano, G.; Buchwald, H.; Scopinaro, N. Bariatric Surgery Worldwide 2013. Obes. Surg. 2015, 25, 1822–1832. [Google Scholar] [CrossRef]
- Shantavasinkul, P.C.; Omotosho, P.; Muehlbauer, M.J.; Natoli, M.; Corsino, L.; Tong, J.; Portenier, D.; Torquati, A. Metabolic profiles, energy expenditures, and body compositions of the weight regain versus sustained weight loss patients who underwent Roux-en-Y gastric bypass. Surg. Obes. Relat. Dis. 2021, 17, 2015–2025. [Google Scholar] [CrossRef]
- Marks, B.L.; Rippe, J.M. The importance of fat free mass maintenance in weight loss programmes. Sport. Med. 1996, 22, 273–281. [Google Scholar] [CrossRef]
- Montague, C.T.; O’Rahilly, S. The perils of portliness: Causes and consequences of visceral adiposity. Diabetes 2000, 49, 883–888. [Google Scholar] [CrossRef]
- Despres, J.P.; Lemieux, I. Abdominal obesity and metabolic syndrome. Nature 2006, 444, 881–887. [Google Scholar] [CrossRef] [PubMed]
- Rao, V.N.; Fudim, M.; Mentz, R.J.; Michos, E.D.; Felker, G.M. Regional adiposity and heart failure with preserved ejection fraction. Eur. J. Heart Fail. 2020, 22, 1540–1550. [Google Scholar] [CrossRef] [PubMed]
- Fontana, L.; Eagon, J.C.; Trujillo, M.E.; Scherer, P.E.; Klein, S. Visceral fat adipokine secretion is associated with systemic inflammation in obese humans. Diabetes 2007, 56, 1010–1013. [Google Scholar] [CrossRef]
- Favre, L.; Marino, L.; Roth, A.; Acierno, J., Jr.; Hans, D.; Demartines, N.; Pitteloud, N.; Suter, M.; Collet, T.H. The Reduction of Visceral Adipose Tissue after Roux-en-Y Gastric Bypass Is more Pronounced in Patients with Impaired Glucose Metabolism. Obes. Surg. 2018, 28, 4006–4013. [Google Scholar] [CrossRef]
- Santini, S.; Vionnet, N.; Pasquier, J.; Suter, M.; Hans, D.; Gonzalez-Rodriguez, E.; Pitteloud, N.; Favre, L. Long-term body composition improvement in post-menopausal women following bariatric surgery: A cross-sectional and case-control study. Eur. J. Endocrinol. 2022, 186, 255–263. [Google Scholar] [CrossRef]
- Ray, K.K.; Colhoun, H.M.; Szarek, M.; Baccara-Dinet, M.; Bhatt, D.L.; Bittner, V.A.; Budaj, A.J.; Diaz, R.; Goodman, S.G.; Hanotin, C.; et al. Effects of alirocumab on cardiovascular and metabolic outcomes after acute coronary syndrome in patients with or without diabetes: A prespecified analysis of the ODYSSEY OUTCOMES randomised controlled trial. Lancet Diabetes Endocrinol. 2019, 7, 618–628. [Google Scholar] [CrossRef]
- Geldsetzer, P.; Manne-Goehler, J.; Marcus, M.E.; Ebert, C.; Zhumadilov, Z.; Wesseh, C.S.; Tsabedze, L.; Supiyev, A.; Sturua, L.; Bahendeka, S.K.; et al. The state of hypertension care in 44 low-income and middle-income countries: A cross-sectional study of nationally representative individual-level data from 1.1 million adults. Lancet 2019, 394, 652–662. [Google Scholar] [CrossRef]
- Katzmarzyk, P.T.; Greenway, F.L.; Heymsfield, S.B.; Bouchard, C. Clinical utility and reproducibility of visceral adipose tissue measurements derived from dual-energy X-ray absorptiometry in White and African American adults. Obesity 2013, 21, 2221–2224. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyere, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 601. [Google Scholar] [CrossRef]
- McLean, R.R.; Shardell, M.D.; Alley, D.E.; Cawthon, P.M.; Fragala, M.S.; Harris, T.B.; Kenny, A.M.; Peters, K.W.; Ferrucci, L.; Guralnik, J.M.; et al. Criteria for clinically relevant weakness and low lean mass and their longitudinal association with incident mobility impairment and mortality: The foundation for the National Institutes of Health (FNIH) sarcopenia project. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 576–583. [Google Scholar] [CrossRef]
- Fielding, R.A.; Vellas, B.; Evans, W.J.; Bhasin, S.; Morley, J.E.; Newman, A.B.; Abellan van Kan, G.; Andrieu, S.; Bauer, J.; Breuille, D.; et al. Sarcopenia: An undiagnosed condition in older adults. Current consensus definition: Prevalence, etiology, and consequences. International working group on sarcopenia. J. Am. Med. Dir. Assoc. 2011, 12, 249–256. [Google Scholar] [CrossRef]
- Roza, A.M.; Shizgal, H.M. The Harris Benedict equation reevaluated: Resting energy requirements and the body cell mass. Am. J. Clin. Nutr. 1984, 40, 168–182. [Google Scholar] [CrossRef]
- Buhler, J.; Rast, S.; Beglinger, C.; Peterli, R.; Peters, T.; Gebhart, M.; Meyer-Gerspach, A.C.; Wolnerhanssen, B.K. Long-Term Effects of Laparoscopic Sleeve Gastrectomy and Roux-en-Y Gastric Bypass on Body Composition and Bone Mass Density. Obes. Facts 2021, 14, 131–140. [Google Scholar] [CrossRef]
- Martinez, M.C.; Meli, E.F.; Candia, F.P.; Filippi, F.; Vilallonga, R.; Cordero, E.; Hernandez, I.; Eguinoa, A.Z.; Burgos, R.; Vila, A.; et al. The Impact of Bariatric Surgery on the Muscle Mass in Patients with Obesity: 2-Year Follow-up. Obes. Surg. 2022, 32, 625–633. [Google Scholar] [CrossRef]
- Golzarand, M.; Toolabi, K.; Djafarian, K. Changes in Body Composition, Dietary Intake, and Substrate Oxidation in Patients Underwent Laparoscopic Roux-en-Y Gastric Bypass and Laparoscopic Sleeve Gastrectomy: A Comparative Prospective Study. Obes. Surg. 2019, 29, 406–413. [Google Scholar] [CrossRef]
- Coluzzi, I.; Raparelli, L.; Guarnacci, L.; Paone, E.; Del Genio, G.; le Roux, C.W.; Silecchia, G. Food Intake and Changes in Eating Behavior After Laparoscopic Sleeve Gastrectomy. Obes. Surg. 2016, 26, 2059–2067. [Google Scholar] [CrossRef]
- Harvie, M.N.; Pegington, M.; Mattson, M.P.; Frystyk, J.; Dillon, B.; Evans, G.; Cuzick, J.; Jebb, S.A.; Martin, B.; Cutler, R.G.; et al. The effects of intermittent or continuous energy restriction on weight loss and metabolic disease risk markers: A randomized trial in young overweight women. Int. J. Obes. 2011, 35, 714–727. [Google Scholar] [CrossRef]
- Tam, C.S.; Frost, E.A.; Xie, W.; Rood, J.; Ravussin, E.; Redman, L.M.; Pennington, C.T. No effect of caloric restriction on salivary cortisol levels in overweight men and women. Metabolism 2014, 63, 194–198. [Google Scholar] [CrossRef]
- Juiz-Valina, P.; Pena-Bello, L.; Cordido, M.; Outeirino-Blanco, E.; Pertega, S.; Varela-Rodriguez, B.; Garcia-Brao, M.J.; Mena, E.; Sangiao-Alvarellos, S.; Cordido, F. Altered GH-IGF-1 Axis in Severe Obese Subjects is Reversed after Bariatric Surgery-Induced Weight Loss and Related with Low-Grade Chronic Inflammation. J. Clin. Med. 2020, 9, 2614. [Google Scholar] [CrossRef]
- Kojta, I.; Chacinska, M.; Blachnio-Zabielska, A. Obesity, Bioactive Lipids, and Adipose Tissue Inflammation in Insulin Resistance. Nutrients 2020, 12, 1305. [Google Scholar] [CrossRef]
- Pellitero, S.; Granada, M.L.; Martinez, E.; Balibrea, J.M.; Guanyabens, E.; Serra, A.; Moreno, P.; Navarro, M.; Romero, R.; Alastrue, A.; et al. IGF1 modifications after bariatric surgery in morbidly obese patients: Potential implications of nutritional status according to specific surgical technique. Eur. J. Endocrinol. 2013, 169, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Ohira, M.; Watanabe, Y.; Yamaguchi, T.; Onda, H.; Yamaoka, S.; Abe, K.; Nakamura, S.; Tanaka, S.; Kawagoe, N.; Nabekura, T.; et al. The Relationship between Serum Insulin-Like Growth Factor-1 Levels and Body Composition Changes after Sleeve Gastrectomy. Obes. Facts 2021, 14, 641–649. [Google Scholar] [CrossRef] [PubMed]
- De Marinis, L.; Bianchi, A.; Mancini, A.; Gentilella, R.; Perrelli, M.; Giampietro, A.; Porcelli, T.; Tilaro, L.; Fusco, A.; Valle, D.; et al. Growth hormone secretion and leptin in morbid obesity before and after biliopancreatic diversion: Relationships with insulin and body composition. J. Clin. Endocrinol. Metab. 2004, 89, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Maimoun, L.; Serrand, C.; Mura, T.; Renard, E.; Nocca, D.; Lefebvre, P.; Boudousq, V.; Avignon, A.; Mariano-Goulart, D.; Sultan, A. Definition of an adapted cut-off for determining low lean tissue mass in older women with obesity: A comparison to current cut-offs. Sci. Rep. 2022, 12, 16905. [Google Scholar] [CrossRef]
- Ruthes, E.M.P.; Lenardt, B.C.C.; Lass, A.D.; Petroski, C.A.; de Mello, M.F.; de Andrade Junior, A.B.; Souza, C.J.F.; de Matos, O.; Castelo-Branco, C. Lean mass and strength profile of women submitted to bariatric surgery: Comparison of the EWGSOP2 and FNIH classification for sarcopenia—ASBS program phase II. Gynecol. Endocrinol. 2022, 38, 868–873. [Google Scholar] [CrossRef]
- Vassilev, G.; Galata, C.; Finze, A.; Weiss, C.; Otto, M.; Reissfelder, C.; Blank, S. Sarcopenia after Roux-en-Y Gastric Bypass: Detection by Skeletal Muscle Mass Index vs. Bioelectrical Impedance Analysis. J. Clin. Med. 2022, 11, 1468. [Google Scholar] [CrossRef]
- Voican, C.S.; Lebrun, A.; Maitre, S.; Lainas, P.; Lamouri, K.; Njike-Nakseu, M.; Gaillard, M.; Tranchart, H.; Balian, A.; Dagher, I.; et al. Predictive score of sarcopenia occurrence one year after bariatric surgery in severely obese patients. PLoS ONE 2018, 13, e0197248. [Google Scholar] [CrossRef]
- Johnstone, A.M.; Murison, S.D.; Duncan, J.S.; Rance, K.A.; Speakman, J.R. Factors influencing variation in basal metabolic rate include fat-free mass, fat mass, age, and circulating thyroxine but not sex, circulating leptin, or triiodothyronine. Am. J. Clin. Nutr. 2005, 82, 941–948. [Google Scholar] [CrossRef]
- Fidilio, E.; Comas, M.; Giribes, M.; Cardenas, G.; Vilallonga, R.; Palma, F.; Pelaez, R.B.; Simo, R.; Ciudin, A. Evaluation of Resting Energy Expenditure in Subjects with Severe Obesity and Its Evolution after Bariatric Surgery. Obes. Surg. 2021, 31, 4347–4355. [Google Scholar] [CrossRef]
- Moize, V.; Andreu, A.; Rodriguez, L.; Flores, L.; Ibarzabal, A.; Lacy, A.; Jimenez, A.; Vidal, J. Protein intake and lean tissue mass retention following bariatric surgery. Clin Nutr 2013, 32, 550–555. [Google Scholar] [CrossRef]
- Hassannejad, A.; Khalaj, A.; Mansournia, M.A.; Rajabian Tabesh, M.; Alizadeh, Z. The Effect of Aerobic or Aerobic-Strength Exercise on Body Composition and Functional Capacity in Patients with BMI ≥35 after Bariatric Surgery: A Randomized Control Trial. Obes. Surg. 2017, 27, 2792–2801. [Google Scholar] [CrossRef]
- Siervo, M.; Faber, P.; Lara, J.; Gibney, E.R.; Milne, E.; Ritz, P.; Lobley, G.E.; Elia, M.; Stubbs, R.J.; Johnstone, A.M. Imposed rate and extent of weight loss in obese men and adaptive changes in resting and total energy expenditure. Metabolism 2015, 64, 896–904. [Google Scholar] [CrossRef]
- Faria, S.L.; Kelly, E.; Faria, O.P. Energy expenditure and weight regain in patients submitted to Roux-en-Y gastric bypass. Obes. Surg. 2009, 19, 856–859. [Google Scholar] [CrossRef]
- Faria, S.L.; Faria, O.P.; Buffington, C.; de Almeida Cardeal, M.; Rodrigues de Gouvea, H. Energy expenditure before and after Roux-en-Y gastric bypass. Obes. Surg. 2012, 22, 1450–1455. [Google Scholar] [CrossRef]
- Bazzocchi, A.; Ponti, F.; Cariani, S.; Diano, D.; Leuratti, L.; Albisinni, U.; Marchesini, G.; Battista, G. Visceral Fat and Body Composition Changes in a Female Population After RYGBP: A Two-Year Follow-Up by DXA. Obes. Surg. 2015, 25, 443–451. [Google Scholar] [CrossRef]
- Heath, M.L.; Kow, L.; Slavotinek, J.P.; Valentine, R.; Toouli, J.; Thomson, C.H. Abdominal adiposity and liver fat content 3 and 12 months after gastric banding surgery. Metabolism 2009, 58, 753–758. [Google Scholar] [CrossRef]
- Zang, Y.; Zhu, C.; Wen, X.; Wang, X.; Li, L.; Rampersad, S.; Lu, L.; Zhou, D.; Qian, C.; Cui, R.; et al. Laparoscopic sleeve gastrectomy improves body composition and alleviates insulin resistance in obesity related acanthosis nigricans. Lipids Health Dis. 2017, 16, 209. [Google Scholar] [CrossRef]
- Gletsu-Miller, N.; Hansen, J.M.; Jones, D.P.; Go, Y.M.; Torres, W.E.; Ziegler, T.R.; Lin, E. Loss of total and visceral adipose tissue mass predicts decreases in oxidative stress after weight-loss surgery. Obesity 2009, 17, 439–446. [Google Scholar] [CrossRef]
- Sun, J.; Lv, H.; Li, M.; Zhao, L.; Liu, Y.; Zeng, N.; Wei, X.; Chen, Q.; Ren, P.; Liu, Y.; et al. How much abdominal fat do obese patients lose short term after laparoscopic sleeve gastrectomy? A quantitative study evaluated with MRI. Quant. Imaging Med. Surg. 2021, 11, 4569–4582. [Google Scholar] [CrossRef]
- Guida, B.; Cartaldi, M.; Busetto, M.; Aiello, M.L.; Musella, M.; Capone, D.; Parolisi, S.; Policastro, V.; Ragozini, G.; Belfiore, A. Predictors of fat-free mass loss 1 years after laparoscopic sleeve gastrectomy. J. Endocrinol. Investig. 2018, 41, 1307–1315. [Google Scholar] [CrossRef]
- Nuijten, M.A.H.; Monpellier, V.M.; Eijsvogels, T.M.H.; Janssen, I.M.C.; Hazebroek, E.J.; Hopman, M.T.E. Rate and Determinants of Excessive Fat-Free Mass Loss after Bariatric Surgery. Obes. Surg. 2020, 30, 3119–3126. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).