Abstract
We aimed to assess the effect of oral probiotic containing the Streptococcus salivarius M18 strain on gingival inflammation, bleeding on probing, and oral biofilm. Sixty-one consenting participants aged between 18 and 25 with gingivitis were recruited in this double-blind, parallel-group study and randomly divided into the probiotic group (n = 31) and the placebo group (n = 30). Fifty-seven participants completed the entire study protocol, 27 in the probiotic group and 30 in the placebo group. The outcomes were assessed after 4 weeks of intervention and 4 weeks of follow-up. There was a significant decrease in the Gingival Index, with the effect size of 0.58 [95%CI 0.05–1.10], and Turesky modification of the Quigley and Hein Plaque Index, with the effect size of 0.55 [95%CI: 0.02–1.07], in the probiotic group after the intervention. However, after a 4-week follow-up, the only significant treatment outcome was improved gingival condition according to the Gingival Index. The Gingival Bleeding Index also decreased significantly in the probiotic group after the intervention period; after the follow-up, this parameter did not differ significantly in both groups from the baseline values. In the placebo group, there were no significant improvements in the assessed parameters throughout this study. No serious side effects were registered. Within the limitations of this study, we conclude that the use of oral probiotic containing the Streptococcus salivarius M18 strain resulted in a significant improvement in gingival condition and oral hygiene level in young adults with gingivitis. Trial registration NCT05727436. Funding: none.
1. Introduction
Periodontal diseases are among the most common chronic diseases worldwide [1]. The prevalence of periodontal diseases is estimated at 78.8%, 90.7%, and 90.3% in adolescents, adults, and the elderly, respectively [2]. Periodontal inflammation can range from mild gingivitis to severe irreversible periodontitis, leading to tooth loss [3], and is considered a risk factor for certain systemic diseases [4]. Gingivitis is a reversible condition, which is believed to be a pre-existing stage of periodontitis. Thus, timely treatment of gingivitis is crucial to prevent periodontitis [5,6].
Current etiological concepts suggest that the dysbiotic growth of virulent microorganisms of dental plaque (biofilm) is a necessary component for the development of gingivitis and periodontitis [7,8,9,10]. The main bacteria causing gingivitis include some species of streptococcus, fusobacterium, actinomyces, veillonella, and treponema. Bacteroides, capnocytophaga, and eikenella are also likely to play a role in the etiology of gingivitis [11]. There are also individual factors such as smoking, endocrine disorders, medications, or systemic diseases that can alter the inflammatory response to plaque and thereby increase susceptibility to gingivitis [12].
Initial therapy for gingivitis consists of motivating and educating the patient, as well as mechanical removal of dental plaque [13,14]. However, most people do not properly control plaque accumulation with tooth brushing and interdental cleaning [5,15]. To overcome this, antimicrobial products have been suggested for their adjuvant efficacy to reduce plaque accumulation and gingivitis development [16]. At the same time, prolonged use of antiseptics can be associated with undesirable side effects, which has led to a search for alternative approaches [16]. Bacteriotherapy is a method in which a commensal strain is introduced into the host microbiome to rebalance the microflora [17]. This approach is of great interest for the treatment of oral diseases [18] due to minimal side effects [17]. It is potentially effective even if there is a lack of mechanical plaque control [19]. Its possible mechanisms of action are not fully understood. However, both direct and indirect effects seem to be evident [20]. Among them are coaggregation with pathogenic microorganisms and growth inhibition, production of bacteriocins and hydrogen peroxide, competition for adhesion sites of nutrient substrates, and systemic immunomodulation by affecting mucosal immune system cells or mucosal epithelial barrier function [21,22,23,24,25,26,27].
A number of studies have assessed the effects of probiotics in the prevention of dental caries [28,29,30,31,32] and the prevention and treatment of oral candidiasis [33,34,35], halitosis [36,37,38,39], and periodontal diseases [17,19,37,40,41,42]. Nase et al. were the first to suggest using probiotics in periodontal patients more than a decade ago [43]. However, the findings of existing studies remain contradictory. Some authors have reported a significant reduction in gingival and periodontal inflammation [40,42] and plaque accumulation, significant improvement in periodontal pocket depth [41], and reduction in pro-inflammatory cytokines [44]. At the same time, there have been reports of no positive effects on clinical and microbiological parameters [37,45] or of positive effects only on the composition of oral microbiota [46]. The heterogeneity of results can be explained by the variability in methodology and the use of different probiotic strains or their combinations [19]. The most popular microorganisms used as probiotics have been strains belonging to lacto- and bifidobacteria [47,48,49]. Streptococcus salivarius strains have been less studied but are also of great interest. The two main strains of Streptococcus salivarius used in dentistry are K12 and M18. S. salivarius K12 has been shown to provide an inhibitory effect on oral biofilm formation [50,51], and the use of S. salivarius M18 has been confirmed to reduce plaque index [52,53] and improve periodontal health indicators [53,54,55].
Still, healthcare professionals may be reluctant to recommend probiotics to patients due to a paucity of the peer-reviewed literature on the topic and the absence of accepted evidence-based recommendations [56,57]. A systematic review by Ausenda et al. concluded that more research on the topic was needed, as significant heterogeneity of the existing studies did not allow researchers to make firm conclusions and develop clinical recommendations regarding probiotics use in the treatment of periodontal diseases [58].
Therefore, the aim of our study was to expand the knowledge on the topic by assessing the effect of oral probiotic containing the Streptococcus salivarius M18 strain on gingival inflammation, bleeding on probing, and oral biofilm.
2. Materials and Methods
2.1. Study Design
This study’s protocol was approved by the local ethics committee (Protocol no. 23-22, 17 November 2022) and was registered on clinicaltrials.gov (NCT05727436, February 2023). This study complied with the 1964 Declaration of Helsinki and its subsequent amendments and the CONSORT 2010 statement.
This was a double-blind, randomized, placebo-controlled, two-arm parallel-group trial evaluating the effect of oral probiotic containing Streptococcus salivarius M18 strain on the gingival inflammation, bleeding on probing, and oral biofilm. This study was conducted between February 2023 and May 2023 at the Therapeutic Dentistry department of Sechenov University, Moscow, Russia.
2.2. Subjects
This study included healthy young adults visiting Sechenov University’s Dental Institute. The enrollment was accomplished by the single trained operator (D.S.). All participants were informed of the purpose and implications of this study and signed written informed consent. At baseline, all patients were instructed to follow a standardized brushing technique (Bass) using a pea-sized amount of toothpaste without antibacterial or anti-plaque components twice a day.
The inclusion criteria were the following:
- -
- men and women aged between 18 and 25;
- -
- permanent bite;
- -
- presence of at least 20 teeth;
- -
- absence of systemic or chronic diseases;
- -
- the diagnosis of gingivitis stated clinically.
The exclusion criteria were the following:
- -
- refusal to provide written informed consent;
- -
- chronic periodontitis;
- -
- use of medication or dietary supplement that contain pro- and prebiotics (within 1 month before the enrollment);
- -
- use of antibiotics (within 3 months before the enrollment);
- -
- allergy to the components of the probiotic/placebo;
- -
- immunodeficiency disorders and use of immunosuppressants;
- -
- use of other hygiene products, immunostimulants, antibacterial drugs, and pre-and probiotics during this study;
- -
- failure to follow the prescription regimen;
- -
- failure to follow the research protocol.
2.3. Randomization, Blinding, and Interventions
Sixty-one participants were randomly assigned to two groups in accordance with a computer-generated sequence prepared by a third-party person. Group 1 received lozenges containing probiotic; Group 2 received lozenges containing placebo. Allocation concealment was performed by a person not involved in this study using numbered unlabeled containers. Subjects and researchers were unaware of which participants received probiotics and who received placebos. The oral probiotic tested in this study was S. salivarius M18 ≥ 5 × 108 CFU per lozenge, mint-flavored gray-and-white lozenges (Dentoblis, MEDICO DOMUS, d.d.o., Nis, Serbia). The placebo lozenges did not contain S. salivarius M18 but had the same flavor, consistency, and appearance.
Participants were instructed to take one lozenge daily in the evening after toothbrushing, let it dissolve, not bite or swallow it, and then avoid eating or drinking for at least one hour. To increase participants’ compliance, they were asked to mark each lozenge intake on Google spreadsheets.
2.4. Endpoints and Assessments
A one-month intervention period was followed by a one-month follow-up.
Primary endpoints included changes in the Gingival Index (GI) and Gingival Bleeding Index (GBI) after the one-month intervention period and after the one-month follow-up. Secondary endpoints were changes in the Turesky modification of the Quigley and Hein Plaque Index (TQHPI) values after the one-month intervention period and after the one-month follow-up. The parameters were assessed as described in previous studies [59,60,61].
2.5. Statistical Analyses
2.5.1. Sample Size Calculation
The sample size was determined for the primary outcome (GBI) according to our pilot study. Sample size calculations were performed using the G*Power calculator (version 3.1.9.6) for the Wilcoxon rank sum test for the two groups—the power was set at 80%, and the alfa-level was set as 0.05. The allocation ratio was equal to 1. The resultant target sample size comprised 30 participants in each group (26 participants according to sample size calculations plus 15% to account for possible dropout), 60 patients in total.
2.5.2. Statistical Methods
Continuous data were presented as means and standard deviations, 95% confidence intervals, and medians and quartiles; categorical variables were presented as counts and percentages. The normality and sphericity of distribution were assessed with a Shapiro–Wilk test and Levene’s test, respectively. Fisher’s exact test was used to compare gender distribution between this study’s groups. A repeated analysis of variance and a paired t-test or Welch t-test were used to compare GI and TQHPI values between this study’s groups. A Wilcoxon rank sum test and a Wilcoxon matched-pairs signed-rank test were used to compare the GBI values between this study’s groups. Hedge’s g was used to calculate the effect size between the groups by comparing mean baseline and post-intervention values. Collected data were analyzed in R version 3.6.0 (26 April 2019) with the following packages: “doBy”, “rstatix”, “stats”, and “effectsize” in RStudio version 1.2.1335 2009–2019. Statistical significance was defined as p < 0.05 (two-tailed).
3. Results
Two hundred and five patients aged between 18 and 25 were assessed for eligibility. Of these, 144 patients were ineligible due to the following reasons: not meeting inclusion criteria (n = 84); meeting exclusion criteria (n = 55); refusing to participate (n = 1); and not being sure they would comply with the protocol (n = 4). Sixty-one patients were enrolled and randomly assigned to the probiotic group (21 females and 10 males) or the placebo group (19 females and 11 males). Four participants from the probiotic group were lost to the follow-up due to a self-reported allergy (n = 1), measles quarantine (n = 2), and failure to check up (n = 1). A total of 57 participants were included in the final analysis, 27 in the probiotic group and 30 in the placebo group (Figure 1). Group comparisons showed no significant differences in age and gender distribution (Table 1).
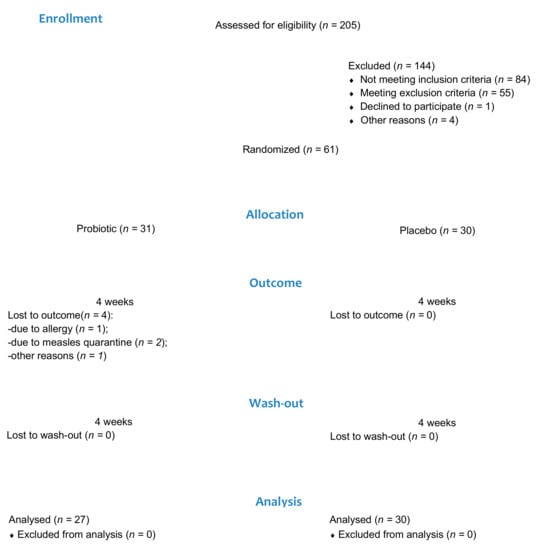
Figure 1.
Patient flow diagram.

Table 1.
Subject demographics.
We assessed the effect of the probiotic intake on gingival inflammation (GI and GBI) and plaque accumulation (TQHPI).
Participants receiving the probiotic showed a significant decrease in gingival bleeding at the post-intervention time point. The mean GBI values were 0.127 ± 0.137 and 0.086 ± 0.086 at baseline and at 4 weeks. Participants receiving the placebo, on the contrary, showed an increase in this parameter from 0.066 ± 0.049 to 0.083 ± 0.052 (Table 2). However, the GBI values after the follow-up period in both groups did not differ significantly from the baseline values.

Table 2.
GBI values.
According to ANOVA, the “visit” (p = 0.012) and interaction of “visit” and “group” factors (p < 0.001) had a significant impact on the GI values (Table 3).

Table 3.
ANOVA table for GI indices values.
The probiotic group showed lower mean post-intervention and post-follow-up GI values than the placebo group (p = 0.045). According to the GI, a 4-week probiotic intake resulted in an improvement of gingival condition with the effect size of 0.58; moreover, post-follow-up GI values did not differ significantly from post-intervention values. The placebo group showed a significant increase in post-intervention GI values, while post-follow-up values did not differ significantly from baseline and post-intervention values (Table 4 and Figure 2).

Table 4.
GI values.
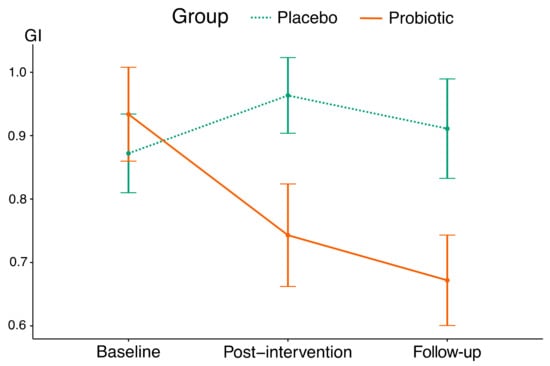
Figure 2.
The mean trajectories of GI values in this study’s groups (vertical lines indicate standard deviations).
The mean trajectory showed a decrease in TQHPI values over the 4-week intervention period in the probiotic group (Figure 3), and interaction between the factors indicated significant differences between the groups (Table 5 and Table 6). According to this parameter, a 4-week probiotic intake resulted in a reduction in plaque scores with the effect size of 0.55. No significant changes in TQHPI values were registered in the placebo group throughout this study.
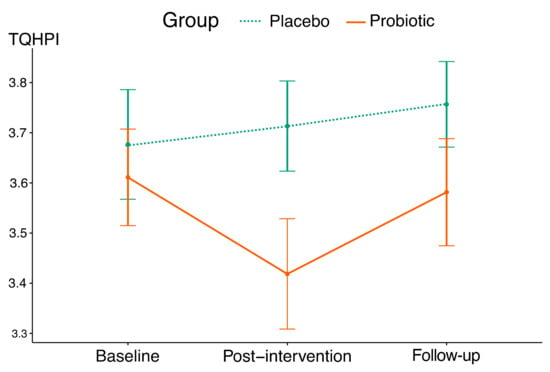
Figure 3.
The mean trajectories of TQHPI values in this study’s groups (vertical lines indicate standard deviations).

Table 5.
ANOVA table for TQHPI indices values.

Table 6.
TQHPI index values.
Adverse Events
No serious adverse events were reported. One patient from the probiotic group canceled the participation in this study due to a self-reported allergy (skin rash). However, the patient did not attend the visit scheduled to assess his condition; thus, the allergic reaction was not confirmed.
4. Discussion
We assessed the effect of oral probiotic containing the Streptococcus salivarius M18 strain on gingival inflammation (GI), bleeding on probing (GBI), and oral biofilm (TQHPI). A 4-week probiotic intake resulted in a significant improvement in gingival condition and oral hygiene level. However, after a 4-week follow-up, the only significant treatment outcome was an improved gingival condition, according to the GI.
Gingivitis is defined as “an inflammatory lesion resulting from interactions between the dental plaque biofilm and the host’s immune–inflammatory response, which remains contained within the gingiva and does not extend to the periodontal attachment” [62]. It is characterized by redness, swelling, and bleeding of the gingiva [63]. Therefore, the assessment of gingival status should include a subjective assessment of the color, form, density, and bleeding tendency of the gingival tissues, as well as oral hygiene performance expressed as qualitative or semi-quantitative indices [64]. In our study, we used the GI to assess gingival changes in color and texture and the GBI to assess bleeding elicited by probing.
Most previous studies assessing the effect of probiotics on gingival health indicators used lactobaccilli or bifidobacteria strains in different formulations. In a study by Toiviainen involving adults, the probiotic lozenges containing a combination of Lactobacillus rhamnosus GG and Bifidobacterium animalis subsp. lactis BB-12 decreased GI values, while no changes were observed in the control group after 4 weeks [65]. In a similar study involving adolescents, a reduction in GI was registered in both groups; however, it was significantly higher in the probiotic group than in the control group [66]. According to Twetman et al., the number of sites with bleeding on probing decreased in the test and placebo groups, but the changes were significant only in the test groups (after one or two weeks of chewing Lactobacillus reuteri-containing gum) [67]. Keller et al. assessed bleeding on probing after a 2- and 4-week probiotic intake (Lactobacillus rhamnosus and Lactobacillus curvatus) and a 2-week follow-up period. They found that the decrease in this parameter was statistically significant after 4 and 6 weeks in the test group compared to baseline [17]. In a study by Schlagenhauf et al., the observed mean GI score in the test group that took Lactobacillus reuteri-containing lozenges for 6 weeks significantly decreased, whereas in the placebo group, it significantly increased compared to baseline values. As the participants were recruited among sailors of a naval ship on a mission at sea, the decrease in the GI could be attributed to a deterioration of oral hygiene [19].
Some of the previous studies confirmed the beneficial effects of lactobaccilli or bifidobacteria strains on periodontal parameters in patients with different health problems. Yuki et al. found that the consumption of milk fermented with Lactobacillus rhamnosus by individuals with intellectual disabilities resulted in a decrease in GI scores during this study, with a tendency for a greater decrease in the test group. Also, there was a significantly greater reduction in PMA index scores in the test group compared to the placebo group [68]. Sabatini et al. assessed the effect of oral probiotics (Lactobacillus reuteri) in the management of gingivitis in diabetic patients. In their study, at 30 days, both groups showed a statistically significant reduction in bleeding on probing; the changes were more pronounced in the probiotic group [69]. In a study by Hambire and Hambire, there was a significant improvement in the gingival status of children undergoing chemotherapy in the test group after the 90-day use of oral probiotics [70].
In other studies, the effects of probiotic use on gingival health were questionable. Benic et al. showed that GI values were almost identical in the test (Streptococcus salivarius M18) group and the placebo group at baseline, at the end of the intervention (1 month), and at a 3-month follow-up [37]. Montero et al. evaluated the potency of oral tablets containing Lactobacillus plantarum, Lactobacillus brevis, and Pediococcus acidilactici in the treatment of gingivitis. Both study groups experienced a statistically significant improvement in mean GI scores. At 6 weeks, despite insignificant differences between the groups in general, GI values for the sites of severe inflammation (GI = 3 at baseline) were significantly lower in the probiotic group [63].
In our study, we used an oral probiotic containing the Streptococcus salivarius M18. To the best of our knowledge, the literature on the use of Streptococci strains for dental purposes is scarce. A recent systematic review on the use of probiotics in the treatment of periodontal diseases, which included 21 studies, showed that the majority of the studies used lactobacilli and bifidobacteria, whereas Streptococci species were used only in one study [71]. Another similar systematic review, which included 64 studies, had similar results, with Streptococci used only in three studies [47].
Burton et al. reported no improvement and no significant differences in GI scores between the probiotic-treated (Streptococcus salivarius M18) children and the controls at 1-, 3-, and 7-month time points [52]. In a study by Babina et al., the authors assessed the effect of probiotic containing the Streptococcus salivarius K12 strain on oral health indicators. Although PMA values tended to decrease in the probiotic group at the end of a 4-week treatment period and after a 2-week washout period, these changes did not reach the level of statistical significance [50]. Similar results were obtained by Ferrer et al., who assessed the effect of a bucco-adhesive gel containing Streptococcus dentisani applied in a dental splint for 5 min every 48 h for a period of 1 month. Oral health indicators were assessed at baseline, 15 and 30 days after the first application, and 15 days after the end of treatment. The GI decreased in both groups throughout this study; however, the changes were more pronounced in the probiotic group than in the placebo at each of the visits, but the differences were insignificant [23].
In our study, we found that a 4-week intake of probiotic resulted in an improvement of the GI with the effect size of 0.58; moreover, this effect remained after a 4-week follow-up period. The probiotic group showed a significant decrease in GI and GBI scores at the post-intervention time point; the placebo group, on the contrary, showed an increase in these parameters. However, after the follow-up period, the GBI values in both groups did not differ significantly from the baseline.
The antigingivitis effect of probiotics demonstrated in a number of studies could be explained by a decrease in oral biofilm accumulation. In clinical settings, the extent of oral biofilm accumulation may be assessed using oral hygiene indices. Many studies reported a reduction in plaque scores according to hygienic indices alongside a reduction in gingival inflammation [18,19,70]. In our study, we observed a significant reduction in TQHPI scores in the probiotic group with the effect size of 0.55, while no significant changes were registered in the placebo group.
Several studies have also evaluated the anti-plaque and direct anti-inflammatory effects of oral probiotics in patients with experimental gingivitis [18,72,73] and reported controversial results. Hallström et al. assessed the effect of probiotic containing Lactobacillus reuteri on gingival health in experimental gingivitis. They concluded that daily intake of probiotic lozenges did not seem to significantly affect plaque accumulation, inflammatory reaction, or composition of the biofilm during experimental gingivitis [72].
Kuru et al. compared gingival indices in the placebo and probiotic (bifidobacterium-containing yogurt) groups at baseline, after 28 days of this study product usage, and subsequently, after plaque accumulation. After plaque accumulation (a 5-day non-brushing period), significantly better results for plaque and gingivitis scores and bleeding on probing were registered in the probiotic group compared to the control group [18]. Lee et al. reported that Lactobacillus brevis CD2 may delay gingivitis development in the model of experimental gingivitis by downregulating the inflammatory cascade. Their study suggests that the antigingivitis effect of probiotics may be associated not only with a decrease in plaque accumulation but also with a direct anti-inflammatory effect [73].
All in all, the studies on the use of probiotics in periodontal patients were different in methodology and, therefore, are very difficult to compare. The reported effects of probiotics could be strain-specific, product-specific, disease-specific, group-specific, etc. However, in general, current evidence is supportive of the use of probiotics in managing gingivitis or periodontitis [58].
We readily acknowledge several limitations to our study. We focused on clinical parameters of gingival health and biofilm accumulation without the assessment of microbiological ones, taking into consideration that clinically relevant outcomes are more important. Also, we enrolled patients of a limited age group (18–25-year-olds), as within this age limit, the majority of patients do not exhibit attachment loss (i.e., have gingivitis, not periodontitis). Further studies with longer intervention periods are needed, as a four-week intake of probiotics is a relatively short period for the colonization of the oral cavity with a tested microorganism [63].
5. Conclusions
According to our findings, the intake of oral probiotic with the Streptococcus salivarius M18 strain resulted in a significant improvement in the gingival condition and oral hygiene levels in young adults with gingivitis. However, taking into consideration the limitations of our study, its results should be generalized with caution, and more studies with broader age groups and longer intervention periods are needed to assess the long-term effect of Streptococcus salivarius M18-containing probiotics.
Author Contributions
Conceptualization, K.B. and N.N.; methodology, K.B. and N.N.; software, M.P. and I.M.; validation, M.P.; formal analysis, M.P. and A.Z.; investigation, D.S.; resources, D.S.; data curation, D.S., V.D., A.Z. and M.U.; writing—original draft preparation, K.B., D.S., N.N., M.P., V.D., I.M., A.Z. and M.U.; writing—review and editing, K.B., M.P., N.N. and A.Z.; visualization, M.P., V.D., I.M. and M.U.; supervision, K.B. and N.N.; project administration, K.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Ethics Committee of I.M. Sechenov First Moscow State Medical University (Sechenov University) (Protocol no. 23-22, 17 November 2022).
Informed Consent Statement
Informed consent was obtained from all subjects involved in this study.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kang, M.; Lee, D.; Lee, S.; Kim, M.; Nam, S. Effects of Probiotic Bacterium Weissella Cibaria CMU on Periodontal Health and Microbiota: A Randomised, Double-Blind, Placebo-Controlled Trial. BMC Oral Health 2020, 20, 243. [Google Scholar] [CrossRef] [PubMed]
- Nazir, M.; Al-Ansari, A.; Al-Khalifa, K.; Alhareky, M.; Gaffar, B.; Almas, K. Global Prevalence of Periodontal Disease and Lack of Its Surveillance. Sci. World J. 2020, 2020, 2146160. [Google Scholar] [CrossRef] [PubMed]
- Kwon, T.; Lamster, I.B.; Levin, L. Current Concepts in the Management of Periodontitis. Int. Dent. J. 2021, 71, 462. [Google Scholar] [CrossRef] [PubMed]
- Cecoro, G.; Annunziata, M.; Iuorio, M.; Nastri, L.; Guida, L. Periodontitis, Low-Grade Inflammation and Systemic Health: A Scoping Review. Med. B Aires 2020, 56, 272. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xu, J.; Li, S.; Wang, X.; Liu, J.; Li, X. The Prevalence of Gingivitis and Related Risk Factors in Schoolchildren Aged 6–12 Years Old. BMC Oral Health 2022, 22, 623. [Google Scholar] [CrossRef] [PubMed]
- Zini, A.; Mazor, S.; Timm, H.; Barker, M.; Grender, J.; Gerlach, R.; Biesbrock, A. Effects of an Oral Hygiene Regimen on Progression of Gingivitis/Early Periodontitis: A Randomized Controlled Trial. Can. J. Dent. Hyg. 2021, 55, 85–94. [Google Scholar] [PubMed]
- Abusleme, L.; Hoare, A.; Hong, B.; Diaz, P. Microbial Signatures of Health, Gingivitis, and Periodontitis. Periodontol. 2000 2021, 86, 57–78. [Google Scholar] [CrossRef] [PubMed]
- Curtis, M.; Diaz, P.; Van Dyke, T. The Role of the Microbiota in Periodontal Disease. Periodontology 2000 2020, 83, 14–25. [Google Scholar] [CrossRef]
- Sedghi, L.; Bacino, M.; Kapila, Y. Periodontal Disease: The Good, The Bad, and The Unknown. Front. Cell. Infect. Microbiol. 2021, 11, 766944. [Google Scholar] [CrossRef]
- Santonocito, S.; Polizzi, A. Oral Microbiota Changes during Orthodontic Treatment. Front. Biosci. Elite 2022, 14, 19. [Google Scholar] [CrossRef]
- Rathee, M.; Jain, P. Gingivitis. Aust. J. Pharm. 2023, 96, 64–67. [Google Scholar]
- Stephens, M.; Wiedemer, J.; Kushner, G. Dental Problems in Primary Care. Am. Fam. Physician 2018, 98, 654–660. [Google Scholar] [PubMed]
- Sanz, M.; Herrera, D.; Kebschull, M.; Chapple, I.; Jepsen, S.; Berglundh, T.; Sculean, A.; Tonetti, M. Treatment of Stage I–III Periodontitis—The EFP S3 Level Clinical Practice Guideline. J. Clin. Periodontol. 2020, 47, 4–60. [Google Scholar] [CrossRef] [PubMed]
- Garyga, V.; Pochelu, F.; Thivichon-Prince, B.; Aouini, W.; Santamaria, J.; Lambert, F.; Maucort-Boulch, D.; Gueyffier, F.; Gritsch, K.; Grosgogeat, B. GoPerio—Impact of a Personalized Video and an Automated Two-Way Text-Messaging System in Oral Hygiene Motivation: Study Protocol for a Randomized Controlled Trial. Trials 2019, 20, 699. [Google Scholar] [CrossRef] [PubMed]
- Tadakamadla, S.; Bharathwaj, V.; Duraiswamy, P.; Sforza, C.; Tartaglia, G. Clinical Efficacy of a New Cetylpyridinium Chloride-hyaluronic Acid–Based Mouthrinse Compared to Chlorhexidine and Placebo Mouthrinses—A 21-day Randomized Clinical Trial. Int. J. Dent. Hyg. 2020, 18, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Barboza, E.; Arriaga, P.; Luz, D.; Montez, C.; Vianna, K. Systematic Review of the Effect of Probiotics on Experimental Gingivitis in Humans. Braz. Oral Res. 2020, 34, e031. [Google Scholar] [CrossRef] [PubMed]
- Keller, M.; Brandsborg, E.; Holmstrøm, K.; Twetman, S. Effect of Tablets Containing Probiotic Candidate Strains on Gingival Inflammation and Composition of the Salivary Microbiome: A Randomised Controlled Trial. Benef. Microbes 2018, 9, 487–494. [Google Scholar] [CrossRef]
- Kuru, B.; Laleman, I.; Yalnızoğlu, T.; Kuru, L.; Teughels, W. The Influence of a Bifidobacterium Animalis Probiotic on Gingival Health: A Randomized Controlled Clinical Trial. J. Periodontol. 2017, 88, 1115–1123. [Google Scholar] [CrossRef]
- Schlagenhauf, U.; Rehder, J.; Gelbrich, G.; Jockel-Schneider, Y. Consumption of Lactobacillus Reuteri-containing Lozenges Improves Periodontal Health in Navy Sailors at Sea: A Randomized Controlled Trial. J. Periodontol. 2020, 91, 1328–1338. [Google Scholar] [CrossRef]
- Kaźmierczyk-Winciorek, M.; Nędzi-Góra, M.; Słotwińska, S. The Immunomodulating Role of Probiotics in the Prevention and Treatment of Oral Diseases. Cent. Eur. J. Immunol. 2021, 46, 99–104. [Google Scholar] [CrossRef]
- Zhang, J.; Li, K.; Bu, X.; Cheng, S.; Duan, Z. Characterization of the Anti-Pathogenic, Genomic and Phenotypic Properties of a Lacticaseibacillus Rhamnosus VHProbi M14 Isolate. PLoS ONE 2023, 18, e0285480. [Google Scholar] [CrossRef] [PubMed]
- Luan, C.; Yan, J.; Jiang, N.; Zhang, C.; Geng, X.; Li, Z.; Li, C. Leuconostoc Mesenteroides LVBH107 Antibacterial Activity against Porphyromonas Gingivalis and Anti-Inflammatory Activity against P. Gingivalis Lipopolysaccharide-Stimulated RAW 264.7 Cells. Nutrients 2022, 14, 2584. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, M.; López-López, A.; Nicolescu, T.; Perez-Vilaplana, S.; Boix-Amorós, A.; Dzidic, M.; Garcia, S.; Artacho, A.; Llena, C.; Mira, A. Topic Application of the Probiotic Streptococcus Dentisani Improves Clinical and Microbiological Parameters Associated with Oral Health. Front. Cell. Infect. Microbiol. 2020, 10, 465. [Google Scholar] [CrossRef] [PubMed]
- Gönczi, N.; Strang, O.; Bagi, Z.; Rákhely, G.; Kovács, K. Interactions between Probiotic and Oral Pathogenic Strains. Biol. Futur. 2021, 72, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Invernici, M.; Furlaneto, F.; Salvador, S.; Ouwehand, A.; Salminen, S.; Mantziari, A.; Vinderola, G.; Ervolino, E.; Santana, S.; Silva, P.; et al. Bifidobacterium Animalis Subsp Lactis HN019 Presents Antimicrobial Potential against Periodontopathogens and Modulates the Immunological Response of Oral Mucosa in Periodontitis Patients. PLoS ONE 2020, 15, e0238425. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque-Souza, E.; Balzarini, D.; Ando-Suguimoto, E.; Ishikawa, K.; Simionato, M.; Holzhausen, M.; Mayer, M. Probiotics Alter the Immune Response of Gingival Epithelial Cells Challenged by Porphyromonas Gingivalis. J. Periodontal Res. 2019, 54, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Esteban-Fernández, A.; Ferrer, M.; Zorraquín-Peña, I.; López-López, A.; Moreno-Arribas, V.; Mira, A. In Vitro Beneficial Effects of Streptococcus Dentisani as Potential Oral Probiotic for Periodontal Diseases. J. Periodontol. 2019, 90, 1346–1355. [Google Scholar] [CrossRef] [PubMed]
- Piwat, S.; Teanpaisan, R.; Manmontri, C.; Wattanarat, O.; Pahumunto, N.; Makeudom, A.; Krisanaprakornkit, S.; Nirunsittirat, A. Efficacy of Probiotic Milk for Caries Regression in Preschool Children: A Multicenter Randomized Controlled Trial. Caries Res. 2020, 54, 491–501. [Google Scholar] [CrossRef]
- Sandoval, F.; Faleiros, S.; Cabello, R.; Díaz-Dosque, M.; Rodríguez, G.; Escobar, A. The Consumption of Milk Supplemented with Probiotics Decreases the Occurrence of Caries and the Salivary Concentration of HβD-3 in Children. Clin. Oral Investig. 2021, 25, 3823–3830. [Google Scholar] [CrossRef]
- Staszczyk, M.; Jamka-Kasprzyk, M.; Kościelniak, D.; Cienkosz-Stepańczak, B.; Krzyściak, W.; Jurczak, A. Effect of a Short-Term Intervention with Lactobacillus Salivarius Probiotic on Early Childhood Caries—An Open Label Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2022, 19, 12447. [Google Scholar] [CrossRef]
- Sakhare, S.; Shantanu, C.; Mopagar, V.; Hadpe, H.; Choughule, K.; Dahapute, S.; Shetty, S.; Joshi, S. A Comparative Evaluation of Probiotic Formulations in Prevention of Dental Caries: A Clinical Study. J. Indian Soc. Pedod. Prev. Dent. 2021, 39, 416. [Google Scholar] [CrossRef] [PubMed]
- Zare Javid, A.; Amerian, E.; Basir, L.; Ekrami, A.; Haghighizadeh, M.; Maghsoumi-Norouzabad, L. Effects of the Consumption of Probiotic Yogurt Containing Bifidobacterium Lactis Bb12 on the Levels of Streptococcus Mutans and Lactobacilli in Saliva of Students with Initial Stages of Dental Caries: A Double-Blind Randomized Controlled Trial. Caries Res. 2020, 54, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Doppalapudi, R.; Vundavalli, S.; Prabhat, M. Effect of Probiotic Bacteria on Oral Candida in Head- and Neck-Radiotherapy Patients. J. Cancer Res. Ther. 2020, 16, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Lee, X.; Vergara, C.; Lozano, C. Severity of Candida-Associated Denture Stomatitis Is Improved in Institutionalized Elders Who Consume Lactobacillus Rhamnosus SP1. Aust. Dent. J. 2019, 64, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Mao, Q.; Zhou, P.; Lv, X.; Hua, H.; Yan, Z. Effects of Streptococcus Salivarius K12 with Nystatin on Oral Candidiasis-RCT. Oral Dis. 2019, 25, 1573–1580. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Lee, S.; Kim, M.; Nam, S.; Kang, M. Reduction of Halitosis by a Tablet Containing Weissella cibaria CMU: A Randomized, Double-Blind, Placebo-Controlled Study. J. Med. Food 2020, 23, 649–657. [Google Scholar] [CrossRef] [PubMed]
- Benic, G.; Farella, M.; Morgan, X.; Viswam, J.; Heng, N.; Cannon, R.; Mei, L. Oral Probiotics Reduce Halitosis in Patients Wearing Orthodontic Braces: A Randomized, Triple-Blind, Placebo-Controlled Trial. J. Breath. Res. 2019, 13, 036010. [Google Scholar] [CrossRef]
- Gurpinar, B.; Yildirim, G.; Kumral, T.; Akgun, M.; Sari, H.; Tutar, B.; Uyar, Y. A Simple Method to Reduce Halitosis; Tongue Scraping with Probiotics. J. Breath Res. 2019, 14, 016008. [Google Scholar] [CrossRef]
- Lee, D.; Kim, M.; Nam, S.; Kang, M.; Lee, S. Effects of Oral Probiotics on Subjective Halitosis, Oral Health, and Psychosocial Health of College Students: A Randomized, Double-Blind, Placebo-Controlled Study. Int. J. Environ. Res. Public Health 2021, 18, 1143. [Google Scholar] [CrossRef]
- Fang, F.; Xu, J.; Li, Q.; Xia, X.; Du, G. Characterization of a Lactobacillus Brevis Strain with Potential Oral Probiotic Properties. BMC Microbiol. 2018, 18, 221. [Google Scholar] [CrossRef]
- Soares, L.; de Carvalho, E.; Tinoco, E. Clinical Effect of Lactobacillus on the Treatment of Severe Periodontitis and Halitosis: A Double-Blinded, Placebo-Controlled, Randomized Clinical Trial. Am. J. Dent. 2019, 32, 9–13. [Google Scholar] [PubMed]
- Invernici, M.; Salvador, S.; Silva, P.; Soares, M.; Casarin, R.; Palioto, D.; Souza, S.; Taba, M.; Novaes, A.; Furlaneto, F.; et al. Effects of Bifidobacterium Probiotic on the Treatment of Chronic Periodontitis: A Randomized Clinical Trial. J. Clin. Periodontol. 2018, 45, 1198–1210. [Google Scholar] [CrossRef] [PubMed]
- Näse, L.; Hatakka, K.; Savilahti, E.; Saxelin, M.; Pönkä, A.; Poussa, T.; Korpela, R.; Meurman, J. Effect of Long–Term Consumption of a Probiotic Bacterium, Lactobacillus Rhamnosus GG, in Milk on Dental Caries and Caries Risk in Children. Caries Res. 2001, 35, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Pahumunto, N.; Duangnumsawang, Y.; Teanpaisan, R. Effects of Potential Probiotics on the Expression of Cytokines and Human β-Defensins in Human Gingival Epithelial Cells and in Vivo Efficacy in a Dog Model. Arch. Oral Biol. 2022, 142, 105513. [Google Scholar] [CrossRef] [PubMed]
- Morales, A.; Contador, R.; Bravo, J.; Carvajal, P.; Silva, N.; Strauss, F.; Gamonal, J. Clinical Effects of Probiotic or Azithromycin as an Adjunct to Scaling and Root Planning in the Treatment of Stage III Periodontitis: A Pilot Randomized Controlled Clinical Trial. BMC Oral Health 2021, 21, 12. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.; Lourenço, T.; Colombo, A. Impact of Systemic Probiotics as Adjuncts to Subgingival Instrumentation on the Oral-gut Microbiota Associated with Periodontitis: A Randomized Controlled Clinical Trial. J. Periodontol. 2022, 93, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Gheisary, Z.; Mahmood, R.; Shivanantham, A.H.; Liu, J.; Lieffers, J.; Papagerakis, P.; Papagerakis, S. The Clinical, Microbiological, and Immunological Effects of Probiotic Supplementation on Prevention and Treatment of Periodontal Diseases: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 1036. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhao, G.; Zhang, H.; Zhu, F. Probiotic Adjuvant Treatment in Combination with Scaling and Root Planing in Chronic Periodontitis: A Systematic Review and Meta-Analysis. Benef. Microbes 2023, 14, 95–107. [Google Scholar] [CrossRef]
- Akram, Z.; Shafqat, S.; Aati, S.; Kujan, O.; Fawzy, A. Clinical Efficacy of Probiotics in the Treatment of Gingivitis: A Systematic Review and Meta-analysis. Aust. Dent. J. 2020, 65, 12–20. [Google Scholar] [CrossRef]
- Babina, K.; Salikhova, D.; Polyakova, M.; Svitich, O.; Samoylikov, R.; Ahmad El-Abed, S.; Zaytsev, A.; Novozhilova, N. The Effect of Oral Probiotics (Streptococcus Salivarius K12) on the Salivary Level of Secretory Immunoglobulin A, Salivation Rate, and Oral Biofilm: A Pilot Randomized Clinical Trial. Nutrients 2022, 14, 1124. [Google Scholar] [CrossRef]
- Kim, H.; Yoo, H. Inhibitory Effects of Streptococcus Salivarius K12 on Formation of Cariogenic Biofilm. J. Dent. Sci. 2023, 18, 65–72. [Google Scholar] [CrossRef]
- Burton, J.; Drummond, B.; Chilcott, C.; Tagg, J.; Thomson, M.; Hale, J.; Wescombe, P. Influence of the Probiotic Streptococcus Salivarius Strain M18 on Indices of Dental Health in Children: A Randomized Double-Blind, Placebo-Controlled Trial. J. Med. Microbiol. 2013, 62, 875–884. [Google Scholar] [CrossRef] [PubMed]
- Kaklamanos, E.; Nassar, R.; Kalfas, S.; Al Halabi, M.; Kowash, M.; Hannawi, H.; Hussein, I.; Salami, A.; Hassan, A.; Senok, A. A Single-Centre Investigator-Blinded Randomised Parallel Group Clinical Trial to Investigate the Effect of Probiotic Strains Streptococcus Salivarius M18 and Lactobacillus acidophilus on Gingival Health of Paediatric Patients Undergoing Treatment with Fixed Orthodontic Appliances: Study Protocol. BMJ Open 2019, 9, e030638. [Google Scholar] [CrossRef] [PubMed]
- Jansen, P.; Abdelbary, M.; Conrads, G. A Concerted Probiotic Activity to Inhibit Periodontitis-Associated Bacteria. PLoS ONE 2021, 16, e0248308. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, K.; Chanyi, R.; Macklaim, J.; Cadieux, P.; Reid, G.; Burton, J. Streptococcus Salivarius Inhibits Immune Activation by Periodontal Disease Pathogens. BMC Oral Health 2021, 21, 245. [Google Scholar] [CrossRef] [PubMed]
- Fijan, S.; Frauwallner, A.; Varga, L.; Langerholc, T.; Rogelj, I.; Lorber, M.; Lewis, P.; Povalej Bržan, P. Health Professionals’ Knowledge of Probiotics: An International Survey. Int. J. Environ. Res. Public Health 2019, 16, 3128. [Google Scholar] [CrossRef] [PubMed]
- Babina, K.; Salikhova, D.; Polyakova, M.; Zaytsev, A.; Egiazaryan, A.; Novozhilova, N. Knowledge and Attitude towards Probiotics among Dental Students and Teachers: A Cross-Sectional Survey. Dent. J. 2023, 11, 119. [Google Scholar] [CrossRef] [PubMed]
- Ausenda, F.; Barbera, E.; Cotti, E.; Romeo, E.; Natto, Z.; Valente, N. Clinical, Microbiological and Immunological Short, Medium and Long-Term Effects of Different Strains of Probiotics as an Adjunct to Non-Surgical Periodontal Therapy in Patients with Periodontitis. Systematic Review with Meta-Analysis. Jpn. Dent. Sci. Rev. 2023, 59, 62–103. [Google Scholar] [CrossRef]
- De Almeida Silva Levi, Y.L.; Ribeiro, M.C.; Silva, P.H.F.; Silva, G.A.; de Souza Salvador, S.L.; de Souza, S.L.S.; Casarin, R.; Júnior, A.B.N.; Júnior, M.T.; Palioto, D.B.; et al. Effects of Oral Administration of Bifidobacterium Animalis Subsp. Lactis HN019 on the Treatment of Plaque-Induced Generalized Gingivitis. Clin. Oral Investig. 2022, 27, 387–398. [Google Scholar] [CrossRef]
- Wiesmüller, V.; Kasslatter, M.; Zengin, B.; Zotz, D.; Offermanns, V.; Steiner, R.; Crismani, A.; Kapferer-Seebacher, I. Cleansing Efficacy of an Oral Irrigator with Microburst Technology in Orthodontic Patients—A Randomized-Controlled Crossover Study. Clin. Oral Investig. 2023, 27, 2089–2095. [Google Scholar] [CrossRef]
- Kim, J.; Yoo, S.; An, J.; Woo, J.; Cho, Y.; Park, H.; Karm, M. Effect of a Multichannel Oral Irrigator on Periodontal Health and the Oral Microbiome. Sci. Rep. 2023, 13, 12043. [Google Scholar] [CrossRef] [PubMed]
- Chapple, I.; Mealey, B.; Van Dyke, T.; Bartold, M.; Dommisch, H.; Eickholz, P.; Geisinger, M.; Genco, R.; Glogauer, M.; Goldstein, M.; et al. Periodontal Health and Gingival Diseases and Conditions on an Intact and a Reduced Periodontium: Consensus Report of Workgroup 1 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Clin. Periodontol. 2018, 45, S68–S77. [Google Scholar] [CrossRef] [PubMed]
- Montero, E.; Iniesta, M.; Rodrigo, M.; Marín, M.; Figuero, E.; Herrera, D.; Sanz, M. Clinical and Microbiological Effects of the Adjunctive Use of Probiotics in the Treatment of Gingivitis: A Randomized Controlled Clinical Trial. J. Clin. Periodontol. 2017, 44, 708–716. [Google Scholar] [CrossRef]
- Carvalho, A.; Moura, M.; Costa, F.; Cota, L. Correlations between Different Plaque Indexes and Bleeding on Probing: A Concurrent Validity Study. J. Clin. Exp. Dent. 2023, 15, e9–e16. [Google Scholar] [CrossRef] [PubMed]
- Toiviainen, A.; Jalasvuori, H.; Lahti, E.; Gursoy, U.; Salminen, S.; Fontana, M.; Flannagan, S.; Eckert, G.; Kokaras, A.; Paster, B.; et al. Impact of Orally Administered Lozenges with Lactobacillus Rhamnosus GG and Bifidobacterium animalis Subsp. Lactis BB-12 on the Number of Salivary Mutans Streptococci, Amount of Plaque, Gingival Inflammation and the Oral Microbiome in Healthy Adults. Clin. Oral Investig. 2015, 19, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Alanzi, A.; Honkala, S.; Honkala, E.; Varghese, A.; Tolvanen, M.; Söderling, E. Effect of Lactobacillus Rhamnosus and Bifidobacterium Lactis on Gingival Health, Dental Plaque, and Periodontopathogens in Adolescents: A Randomised Placebocontrolled Clinical Trial. Benef. Microbes 2018, 9, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Twetman, S.; Derawi, B.; Keller, M.; Ekstrand, K.; Yucel-Lindberg, T.; Stecksén-Blicks, C. Short-Term Effect of Chewing Gums Containing Probiotic Lactobacillus reuteri on the Levels of Inflammatory Mediators in Gingival Crevicular Fluid. Acta Odontol. Scand. 2009, 67, 19–24. [Google Scholar] [CrossRef]
- Yuki, O.; Furutani, C.; Mizota, Y.; Wakita, A.; Mimura, S.; Kihara, T.; Ohara, M.; Okada, Y.; Okada, M.; Nikawa, H. Effect of Bovine Milk Fermented with Lactobacillus Rhamnosus L8020 on Periodontal Disease in Individuals with Intellectual Disability: A Randomized Clinical Trial. J. Appl. Oral Sci. 2019, 27, e20180564. [Google Scholar] [CrossRef]
- Sabatini, S.; Lauritano, D.; Candotto, V.; Silvestre, F.; Nardi, G. Oral Probiotics in the Management of Gingivitis in Diabetic Patients: A Double Blinded Randomized Controlled Study. J. Biol. Regul. Homeost. Agents 2017, 31, 197–202. [Google Scholar]
- Hambire, C.; Hambire, U. Evaluation of Effect of Consumption of Probiotics on the Gingival and Periodontal Health Status in Children Undergoing Chemotherapy. Indian J. Cancer, 2022; ahead of print. [Google Scholar] [CrossRef]
- Hardan, L.; Bourgi, R.; Cuevas-Suárez, C.; Flores-Rodríguez, M.; Omaña-Covarrubias, A.; Nicastro, M.; Lazarescu, F.; Zarow, M.; Monteiro, P.; Jakubowicz, N.; et al. The Use of Probiotics as Adjuvant Therapy of Periodontal Treatment: A Systematic Review and Meta-Analysis of Clinical Trials. Pharmaceutics 2022, 14, 1017. [Google Scholar] [CrossRef] [PubMed]
- Hallström, H.; Lindgren, S.; Yucel-Lindberg, T.; Dahlén, G.; Renvert, S.; Twetman, S. Effect of Probiotic Lozenges on Inflammatory Reactions and Oral Biofilm during Experimental Gingivitis. Acta Odontol. Scand. 2013, 71, 828–833. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, S.; Ko, S.; Ouwehand, A.; Ma, D. Modulation of the Host Response by Probiotic Lactobacillus Brevis CD2 in Experimental Gingivitis. Oral Dis. 2015, 21, 705–712. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).