Abstract
The aim of this study was to investigate the effects of 12 weeks of high-intensity training with astaxanthin supplementation on adipokine levels, insulin resistance and lipid profiles in males with obesity. Sixty-eight males with obesity were randomly stratified into four groups of seventeen subjects each: control group (CG), supplement group (SG), training group (TG), and training plus supplement group (TSG). Participants underwent 12 weeks of treatment with astaxanthin or placebo (20 mg/d capsule daily). The training protocol consisted of 36 sessions of high-intensity functional training (HIFT), 60 min/sessions, and three sessions/week. Metabolic profiles, body composition, anthropometrical measurements, cardio-respiratory indices and adipokine [Cq1/TNF-related protein 9 and 2 (CTRP9 and CTRP2) levels, and growth differentiation factors 8 and 15 (GDF8 and GDF15)] were measured. There were significant differences for all indicators between the groups (p < 0.05). Post-hoc analysis indicated that the levels of CTRP9, CTRP2, and GDF8 were different from CG (p < 0.05), although levels of GDF15 were similar to CG (p > 0.05). Levels of GDF8 were similar in the SG and TG groups (p > 0.05), with reductions of GDF15 levels in both training groups (p < 0.05). A total of 12 weeks of astaxanthin supplementation and exercise training decreased adipokines levels, body composition (weight, %fat), anthropometrical factors (BMI), and improved lipid and metabolic profiles. These benefits were greater for men with obesity in the TSG group.
1. Introduction
Obesity promotes co-morbid diseases such as cardiovascular diseases, type 2 diabetes, and metabolic syndrome [1,2], and is associated with increased adipocyte size, increased production of reactive oxygen species (ROS), secretion of pro-inflammatory cytokines, and lipid deposition to promote insulin resistance in peripheral tissues [3,4,5,6]. Adipocytes release proteins such as adiponectin, leptin, resistin, and visfatin into the bloodstream [1], in addition to others such as Cq1/TNF-related proteins (CTRPs) and growth differentiation factors (GDFs) [7,8]. CTRPs released by adipose tissue regulate lipid and glucose metabolism [9]. The CTRP adipokine family consists of 15 members (CTRP-1 to CTRP-15) with localized tissue expression, where CTRP-2 and CTRP-9 are highly expressed in adipocytes. The secretion of CTRP-2 and CTRP-9 is increased during obesity in humans and in animal models [10,11], making CTRP-2 and CTRP-9 targets for the medical management of obesity [7,12]. GDFs, including GDF-8 and GDF-15, are secretory proteins of the transforming growth factor (TGF)-β family [13]. GDF-8 or myostatin plays a critical role in skeletal muscle homeostasis and reduced expression of GDF-8 is associated with fat loss, increased insulin sensitivity, and increased glucose uptake [14]. Levels of GDF-15, also known as macrophage inhibitory cytokine (MIC-1) released by adipocytes, positively correlate with obesity [15], with a suggested role in lipid metabolism [16]. Treatments to reduce obesity and its related health problems remain elusive. Pharmacological and surgical approaches in obese patients are associated with side effects and have variable effectiveness [17,18]. Reducing energy consumption, increasing energy expenditure, and increasing muscle mass hold promise in the management of obesity and associated complications [19,20]. However, the effects of regular exercise training to manage obesity and related diseases are related to the modes of exercise [21]. For example, CrossFit training (a high-intensity mixed exercise model of concurrent strength and endurance performance) reduces lipid oxidation in obese individuals [22,23]. This high-intensity functional training (HIFT) exercise modality consists of exercise sets with or without rest times between sets [24] and has increased IL-6 and IL-10 activity [25], elevated aerobic capacity, improved muscular endurance, increased lean body mass, and reduced body fat [26]. Another way to reduce or prevent obesity is to use natural antioxidant supplements. Astaxanthin (3, 3′-dihydroxy-β, β-carotene-4, 4′-dione) is derived from Haematoccus pluvialis algae and has health benefits in treating some cancers, chronic inflammatory disease, diabetes, obesity, cardiovascular diseases, and neurodegenerative disorders [27,28]. Astaxanthin reduces the effects of oxidative stress on lipid metabolism [29]. Using astaxanthin as a dietary supplementation can expedite lipid metabolism in muscles during exercise [29]. Despite the well-accepted benefits of exercise training on lipid oxidation and metabolic disorders in obese patients, astaxanthin, as a supplement rich in antioxidants, can also improve metabolism and reduce the inflammation caused by obesity. There is limited information about the benefits of supplementing HIFT with astaxanthin on cardiovascular risk factors and adipokine levels for individuals with obesity. We hypothesized that supplementing astaxanthin with HIFT increases weight loss and attenuates the effects of CTRPs and GDFs and fat loss in individuals with obesity. To this end, we examined the effects of 12 weeks of HIFT supplemented with astaxanthin on body composition, cardio-respiratory fitness, adipokine levels, insulin resistance and lipid profiles in males with obesity.
2. Materials and Methods
After calling in public places such as gyms, medical clinics, hospitals and social networks, there were 101 participants who initially volunteered for the study, of whom 33 were ineligible, leaving 68 participants in the study (mean age: 27.6 ± 8.4 yrs; mean height: 167.8 ± 3.1 cm; mean weight: 94.7± 2.0 kg; mean BMI: 33.6 ± 1.4 kg/m2), who were divided into 4 groups of 17 per group. The inclusion criteria for the study were: BMI > 30 kg/m2, not involved in regular physical activity during the last six months, without endocrine, metabolic and cardiovascular diseases, and without alcohol consumption. Participants taking supplements and medications that may have an effect on adipose and muscle tissue or presenting joint disorders or physical disabilities were also excluded from the study. During the first visit, all participants undertook a physical examination performed by a physician and a clinical exercise physiologist. All the participants provided written informed consent forms and completed a Physical Activity Readiness Questionnaire (PAR-Q) [30]. Study procedures were explained during the first visit and the Research and Ethics Committee of the Islamic Azad University approved all procedures of this study (Ethics code: IR-IAU1400-47). All procedures were performed according to the latest revision of the Declaration of Helsinki [31].
2.1. Experimental Design
One week prior to the start of the training programs, the study procedures were explained and a familiarization session was completed by all participants. Height, weight, and body composition were assessed for all the participants, who were then randomly assigned into one of four equal groups (n = 17 per group): Control group (CG), Supplement group (SG), Training group (TG), and Training + supplement group (TSG). During the experiment period, 8 participants from different groups withdrew from the study due to medical reasons, work-related difficulties and no interest to continue the research, leaving 15 participants in each group. During the third session, body composition and VO2peak were determined and instructions on how to perform the training programs were given to each group. The two training groups (TG and TSG) performed the exercise training program for 12 weeks, 3 sessions/week. The CG was instructed to not change their current lifestyles during the experiment. All the measurements were performed at the same time of day (within ~1 h) and under the same environmental conditions (~20 °C and ~55% humidity). Baseline assessments were obtained 48 h before the start of the training protocols and post-test measurements were performed 48 h after the last session in all groups.
2.2. Body Composition and Cardio-Respiratory Fitness Assessments
Body weights and heights were measured using a calibrated scale (Seca GmbH & Co., Hamburg, Germany) and stadiometer (Seca GmbH & Co., Hamburg, Germany), respectively. Then, the body mass index (BMI, kg/m2) was calculated. A bio-impedance analyzer (Medigate Company Inc., Gyeonggi-do, Korea) was used to determine fat-free mass (FFM) and fat mass (FM). In a temperature-controlled room (21–23 °C), a modified Bruce protocol [32,33] using an electronically motorized treadmill (H/P/Cosmos, Pulsar med 3p- Sports and Medical, Nussdorf-Traunstein Germany) allowed for measuring VO2peak. The criteria used to determine VO2peak were based on the American College of Sports Medicine (ACSM) guidelines [34]. During this test, blood pressure was measured using an electronic sphygmomanometer (Kenz BPM AM 300P CE, Suzuken Company, Nagoya, Japan), and heart rate was monitored with a Polar V800 heart monitor (Kempele, Finland). Gas analysis was performed using a gas analyzer system (Metalyzer 3B analyzer, Cortex: biophysik, GMbH, Leipzig, Germany) which was calibrated before each test.
2.3. Training Protocols
In the current study, a total of 36 sessions lasting up to 60 min each were included in the HIFT program using CrossFit. Crossfit training is stratified as high-intensity functional training with a mixed model of concurrent strength and endurance performance often containing various fitness components [26]. This modality of training consists of some workout sets that may or may not include rest between sets [25]. This type of training is associated with some physiological effects, such as increased blood lactate [35,36], elevated testosterone levels, increased cortisol [37], and increased IL-6 and IL-10 activity [25], as well as some adaptations in elevating aerobic capacity, muscular endurance, lean body mass, utilization of body fat or reducing body fat [26]. All HIFT sessions were led by a trainer with a Level 1 CrossFit certificate according to the methods described earlier [38,39]. Average times for each workout of the day (WOD) and total average WOD time per week were calculated for the HIFT group as a whole (i.e., participants in the TG and TSG).
2.4. Supplementation of Astaxanthin and Placebo
Eligible participants in the SG and TSG received 20 mg/day of astaxanthin capsule (Marine Product Tech. Inc., Seongnam, Republic of Korea) or matching placebo capsule (20 mg/day of raw corn starch) once daily with breakfast for 12 weeks. Participants were considered adherent when ≥80% of their prescribed supplements were consumed.
2.5. Nutrient Intake and Dietary Analysis
Before and after the experiment changes in habitual dietary intake over time were determined using three-day food records (two weekdays and one weekend day) [40]. Nutritional intakes were analyzed using Diet Analysis Plus version 10 (Cengage, Boston, MA, USA) (Table 1).

Table 1.
Nutritional intakes in the four study groups.
2.6. Assessment of Blood Markers
Fasting blood samples were taken under standard conditions between 8 and 10 am from the right arm 12 h before the first exercise session and 72 h after the last session. Blood samples were transferred to EDTA-containing tubes, centrifuged for 10 min at 3000 rpm, and stored at −70 °C for later use for the following measurements:
Plasma CTRP-9 was measured with an ELISA kit (Aviscera Bioscience, Santa Clara, CA, USA). Catalogue No: SK00081-02. Sensitivity: 1 ng/mL. Intra-coefficients of variation (CV) = 4%, inter-CV = 8%.
CTRP-2 was measured using ELISA kits obtained from MyBioSource (San Diego, CA, USA) with a minimum detectable dose (MDD) of 0.039 ng/mL and detection range of 0.156–10 ng/mL (intra-assay CV: <8% and inter-assay < 10%).
GDF-15 was measured using ELISA kits obtained from Thermo Scientific (Frederick, MD, USA) with a sensitivity of 2 pg/mL and detection range of 1.10–800 pg/mL (intra-assay CV < 10% and inter-assay CV < 12%).
Plasma GDF8 was measured with an ELISA kit (R&D Systems, Minneapolis, MN, USA) Catalogue No: DGDF80. The sensitivity was 5.32 pg/mL. Intra-CV = 5.4%, inter-CV = 6%.
Plasma total cholesterol (TC) and triglyceride (TG) were measured by enzymatic methods (CHOD-PAP). High-density cholesterol (HDL-C) and low-density cholesterol (LDL-C) were determined using a photometric method (Pars Testee’s Quantitative Detection kit, Tehran, Iran) with a coefficient and sensitivity of 1.8% and 1 mg/dL and 1.2% and 1 mg/dL, respectively.
Insulin levels were measured with an ELISA kit (Demeditec, Kiel, Germany) with a sensitivity of 1 ng/mL and between and within CV of 5.1% and 8.4%, respectively.
Glucose levels were measured with a colorimetric enzymatic kit (Paracetamol Glucose, Colorimetric Enzymatic kit, Parsazmun, Tehran, Iran) with a sensitivity of 5 mmol/L.
HOMA-insulin resistance (HOMA-IR) was calculated from the ratio of insulin to glucose and the HOMA-IR index [HOMA-IR = fasting insulin (mU/L) × glucose (mmol/L)/22.5] [41].
2.7. Statistical Analysis
Descriptive statistics (means ± standard deviation) were used to summarize all data. The normality of the data was assessed by the Shapiro–Wilk test. A two-way ANOVA repeated measures test was used to determine group X time interactions. One-way ANOVA and Fisher LSD post-hoc tests were used for evaluation of baseline data for the four groups. When a significant difference was detected by ANOVA, mean differences were determined by pairwise comparisons. The sample size needed to detect a statistical difference between study variables with a 95% confidence interval (CI) and equal to or greater than 80% of the power value was calculated. Effect sizes (ES) were reported as partial eta-squared, where ES were considered trivial (<0.2), small (0.2–0.6), moderate (0.6–1.2), large (1.2–2.0) and very large (2.0–4.0). A p-value of <0.05 was used to indicate statistical significance. All data were analyzed using SPSS software (version 24; Armonk, NY, USA).
3. Results
3.1. Anthropometric Characteristics and Cardio-Respiratory Parameters
There were no between-group differences in baseline values for weight (p = 0.46), BMI (p = 0.57), body fat (p = 0.33), FFM (p = 0.59), or VO2peak (p = 0.98). There were significant group X time interactions for weight (p = 0.0001, η2 = 0.46), BMI (p = 0.002, η2 = 0.30), fat percent (p = 0.0001, η2 = 0.51), FFM (p = 0.0001, η2 = 0.43), and VO2peak (p = 0.0001, η2 = 0.61) (Table 2). Body weight reductions after 12 weeks were significant in the SG (p = 0.008), TG (p = 0.0001), and TSG (p = 0.0001) but not in the CG (p = 0.32). Furthermore, the post-hoc test for body weight shows that after 12 weeks there were significant changes in the CG compared to the TG (p = 0.004) and TSG (p = 0.0001), and in the TSG compared to the TG (p = 0.01) and SG (p = 0.0001), while other changes were not significant (p > 0.05) (Table 2).

Table 2.
Mean (±SD) values of glucose, insulin, lipid profile, body composition, and VO2peak.
Changes in BMI after 12 weeks were significantly decreased in the SG (p = 0.019), TG (p = 0.0001) and TSG (p = 0.0001) but not in the CG (p = 0.37). BMI changes after 12 weeks were significantly decreased in the TG (p = 0.016) and the TSG (p = 0.0001) compared to the CG. The differences induced by training were significant between the TG and TSG (p = 0.007) and between the SG and TSG (p = 0.007), while all other differences in BMI between the groups were not significant (p > 0.05) (Table 2). Increases in post-test values of FFM were significant in comparison to pre-test values in the SG (p = 0.0001), TG (p = 0.0001) and TSG (p = 0.0001) but not for the CG (p = 0.08). Between-group differences in FFM were significant in the CG compared to the SG (p = 0.0001), TG (p = 0.0001) and TSG (p = 0.0001) but all other between-group differences were not significant (p > 0.05) (Table 2). The decrease in body fat percent after 12 weeks was significant in the SG (p = 0.004), TG (p = 0.0001) and TSG (p = 0.0001) but not in the CG (p = 0.28). Between-group differences in body fat percent were significant in the CG compared to the SG (p = 0.006), TG (p = 0.0001) and TSG (p = 0.0001), and also between the SG and TSG (p = 0.013), while all other differences were not significant (p > 0.05) (Table 2). Increases in the VO2peak after 12 weeks of exercise were significant in the TG (p = 0.0001) and TSG (p = 0.0001) but not in the CG (p = 0.32) and SG (p = 0.21). Between-group differences in VO2peak were significant in the CG compared to the TG (p = 0.0001) and TSG (p = 0.0001), and also in the SG compared to the TG (p = 0.0001) and TSG (p = 0.0001), while all other differences were not significant (p > 0.05) (Table 2).
3.2. Lipid Profiles
There were no significant differences in the baseline levels of HDL (p = 0.57), LDL (p = 0.71), TC (p = 0.17), and TGs (p = 0.47) in the four groups. There were significant group X time interactions for HDL (p = 0.0001, η2 = 0.89), LDL (p = 0.0001, η2 = 0.86), TC (p = 0.0001, η2 = 0.95), and TGs (p = 0.0001, η2 = 0.95) (Table 2). Post-test HDL levels increased in the SG (p = 0.0001), TG (p = 0.0001) and TSG (p = 0.0001) groups compared to pre-test values but were unchanged in the CG (p = 0.88). Bonferroni’s post-hoc test showed that increases in HDL after 12 weeks of training were significant in all groups (p = 0.0001) except for differences between the TG and TSG (p = 0.06) (Table 2). Post-test levels of LDL decreased in the SG (p = 0.0001), TG (p = 0.0001) and TSG (p = 0.0001) but not in the CG (p = 0.82). Differences in LDL after 12 weeks of training were significant among all groups (p = 0.0001) except for differences between the TG and TSG (p = 0.39) (Table 2). Post-test levels of TC decreased in the SG (p = 0.0001), TG (p = 0.0001) and TSG (p = 0.0001) but not in the CG (p = 0.88). Differences in TC after 12 weeks of training were significant in all groups (p = 0.0001) (Table 2). Post-test levels of TGs were reduced in the SG (p = 0.0001), TG (p = 0.0001) and TSG (p = 0.0001) but not in the CG (p = 0.47). Differences in TGs after 12 weeks of training were significant in all groups (p = 0.0001) except for differences between the TG and TSG (p = 0.64) (Table 2).
3.3. Metabolic Factors
There were no significant differences in baseline levels of glucose (p = 0.66), insulin (p = 0.53), and HOMA (p = 0.49) in the four groups. There were significant group X time interactions for glucose (p = 0.0001, η2 = 0.49), insulin (p = 0.0001, η2 = 0.78) and HOMA (p = 0.0001, η2 = 0.69) (Table 2). Glucose levels decreased significantly in the SG (p = 0.0001), TG (p = 0.0001), and TSG (p = 0.0001), yet did not significantly change in the CG (p = 0.06). Post-hoc tests revealed significant between-group differences (p = 0.0001) except for the CG and SG (p = 0.056) and the TSG and TG (p = 0.26) (Table 2). Insulin levels significantly decreased in the SG (p = 0.0001), TG (p = 0.0001), and TSG (p = 0.0001), with no significant changes in the CG (p = 0.21). Post-hoc between-group differences were significant for all groups (p = 0.0001) (Table 2). Levels of HOMA-IR decreased following 12 weeks of training in the SG (p = 0.0001), TG (p = 0.0001), and TSG (p = 0.0001), while the difference in HOMA-IR in the CG was not significant (p = 0.17). Post-hoc between-group differences were significant for all groups (p = 0.0001) except for the TG and TSG (p = 0.065) (Table 2).
3.4. Adipokines and Growth Differentiation Factors
There were no significant differences in baseline levels of CTRP9 (p = 0.22), CTRP2 (p = 0.18), GDF8 (p = 0.75) and GDF15 (p = 0.59) between the four study groups. There were statistically significant group X time interactions for CTRP9 (p = 0.001, η2 = 0.62), CTRP2 (p = 0.0001, η2 = 0.71), GDF8 (p = 0.0001, η2 = 0.38) and GDF15 (p = 0.0001, η2 = 0.75).
A comparison of pre-test and post-test values indicated no differences in CTRP9 (p = 0.30), CTRP2 (p = 0.30), GDF8 (p = 0.30) and GDF15 (p = 0.30) for CG, the result was also not significant in the SG for GDF15 (p = 0.07). Differences from the pre-test to post-test were that GDF15 was significantly lower in the TG (p = 0.0001) and TSG (p = 0.0001), SG (p = 0.0001), and CTRP9, GDF8 and GDF15 were significantly lower in the TG (p = 0.0001) and TSG (p = 0. 0.0001).
The analysis of between-group differences for CTRP9 indicated a significance in TG (p = 0.0001) and TSG (p = 0.0001) compared to the CG. The decrease in CTRP9 in the TSG following 12 weeks of training was significantly greater than the decreases in the SG (p = 0.0001) and TG (p = 0.001) (Figure 1).
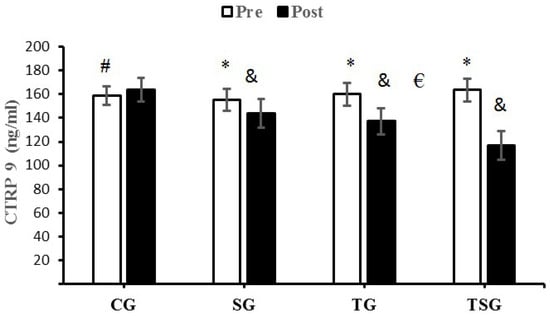
Figure 1.
Pre- and post-training values (mean ± SD) for CTRP9 in the control (CG), supplement (SG), training (TG), training + supplement (TSG) groups. & Significant differences with pretest values (p < 0.05). * Significant differences with the control group (p < 0.05). # Significant interaction between time and group (p < 0.05). € Significant time X group interaction (p < 0.05).
The decreases of CTRP2 in the SG (p = 0.024), TG (p = 0.0001) and TSG (p = 0.0001) were significantly greater than the changes in the CG but the differences between the TG and TSG were not significant (p = 0.11). The decrease in CTRP2 in TG (p = 0.0001) and TSG (p = 0.001) were significantly different from the changes in the SG following 12 weeks of training (Figure 2).
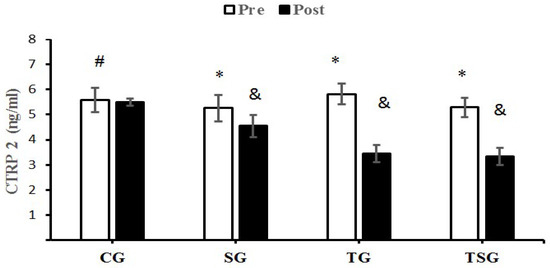
Figure 2.
Pre- and post-training values (mean ± SD) for CTRP2 in the control (CG), supplement (SG), training (TG), training + supplement (TSG) groups. & Significantly different from pretest values (p < 0.05). * Significantly different from control group (p < 0.05). # Significant time X group interaction (p < 0.05).
GDF8 decreased significantly in the SG (p = 0.0001), TG (p = 0.0001) and TSG (p = 0.0001) compared to the CG. Additionally, Bonferroni’s post-hoc test showed that the decrease in GDF8 in TSG following 12 weeks of training was different from the changes in TG (p = 0.001) and SG (p = 0.0001) (Figure 3).
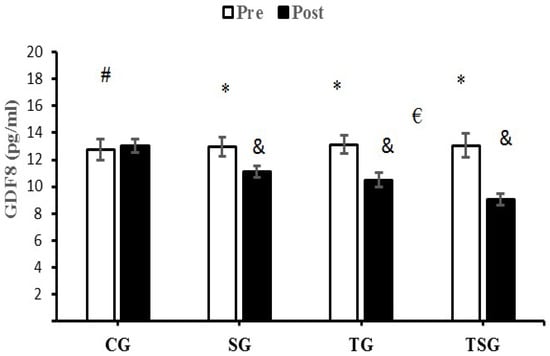
Figure 3.
Pre- and post-training values (mean ± SD) for GDF8 in the control (CG), supplement (SG), training (TG), training + supplement (TSG) groups. & Significantly different from pretest values (p < 0.05). * Significantly different from CG (p < 0.05). # Significant interaction between time and group (p < 0.05). € Significant time X group interaction (p < 0.05).
GDF15 significantly decreased in the TG (p = 0.004) and TSG (p = 0.0001) compared to the CG. Additionally, Bonferroni’s post-hoc test showed that the decrease in GDF15 in TG (p = 0.02) and TSG (p = 0.001) following 12 weeks of training were significantly different from the SG (Figure 4).
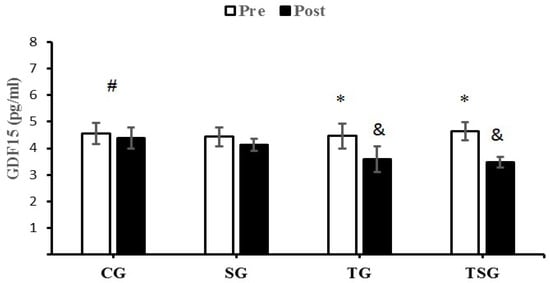
Figure 4.
Pre- and post-training values (mean ± SD) for GDF15 in the control (CG), supplement (SG), training (TG), training + supplement (TSG) groups. & Significantly different from pretest values (p < 0.05). * Significantly different from CG (p < 0.05). # Significant time X group interaction (p < 0.05).
4. Discussion
We examined the effects of 12 weeks of HIFT that was supplemented with astaxanthin on adipokine levels, insulin resistance and lipid profiles in males with obesity. Our findings indicate that 12 weeks of CrossFit exercise training supplemented with astaxanthin: (A) reduced anthropometric indices (body weight, BMI, FFM, body fat percent), (B) improved cardio-respiratory fitness (measured by VO2peak), (C) improved lipid profiles (HDL-C LDL-C, TC, and TGs), (D) improved post-test metabolic markers (glucose, insulin, and HOMA-IR, and (E) reduced adipokine (CTRP2, CTRP9) and growth differentiation factor (GDF8, and GDF15) levels. Obesity is associated with increased adipocyte hypertrophy and hyperplasia. Increased fat accumulation by adipocytes is accompanied by the inhibition of lipolysis and promotion of lipogenesis by inhibiting mitochondrial β-oxidation [42], AMPK activity, and increasing the activity of enzymes involved in lipogenesis [43,44]. An accumulation of fat in the liver, skeletal muscle, and adipocytes leads to insulin resistance (IR) by activating protein kinase C θ (PKC θ) [45]; IR increases fatty acid mobilization from adipose tissue to circulation [46] and triggers oxidative-stress mediated inflammation [47]. Adipocyte hypertrophy increases macrophage accumulation and the production of proinflammatory M1 phenotypes that damages pancreatic β-cells [48,49]. The combined effects of these changes increase body weight, FFM, BMI, and exacerbate insulin resistance and related metabolic disorders. Antioxidants such as astaxanthin can improve dyslipidemia and reduce metabolic disorders [50,51]. Our study indicates that HIFT, supplemented astaxanthin and exercise supplemented with astaxanthin reduced circulatory lipid levels and markers of metabolic disorders, and improved body composition and HDL-C levels. This is supported by other findings that astaxanthin prevents body weight gain and improves lipid profiles and fatty acid utilization related to its anti-oxidative and anti-inflammatory properties [52,53]. Astaxanthin increases plasma total antioxidant capacity (TAC) and superoxide dismutase (SOD) levels [54]. Changes in inflammatory conditions may be another pathway through which astaxanthin can improve metabolic changes induced by obesity, as astaxanthin inhibits the activation of the transcriptional factor of NF-κB to reduce the actions of pro-inflammatory cytokines [55]. Additionally, astaxanthin reduces the infiltration of inflammatory M1 macrophages into hypertrophied adipocytes, which mitigates the release of pro-inflammatory cytokines by macrophages and reduces the release of free fatty acids into circulation and improves insulin sensitivity [56,57]. Astaxanthin accelerates fatty acid oxidation during physical exercise [29,58]. Our study showed that the percentage of body fat in the SG and TG decreased, and this improvement was greater in the TSG. Notably, the significant differences between TG and TSG may be due to the intensity of exercise. Our finding of increased fat utilization is consistent with previous reports indicating that HIFT reduced body fat levels in active healthy individuals [59,60]. Increased fat reduction induced by this type of training reflects changes in aerobic capacity, as measured by the VO2peak in our study, and supported by other studies that CrossFit exercise increases muscle mass and improves insulin sensitivity. Our findings are supported by other reports that HIIT improves insulin sensitivity [61,62]. Adipokine levels correlate positively with adipose tissue levels in obese animal models and humans [11]. The changes in both CTRPs in our study may be related to changes in body weight and lipid profiles [8,11]. Our findings indicate that HIFT and astaxanthin reduced body weight and lipid profiles, while increasing HDL-C levels; these changes were greater when exercise was supplemented with astaxanthin (Figure 1 and Figure 2). In an investigation that used apoE-knocked out mice fed with a high-fat diet, astaxanthin increased the hepatic levels of LDL receptors, reduced HMG-CoA reductase, and increased Sterol regulatory element-binding protein 2 (SREBP-2), which were associated with hypocholesterolemia effects [63]. Another study that utilized two doses of astaxanthin indicated that the level of TG reduced, while HDL-C increased [64]. Our results are supported by other 12-week studies of combined aerobic and resistance training reduced body weight and CTRP5 and CTRP3 levels in obese women [65].
The release of GDF8 is increased in overweight and obese individuals, suggesting a role for GDF8 in the regulation of body fat and total energy metabolism [19]. GDF8 is a potentially negative regulator of skeletal muscle mass [66,67]. Our study found lower circulatory levels of GDF8 to a greater extent in the group receiving both astaxanthin and HIFT training than when either intervention was studied in isolation. Circulating levels of GDF15 are increased with obesity, chronic inflammation [68,69,70,71], and reduced exercise training [72,73]. Contrary to our findings, the majority of other studies report increases in GDF15 levels following exercise in healthy and obese individuals [73,74,75,76], likely due to acute episodes of metabolic and inflammatory stress [77,78]. The conflicting results between our findings (of reduced GDF15 levels following HIFT training) and those of previous studies (of increases in GDF15 levels with exercise) may be related to differences in training modalities [79].
5. Conclusions
Our findings indicate that 12 weeks of astaxanthin supplementation and exercise training decreased adipokines levels, body composition (weight, %fat), anthropometrical factors (BMI), and improved lipid and metabolic profiles. These benefits were greater in men with obesity who exercised and used astaxanthin supplementation.
Author Contributions
All authors contributed equally to data collection. They read and approved the final manuscript. Finally, all authors agreed to be accountable for all aspects of the work. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
All procedures were performed according to the latest revision of the Declaration of Helsinki. The Research and Ethics Committee of the Islamic Azad University approved all procedures of this study (Ethics code: IR-IAU1400-47, 8 July 2021).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available within the manuscript.
Acknowledgments
The authors thank all the volunteers for their enthusiastic participation in this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Saeidi, A.; Tayebi, S.M.; Khosravi, A.; Razi, O.; Sellami, M.; Abderrahman, A.B.; Zouhal, H. Obesity, Fat Mass, Osteopontin and Exercise Training. Int. J. Appl. Exerc. Physiol. 2019, 8, 177–179. [Google Scholar]
- Haslam, D.W.; James, W.P. Obesity. Lancet 2005, 366, 1197–1209. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, S.; Fujita, T.; Shimabukuro, M.; Iwaki, M.; Yamada, Y.; Nakajima, Y.; Nakayama, O.; Makishima, M.; Matsuda, M.; Shimomura, I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Investig. 2017, 114, 1752–1761. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S. Inflammation and metabolic disorders. Nature 2006, 444, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Shoelson, S.E.; Herrero, L.; Naaz, A. Obesity, inflammation, and insulin resistance. Gastroenterology 2007, 132, 2169–2180. [Google Scholar] [CrossRef] [PubMed]
- Lumeng, C.N.; Saltiel, A.R. Inflammatory links between obesity and metabolic disease. J. Clin. Investig. 2011, 121, 2111–2117. [Google Scholar] [CrossRef] [PubMed]
- Peterson, J.M.; Wei, Z.; Seldin, M.M.; Byerly, M.S.; Aja, S.; Wong, G.W. CTRP9 transgenic mice are protected from diet-induced obesity and metabolic dysfunction. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 305, R522–R533. [Google Scholar] [CrossRef]
- Jerobin, J.; Ramanjaneya, M.; Bettahi, I.; Parammal, R.; Siveen, K.S.; Alkasem, M.; Aye, M.; Sathyapalan, T.; Skarulis, M.; Atkin, S.L. Regulation of circulating CTRP-2/CTRP-9 and GDF-8/GDF-15 by intralipids and insulin in healthy control and polycystic ovary syndrome women following chronic exercise training. Lipids Health Dis. 2021, 20, 1–11. [Google Scholar] [CrossRef]
- Seldin, M.M.; Tan, S.Y.; Wong, G.W. Metabolic function of the CTRP family of hormones. Rev. Endocr. Metab. Disord. 2014, 15, 111–123. [Google Scholar] [CrossRef]
- Wong, G.W.; Krawczyk, S.A.; Kitidis-Mitrokostas, C.; Revett, T.; Gimeno, R.; Lodish, H.F. Molecular, biochemical and functional characterizations of C1q/TNF family members: Adipose-tissue-selective expression patterns, regulation by PPAR-γ agonist, cysteine-mediated oligomerizations, combinatorial associations and metabolic functions. Biochem. J. 2008, 416, 161–177. [Google Scholar] [CrossRef]
- Wolf, R.M.; Steele, K.E.; Peterson, L.A.; Zeng, X.; Jaffe, A.E.; Schweitzer, M.A.; Magnuson, T.H.; Wong, G.W. C1q/TNF-related protein-9 (CTRP9) levels are associated with obesity and decrease following weight loss surgery. J. Clin. Endocrinol. Metab. 2016, 101, 2211–2217. [Google Scholar] [CrossRef] [PubMed]
- Peterson, J.M.; Seldin, M.M.; Tan, S.Y.; Wong, G.W. CTRP2 overexpression improves insulin and lipid tolerance in diet-induced obese mice. PLoS ONE 2014, 9, e88535. [Google Scholar] [CrossRef] [PubMed]
- Hanna, A.; Frangogiannis, N.G. The role of the TGF-β superfamily in myocardial infarction. Front. Cardiovasc. Med. 2019, 6, 140. [Google Scholar] [CrossRef]
- Guo, T.; Jou, W.; Chanturiya, T.; Portas, J.; Gavrilova, O.; McPherron, A.C. Myostatin inhibition in muscle, but not adipose tissue, decreases fat mass and improves insulin sensitivity. PLoS ONE 2009, 4, e4937. [Google Scholar] [CrossRef] [PubMed]
- Ding, Q.; Mracek, T.; Gonzalez-Muniesa, P.; Kos, K.; Wilding, J.; Trayhurn, P.; Bing, C. Identification of macrophage inhibitory cytokine-1 in adipose tissue and its secretion as an adipokine by human adipocytes. Endocrinology 2009, 150, 1688–1696. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.; Yang, J.; Gardner, J.; Xiong, Y. GDF15 deficiency promotes high fat diet-induced obesity in mice. PLoS ONE 2018, 13, e0201584. [Google Scholar] [CrossRef] [PubMed]
- Hale, C.; Véniant, M.M. Growth differentiation factor 15 as a potential therapeutic for treating obesity. Mol. Metab. 2021, 46, 101117. [Google Scholar] [CrossRef]
- Lu, J.F.; Zhu, M.Q.; Xie, B.C.; Shi, X.C.; Liu, H.; Zhang, R.X.; Xia, B.; Wu, J.W. Camptothecin effectively treats obesity in mice through GDF15 induction. PLoS Biol. 2022, 20, e3001517. [Google Scholar] [CrossRef]
- Pervin, S.; Reddy, S.T.; Singh, R. Novel Roles of Follistatin/Myostatin in Transforming Growth Factor-β Signaling and Adipose Browning: Potential for Therapeutic Intervention in Obesity Related Metabolic Disorders. Front. Endocrinol. 2021, 12, 339. [Google Scholar] [CrossRef]
- Koncarevic, A.; Kajimura, S.; Cornwall-Brady, M.; Andreucci, A.; Pullen, A.; Sako, D.; Kumar, R.; Grinberg, A.V.; Liharska, K.; Ucran, J.A. A novel therapeutic approach to treating obesity through modulation of TGFβ signaling. Endocrinology 2012, 153, 3133–3146. [Google Scholar] [CrossRef] [PubMed]
- Kolnes, K.J.; Petersen, M.H.; Lien-Iversen, T.; Højlund, K.; Jensen, J. Effect of Exercise Training on Fat Loss—Energetic Perspectives and the Role of Improved Adipose Tissue Function and Body Fat Distribution. Front. Physiol. 2021, 1634. [Google Scholar] [CrossRef]
- Durkalec-Michalski, K.; Nowaczyk, P.M.; Siedzik, K. Effect of a four-week ketogenic diet on exercise metabolism in CrossFit-trained athletes. J. Int. Soc. Sport. Nutr. 2019, 16, 16. [Google Scholar] [CrossRef]
- Kliszczewicz, B.; Quindry, C.J.; Blessing, L.D.; Oliver, D.G.; Esco, R.M.; Taylor, J.K. Acute exercise and oxidative stress: CrossFit™ vs. treadmill bout. J. Hum. Kinet. 2015, 47, 81. [Google Scholar] [CrossRef] [PubMed]
- Feito, Y.; Heinrich, K.M.; Butcher, S.J.; Poston, W.S.C. High-intensity functional training (HIFT): Definition and research implications for improved fitness. Sports 2018, 6, 76. [Google Scholar] [CrossRef] [PubMed]
- Tibana, R.A.; De Almeida, L.M.; de Sousa, N.M.F.; Dda, C.N.; Neto, I.; De Almeida, J.A.; De Souza, V.C.; Mde, F.L.; Ode, T.N.; Vieira, D. Two consecutive days of crossfit training affects pro and anti-inflammatory cytokines and osteoprotegerin without impairments in muscle power. Front. Physiol. 2016, 7, 260. [Google Scholar] [CrossRef] [PubMed]
- Schlegel, P. CrossFit® training strategies from the perspective of concurrent training: A systematic review. J. Sport. Sci. Med. 2020, 19, 670. [Google Scholar]
- Xia, W.; Tang, N.; Kord-Varkaneh, H.; Low, T.Y.; Tan, S.C.; Wu, X.; Zhu, Y. The effects of astaxanthin supplementation on obesity, blood pressure, CRP, glycemic biomarkers, and lipid profile: A meta-analysis of randomized controlled trials. Pharmacol. Res. 2020, 161, 105113. [Google Scholar] [CrossRef]
- Fakhri, S.; Abbaszadeh, F.; Dargahi, L.; Jorjani, M. Astaxanthin: A mechanistic review on its biological activities and health benefits. Pharmacol. Res. 2018, 136, 1–20. [Google Scholar] [CrossRef]
- Aoi, W.; Naito, Y.; Takanami, Y.; Ishii, T.; Kawai, Y.; Akagiri, S.; Kato, Y.; Osawa, T.; Yoshikawa, T. Astaxanthin improves muscle lipid metabolism in exercise via inhibitory effect of oxidative CPT I modification. Biochem. Biophys. Res. Commun. 2008, 366, 892–897. [Google Scholar] [CrossRef]
- Thomas, S.; Reading, J.; Shephard, R.J. Revision of the physical activity readiness questionnaire (PAR-Q). Can. J. Sport Sci. 1992, 17, 338–445. [Google Scholar]
- Nathanson, V. Revising the Declaration of Helsinki; BMJ Publishing Group Ltd.: London, UK, 2013; Volume 346. [Google Scholar]
- Ghroubi, S.; Elleuch, H.; Chikh, T.; Kaffel, N.; Abid, M.; Elleuch, M. Physical training combined with dietary measures in the treatment of adult obesity. A comparison of two protocols. Ann. Phys. Rehabil. Med. 2009, 52, 394–413. [Google Scholar] [CrossRef] [PubMed]
- Hunter, G.R.; Byrne, N.M.; Sirikul, B.; Fernández, J.R.; Zuckerman, P.A.; Darnell, B.E.; Gower, B.A. Resistance training conserves fat-free mass and resting energy expenditure following weight loss. Obesity 2008, 16, 1045–1051. [Google Scholar] [CrossRef] [PubMed]
- Nelson, M.E.; Rejeski, W.J.; Blair, S.N.; Duncan, P.W.; Judge, J.O.; King, A.C.; Macera, C.A.; Castaneda-Sceppa, C. Physical activity and public health in older adults: Recommendation from the American College of Sports Medicine and the American Heart Association. Circulation 2007, 116, 1094. [Google Scholar] [CrossRef] [PubMed]
- Perciavalle, V.; Marchetta, N.S.; Giustiniani, S.; Borbone, C.; Perciavalle, V.; Petralia, M.C.; Buscemi, A.; Coco, M. Attentive processes, blood lactate and CrossFit®. Physician Sportsmed. 2016, 44, 403–406. [Google Scholar] [CrossRef] [PubMed]
- Timón, R.; Olcina, G.; Camacho-Cardeñosa, M.; Camacho-Cardenosa, A.; Martinez-Guardado, I.; Marcos-Serrano, M. 48-hour recovery of biochemical parameters and physical performance after two modalities of CrossFit workouts. Biol. Sport 2019, 36, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Mangine, G.T.; Van Dusseldorp, T.A.; Feito, Y.; Holmes, A.J.; Serafini, P.R.; Box, A.G.; Gonzalez, A.M. Testosterone and cortisol responses to five high-intensity functional training competition workouts in recreationally active adults. Sports 2018, 6, 62. [Google Scholar] [CrossRef] [PubMed]
- Glassman, G. A theoretical template for crossfit’s programming. CrossFit J. 2003, 6, 1–5. [Google Scholar]
- Heinrich, K.M.; Patel, P.M.; O’Neal, J.L.; Heinrich, B.S. High-intensity compared to moderate-intensity training for exercise initiation, enjoyment, adherence, and intentions: An intervention study. BMC Public Health 2014, 14, 789. [Google Scholar] [CrossRef]
- Thomas, D.T.; Erdman, K.A.; Burke, L.M. Position of the Academy of Nutrition and Dietetics, Dietitians of Canada, and the American College of Sports Medicine: Nutrition and athletic performance. J. Acad. Nutr. Diet. 2016, 116, 501–528. [Google Scholar] [CrossRef]
- Hsing, A.W.; Gao, Y.-T.; Chua, S.; Deng, J.; Stanczyk, F.Z. Insulin resistance and prostate cancer risk. J. Natl. Cancer Inst. 2003, 95, 67–71. [Google Scholar] [CrossRef]
- Serra, D.; Mera, P.; Malandrino, M.I.; Mir, J.F.; Herrero, L. Mitochondrial fatty acid oxidation in obesity. Antioxid. Redox Signal. 2013, 19, 269–284. [Google Scholar] [CrossRef] [PubMed]
- Rocha-Rodrigues, S.; Rodríguez, A.; Gouveia, A.M.; Gonçalves, I.O.; Becerril, S.; Ramírez, B.; Beleza, J.; Frühbeck, G.; Ascensão, A.; Magalhães, J. Effects of physical exercise on myokines expression and brown adipose-like phenotype modulation in rats fed a high-fat diet. Life Sci. 2016, 165, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Cheema, S.K.; Clandinin, M.T. Diet fat alters expression of genes for enzymes of lipogenesis in lean and obese mice. Biochim. Biophys. Acta (BBA) Lipids Lipid Metab. 1996, 1299, 284–288. [Google Scholar] [CrossRef]
- Itani, S.I.; Ruderman, N.B.; Schmieder, F.; Boden, G. Lipid-induced insulin resistance in human muscle is associated with changes in diacylglycerol, protein kinase C, and IκB-α. Diabetes 2002, 51, 2005–2011. [Google Scholar] [CrossRef] [PubMed]
- Coppack, S.; Evans, R.; Fisher, R.; Frayn, K.; Gibbons, G.; Humphreys, S.; Kirk, M.; Potts, J.; Hockaday, T. Adipose tissue metabolism in obesity: Lipase action in vivo before and after a mixed meal. Metabolism 1992, 41, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.L.; Goldfine, I.D.; Maddux, B.A.; Grodsky, G.M. Oxidative stress and stress-activated signaling pathways: A unifying hypothesis of type 2 diabetes. Endocr. Rev. 2002, 23, 599–622. [Google Scholar] [CrossRef]
- Murano, I.; Barbatelli, G.; Parisani, V.; Latini, C.; Muzzonigro, G.; Castellucci, M.; Cinti, S. Dead adipocytes, detected as crown-like structures, are prevalent in visceral fat depots of genetically obese mice. J. Lipid Res. 2008, 49, 1562–1568. [Google Scholar] [CrossRef]
- Nishimura, S.; Manabe, I.; Nagasaki, M.; Eto, K.; Yamashita, H.; Ohsugi, M.; Otsu, M.; Hara, K.; Ueki, K.; Sugiura, S. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat. Med. 2009, 15, 914–920. [Google Scholar] [CrossRef]
- Tsuda, T.; Horio, F.; Uchida, K.; Aoki, H.; Osawa, T. Dietary cyanidin 3-O-β-D-glucoside-rich purple corn color prevents obesity and ameliorates hyperglycemia in mice. J. Nutr. 2003, 133, 2125–2130. [Google Scholar] [CrossRef]
- Wolfram, S.; Wang, Y.; Thielecke, F. Anti-obesity effects of green tea: From bedside to bench. Mol. Nutr. Food Res. 2006, 50, 176–187. [Google Scholar] [CrossRef]
- Kobayashi, M. In vivo antioxidant role of astaxanthin under oxidative stress in the green alga Haematococcus pluvialis. Appl. Microbiol. Biotechnol. 2000, 54, 550–555. [Google Scholar] [CrossRef] [PubMed]
- Bennedsen, M.; Wang, X.; Willén, R.; Wadström, T.; Andersen, L.P. Treatment of H. pylori infected mice with antioxidant astaxanthin reduces gastric inflammation, bacterial load and modulates cytokine release by splenocytes. Immunol. Lett. 2000, 70, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.D.; Kim, J.H.; Chang, M.J.; Kyu-Youn, Y.; Shin, W.G. Effects of astaxanthin on oxidative stress in overweight and obese adults. Phytother. Res. 2011, 25, 1813–1818. [Google Scholar] [CrossRef]
- Speranza, L.; Pesce, M.; Patruno, A.; Franceschelli, S.; De Lutiis, M.A.; Grilli, A.; Felaco, M. Astaxanthin treatment reduced oxidative induced pro-inflammatory cytokines secretion in U937: SHP-1 as a novel biological target. Mar. Drugs 2012, 10, 890–899. [Google Scholar] [CrossRef]
- Nawaz, A.; Nishida, Y.; Takikawa, A.; Fujisaka, S.; Kado, T.; Aminuddin, A.; Bilal, M.; Jeelani, I.; Aslam, M.R.; Nishimura, A. Astaxanthin, a Marine Carotenoid, Maintains the Tolerance and Integrity of Adipose Tissue and Contributes to Its Healthy Functions. Nutrients 2021, 13, 4374. [Google Scholar] [CrossRef]
- Lee, S.J.; Bai, S.K.; Lee, K.S.; Namkoong, S.; Na, H.J.; Ha, K.S.; Han, J.A.; Yim, S.V.; Chang, K.; Kwon, Y.G.; et al. Astaxanthin Inhibits Nitric Oxide Production and Inflammatory Gene Expression by Suppressing I (kappa) B Kinase-dependent NF-(kappa) B Activation. Mol. Cells 2003, 16, 97–105. [Google Scholar]
- Ikeuchi, M.; Koyama, T.; Takahashi, J.; Yazawa, K. Effects of astaxanthin supplementation on exercise-induced fatigue in mice. Biol. Pharm. Bull. 2006, 29, 2106–2110. [Google Scholar] [CrossRef] [PubMed]
- Feito, Y.; Hoffstetter, W.; Serafini, P.; Mangine, G. Changes in body composition, bone metabolism, strength, and skill-specific performance resulting from 16-weeks of HIFT. PLoS ONE 2018, 13, e0198324. [Google Scholar] [CrossRef]
- Murawska-Cialowicz, E.; Wojna, J.; Zuwala-Jagiello, J. Crossfit training changes brain-derived neurotrophic factor and irisin levels at rest, after wingate and progressive tests, and improves aerobic capacity and body composition of young physically active men and women. J. Physiol. Pharmacol. 2015, 66, 811–821. [Google Scholar]
- Cocks, M.; Shaw, C.S.; Shepherd, S.O.; Fisher, J.P.; Ranasinghe, A.M.; Barker, T.A.; Tipton, K.D.; Wagenmakers, A.J. Sprint interval and endurance training are equally effective in increasing muscle microvascular density and eNOS content in sedentary males. J. Physiol. 2013, 591, 641–656. [Google Scholar] [CrossRef]
- Shepherd, S.O.; Cocks, M.; Tipton, K.; Ranasinghe, A.M.; Barker, T.A.; Burniston, J.G.; Wagenmakers, A.J.; Shaw, C.S. Sprint interval and traditional endurance training increase net intramuscular triglyceride breakdown and expression of perilipin 2 and 5. J. Physiol. 2013, 591, 657–675. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Seo, J.M.; Nguyen, A.; Pham, T.X.; Park, H.J.; Park, Y.; Kim, B.; Bruno, R.S.; Lee, J. Astaxanthin-rich extract from the green alga Haematococcus pluvialis lowers plasma lipid concentrations and enhances antioxidant defense in apolipoprotein E knockout mice. J. Nutr. 2011, 141, 1611–1617. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, Y.; Yoshida, H.; Kondo, K. Potential anti-atherosclerotic properties of astaxanthin. Mar. Drugs 2016, 14, 35. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.Y.; Park, J.W.; Lee, N.; Hwang, S.Y.; Cho, G.J.; Hong, H.C.; Yoo, H.J.; Hwang, T.G.; Kim, S.M.; Baik, S.H. Effects of a combined aerobic and resistance exercise program on C1q/TNF-related protein-3 (CTRP-3) and CTRP-5 levels. Diabetes Care 2013, 36, 3321–3327. [Google Scholar] [CrossRef] [PubMed]
- McPherron, A.C.; Lawler, A.M.; Lee, S.-J. Regulation of skeletal muscle mass in mice by a new TGF-p superfamily member. Nature 1997, 387, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Schuelke, M.; Wagner, K.R.; Stolz, L.E.; Hübner, C.; Riebel, T.; Kömen, W.; Braun, T.; Tobin, J.F.; Lee, S.-J. Myostatin mutation associated with gross muscle hypertrophy in a child. N. Engl. J. Med. 2004, 350, 2682–2688. [Google Scholar] [CrossRef]
- Keipert, S.; Ost, M. Stress-induced FGF21 and GDF15 in obesity and obesity resistance. Trends Endocrinol. Metab. 2021, 32, 904–915. [Google Scholar] [CrossRef]
- Sarkar, S.; Legere, S.; Haidl, I.; Marshall, J.; MacLeod, J.B.; Aguiar, C.; Lutchmedial, S.; Hassan, A.; Brunt, K.R.; Kienesberger, P. Serum GDF15, a promising biomarker in obese patients undergoing heart surgery. Front. Cardiovasc. Med. 2020, 7, 103. [Google Scholar] [CrossRef]
- Kempf, T.; Guba-Quint, A.; Torgerson, J.; Magnone, M.C.; Haefliger, C.; Bobadilla, M.; Wollert, K.C. Growth differentiation factor 15 predicts future insulin resistance and impaired glucose control in obese nondiabetic individuals: Results from the XENDOS trial. Eur. J. Endocrinol. 2012, 167, 671. [Google Scholar] [CrossRef]
- Vila, G.; Riedl, M.; Anderwald, C.; Resl, M.; Handisurya, A.; Clodi, M.; Prager, G.; Ludvik, B.; Krebs, M.; Luger, A. The relationship between insulin resistance and the cardiovascular biomarker growth differentiation factor-15 in obese patients. Clin. Chem. 2011, 57, 309–316. [Google Scholar] [CrossRef]
- Cai, L.; Li, C.; Wang, Y.; Mo, Y.; Yin, J.; Ma, X. Increased serum GDF15 related to improvement in metabolism by lifestyle intervention among young overweight and obese adults. Diabetes Metab. Syndr. Obes. Targets Ther. 2021, 14, 1195. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Fealy, C.E.; Kirwan, J.P. Exercise training promotes a GDF15-associated reduction in fat mass in older adults with obesity. Am. J. Physiol. Endocrinol. Metab. 2019, 316, E829–E836. [Google Scholar] [CrossRef] [PubMed]
- Klein, A.B.; Nicolaisen, T.S.; Ørtenblad, N.; Gejl, K.D.; Jensen, R.; Fritzen, A.M.; Larsen, E.L.; Karstoft, K.; Poulsen, H.E.; Morville, T. Pharmacological but not physiological GDF15 suppresses feeding and the motivation to exercise. Nat. Commun. 2021, 12, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Laurens, C.; Parmar, A.; Murphy, E.; Carper, D.; Lair, B.; Maes, P.; Vion, J.; Boulet, N.; Fontaine, C.; Marquès, M. Growth and differentiation factor 15 is secreted by skeletal muscle during exercise and promotes lipolysis in humans. JCI Insight 2020, 5, e131870. [Google Scholar] [CrossRef] [PubMed]
- Kleinert, M.; Clemmensen, C.; Sjøberg, K.A.; Carl, C.S.; Jeppesen, J.F.; Wojtaszewski, J.F.; Kiens, B.; Richter, E.A. Exercise increases circulating GDF15 in humans. Mol. Metab. 2018, 9, 187–191. [Google Scholar] [CrossRef] [PubMed]
- de Freitas, M.C.; Gerosa-Neto, J.; Zanchi, N.E.; Lira, F.S.; Rossi, F.E. Role of metabolic stress for enhancing muscle adaptations: Practical applications. World J. Methodol. 2017, 7, 46. [Google Scholar] [CrossRef]
- Kaspar, F.; Jelinek, H.F.; Perkins, S.; Al-Aubaidy, H.A.; Dejong, B.; Butkowski, E. Acute-phase inflammatory response to single-bout HIIT and endurance training: A comparative study. Mediat. Inflamm. 2016, 2016, 5474837. [Google Scholar] [CrossRef][Green Version]
- Xiong, Y.; Walker, K.; Min, X.; Hale, C.; Tran, T.; Komorowski, R.; Yang, J.; Davda, J.; Nuanmanee, N.; Kemp, D. Long-acting MIC-1/GDF15 molecules to treat obesity: Evidence from mice to monkeys. Sci. Transl. Med. 2017, 9, eaan8732. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).