Associations between Plasma Essential Metals Levels and the Risks of All-Cause Mortality and Cardiovascular Disease Mortality among Individuals with Type 2 Diabetes
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Subjects
2.2. Measurement of Metals Exposure
2.3. Assessment of Covariates
2.4. Ascertainment of Type 2 Diabetes
2.5. Ascertainment of All-Cause and CVD Mortality
2.6. Statistical Analyses
3. Results
3.1. Characteristics of Study Participants
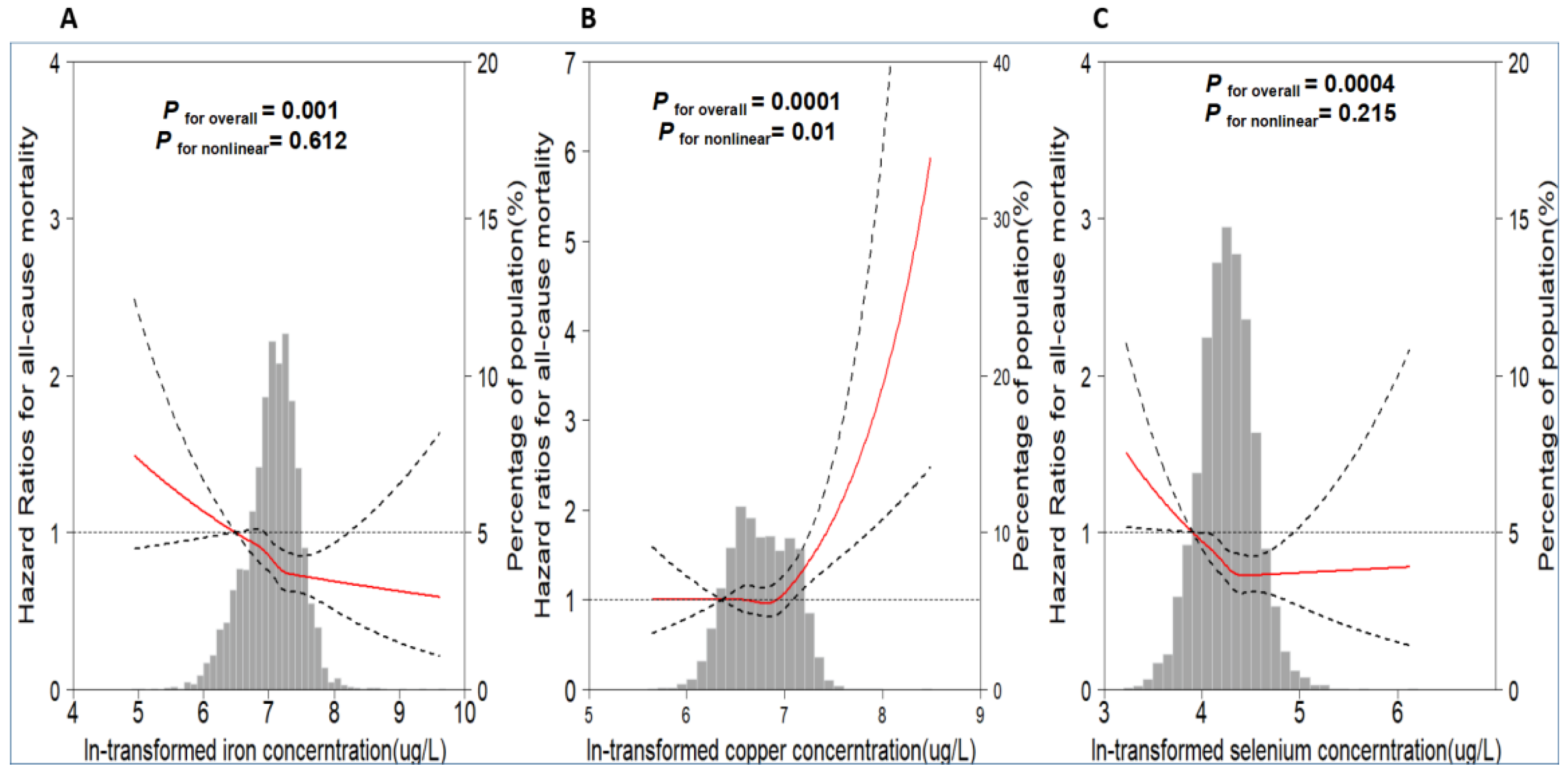
3.2. Association between Plasma Metals Levels and All-Cause Mortality
3.3. Association between Plasma Metals and CVD Mortality
4. Discussion
4.1. Copper
4.2. Iron
4.3. Selenium
4.4. Other Essential Elements
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, L.; Peng, W.; Zhao, Z.; Zhang, M.; Shi, Z.; Song, Z.; Zhang, X.; Li, C.; Huang, Z.; Sun, X.; et al. Prevalence and Treatment of Diabetes in China, 2013–2018. JAMA 2021, 326, 2498–2506. [Google Scholar] [CrossRef] [PubMed]
- Harding, J.L.; Pavkov, M.E.; Magliano, D.J.; Shaw, J.E.; Gregg, E.W. Global trends in diabetes complications: A review of current evidence. Diabetologia 2019, 62, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Xu, Y.; Pan, X.; Xu, J.; Ding, Y.; Sun, X.; Song, X.; Ren, Y.; Shan, P.F. Global, regional, and national burden and trend of diabetes in 195 countries and territories: An analysis from 1990 to 2025. Sci. Rep. 2020, 10, 14790. [Google Scholar] [CrossRef] [PubMed]
- The World Health Organization. Available online: https://www.who.int/news-room/fact-sheets/detail/diabetes (accessed on 11 January 2023).
- Mertz, W. The essential trace elements. Science 1981, 213, 1332–1338. [Google Scholar] [CrossRef]
- Zoroddu, M.A.; Aaseth, J.; Crisponi, G.; Medici, S.; Peana, M.; Nurchi, V.M. The essential metals for humans: A brief overview. J. Inorg. Biochem. 2019, 195, 120–129. [Google Scholar] [CrossRef]
- Shi, L.; Yuan, Y.; Xiao, Y.; Long, P.; Li, W.; Yu, Y.; Liu, Y.; Liu, K.; Wang, H.; Zhou, L.; et al. Associations of plasma metal concentrations with the risks of all-cause and cardiovascular disease mortality in Chinese adults. Environ. Int. 2021, 157, 106808. [Google Scholar] [CrossRef]
- Long, T.; Wang, R.; Wang, J.; Wang, F.; Xu, Y.; Wei, Y.; Zhou, L.; Zhang, X.; Yuan, J.; Yao, P.; et al. Plasma metals and cardiovascular disease in patients with type 2 diabetes. Environ. Int. 2019, 129, 497–506. [Google Scholar] [CrossRef]
- Qiu, Z.; Geng, T.; Wan, Z.; Lu, Q.; Guo, J.; Liu, L.; Pan, A.; Liu, G. Serum selenium concentrations and risk of all-cause and heart disease mortality among individuals with type 2 diabetes. Am. J. Clin. Nutr. 2022, 115, 53–60. [Google Scholar] [CrossRef]
- Sanjeevi, N.; Freeland-Graves, J.; Beretvas, S.N.; Sachdev, P.K. Trace element status in type 2 diabetes: A meta-analysis. J. Clin. Diagn. Res. 2018, 12, OE01–OE08. [Google Scholar] [CrossRef]
- Wang, F.; Zhu, J.; Yao, P.; Li, X.; He, M.; Liu, Y.; Yuan, J.; Chen, W.; Zhou, L.; Min, X.; et al. Cohort Profile: The Dongfeng-Tongji cohort study of retired workers. J. Clin. Diagn. Res. 2013, 42, 731–740. [Google Scholar] [CrossRef]
- Ma, Y.C.; Zuo, L.; Chen, J.H.; Luo, Q.; Yu, X.Q.; Li, Y.; Xu, J.-S.; Huang, S.-M.; Wang, L.-N.; Huang, W.; et al. Modified glomerular filtration rate estimating equation for Chinese patients with chronic kidney disease. J. Am. Soc. Nephrol. 2006, 17, 2937–2944. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2011, 34 (Suppl. 1), S62–S69. [Google Scholar] [CrossRef] [PubMed]
- Barceloux, D.G. Copper. J. Toxicol. Clin. Toxicol. 1999, 37, 217–230. [Google Scholar] [CrossRef] [PubMed]
- Lan, Y.; Wu, S.; Wang, Y.; Chen, S.; Liao, W.; Zhang, X.; Pan, L.; Jiang, X.; Zhang, Y.; Wang, L. Association between blood copper and nonalcoholic fatty liver disease according to sex. Clin. Nutr. 2021, 40, 2045–2052. [Google Scholar] [CrossRef]
- Tisato, F.; Marzano, C.; Porchia, M.; Pellei, M.; Santini, C. Copper in diseases and treatments, and copper-based anticancer strategies. Med. Res. Rev. 2010, 30, 708–749. [Google Scholar] [CrossRef]
- Bates, C.J.; Hamer, M.; Mishra, G.D. Redox-modulatory vitamins and minerals that prospectively predict mortality in older British people: The National Diet and Nutrition Survey of people aged 65 years and over. Br. J. Nutr. 2011, 105, 123–132. [Google Scholar] [CrossRef]
- Mursu, J.; Robien, K.; Harnack, L.J.; Park, K.; Jacobs, D.R., Jr. Dietary supplements and mortality rate in older women: The Iowa Women’s Health Study. Arch. Intern. Med. 2011, 171, 1625–1633. [Google Scholar] [CrossRef]
- Mocchegiani, E.; Malavolta, M.; Lattanzio, F.; Piacenza, F.; Basso, A.; Abbatecola, A.M.; Russo, A.; Giovannini, S.; Capoluongo, E.; Bustacchini, S.; et al. Cu to Zn ratio, physical function, disability, and mortality risk in older elderly (ilSIRENTE study). Age 2012, 34, 539–552. [Google Scholar] [CrossRef]
- Grammer, T.B.; Kleber, M.E.; Silbernagel, G.; Pilz, S.; Scharnagl, H.; Lerchbaum, E.; Tomaschitz, A.; Koenig, W.; Marz, W. Copper, ceruloplasmin, and long-term cardiovascular and total mortality (the Ludwigshafen Risk and Cardiovascular Health Study). Free Radic. Res. 2014, 48, 706–715. [Google Scholar] [CrossRef]
- Calderon Moreno, R.; Navas-Acien, A.; Escolar, E.; Nathan, D.M.; Newman, J.; Schmedtje, J.F.; Diaz, D.; Diaz, D.; A Lamas, G.; Fonseca, V. Potential Role of Metal Chelation to Prevent the Cardiovascular Complications of Diabetes. J. Clin. Endocrinol. Metab. 2019, 104, 2931–2941. [Google Scholar] [CrossRef]
- Argirova, M.D.; Ortwerth, B. Activation of protein-bound copper ions during early glycation: Study on two proteins. Arch. Biochem. Biophys. 2003, 420, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Svensson, P.A.; Englund, M.C.; Markström, E.; Ohlsson, B.G.; Jernås, M.; Billig, H.; Torgerson, J.S.; Wiklund, O.; Carlsson, L.M.; Carlsson, B. Copper induces the expression of cholesterogenic genes in human macrophages. Atherosclerosis 2003, 169, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Repiščák, P.; Erhardt, S.; Rena, G.; Paterson, M.J. Biomolecular mode of action of metformin in relation to its copper binding properties. Biochemistry 2014, 53, 787–795. [Google Scholar] [CrossRef]
- The National Institutes of Health. Available online: https://ods.od.nih.gov/factsheets/Copper-HealthProfessional/#:~:Text=Normal%20serum%20concentrations%20are%2010%E2%80%9325%20mcmol%2FL%20%2863.5%E2%80%93158.9%20mcg%2FdL%29,mg%2FL%20for%20CP%20%5B%2010%20%5D.%20Recommended%20Intakes (accessed on 11 January 2023).
- Ghouse, J.; Isaksen, J.L.; Skov, M.W.; Lind, B.; Svendsen, J.H.; Kanters, J.K.; Olesen, M.S.; Holst, A.G.; Nielsen, J.B. Effect of diabetes duration on the relationship between glycaemic control and risk of death in older adults with type 2 diabetes. Diabetes Obes. Metab. 2020, 22, 231–242. [Google Scholar] [CrossRef]
- The Global BMI Mortality Collaboration; Di Angelantonio, E.; Bhupathiraju Sh, N.; Wormser, D.; Gao, P.; Kaptoge, S.; de Gonzalez, A.B.; Cairns, B.J.; Huxley, R.; Jackson, C.L.; et al. Body-mass index and all-cause mortality: Individual-participant-data meta-analysis of 239 prospective studies in four continents. Lancet 2016, 388, 776–786. [Google Scholar] [CrossRef]
- Nitsch, D.; Grams, M.; Sang, Y.; Black, C.; Cirillo, M.; Djurdjev, O.; Iseki, K.; Jassal, S.K.; Kimm, H.; Kronenberg, F.; et al. Associations of estimated glomerular filtration rate and albuminuria with mortality and renal failure by sex: A meta-analysis. BMJ 2013, 346, f324. [Google Scholar] [CrossRef]
- Ahmad, S.; Ärnlöv, J.; Larsson, S.C. Genetically Predicted Circulating Copper and Risk of Chronic Kidney Disease: A Mendelian Randomization Study. Nutrients 2022, 14, 509. [Google Scholar] [CrossRef]
- Andrews, N.C.; Schmidt, P.J. Iron homeostasis. Annu. Rev. Physiol. 2007, 69, 69–85. [Google Scholar] [CrossRef]
- Mainous, A.G., 3rd; Gill, J.M.; Carek, P.J. Elevated serum transferrin saturation and mortality. Ann. Fam. Med. 2004, 2, 133–138. [Google Scholar] [CrossRef]
- Menke, A.; Muntner, P.; Fernández-Real, J.M.; Guallar, E. The association of biomarkers of iron status with mortality in US adults. Nutr. Metab. Cardiovasc. Dis. 2012, 22, 734–740. [Google Scholar] [CrossRef]
- Ponikowska, B.; Suchocki, T.; Paleczny, B.; Olesinska, M.; Powierza, S.; Borodulin-Nadzieja, L.; Reczuch, K.; von Haehling, S.; Doehner, W.; Anker, S.D.; et al. Iron status and survival in diabetic patients with coronary artery disease. Diabetes Care 2013, 36, 4147–4156. [Google Scholar] [CrossRef] [PubMed]
- Mainous, A.G., 3rd; Tanner, R.J.; Coates, T.D.; Baker, R. Prediabetes, elevated iron and all-cause mortality: A cohort study. BMJ Open 2014, 4, e006491. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, M.E.; Sharif, M.U.; Stack, A.G. Transferrin Saturation: A Body Iron Biomarker. Adv. Clin. Chem. 2016, 75, 71–97. [Google Scholar] [CrossRef] [PubMed]
- Zakai, N.A.; French, B.; Arnold, A.M.; Newman, A.B.; Fried, L.F.; Robbins, J.; Chaves, P.; Cushman, M. Hemoglobin decline, function, and mortality in the elderly: The cardiovascular health study. Am. J. Hematol. 2013, 88, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Zhen, S.; Zhou, Y.; Taylor, A.W. Hb level, iron intake and mortality in Chinese adults: A 10-year follow-up study. Br. J. Nutr. 2017, 117, 572–581. [Google Scholar] [CrossRef]
- Zhang, W.; Iso, H.; Ohira, T.; Date, O.C.; Tanabe, N.; Kikuchi, S.; Tamakoshi, A. Associations of dietary iron intake with mortality from cardiovascular disease: The JACC study. J. Epidemiol. 2012, 22, 484–493. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.L.; Bryant, C.; Cook, R.; O’Connor, H.; Rooney, K.; Steinbeck, K. The relationship between obesity and hypoferraemia in adults: A systematic review. Obes. Rev. 2012, 13, 150–161. [Google Scholar] [CrossRef]
- Pilar Vaquero, M.; Martínez-Suárez, M.; García-Quismondo, Á.; Del Cañizo, F.J.; Sánchez-Muniz, F.J. Diabesity negatively affects transferrin saturation and iron status. The DICARIVA study. Diabetes Res. Clin. Pract. 2021, 172, 108653. [Google Scholar] [CrossRef]
- Oparil, S.; Acelajado, M.C.; Bakris, G.L.; Berlowitz, D.R.; Cífková, R.; Dominiczak, A.F.; Grassi, G.; Jordan, J.; Poulter, N.R.; Rodgers, A.; et al. Hypertension. Nat. Rev. Dis. Prim. 2018, 4, 18014. [Google Scholar] [CrossRef]
- Xiang, S.; Dai, Z.; Man, C.; Fan, Y. Circulating Selenium and Cardiovascular or All-Cause Mortality in the General Population: A Meta-Analysis. Biol. Trace Elem. Res. 2020, 195, 55–62. [Google Scholar] [CrossRef]
- Jenkins, D.J.A.; Kitts, D.; Giovannucci, E.L.; Sahye-Pudaruth, S.; Paquette, M.; Blanco Mejia, S.; Patel, D.; Kavanagh, M.; Tsirakis, T.; Kendall, C.W.C.; et al. Selenium, antioxidants, cardiovascular disease, and all-cause mortality: A systematic review and meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2020, 112, 1642–1652. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Zhang, J.; Li, H. Selenium, aging and aging-related diseases. Aging Clin. Exp. Res. 2019, 31, 1035–1047. [Google Scholar] [CrossRef] [PubMed]
- Vural, Z.; Avery, A.; Kalogiros, D.I.; Coneyworth, L.J.; Welham, S.J.M. Trace Mineral Intake and Deficiencies in Older Adults Living in the Community and Institutions: A Systematic Review. Nutrients 2020, 12, 1072. [Google Scholar] [CrossRef]
- Reinhardt, W.; Dolff, S.; Benson, S.; Broecker-Preuß, M.; Behrendt, S.; Hög, A.; Führer, D.; Schomburg, L.; Köhrle, J. Chronic Kidney Disease Distinctly Affects Relationship Between Selenoprotein P Status and Serum Thyroid Hormone Parameters. Thyroid 2015, 25, 1091–1096. [Google Scholar] [CrossRef] [PubMed]
- Dubey, P.; Thakur, V.; Chattopadhyay, M. Role of Minerals and Trace Elements in Diabetes and Insulin Resistance. Nutrients 2020, 12, 1864. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Rayman, M.P. Multiple Nutritional Factors and the Risk of Hashimoto’s Thyroiditis. Thyroid 2017, 27, 597–610. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, D.M. Copper, iron, and selenium dietary deficiencies negatively impact skeletal integrity: A review. Exp. Biol. Med. 2016, 241, 1316–1322. [Google Scholar] [CrossRef]
- Duque Rodriguez, M.; Cittadini, C.O.; Teplitz, G.M.; De Stefano, A.; Lombardo, D.M.; Salamone, D.F. Canine IVM with SOF Medium, Insulin-Transferrin-Selenium, and Low O(2) Tension Improves Oocyte Meiotic Competence and Decreases Reactive Oxygen Species Levels. Front. Cell Dev. Biol. 2021, 9, 694889. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, T.; Wang, R.; Shi, P.; Pan, B.; Pang, X. Insulin-Transferrin-Selenium as a Novel Serum-free Media Supplement for the Culture of Human Amnion Mesenchymal Stem Cells. Ann. Clin. Lab. Sci. 2019, 49, 63–71. [Google Scholar]
- Soinio, M.; Marniemi, J.; Laakso, M.; Pyörälä, K.; Lehto, S.; Rönnemaa, T. Serum zinc level and coronary heart disease events in patients with type 2 diabetes. Diabetes Care 2007, 30, 523–528. [Google Scholar] [CrossRef]
- Henze, L.A.; Estepa, M.; Pieske, B.; Lang, F.; Eckardt, K.U.; Alesutan, I.; Voelkl, J. Zinc Ameliorates the Osteogenic Effects of High Glucose in Vascular Smooth Muscle Cells. Cells 2021, 10, 3083. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Luo, D.; He, W.; Chen, J.; Su, X.; Huang, H. Diabetes and calcification: The potential role of anti-diabetic drugs on vascular calcification regression. Pharmacol. Res. 2020, 158, 104861. [Google Scholar] [CrossRef] [PubMed]
- Lowe, N.M. Assessing zinc in humans. Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 321–327. [Google Scholar] [CrossRef]
- Knez, M.; Pantovic, A.; Tako, E.; Boy, E. FADS1 and FADS2 as biomarkers of Zn status—A systematic review and meta-analysis. Crit. Rev. Food Sci. Nutr. 2022, 26, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Blot, W.J.; Li, J.Y.; Taylor, P.R.; Guo, W.; Dawsey, S.; Wang, G.Q.; Yang, C.S.; Zheng, S.-F.; Gail, M.; Li, G.-Y.; et al. Nutrition intervention trials in Linxian, China: Supplementation with specific vitamin/mineral combinations, cancer incidence, and disease-specific mortality in the general population. J. Natl. Cancer Inst. 1993, 85, 1483–1492. [Google Scholar] [CrossRef] [PubMed]
- Panneerselvam, S.R.; Govindasamy, S. Effect of sodium molybdate on the status of lipids, lipid peroxidation and antioxidant systems in alloxan-induced diabetic rats. Clin. Chim. Acta 2004, 345, 93–98. [Google Scholar] [CrossRef]
- Musoro, J.Z.; Zwinderman, A.H.; Puhan, M.A.; ter Riet, G.; Geskus, R.B. Validation of prediction models based on lasso regression with multiply imputed data. BMC Med. Res. Methodol. 2014, 14, 116. [Google Scholar] [CrossRef] [PubMed]
- Noisel, N.; Carrier, G.; Bouchard, M. Study of selenium intake and disposition in various matrices based on mathematical algorithms derived from pooled biomonitoring data. Int. J. Hyg. Environ. Health 2014, 217, 796–804. [Google Scholar] [CrossRef]
- Yuan, Y.; Xiao, Y.; Feng, W.; Liu, Y.; Yu, Y.; Zhou, L.; Qiu, G.; Wang, H.; Liu, B.; Liu, K.; et al. Plasma Metal Concentrations and Incident Coronary Heart Disease in Chinese Adults: The Dongfeng-Tongji Cohort. Environ. Health Perspect. 2017, 125, 107007. [Google Scholar] [CrossRef]
| Death (n = 890) | Survivors (n = 4388) | p | |
|---|---|---|---|
| Age (years) | 69.41 ± 7.15 | 64.04 ± 7.12 | <0.001 |
| Gender (man, %) | 61 | 43 | <0.001 |
| BMI (kg/m2) | 25.30 ± 3.50 | 25.54 ± 3.36 | 0.054 |
| Education | <0.001 | ||
| Primary or below | 42.8 | 30.7 | |
| Junior high school | 29.3 | 38.2 | |
| High school | 18.5 | 21.5 | |
| College or above | 9.3 | 9.4 | |
| Smoking (%) | <0.001 | ||
| Current smoker | 23.8 | 13.9 | |
| Ex-smoker | 19.2 | 12.4 | |
| Never smoker | 57 | 73.7 | |
| Alcohol consumption (%) | <0.001 | ||
| Current drinker | 18.5 | 18.3 | |
| Ex-drinker | 11.1 | 6.6 | |
| Never drinker | 70.3 | 75.1 | |
| Physical activity (yes, %) | 86.2 | 89.8 | 0.002 |
| Hypertension (yes, %) | 79.9 | 70.9 | <0.001 |
| Hyperlipidemia (yes, %) | 70.4 | 66.8 | 0.035 |
| Family history of CVD (yes, %) | 6.4 | 11.3 | <0.001 |
| Antidiabetic drugs (yes, %) | 50.8 | 38.5 | <0.001 |
| Systolic blood pressure (mmHg) | 136.97 ± 20.06 | 135.89 ± 19.88 | 0.139 |
| Diastolic blood pressure (mmHg) | 77.17 ± 12.29 | 78.51 ± 11.34 | <0.001 |
| Fast blood glucose (mmol/L) | 7.70 (6.70,9.68) | 7.40 (6.63,8.60) | <0.001 |
| Duration of diabetes (years) | 3.27 (0.00,11.23) | 0.05 (0.00,7.19) | <0.001 |
| Triglyceride (mmol/L) | 1.80 ± 1.73 | 1.77 ± 1.59 | 0.61 |
| Total cholesterol (mmol/L) | 5.18 ± 1.19 | 5.17 ± 1.09 | 0.613 |
| High-density lipoprotein (mmol/L) | 1.32 ± 0.38 | 1.38 ± 0.39 | <0.001 |
| Low-density lipoprotein (mmol/L) | 3.01 ± 0.95 | 2.99 ± 0.89 | 0.593 |
| Estimated glomerular filtration rate (mL/min/1.73 m2) | 70.19 ± 19.66 | 78.39 ± 17.85 | <0.001 |
| Iron (μg/L) | 1192.78 (895.34,1517.69) | 1209.31 (928.19,1519.08) | 0.366 |
| Copper (μg/L) | 856.50 (674.58,1174.97) | 855.12 (682.26,1110.23) | 0.254 |
| Zinc (μg/L) | 1302.97 (1055.19,2666.08) | 1511.75 (1158.21,2435.45) | <0.001 |
| Selenium (μg/L) | 67.34 (56.33,82.30) | 71.65 (59.87,85.26) | <0.001 |
| Manganese (μg/L) | 3.28 (2.37,4.82) | 2.95 (2.13,4.45) | <0.001 |
| Molybdenum (μg/L) | 1.62 (1.22,2.18) | 1.41 (1.10,1.85) | <0.001 |
| Vanadium (μg/L) | 3.19 (1.68,4.14) | 2.86 (1.13,4.09) | <0.001 |
| Cobalt (μg/L) | 0.25 (0.19,0.32) | 0.24 (0.17,0.31) | <0.001 |
| Chromium (μg/L) | 5.41 (3.06,7.91) | 5.07 (3.12,7.56) | 0.289 |
| Nickel (μg/L) | 3.32 (2.08,5.12) | 3.09 (1.91,4.55) | 0.001 |
| Tin (μg/L) | 0.16 (0.07,0.29) | 0.16 (0.07,0.28) | 0.031 |
| Metals (μg/L) | Hazard Ratio (95%CIs) by Continuous Metals | p | Hazard Ratio (95%CIs) by Quartiles of Metals | p Trend * | |||
|---|---|---|---|---|---|---|---|
| Quartile1 | Quartile2 | Quartile3 | Quartile4 | ||||
| All-cause mortality # | |||||||
| Iron | 0.83 (0.70,0.98) | 0.031 | 1.00 (ref.) | 0.91 (0.75,1.09) | 0.81 (0.67,0.98) | 0.79 (0.64,0.96) | 0.006 |
| Copper | 1.60 (1.30,1.97) | <0.001 | 1.00 (ref.) | 1.06 (0.87,1.28) | 1.05 (0.86,1.28) | 1.50 (1.23,1.82) | <0.001 |
| Zinc | 0.96 (0.89,1.03) | 0.289 | 1.00 (ref.) | 0.80 (0.66,0.96) | 0.96 (0.78,1.17) | 0.94 (0.79,1.13) | 0.963 |
| Selenium | 0.60 (0.46,0.77) | <0.001 | 1.00 (ref.) | 0.82 (0.68,0.98) | 0.72 (0.59,0.88) | 0.72 (0.58,0.88) | <0.001 |
| Molybdenum | 1.08 (0.97,1.20) | 0.141 | 1.00 (ref.) | 0.92 (0.74,1.14) | 1.10 (0.90,1.34) | 1.17 (0.96,1.43) | 0.023 |
| Cardiovascular mortality & | |||||||
| Iron | 0.61 (0.49,0.78) | <0.001 | 1.00 (ref.) | 0.70 (0.51,0.95) | 0.69 (0.51,0.93) | 0.52 (0.38,0.72) | <0.001 |
| Metals # | n (Death/Survivors) | Hazard Ratio (95%CIs) & | Pinteraction |
|---|---|---|---|
| Iron-Selenium | |||
| Low Fe + Low Se | 277/1242 | 1.00 (ref) | |
| Low Fe + High Se | 179/941 | 0.94 (0.77,1.13) | 0.285 |
| High Fe + Low Se | 227/893 | 0.89 (0.74,1.06) | |
| High Fe + High Se | 207/1312 | 0.66 (0.55,0.79) | |
| Iron-Copper | |||
| Low Fe + Low Cu | 212/1059 | 1.00 (ref) | |
| Low Fe + High Cu | 244/1124 | 1.21 (1.00,1.46) | |
| High Fe + Low Cu | 232/1136 | 0.83 (0.69,1.00) | 0.575 |
| High Fe + High Cu | 202/1069 | 0.90 (0.74,1.09) | |
| Selenium-Copper | |||
| Low Se + Low Cu | 286/1272 | 1.00 (ref) | |
| Low Se + High Cu | 218/863 | 1.17 (0.98,1.40) | |
| High Se + Low Cu | 158/923 | 0.77 (0.63,0.94) | 0.663 |
| High Se + High Cu | 228/1330 | 0.94 (0.79,1.13) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Wang, R.; Long, T.; Xu, Y.; Guo, H.; Zhang, X.; He, M. Associations between Plasma Essential Metals Levels and the Risks of All-Cause Mortality and Cardiovascular Disease Mortality among Individuals with Type 2 Diabetes. Nutrients 2023, 15, 1198. https://doi.org/10.3390/nu15051198
Li Z, Wang R, Long T, Xu Y, Guo H, Zhang X, He M. Associations between Plasma Essential Metals Levels and the Risks of All-Cause Mortality and Cardiovascular Disease Mortality among Individuals with Type 2 Diabetes. Nutrients. 2023; 15(5):1198. https://doi.org/10.3390/nu15051198
Chicago/Turabian StyleLi, Zhaoyang, Ruixin Wang, Tengfei Long, Yali Xu, Huan Guo, Xiaomin Zhang, and Meian He. 2023. "Associations between Plasma Essential Metals Levels and the Risks of All-Cause Mortality and Cardiovascular Disease Mortality among Individuals with Type 2 Diabetes" Nutrients 15, no. 5: 1198. https://doi.org/10.3390/nu15051198
APA StyleLi, Z., Wang, R., Long, T., Xu, Y., Guo, H., Zhang, X., & He, M. (2023). Associations between Plasma Essential Metals Levels and the Risks of All-Cause Mortality and Cardiovascular Disease Mortality among Individuals with Type 2 Diabetes. Nutrients, 15(5), 1198. https://doi.org/10.3390/nu15051198






