Urolithins: A Prospective Alternative against Brain Aging
Abstract
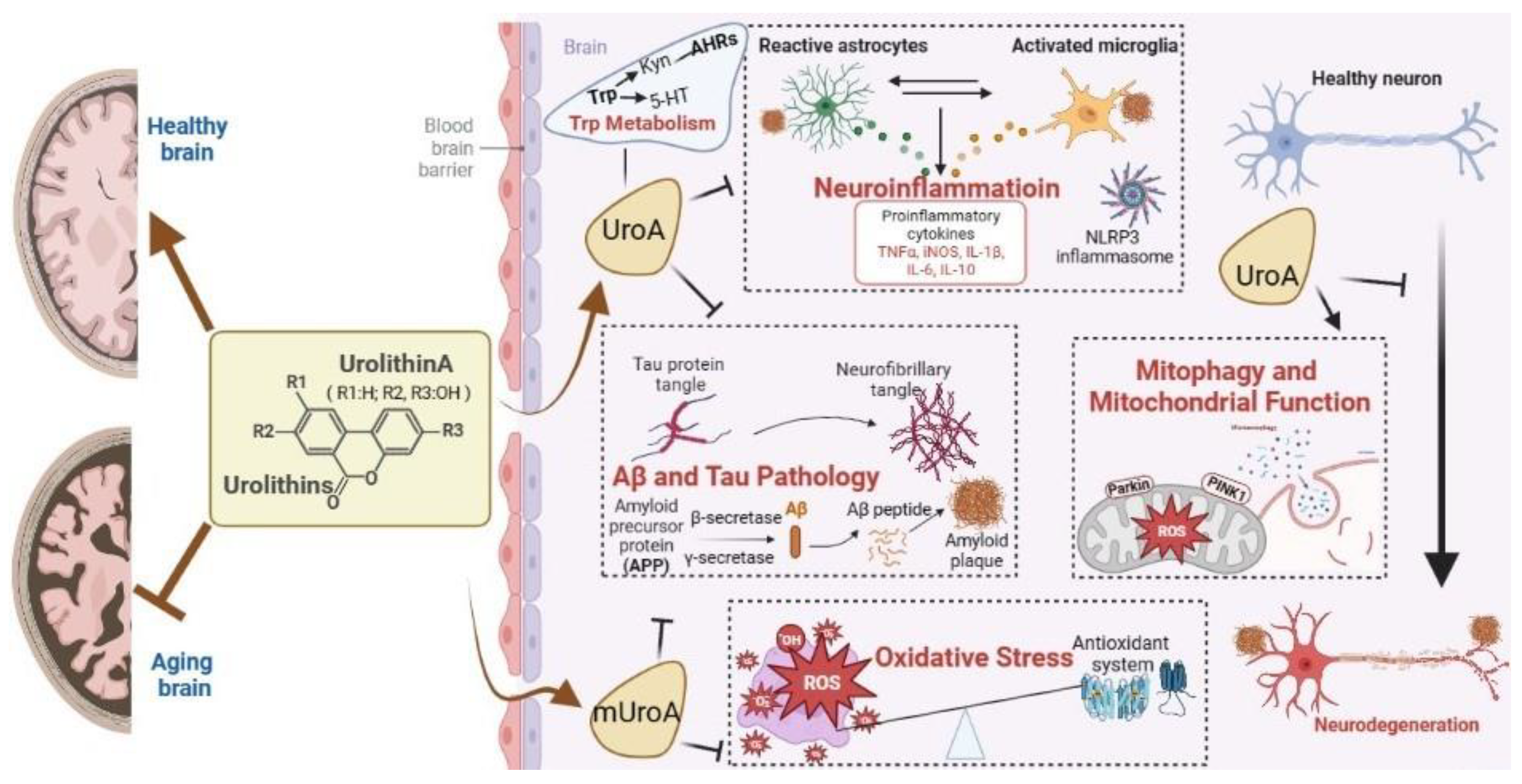
:1. Introduction
2. Overview and Advantage of Uros
3. Preclinical and Clinical Studies
| Uros | Cells | Pharmacological or Genetic Interventions | Treatment (Dosage and Time) | Effects | Findings | Refs. |
|---|---|---|---|---|---|---|
| UroA | Neuro-2a cells | H2O2 (250 μM) for 45 min | 0.5 μM, 1 μM, 2 μM, 4 μM pretreatment for 24 h | antioxidation | ↑ cells viability, ↓ MAO-A and Tyrosinase, ↑ free radical (O2− and DPPH), ↓ ROS, lipid peroxidation, ↑ peroxiredoxins expression, ↑ CAT, SOD, GR, GSH-Px, | [54] |
| UroA | PC12 cells | H2O2 (100 μM) for 2 h | 10 μg/mL, 30 μg/mL, and 50 μg/mL pretreatment for 24 h | antioxidation | ↑ cells viability, ↓ LDH release, ↓ apoptosis, ↓ caspase 3 and Bcl-2 | [17] |
| UroA | SK-N-MC cells | H2O2 (300 μM) for 18 h | 1.25 μM, 2.5 μM, and 5 μM pretreatment for 6 h | antioxidation | ↑ cells viability, ↓ apoptosis, ↓ ROS, Bax/Bcl-2, PARP, cytochrome c, caspase 3/9, p38 MAPK | [55] |
| UroA | SH-SY5Y cells | H2O2 (100 μM) for 24 h | 10 μM treatment for 2 h, 6 h or 24 h | antioxidation | ↑ REDOX activity, ↓ cytotoxicity, ↓ ROS, ↓ apoptosis, ↓ caspase 3/8 and 9 | [20] |
| UroA | SH-SY5Y cells | H2O2(200 μM) for 6 h | 5 μM, 7.5 μM, 10 μM, 15 μM pretreatment for 12 h | antioxidation | ↑ cells viability, ↓ ROS, ↑ SOD, CAT, ↑ PKA/CREB/BDNF | [56] |
| UroB | Neuro-2a cells | H2O2 (250 μM) for 2 h | 20 μg/mL, 40 μg/mL, and 60 μg/mL pretreatment for 24 h | antioxidation | ↑ cells viability, ↓ ROS, ↓ apoptosis, cytotoxicity, ↓ caspase 3, ↑ Bcl-2 | [57] |
| UroB | BV-2 cells | LPS (100 ng/mL) or LTA (10 μg/mL) or poly(I:C) (25 μg/mL) for 16 h | 30 μM, 50 μM, or 100 μM pretreatment for 1 h | antioxidation, anti- inflamation | ↓ NO, ROS, TNF-α, IL-6, IL-1β, iNOS, COX-2, ↓ NF-κB, p-JNK, p-ERK, p-Akt, AP-1, ↑ IL-10, pAMPK, p47phox, gp91phox | [58] |
| UroA mUroA UroB mUrOA | BV-2 cells, SH-SY5Y cells | H2O2 (100 μM) for 6 h; LPS (1 μg/mL) for 24 h | 0.1 μM, 0.5 μM, 5 μM, 10 μM pretreatment for 1 h, 24 h, or 48 h | antioxidation, anti- inflammation | ↓ apoptosis, ↓ NO, TNF-α,NO, COX-2, IL-1, IL-6, PGE2, ↓ caspase 3/7 and 9, ↓ oxidative stress | [19] |
| UroA | BV-2 cells | LPS (500 ng/mL) for 3 h, 12 h, 24 h; IL-4 (100 ng/mL), IL-13 (10 ng/mL) for 24 h | 10 μM pretreatment for 12 h | anti- inflammation | ↓ IL-6, IL-1β, TNF-α, ↓ NOS, ↓ JNK/c-Jun, ↑ M2 microglia polarization | [59] |
| UroA | BV-2 cells | LPS (1 μg/mL) for 6 h, 12 h or 24 h | 2.5 μM, 5 μM, and 10μM pretreatment for 2 h | anti- inflammation improved mitochondrial function | ↓ IL-1β, iNOS, COX-2, ↓ ROS, ↑ MMP, ↑ p62, ↓ LC3-II, ↑ Parkin, PINK, ↓ caspase 1, NLRP3, ↓ TOM20, Tim23,↑ mitophagy, ↑ OXPHOS | [14] |
| UroA, UroB, UroC, mUroA dmUroC | BV-2 cells | LPS (100 ng/mL) for 30 min, 16 h or 24 h | 3 μM, 10 μM, 30 μM treatment for 30 min, 1 h, 16 h or 24 h | anti- inflammation | ↓ NO, TNF-α, IL-6, IL-1β, iNOS, COX-2, ↓ pAkt,↓ pERK1/2, p38 MAPK, ↓ NF-κB | [60] |
| UroA | BV-2 cells, ReNcell VM cells | LPS (100 ng/mL) for 1 h, 24 h, or transfected with APPSwe | 2 μM, 5 μM, 10 μM treatment for 30 min, 6 h or 48 h | anti- inflammation, anti-Aβ | ↑ cells viability, ↓ NO, TNFα, IL-6, ↓ Aβ, ↑ SIRT1, ↓ NF-Κb, ↑ induction of autophagic flux | [21] |
| UroA | SH-SY5Y cells, iPSC-ND cells | D-glucose 25 mM for 24 h, 48 h, and 72 h, Aβ (1–42) for 24 h, 48 h, and 72 h | 100 nM pretreatment for 30 min | anti-Aβ, improved mitochondrial function | ↓ APP, BACE1, TGM2, Aβ(1–42), mitochondrial calcium influx, AhR, mtROS, ↓ LDH release | [61] |
| UroA | SH-SY5Y cells | transfected with the APP 695 | 1 µM,10 µM treatment for 1 h or 24 h | improved mitochondrial function | ↑ MMP, ATP, ROS, OXPHOS, mitochondrial biogenesis | [62] |
| UroA | PC12 cells | 6-OHDA (150 μM) for 18 h or 24 h | 2.5 μM, 5 μM, 10 mM treatment for 2 h | improved mitochondrial function | ↑ cells viability, MPP PGC-1α, SIRT1, TFAM, ↓ apoptosis, ↑ APP, ↓ ROS, ↓ mitochondria damage | [63] |
| UroA DHA+ LUT+ UroA | BE(2)-M17 cells | oligomeric Aβ1–42 (20 μM) for 72 h | 5 μM to 40 μM pretreatment for 24 h, 5 μM (combination) pretreatment for 24 h | anti-Aβ | ↑ cells viability, ↓ LDH release | [64] |
| UroA, UroA+ EGCG | HT22 cells | transfected with APP cDNA for 24 h | no concentration mentioned, treatment for 24 h | improved mitochondrial function | ↑ mitochondrial respiration | [65] |
| UroA, UroA+ EGCG | HT22 cells | transfected with Tau cDNA for 24 h | 1 μM or 10 mM treatment for 24 h | improved mitochondrial function | ↓ Drp1 and Fis1, ↑ PGC-1α, Nrf1, Nrf2, ↑ TFAM, PINK1, Parkin, ↑ Mfn1, Mfn2, and Opa1 | [66] |
| UroA | HT22 cells | transfected with APP cDNA for 24 h | 1 μM, 2 μM, 5 μM, 10 mM treatment for 24 h | improved mitochondrial function | ↓ Drp1 and Fis1, ↑ PGC-1α, Nrf1, Nrf2, ↑ TFAM, PINK1, Parkin, ↑ Mfn1, Mfn2, and Opa1 | [67] |
| Uros | Animal | Pharmacological or Genetic Interventions | Route of Administration | Treatment (Dosage and Time) | Effects | Findings | Refs. |
|---|---|---|---|---|---|---|---|
| UroA | male ICR mice (4–6 weeks, 18–22 g) | D-gal 150 mg/kg/d s.c. for 8 weeks | i.g. | 50, 100, 150 mg/kg b.w./day for 8 weeks | anti-brain aging, anti- inflammation, antioxidation | ↑ spontaneous locomotion, object recognition learning, ↓ AchE, MAO, ↑ SOD, CAT, GSH-Px, ↓ p53/p21, TEAC, ↑ SIRT1, ↓ TNF-α, IL-1β, and IL-6, ↑ Bcl-2, ↓ caspase 3, mTOR, ↓ dysfunctional autophagy, astrocyte activation, ↓ apoptosis | [17] |
| UroB | male C57BL/6 mice (6–8 weeks, 18–22 g) | D-gal 150 mg/kg/d s.c. for 8 weeks | i.g. | 50, 100, 150 mg/kg b.w./day for 8 weeks | anti-brain aging, anti- inflammation, antioxidation | ↓ cognitive deficits, ↑ pAkt, ↑ hippocampal LTP,↑ CAT, GSH-Px, TEAC, SOD, ↓ MDA, ↓ TNF-α, IL-6, IL-1β, AGEs, cytotoxicity, ↓ the activation of microglia and astrocytes, ↓ AchE, ↑ number of neuron, ↓ MAO | [57] |
| UroA | female APP/PS1 transgenic mice (28 weeks) | transgenic AD mice | i.g. | 300 mg/kg b.w./day for 14 days | anti- inflammation | ↓ spatial learning deficits, ↑ neurogenesis, ↓ neuronal apoptosis, ↓ reactive gliosis, ↓ Aβ, IL-1β, TNF-α, ↑ AMPK, ↓ p-P65, NF-κB, p-P38, MAPK, BACE1 | [22] |
| UroA | APP/PS1 transgenic mice (13 months) C.elegans | transgenic AD mice | i.g. | 200 mg/kg b.w./day for 1 month or 0.1 mM (C.elegans) | anti- inflammation | ↑ learning and memory retention, ↑ OCR, ↓ ROS, ↓ Aβ1–42, Aβ1–40, ↑ IL-10, ↓ autophagy, ↓ IL-6, TNF-α, ↓ NLRP3, IL-1β, ↓ p-tau, caspase 1 | [68] |
| UroA | CX3CR1- Cre mice | MPTP 15 mg/kg/d, i.p. 4 times a day every2 h | i.g. | 20 mg/kg b.w./day for 7 days | anti- inflammation | ↓ motor deficits, ↑ TH, ↓ caspase 1, NLRP3, ↓ astrogliosis | [14] |
| UroB | Male ICR mice (7 weeks, 2–37 g) | LPS 5 mg/kg/d, i.p. | i.p. | 50 mg/kg b.w./day for4 days | anti- inflammation | ↓ microglia activation, ↓ NADPH, Akt, JNK, ERK, ↑ AMPK, HO-1 | [58] |
| UroA | male C57BL/6J mice (8–10 weeks) | 6-OHDA 9 µg | i.p. | 10 mg/kg b.w./day for 7 days | improved mitochondria function | ↓ neurotoxicity, mitochondria damage, OXPHOS, ↑ PGC-1α, TFAM, ↑ SIRT1 | [63] |
| UroA | mice | STZ 75 mg/kg/d i.p. for 3 days | i.p. | 2.5 mg/kg b.w./day for 8 weeks | anti-Aβ, improved mitochondria function | ↓ APP, BACE1, p-tau, Aβ(1–42), TGM2 | [61] |
| UroA UroB mUroA mUroB | C. elegans | transgenic AD C. elegans (CL4176) | feeding | 10 μg/mL pretreatment for 20 h | anti-Aβ | ↑ C. elegans survival and mobility | [69] |
| UroA UroA + EGCG | hAbKI mice (3 months) | humanized homozygous Aβ knockin (hAbKI) AD mice | i.p. | UroA 2.5 mg/kg b.w., EGCG 25 mg/kg b.w., 3 times per week for 4 months | anti-Aβ, improved mitochondria function | ↑ mitochondrial fusion, synaptic, ↓ Aβ(1–40) and Aβ(1–42), ↑ mitophagy, autophagy genes, ↓ mitochondrial fission genes, mitochondrial dysfunction, ↑ dendritic spines, ↓ fragmented mitochondria number, ↑ mitochondrial length, mitophagosomal formations | [65] |
| UroA | male C57BL/6J mice (5 weeks, 18–22 g) | STZ 30 mg/kg b.w./day i.p. for 4 days | i.g. | 200 mg/kg b.w. | anti-brain aging, anti- inflammation | ↓ hyperglycemia, ↑ learning and memory, ↓ IL-6, IL-1β, TNF-α, IL-1β, COX-2, iNOS-2, ↑ IL-10, ↓ NLRP3 | [70] |
| Source | Subjects | Clinical Trial Procedure | Treatment (Dosage and Time) | Effects | Fundings | Refs |
|---|---|---|---|---|---|---|
| Pomegranate juice | Age: 54–72 years; Cognition and memory: age-related memory decline; Other heath state: no neurological, psychiatric and major medical conditions | Randomized, placebo controlled, double blind trial | Dosage: 240 mL/day of pomegranate juice (n = 15) or placebo drink (n = 13) Time: 4 weeks | anti-age-related memory decline | ↑ fMRI activity during verbal and visual memory tasks, ↑ memory ability, ↑ plasma antioxidant status | [42] |
| Pomegranate juice | Age: 50–75 years; Cognition and memory: age-related memory decline; other heath state: no cerebrovascular disease, neurological or physical illnesses associated with cognitive deterioration | Randomized, placebo controlled, double blind trial | Dosage: 236.5 mL/day of pomegranate juice (n = 98) or placebo drink (n = 102) Time: 48 weeks | anti-age-related memory decline | ↑ visual memory, ↑ visual learning and recall, ↑ verbal memory, words recall | [49] |
| Nuts | Age: 55–80 years; Cognition and memory: healthy; other heath state: no diabetes, smoking, hypertension, dyslipidemia, overweight and cardiovascular disease | Randomized, placebo controlled trial | Dosage: MedDiet + EVOO 1 L/week (n = 224); MedDiet + nuts 30 g/day (n = 166); or low-fat diet (n = 132) Time: 6.5 years | anti-age-related memory decline | ↑ orientation to time and place, ↑ registration, attention and calculation, ↑ recall, language, and visual construction, ↑ visuospatial abilities, working memory, attention, ↑ abstract thinking, language comprehension | [71] |
| Walnuts | Age: 63–79 years; Cognition and memory: healthy; other heath state: no neurodegenerative disease, stroke, head trauma, brain surgery, psychiatric illness, depression, obesity, diabetes, hypertension and chemotherapy | Randomized controlled trial | Dosage: Walnuts 30–60 g/day (n = 336) or control diet (abstention from walnuts) (n = 321) Time: 2 years | anti-age-related memory decline | ↑ global cognition and perception | [51] |
| Strawberry | Age: 60–75 years; Cognition and memory: age-related motor and cognitive decline; other heath state: BMI (18.5–29.9), no psychological or psychiatric disorders and chronic disease | Randomized, placebo controlled, double blind trial | Dosage: Strawberry 24 g/day (n = 18) or placebo (n = 19) Time: 45 or 90 days | anti-age-related memory decline | ↑ words recalled, verbal learning | [72] |
| Mixture of berries | Age: 50–70 years; Cognition and memory: healthy; other heath state: no metabolic disorders, food allergies and, gastrointestinal disorder | Randomized cross-over trial | Dosage: mixture of berries (150 g blueberries, 50 g blackcurrant, 50 g elderberry, 50 g lingonberries, 50 g strawberry, and 100 g tomatoes/day) (n = 20); or placebo drink (n = 21) Time: 5 weeks | anti-age-related memory decline | ↑ verbal working memory, ↑ selective attention, ↓ total- and LDL cholesterol, ↑ insulin concentrations | [73] |
| Grape and blueberry extract | Age: 60–70 years; Cognition and memory: age-related memory decline; other heath state: BMI (20–30) | Randomized, placebo controlled, double blind trial | Dosage: grape and blueberry extract 600 mg/day (n = 91) or placebo (n = 98) Time: 6 months | anti-age-related memory decline | ↑ verbal episodic, ↑ recognition memory, ↑ working memory | [74] |
| Blueberry and blueberry extract | Age: 65–80 years; Cognition and memory: age-related memory decline; other heath state: no metabolic disorders and diabetes | Randomized, placebo controlled, double blind trial | Dosage: blueberry 500 mg/day (n = 28); blueberry 1000 mg/day (n = 29); blueberry extract 111 mg/day (n = 28); or placebo (n = 27) Time: 6 months | anti-age-related memory decline | ↑ word recognition, ↑ total number of sequences correctly recalled, ↓ systolic blood pressure | [75] |
| Blueberry | Age: >65 years; Cognition and memory: age-related memory decline other heath state: healthy | Pilot, single-blind, one-arm trial | Dosage: blueberry 444 mL/day (weighing 54–64 kg); 532 mL/day (weighing 54–64 kg); 621 mL/day (weighing 77–91 kg); (n = 9) Time: 12 weeks | anti-age-related memory decline; antidepressant | ↑ paired associate learning, ↑ word list recall, ↓ depressive symptoms, ↓ fasting glucose levels | [50] |
| Blueberry | Age: <65 years; Cognition and memory: healthy other heath state: no contraindications to fMRI | Randomized, placebo controlled, double blind trial | Dosage: blueberry 30 mL/day (n = 12) or placebo drink (n = 14) Time: 12 weeks | anti-age-related memory decline | ↑ brain perfusion and activation, ↑ psychomotor function, visual processing, executive function, verbal and spatial memory | [52] |
| Blueberry | Age: 62–80 years; Cognition and memory: age-related memory decline; other heath state: no diabetes, kidney disease, liver disease, hematological coagulation disorder | Randomized, parallel groups, placebo controlled, double blind trial | Dosage: fish oil (1.6 g EPA + 0.8 g DHA/day) (n = 15); blueberry 25 g/day (n = 16); fish oil + blueberry 24 g/day (n = 17); or placebo (n = 17) Time: 24 weeks | anti-age-related memory decline | ↑ psychomotor speed, working memory, ↑ lexical access, ↑ long-term memory | [53] |
| Frozen blueberry | Age: 60–75 years; Cognition and memory: healthy; other heath state: BMI (18.5–29.9), no smoking or use of medications | Randomized, placebo controlled, double blind trial | Dosage: frozen blueberry 24 g/day (n = 19) or placebo (n = 19) Time: 90 days | anti-age-related memory decline | ↑ executive function, ↑ long-term memory, short term memory, ↑ spatial cognition, and attention | [76] |
| Frozen blueberry | Age: 68–92 years; Cognition and memory: age-related memory decline; other heath state: no serious psychiatric disorder, substance abuse, and claustrophobia | Randomized, placebo controlled, double blind trial | Dosage: frozen blueberry 25 g/day (n = 8) or placebo (n = 8) Time: 16 weeks | anti-age-related memory decline | ↑ working memory, accuracy, ↑ blood oxygen level dependent activation | [77] |
| Frozen blueberry | Age: >68 years; Cognition and memory: age- related memory decline; other heath state: no dementia, serious psychiatric condition, substance abuse | Randomized, placebo controlled, double blind trial | Dosage: frozen blueberry 24 g/day (n = 16) or placebo 20 g/day (n =21) Time: 16 weeks | anti-age-related memory decline | ↑ lexical access for semantic information, ↑ speed of processing and working memory, ↑ verbal and nonverbal long-term memory | [78] |
4. Mechanisms of Action
4.1. Antioxidant Activity in CNS
4.2. Mitigation of Neuroinflammatioin
4.3. Promotion of Mitophagy and Mitochondrial Function
4.4. Inhibition of Aβ and Tau Pathology
4.5. Regulation of Trp Metabolism
4.6. Others
5. Knowledge Gaps
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Livingston, G.; Huntley, J.; Sommerlad, A.; Ames, D.; Ballard, C.; Banerjee, S.; Brayne, C.; Burns, A.; Cohen-Mansfield, J.; Cooper, C.; et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 2020, 396, 413–446. [Google Scholar] [CrossRef] [PubMed]
- Wahl, D.; Cogger, V.C.; Solon-Biet, S.M.; Waern, R.V.; Gokarn, R.; Pulpitel, T.; Cabo, R.; Mattson, M.P.; Raubenheimer, D.; Simpson, S.J.; et al. Nutritional strategies to optimise cognitive function in the aging brain. Ageing Res. Rev. 2016, 31, 80–92. [Google Scholar] [CrossRef]
- D’Archivio, M.; Filesi, C.; Varì, R.; Scazzocchio, B.; Masella, R. Bioavailability of the polyphenols: Status and controversies. Int. J. Mol. Sci. 2010, 11, 1321–1342. [Google Scholar] [CrossRef] [PubMed]
- Walle, T.; Hsieh, F.; DeLegge, M.H.; Oatis, J.E., Jr.; Walle, U.K. High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab. Dispos. Biol. Fate Chem. 2004, 32, 1377–1382. [Google Scholar] [CrossRef] [PubMed]
- Gentile, C.L.; Weir, T.L. The gut microbiota at the intersection of diet and human health. Science 2018, 362, 776–780. [Google Scholar] [CrossRef]
- Pistollato, F.; Cano, S.S.; Elio, I.; Vergara, M.M.; Giampieri, F.; Battino, M. Role of gut microbiota and nutrients in amyloid formation and pathogenesis of Alzheimer disease. Nutr. Rev. 2016, 74, 624–634. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The Microbiota-Gut-Brain Axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef] [PubMed]
- Megur, A.; Baltriukienė, D.; Bukelskienė, V.; Burokas, A. The Microbiota-Gut-Brain Axis and Alzheimer’s Disease: Neuroinflammation Is to Blame? Nutrients 2020, 13, 37. [Google Scholar] [CrossRef]
- Jie, Z.; Xia, H.; Zhong, S.L.; Feng, Q.; Li, S.; Liang, S.; Zhong, H.; Liu, Z.; Gao, Y.; Zhao, H.; et al. The gut microbiome in atherosclerotic cardiovascular disease. Nat. Commun. 2017, 8, 845. [Google Scholar] [CrossRef]
- Luca, S.V.; Macovei, I.; Bujor, A.; Miron, A.; Skalicka-Woźniak, K.; Aprotosoaie, A.C.; Trifan, A. Bioactivity of dietary polyphenols: The role of metabolites. Crit. Rev. Food Sci. Nutr. 2020, 60, 626–659. [Google Scholar] [CrossRef]
- Reddy, V.P.; Aryal, P.; Robinson, S.; Rafiu, R.; Obrenovich, M.; Perry, G. Polyphenols in Alzheimer’s Disease and in the Gut-Brain Axis. Microorganisms 2020, 8, 199. [Google Scholar] [CrossRef]
- Aravind, S.M.; Wichienchot, S.; Tsao, R.; Ramakrishnan, S.; Chakkaravarthi, S. Role of dietary polyphenols on gut microbiota, their metabolites and health benefits. Food Res. Int. 2021, 142, 110189. [Google Scholar] [CrossRef]
- Hasheminezhad, S.H.; Boozari, M.; Iranshahi, M.; Yazarlu, O.; Sahebkar, A.; Hasanpour, M.; Iranshahy, M. A mechanistic insight into the biological activities of urolithins as gut microbial metabolites of ellagitannins. Phytother. Res. 2022, 36, 112–146. [Google Scholar] [CrossRef]
- Qiu, J.; Chen, Y.; Zhuo, J.; Zhang, L.; Liu, J.; Wang, B.; Sun, D.; Yu, S.; Lou, H. Urolithin A promotes mitophagy and suppresses NLRP3 inflammasome activation in lipopolysaccharide-induced BV2 microglial cells and MPTP-induced Parkinson’s disease model. Neuropharmacology 2022, 207, 108963. [Google Scholar] [CrossRef]
- D’Amico, D.; Andreux, P.A.; Valdes, P.; Singh, A.; Rinsch, C.; Auwerx, J. Impact of the Natural Compound Urolithin A on Health, Disease, and Aging. Trends Mol. Med. 2021, 27, 687–699. [Google Scholar] [CrossRef]
- Adachi, S.-i.; Sasaki, K.; Kondo, S.; Komatsu, W.; Yoshizawa, F.; Isoda, H.; Yagasaki, K. Antihyperuricemic Effect of Urolithin A in Cultured Hepatocytes and Model Mice. Molecules 2020, 25, 5136. [Google Scholar] [CrossRef]
- Chen, P.; Chen, F.; Lei, J.; Li, Q.; Zhou, B. Activation of the miR-34a-Mediated SIRT1/mTOR Signaling Pathway by Urolithin A Attenuates d-Galactose-Induced Brain Aging in Mice. Neurotherapeutics 2019, 16, 1269–1282. [Google Scholar] [CrossRef]
- Lv, M.Y.; Shi, C.J.; Pan, F.F.; Shao, J.; Feng, L.; Chen, G.; Ou, C.; Zhang, J.F.; Fu, W.M. Urolithin B suppresses tumor growth in hepatocellular carcinoma through inducing the inactivation of Wnt/β-catenin signaling. J. Cell. Biochem. 2019, 120, 17273–17282. [Google Scholar] [CrossRef]
- DaSilva, N.A.; Nahar, P.P.; Ma, H.; Eid, A.; Wei, Z.; Meschwitz, S.; Zawia, N.H.; Slitt, A.L.; Seeram, N.P. Pomegranate ellagitannin-gut microbial-derived metabolites, urolithins, inhibit neuroinflammation in vitro. Nutr. Neurosci. 2019, 22, 185–195. [Google Scholar] [CrossRef]
- Gonzalez-Sarrias, A.; Angeles Nunez-Sanchez, M.; Tomas-Barberan, F.A.; Carlos Espin, J. Neuroprotective Effects of Bioavailable Polyphenol-Derived Metabolites against Oxidative Stress-Induced Cytotoxicity in Human Neuroblastoma SH-SY5Y Cells. J. Agric. Food Chem. 2017, 65, 752–758. [Google Scholar] [CrossRef]
- Velagapudi, R.; Lepiarz, I.; El-Bakoush, A.; Katola, F.O.; Bhatia, H.; Fiebich, B.L.; Olajide, O.A. Induction of autophagy and activation of SIRT-1 deacetylation mechanisms mediate neuroprotection by the pomegranate metabolite urolithin A in BV2 microglia and differentiated 3D human neural progenitor cells. Mol. Nutr. Food Res. 2019, 63, 1801237. [Google Scholar] [CrossRef]
- Gong, Z.; Huang, J.; Xu, B.; Ou, Z.; Zhang, L.; Lin, X.; Ye, X.; Kong, X.; Long, D.; Sun, X.; et al. Urolithin A attenuates memory impairment and neuroinflammation in APP/PS1 mice. J. Neuroinflammation 2019, 16, 62. [Google Scholar] [CrossRef]
- Romo-Vaquero, M.; Fernández-Villalba, E.; Gil-Martinez, A.L.; Cuenca-Bermejo, L.; Espín, J.C.; Herrero, M.T.; Selma, M.V. Urolithins: Potential biomarkers of gut dysbiosis and disease stage in Parkinson’s patients. Food Funct. 2022, 13, 6306–6316. [Google Scholar] [CrossRef]
- Sanchez-Gonzalez, C.; Ciudad, C.J.; Noe, V.; Izquierdo-Pulido, M. Health benefits of walnut polyphenols: An exploration beyond their lipid profile. Crit. Rev. Food Sci. Nutr. 2017, 57, 3373–3383. [Google Scholar] [CrossRef]
- Garcia-Munoz, C.; Vaillant, F. Metabolic Fate of Ellagitannins: Implications for Health, and Research Perspectives for Innovative Functional Foods. Crit. Rev. Food Sci. Nutr. 2014, 54, 1584–1598. [Google Scholar] [CrossRef]
- Gonzalez-Barrio, R.; Edwards, C.A.; Crozier, A. Colonic Catabolism of Ellagitannins, Ellagic Acid, and Raspberry Anthocyanins: In Vivo and In Vitro Studies. Drug Metab. Dispos. 2011, 39, 1680–1688. [Google Scholar] [CrossRef]
- Tomas-Barberan, F.A.; Garcia-Villalba, R.; Gonzalez-Sarrias, A.; Selma, M.V.; Espin, J.C. Ellagic Acid Metabolism by Human Gut Microbiota: Consistent Observation of Three Urolithin Phenotypes in Intervention Trials, Independent of Food Source, Age, and Health Status. J. Agric. Food Chem. 2014, 62, 6535–6538. [Google Scholar] [CrossRef]
- Piwowarski, J.P.; Granica, S.; Stefanska, J.; Kisst, A.K. Differences in Metabolism of Ellagitannins by Human Gut Microbiota Ex Vivo Cultures. J. Nat. Prod. 2016, 79, 3022–3030. [Google Scholar] [CrossRef]
- Nunez-Sanchez, M.A.; Garcia-Villalba, R.; Monedero-Saiz, T.; Garcia-Talavera, N.V.; Gomez-Sanchez, M.B.; Sanchez-Alvarez, C.; Garcia-Albert, A.M.; Rodriguez-Gil, F.J.; Ruiz-Marin, M.; Pastor-Quirante, F.A.; et al. Targeted metabolic profiling of pomegranate polyphenols and urolithins in plasma, urine and colon tissues from colorectal cancer patients. Mol. Nutr. Food Res. 2014, 58, 1199–1211. [Google Scholar] [CrossRef]
- Cerdá, B.; Espín, J.C.; Parra, S.; Martínez, P.; Tomás-Barberán, F.A. The potent in vitro antioxidant ellagitannins from pomegranate juice are metabolised into bioavailable but poor antioxidant hydroxy-6H-dibenzopyran-6-one derivatives by the colonic microflora of healthy humans. Eur. J. Nutr. 2004, 43, 205–220. [Google Scholar] [CrossRef]
- Tomas-Barberan, F.A.; Gonzalez-Sarrias, A.; Garcia-Villalba, R.; Nunez-Sanchez, M.A.; Selma, M.V.; Garcia-Conesa, M.T.; Espin, J.C. Urolithins, the rescue of “old” metabolites to understand a “new” concept: Metabotypes as a nexus among phenolic metabolism, microbiota dysbiosis, and host health status. Mol. Nutr. Food Res. 2017, 61. [Google Scholar] [CrossRef] [PubMed]
- Selma, M.V.; Gonzalez-Sarrias, A.; Salas-Salvado, J.; Andres-Lacueva, C.; Alasalvar, C.; Orem, A.; Tomas-Barberan, F.A.; Espin, J.C. The gut microbiota metabolism of pomegranate or walnut ellagitannins yields two urolithin-metabotypes that correlate with cardiometabolic risk biomarkers: Comparison between normoweight, overweight-obesity and metabolic syndrome. Clin. Nutr. 2018, 37, 897–905. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Cui, S.; Mao, B.; Zhang, Q.; Zhao, J.; Zhang, H.; Tang, X.; Chen, W. Ellagic acid and intestinal microflora metabolite urolithin A: A review on its sources, metabolic distribution, health benefits, and biotransformation. Crit. Rev. Food Sci. Nutr. 2022; online ahead of print. [Google Scholar] [CrossRef]
- Cortes-Martin, A.; Garcia-Villalba, R.; Gonzalez-Sarrias, A.; Romo-Vaquero, M.; Loria-Kohen, V.; Ramirez-de-Molina, A.; Tomas-Barberan, F.A.; Selma, M.V.; Espin, J.C. The gut microbiota urolithin metabotypes revisited: The human metabolism of ellagic acid is mainly determined by aging. Food Funct. 2018, 9, 4100–4106. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; D’Amico, D.; Andreux, P.A.; Dunngalvin, G.; Kern, T.; Blanco-Bose, W.; Auwerx, J.; Aebischer, P.; Rinsch, C. Direct supplementation with Urolithin A overcomes limitations of dietary exposure and gut microbiome variability in healthy adults to achieve consistent levels across the population. Eur. J. Clin. Nutr. 2022, 76, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Kujawska, M.; Jourdes, M.; Kurpik, M.; Szulc, M.; Szaefer, H.; Chmielarz, P.; Kreiner, G.; Krajka-Kuzniak, V.; Mikolajczak, P.L.; Teissedre, P.-L.; et al. Neuroprotective Effects of Pomegranate Juice against Parkinson’s Disease and Presence of Ellagitannins-Derived Metabolite-Urolithin A-In the Brain. Int. J. Mol. Sci. 2020, 21, 202. [Google Scholar] [CrossRef] [PubMed]
- Andreux, P.A.; Blanco-Bose, W.; Ryu, D.; Burdet, F.; Ibberson, M.; Aebischer, P.; Auwerx, J.; Singh, A.; Rinsch, C. The mitophagy activator urolithin A is safe and induces a molecular signature of improved mitochondrial and cellular health in humans. Nat. Metab. 2019, 1, 595–603. [Google Scholar] [CrossRef]
- Singh, A.; D’Amico, D.; Andreux, P.A.; Fouassier, A.M.; Blanco-Bose, W.; Evans, M.; Aebischer, P.; Auwerx, J.; Rinsch, C. Urolithin A improves muscle strength, exercise performance, and biomarkers of mitochondrial health in a randomized trial in middle-aged adults. Cell Rep. Med. 2022, 3, 100633. [Google Scholar] [CrossRef]
- Liu, S.; D’Amico, D.; Shankland, E.; Bhayana, S.; Garcia, J.M.; Aebischer, P.; Rinsch, C.; Singh, A.; Marcinek, D.J. Effect of Urolithin A Supplementation on Muscle Endurance and Mitochondrial Health in Older Adults A Randomized Clinical Trial. JAMA Netw. Open 2022, 5, e2144279. [Google Scholar] [CrossRef]
- García-Villalba, R.; Giménez-Bastida, J.A.; Cortés-Martín, A.; Ávila-Gálvez, M.; Tomás-Barberán, F.A.; Selma, M.V.; Espín, J.C.; González-Sarrías, A. Urolithins: A Comprehensive Update on their Metabolism, Bioactivity, and Associated Gut Microbiota. Mol. Nutr. Food Res. 2022, 66, e2101019. [Google Scholar] [CrossRef]
- Gasperotti, M.; Passamonti, S.; Tramer, F.; Masuero, D.; Guella, G.; Mattivi, F.; Vrhovsek, U. Fate of microbial metabolites of dietary polyphenols in rats: Is the brain their target destination? ACS Chem. Neurosci. 2015, 6, 1341–1352. [Google Scholar] [CrossRef]
- Bookheimer, S.Y.; Renner, B.A.; Ekstrom, A.; Li, Z.; Henning, S.M.; Brown, J.A.; Jones, M.; Moody, T.; Small, G.W. Pomegranate juice augments memory and FMRI activity in middle-aged and older adults with mild memory complaints. Evid.-Based Complement. Altern. Med. 2013, 2013, 946298. [Google Scholar] [CrossRef] [PubMed]
- Suez, J.; Elinav, E. The path towards microbiome-based metabolite treatment. Nat. Microbiol. 2017, 2, 17075. [Google Scholar] [CrossRef]
- Gaya, P.; Peiroten, A.; Medina, M.; Alvarez, I.; Landete, J.M. Bifidobacterium pseudocatenulatum INIA P815: The first bacterium able to produce urolithins A and B from ellagic acid. J. Funct. Foods 2018, 45, 95–99. [Google Scholar] [CrossRef]
- Selma, M.V.; Beltran, D.; Garcia-Villalba, R.; Espin, J.C.; Tomas-Barberan, F.A. Description of urolithin production capacity from ellagic acid of two human intestinal Gordonibacter species. Food Funct. 2014, 5, 1779–1784. [Google Scholar] [CrossRef] [PubMed]
- Selma, M.V.; Beltran, D.; Luna, M.C.; Romo-Vaquero, M.; Garcia-Villalba, R.; Mira, A.; Espin, J.C.; Tomas-Barberan, F.A. Isolation of Human Intestinal Bacteria Capable of Producing the Bioactive Metabolite Isourolithin A from Ellagic Acid. Front. Microbiol. 2017, 8, 1521. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, H.; Kishino, S.; Kudoh, M.; Yamamoto, H.; Ogawa, J. Evaluation of electron-transferring cofactor mediating enzyme systems involved in urolithin dehydroxylation in Gordonibacter urolithinfaciens DSM 27213. J. Biosci. Bioeng. 2020, 129, 552–557. [Google Scholar] [CrossRef]
- Connell, E.; Le Gall, G.; Pontifex, M.G.; Sami, S.; Cryan, J.F.; Clarke, G.; Müller, M.; Vauzour, D. Microbial-derived metabolites as a risk factor of age-related cognitive decline and dementia. Mol. Neurodegener. 2022, 17, 43. [Google Scholar] [CrossRef]
- Siddarth, P.; Li, Z.; Miller, K.J.; Ercoli, L.M.; Merril, D.A.; Henning, S.M.; Heber, D.; Small, G.W. Randomized placebo-controlled study of the memory effects of pomegranate juice in middle-aged and older adults. Am. J. Clin. Nutr. 2020, 111, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Krikorian, R.; Shidler, M.D.; Nash, T.A.; Kalt, W.; Vinqvist-Tymchuk, M.R.; Shukitt-Hale, B.; Joseph, J.A. Blueberry supplementation improves memory in older adults. J. Agric. Food Chem. 2010, 58, 3996–4000. [Google Scholar] [CrossRef] [PubMed]
- Sala-Vila, A.; Valls-Pedret, C.; Rajaram, S.; Coll-Padrós, N.; Cofán, M.; Serra-Mir, M.; Pérez-Heras, A.M.; Roth, I.; Freitas-Simoes, T.M.; Doménech, M.; et al. Effect of a 2-year diet intervention with walnuts on cognitive decline. The Walnuts And Healthy Aging (WAHA) study: A randomized controlled trial. Am. J. Clin. Nutr. 2020, 111, 590–600. [Google Scholar] [CrossRef]
- Bowtell, J.L.; Aboo-Bakkar, Z.; Conway, M.E.; Adlam, A.R.; Fulford, J. Enhanced task-related brain activation and resting perfusion in healthy older adults after chronic blueberry supplementation. Appl. Physiol. Nutr. Metab. 2017, 42, 773–779. [Google Scholar] [CrossRef]
- McNamara, R.K.; Kalt, W.; Shidler, M.D.; McDonald, J.; Summer, S.S.; Stein, A.L.; Stover, A.N.; Krikorian, R. Cognitive response to fish oil, blueberry, and combined supplementation in older adults with subjective cognitive impairment. Neurobiol. Aging 2018, 64, 147–156. [Google Scholar] [CrossRef]
- Cásedas, G.; Les, F.; Choya-Foces, C.; Hugo, M.; López, V. The Metabolite Urolithin-A Ameliorates Oxidative Stress in Neuro-2a Cells, Becoming a Potential Neuroprotective Agent. Antioxidants 2020, 9, 177. [Google Scholar] [CrossRef]
- Kim, K.B.; Lee, S.; Kim, J.H. Neuroprotective effects of urolithin A on H2O2-induced oxidative stress-mediated apoptosis in SK-N-MC cells. Nutr Res Prac. 2020, 14, 3–11. [Google Scholar] [CrossRef]
- An, L.; Li, M.; Zou, C.; Wang, K.; Zhang, W.; Huang, X.; Wang, Y. Walnut polyphenols and the active metabolite urolithin A improve oxidative damage in SH-SY5Y cells by up-regulating PKA/CREB/BDNF signaling. Food Funct. 2023, 14, 2698–2709. [Google Scholar] [CrossRef]
- Chen, P.; Chen, F.; Lei, J.; Wang, G.; Zhou, B. The Gut Microbiota Metabolite Urolithin B Improves Cognitive Deficits by Inhibiting Cyt C-Mediated Apoptosis and Promoting the Survival of Neurons through the PI3K Pathway in Aging Mice. Front. Pharmacol. 2021, 12, 768097. [Google Scholar] [CrossRef]
- Lee, G.; Park, J.S.; Lee, E.J.; Ahn, J.H.; Kim, H.S. Anti-inflammatory and antioxidant mechanisms of urolithin B in activated microglia. Phytomedicine Int. J. Phytother. Phytopharm. 2019, 55, 50–57. [Google Scholar] [CrossRef]
- Toney, A.M.; Albusharif, M.; Works, D.; Polenz, L.; Schlange, S.; Chaidez, V.; Ramer-Tait, A.E.; Chung, S. Differential Effects of Whole Red Raspberry Polyphenols and Their Gut Metabolite Urolithin A on Neuroinflammation in BV-2 Microglia. Int. J. Environ. Res. Public Health 2020, 18, 68. [Google Scholar] [CrossRef]
- Xu, J.; Yuan, C.; Wang, G.; Luo, J.; Ma, H.; Xu, L.; Mu, Y.; Li, Y.; Seeram, N.P.; Huang, X.; et al. Urolithins Attenuate LPS-Induced Neuroinflammation in BV2Microglia via MAPK, Akt, and NF-κB Signaling Pathways. J. Agric. Food Chem. 2018, 66, 571–580. [Google Scholar] [CrossRef]
- Lee, H.J.; Jung, Y.H.; Choi, G.E.; Kim, J.S.; Chae, C.W.; Lim, J.R.; Kim, S.Y.; Yoon, J.H.; Cho, J.H.; Lee, S.-J.; et al. Urolithin A suppresses high glucose-induced neuronal amyloidogenesis by modulating TGM2-dependent ER-mitochondria contacts and calcium homeostasis. Cell Death Differ. 2021, 28, 184–202. [Google Scholar] [CrossRef]
- Esselun, C.; Theyssen, E.; Eckert, G.P. Effects of Urolithin A on Mitochondrial Parameters in a Cellular Model of Early Alzheimer Disease. Int. J. Mol. Sci. 2021, 22, 8333. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Jiang, J.; Qiu, J.; Wang, L.; Zhuo, J.; Wang, B.; Sun, D.; Yu, S.; Lou, H. Urolithin A protects dopaminergic neurons in experimental models of Parkinson’s disease by promoting mitochondrial biogenesis through the SIRT1/PGC-1 alpha signaling pathway. Food Funct. 2022, 13, 375–385. [Google Scholar] [CrossRef] [PubMed]
- Jayatunga, D.P.W.; Hone, E.; Fernando, W.M.A.D.B.; Garg, M.L.; Verdile, G.; Martins, R.N. A Synergistic Combination of DHA, Luteolin, and Urolithin A Against Alzheimer’s Disease. Front. Aging Neurosci. 2022, 14, 780602. [Google Scholar] [CrossRef]
- Kshirsagar, S.; Alvir, R.V.; Pradeepkiran, J.A.; Hindle, A.; Vijayan, M.; Ramasubramaniam, B.; Kumar, S.; Reddy, A.P.; Reddy, P.H. A Combination Therapy of Urolithin A plus EGCG Has Stronger Protective Effects than Single Drug Urolithin A in a Humanized Amyloid Beta Knockin Mice for Late-Onset Alzheimer’s Disease. Cells 2022, 11, 2660. [Google Scholar] [CrossRef]
- Kshirsagar, S.; Sawant, N.; Morton, H.; Reddy, A.P.; Reddy, P.H. Mitophagy enhancers against phosphorylated Tau-induced mitochondrial and synaptic toxicities in Alzheimer disease. Pharmacol. Res. 2021, 174, 105973. [Google Scholar] [CrossRef] [PubMed]
- Kshirsagar, S.; Sawant, N.; Morton, H.; Reddy, A.P.; Reddy, P.H. Protective effects of mitophagy enhancers against amyloid beta-induced mitochondrial and synaptic toxicities in Alzheimer disease. Hum. Mol. Genet. 2022, 31, 423–439. [Google Scholar] [CrossRef]
- Fang, E.F.; Hou, Y.; Palikaras, K.; Adriaanse, B.A.; Kerr, J.S.; Yang, B.; Lautrup, S.; Hasan-Olive, M.M.; Caponio, D.; Dan, X.; et al. Mitophagy inhibits amyloid-β and tau pathology and reverses cognitive deficits in models of Alzheimer’s disease. Nat. Neurosci. 2019, 22, 401–412. [Google Scholar] [CrossRef]
- Yuan, T.; Ma, H.; Liu, W.; Niesen, D.B.; Shah, N.; Crews, R.; Rose, K.N.; Vattem, D.A.; Seeram, N.P. Pomegranate’s Neuroprotective Effects against Alzheimer’s Disease Are Mediated by Urolithins, Its Ellagitannin-Gut Microbial Derived Metabolites. ACS Chem. Neurosci. 2016, 7, 26–33. [Google Scholar] [CrossRef]
- Yao, X.; Kailin, L.; Ji, B.; Hang, L.; Xiaotong, Z.; El-Omar, E.; Lin, H.; Lan, G.; Min, W. Urolithin a attenuates diabetes-associated cognitive impairment by ameliorating intestinal barrier dysfunction via n-glycan biosynthesis pathway. Mol. Nutr. Food Res. 2022, 66, 2100863. [Google Scholar] [CrossRef]
- Martínez-Lapiscina, E.H.; Clavero, P.; Toledo, E.; Estruch, R.; Salas-Salvadó, J.; San Julián, B.; Sanchez-Tainta, A.; Ros, E.; Valls-Pedret, C.; Martinez-Gonzalez, M. Mediterranean diet improves cognition: The PREDIMED-NAVARRA randomised trial. J. Neurol. Neurosurg. Psychiatry 2013, 84, 1318–1325. [Google Scholar] [CrossRef]
- Miller, M.G.; Thangthaeng, N.; Rutledge, G.A.; Scott, T.M.; Shukitt-Hale, B. Dietary strawberry improves cognition in a randomised, double-blind, placebo-controlled trial in older adults. Br. J. Nutr. 2021, 126, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, A.; Salo, I.; Plaza, M.; Björck, I. Effects of a mixed berry beverage on cognitive functions and cardiometabolic risk markers; A randomized cross-over study in healthy older adults. PLoS ONE 2017, 12, e0188173. [Google Scholar] [CrossRef] [PubMed]
- Bensalem, J.; Dudonné, S.; Etchamendy, N.; Pellay, H.; Amadieu, C.; Gaudout, D.; Dubreuil, S.; Paradis, M.E.; Pomerleau, S.; Capuron, L.; et al. Polyphenols From Grape and Blueberry Improve Episodic Memory in Healthy Elderly with Lower Level of Memory Performance: A Bicentric Double-Blind, Randomized, Placebo-Controlled Clinical Study. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2019, 74, 996–1007. [Google Scholar] [CrossRef]
- Whyte, A.R.; Cheng, N.; Fromentin, E.; Williams, C.M. A Randomized, Double-Blinded, Placebo-Controlled Study to Compare the Safety and Efficacy of Low Dose Enhanced Wild Blueberry Powder and Wild Blueberry Extract (ThinkBlue™) in Maintenance of Episodic and Working Memory in Older Adults. Nutrients 2018, 10, 660. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.G.; Hamilton, D.A.; Joseph, J.A.; Shukitt-Hale, B. Dietary blueberry improves cognition among older adults in a randomized, double-blind, placebo-controlled trial. Eur. J. Nutr. 2018, 57, 1169–1180. [Google Scholar] [CrossRef]
- Boespflug, E.L.; Eliassen, J.C.; Dudley, J.A.; Shidler, M.D.; Kalt, W.; Summer, S.S.; Stein, A.L.; Stover, A.N.; Krikorian, R. Enhanced neural activation with blueberry supplementation in mild cognitive impairment. Nutr. Neurosci. 2018, 21, 297–305. [Google Scholar] [CrossRef]
- Krikorian, R.; Kalt, W.; McDonald, J.E.; Shidler, M.D.; Summer, S.S.; Stein, A.L. Cognitive performance in relation to urinary anthocyanins and their flavonoid-based products following blueberry supplementation in older adults at risk for dementia. J. Funct. Foods 2020, 64, 103667. [Google Scholar] [CrossRef]
- Halliwell, B. Reactive oxygen species and the central nervous system. J. Neurochem. 1992, 59, 1609–1623. [Google Scholar] [CrossRef]
- Shohami, E.; Beit-Yannai, E.; Horowitz, M.; Kohen, R. Oxidative stress in closed-head injury: Brain antioxidant capacity as an indicator of functional outcome. J. Cereb. Blood Flow Metab. 1997, 17, 1007–1019. [Google Scholar] [CrossRef]
- Shi, P.Z.; Wang, J.W.; Wang, P.C.; Han, B.; Lu, X.H.; Ren, Y.X.; Feng, X.M.; Cheng, X.F.; Zhang, L. Urolithin a alleviates oxidative stress-induced senescence in nucleus pulposus-derived mesenchymal stem cells through SIRT1/PGC-1α pathway. World J. Stem Cells 2021, 13, 1928–1946. [Google Scholar] [CrossRef]
- Mazumder, M.K.; Choudhury, S.; Borah, A. An in silico investigation on the inhibitory potential of the constituents of Pomegranate juice on antioxidant defense mechanism: Relevance to neurodegenerative diseases. IBRO Rep. 2019, 6. [Google Scholar] [CrossRef]
- Singh, R.; Chandrashekharappa, S.; Vemula, P.K.; Haribabu, B.; Jala, V.R. Microbial Metabolite Urolithin B Inhibits Recombinant Human Monoamine Oxidase A Enzyme. Metabolites 2020, 10, 258. [Google Scholar] [CrossRef] [PubMed]
- Saha, P.; Yeoh, B.S.; Singh, R.; Chandrasekar, B.; Vemula, P.K.; Haribabu, B.; Vijay-Kumar, M.; Jala, V.R. Gut Microbiota Conversion of Dietary Ellagic Acid into Bioactive Phytoceutical Urolithin A Inhibits Heme Peroxidases. PLoS ONE 2016, 11, e0156811. [Google Scholar] [CrossRef] [PubMed]
- Priyadarsini, K.I.; Khopde, S.M.; Kumar, S.S.; Mohan, H. Free radical studies of ellagic acid, a natural phenolic antioxidant. J Agric. Food Chem. 2002, 50, 2200–2206. [Google Scholar] [CrossRef] [PubMed]
- Galano, A.; Francisco Marquez, M.; Pérez-González, A. Ellagic acid: An unusually versatile protector against oxidative stress. Chem. Res. Toxicol. 2014, 27, 904–918. [Google Scholar] [CrossRef] [PubMed]
- Ríos, J.L.; Giner, R.M.; Marín, M.; Recio, M.C. A Pharmacological Update of Ellagic Acid. Planta Medica 2018, 84, 1068–1093. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Gong, L.F.; Wu, Y.F.; Lin, Z.; Jiang, B.J.; Wu, L.; Yu, K.H. Urolithin A targets the PI3K/Akt/NF-κB pathways and prevents IL-1β-induced inflammatory response in human osteoarthritis: In vitro and in vivo studies. Food Funct. 2019, 10, 6135–6146. [Google Scholar] [CrossRef]
- Yan, C.; Ma, Z.; Ma, H.; Li, Q.; Zhai, Q.; Jiang, T.; Zhang, Z.; Wang, Q. Mitochondrial Transplantation Attenuates Brain Dysfunction in Sepsis by Driving Microglial M2 Polarization. Mol. Neurobiol. 2020, 57, 3875–3890. [Google Scholar] [CrossRef]
- Lin, X.H.; Ye, X.J.; Li, Q.F.; Gong, Z.; Cao, X.; Li, J.H.; Zhao, S.T.; Sun, X.D.; He, X.S.; Xuan, A.G. Urolithin A Prevents Focal Cerebral Ischemic Injury via Attenuating Apoptosis and Neuroinflammation in Mice. Neuroscience 2020, 448, 94–106. [Google Scholar] [CrossRef]
- Komatsu, W.; Kishi, H.; Yagasaki, K.; Ohhira, S. Urolithin A attenuates pro-inflammatory mediator production by suppressing PI3-K/Akt/NF-κB and JNK/AP-1 signaling pathways in lipopolysaccharide-stimulated RAW264 macrophages: Possible involvement of NADPH oxidase-derived reactive oxygen species. Eur. J. Pharmacol. 2018, 833, 411–424. [Google Scholar] [CrossRef]
- Zhang, Y.; Aisker, G.; Dong, H.; Halemahebai, G.; Zhang, Y.; Tian, L. Urolithin A suppresses glucolipotoxicity-induced ER stress and TXNIP/NLRP3/IL-1β inflammation signal in pancreatic β cells by regulating AMPK and autophagy. Phytomed. Int. J. Phytother. Phytopharm. 2021, 93, 153741. [Google Scholar] [CrossRef]
- Ding, S.L.; Pang, Z.Y.; Chen, X.M.; Li, Z.; Liu, X.X.; Zhai, Q.L.; Huang, J.M.; Ruan, Z.Y. Urolithin a attenuates IL-1β-induced inflammatory responses and cartilage degradation via inhibiting the MAPK/NF-κB signaling pathways in rat articular chondrocytes. J. Inflamm. 2020, 17, 13. [Google Scholar] [CrossRef] [PubMed]
- Toney, A.M.; Fan, R.; Xian, Y.; Chaidez, V.; Ramer-Tait, A.E.; Chung, S. Urolithin A, a Gut Metabolite, Improves Insulin Sensitivity Through Augmentation of Mitochondrial Function and Biogenesis. Obesity 2019, 27, 612–620. [Google Scholar] [CrossRef] [PubMed]
- Cordero, M.D.; Williams, M.R.; Ryffel, B. AMP-Activated Protein Kinase Regulation of the NLRP3 Inflammasome during Aging. Trends Endocrinol. Metab. TEM 2018, 29, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Salt, I.P.; Palmer, T.M. Exploiting the anti-inflammatory effects of AMP-activated protein kinase activation. Expert Opin. Investig. Drugs 2012, 21, 1155–1167. [Google Scholar] [CrossRef]
- Carling, D. AMPK signalling in health and disease. Curr. Opin. Cell Biol. 2017, 45, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Salminen, A.; Kaarniranta, K. AMP-activated protein kinase (AMPK) controls the aging process via an integrated signaling network. Ageing Res. Rev. 2012, 11, 230–241. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, Y.; Xu, Y.; Ruan, W.; Wang, H.; Zhang, Y.; Saavedra, J.M.; Zhang, L.; Huang, Z.; Pang, T. A Dual AMPK/Nrf2 Activator Reduces Brain Inflammation After Stroke by Enhancing Microglia M2 Polarization. Antioxid. Redox Signal. 2018, 28, 141–163. [Google Scholar] [CrossRef]
- Jęśko, H.; Wencel, P.; Strosznajder, R.P.; Strosznajder, J.B. Sirtuins and Their Roles in Brain Aging and Neurodegenerative Disorders. Neurochem. Res. 2017, 42, 876–890. [Google Scholar] [CrossRef]
- Cho, S.H.; Chen, J.A.; Sayed, F.; Ward, M.E.; Gao, F.; Nguyen, T.A.; Krabbe, G.; Sohn, P.D.; Lo, I.; Minami, S.; et al. SIRT1 deficiency in microglia contributes to cognitive decline in aging and neurodegeneration via epigenetic regulation of IL-1β. J. Neurosci. 2015, 35, 807–818. [Google Scholar] [CrossRef]
- Ye, J.; Liu, Z.; Wei, J.; Lu, L.; Huang, Y.; Luo, L.; Xie, H. Protective effect of SIRT1 on toxicity of microglial-derived factors induced by LPS to PC12 cells via the p53-caspase-3-dependent apoptotic pathway. Neurosci. Lett. 2013, 553, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Rizzi, L.; Roriz-Cruz, M. Sirtuin 1 and Alzheimer’s disease: An up-to-date review. Neuropeptides 2018, 71, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhang, J.; Yan, C.; Zhou, J.; Lin, R.; Lin, Q.; Wang, W.; Zhang, K.; Yang, G.; Bian, X.; et al. SIRT1 regulates CD40 expression induced by TNF-α via NF-ĸB pathway in endothelial cells. Cell. Physiol. Biochem. 2012, 30, 1287–1298. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, É.; Marques, C.C.; Pimenta, J.; Jorge, J.; Baptista, M.C.; Gonçalves, A.C.; Pereira, R. Anti-Aging Effect of Urolithin A on Bovine Oocytes In Vitro. Animals 2021, 11, 2048. [Google Scholar] [CrossRef] [PubMed]
- Ryu, D.; Mouchiroud, L.; Andreux, P.A.; Katsyuba, E.; Moullan, N.; Nicolet-Dit-Félix, A.A.; Williams, E.G.; Jha, P.; Lo Sasso, G.; Huzard, D.; et al. Urolithin A induces mitophagy and prolongs lifespan in C. elegans and increases muscle function in rodents. Nat. Med. 2016, 22, 879–888. [Google Scholar] [CrossRef]
- Fivenson, E.M.; Lautrup, S.; Sun, N.; Scheibye-Knudsen, M.; Stevnsner, T.; Nilsen, H.; Bohr, V.A.; Fang, E.F. Mitophagy in neurodegeneration and aging. Neurochem. Int. 2017, 109, 202–209. [Google Scholar] [CrossRef]
- Ahsan, A.; Zheng, Y.R.; Wu, X.L.; Tang, W.D.; Liu, M.R.; Ma, S.J.; Jiang, L.; Hu, W.W.; Zhang, X.N.; Chen, Z. Urolithin A-activated autophagy but not mitophagy protects against ischemic neuronal injury by inhibiting ER stress in vitro and in vivo. CNS Neurosci. Ther. 2019, 25, 976–986. [Google Scholar] [CrossRef]
- Iorio, R.; Celenza, G.; Petricca, S. Mitophagy: Molecular Mechanisms, New Concepts on Parkin Activation and the Emerging Role of AMPK/ULK1 Axis. Cells 2022, 11, 30. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, E.; Musich, P.R.; Lin, F. Mitochondrial dysfunction in neurodegenerative diseases and the potential countermeasure. CNS Neurosci. Ther. 2019, 25, 816–824. [Google Scholar] [CrossRef]
- Wang, S.; Kandadi, M.R.; Ren, J. Double knockout of Akt2 and AMPK predisposes cardiac aging without affecting lifespan: Role of autophagy and mitophagy. Biochim. Biophys. Acta. Mol. Basis Dis. 2019, 1865, 1865–1875. [Google Scholar] [CrossRef]
- Atherton, P.J.; Babraj, J.A.; Smith, K.; Singh, J.; Rennie, M.J.; Wackerhage, H. Selective activation of AMPK-PGC-1 alpha or PKB-TSC2-mTOR signaling can explain specific adaptive responses to endurance or resistance training-like electrical muscle stimulation. FASEB J. 2005, 19, 786–788. [Google Scholar] [CrossRef] [PubMed]
- Laker, R.C.; Drake, J.C.; Wilson, R.J.; Lira, V.A.; Lewellen, B.M.; Ryall, K.A.; Fisher, C.C.; Zhang, M.; Saucerman, J.J.; Goodyear, L.J.; et al. Ampk phosphorylation of Ulk1 is required for targeting of mitochondria to lysosomes in exercise-induced mitophagy. Nat. Commun. 2017, 8, 548. [Google Scholar] [CrossRef] [PubMed]
- Egan, D.F.; Shackelford, D.B.; Mihaylova, M.M.; Gelino, S.; Kohnz, R.A.; Mair, W.; Vasquez, D.S.; Joshi, A.; Gwinn, D.M.; Taylor, R.; et al. Phosphorylation of ULK1 (hATG1) by AMP-Activated Protein Kinase Connects Energy Sensing to Mitophagy. Science 2011, 331, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kundu, M.; Viollet, B.; Guan, K.-L. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat. Cell Biol. 2011, 13, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Pillai, V.B.; Sundaresan, N.R.; Kim, G.; Gupta, M.; Rajamohan, S.B.; Pillai, J.B.; Samant, S.; Ravindra, P.V.; Isbatan, A.; Gupta, M.P. Exogenous NAD Blocks Cardiac Hypertrophic Response via Activation of the SIRT3-LKB1-AMP-activated Kinase Pathway. J. Biol. Chem. 2010, 285, 3133–3144. [Google Scholar] [CrossRef]
- Lin, J.; Zhuge, J.; Zheng, X.; Wu, Y.; Zhang, Z.; Xu, T.; Meftah, Z.; Xu, H.; Wu, Y.; Tian, N.; et al. Urolithin A-induced mitophagy suppresses apoptosis and attenuates intervertebral disc degeneration via the AMPK signaling pathway. Free Radic. Biol. Med. 2020, 150, 109–119. [Google Scholar] [CrossRef]
- Han, Q.-a.; Su, D.; Shi, C.; Liu, P.; Wang, Y.; Zhu, B.; Xia, X. Urolithin A attenuated ox-LDL-induced cholesterol accumulation in macrophages partly through regulating miR-33a and ERK/AMPK/SREBP1 signaling pathways. Food Funct. 2020, 11, 3432–3440. [Google Scholar] [CrossRef]
- Rodriguez, J.; Pierre, N.; Naslain, D.; Bontemps, F.; Ferreira, D.; Priem, F.; Deldicque, L.; Francaux, M. Urolithin B, a newly identified regulator of skeletal muscle mass. J. Cachexia Sarcopenia Muscle 2017, 8, 583–597. [Google Scholar] [CrossRef]
- Zhao, C.; Sakaguchi, T.; Fujita, K.; Ito, H.; Nishida, N.; Nagatomo, A.; Tanaka-Azuma, Y.; Katakura, Y. Pomegranate-Derived Polyphenols Reduce Reactive Oxygen Species Production via SIRT3-Mediated SOD2 Activation. Oxidative Med. Cell. Longev. 2016, 2016, 2927131. [Google Scholar] [CrossRef]
- Ghosh, N.; Das, A.; Biswas, N.; Gnyawali, S.; Singh, K.; Gorain, M.; Polcyn, C.; Khanna, S.; Roy, S.; Sen, C.K. Urolithin A augments angiogenic pathways in skeletal muscle by bolstering NAD+ and SIRT1. Sci. Rep. 2020, 10, 20184. [Google Scholar] [CrossRef]
- Sebastián, D.; Sorianello, E.; Segalés, J.; Irazoki, A.; Ruiz-Bonilla, V.; Sala, D.; Planet, E.; Berenguer-Llergo, A.; Muñoz, J.P.; Sánchez-Feutrie, M.; et al. Mfn2 deficiency links age-related sarcopenia and impaired autophagy to activation of an adaptive mitophagy pathway. EMBO J. 2016, 35, 1677–1693. [Google Scholar] [CrossRef] [PubMed]
- Qiao, H.; Ren, H.; Du, H.; Zhang, M.; Xiong, X.; Lv, R. Liraglutide repairs the infarcted heart: The role of the SIRT1/Parkin/mitophagy pathway. Mol. Med. Rep. 2018, 17, 3722–3734. [Google Scholar] [CrossRef] [PubMed]
- Kwak, H.M.; Jeon, S.Y.; Sohng, B.H.; Kim, J.G.; Lee, J.M.; Lee, K.B.; Jeong, H.H.; Hur, J.M.; Kang, Y.H.; Song, K.S. Beta-Secretase (BACE1) inhibitors from pomegranate (Punica granatum) husk. Arch. Pharmacal Res. 2005, 28, 1328–1332. [Google Scholar] [CrossRef] [PubMed]
- Youn, K.; Jun, M. In Vitro BACE1 inhibitory activity of geraniin and corilagin from Geranium thunbergii. Planta Medica 2013, 79, 1038–1042. [Google Scholar] [CrossRef]
- Iaccarino, H.F.; Singer, A.C.; Martorell, A.J.; Rudenko, A.; Gao, F.; Gillingham, T.Z.; Mathys, H.; Seo, J.; Kritskiy, O.; Abdurrob, F.; et al. Gamma frequency entrainment attenuates amyloid load and modifies microglia. Nature 2016, 540, 230–235. [Google Scholar] [CrossRef]
- Keren-Shaul, H.; Spinrad, A.; Weiner, A.; Matcovitch-Natan, O.; Dvir-Szternfeld, R.; Ulland, T.K.; David, E.; Baruch, K.; Lara-Astaiso, D.; Toth, B.; et al. A Unique Microglia Type Associated with Restricting Development of Alzheimer’s Disease. Cell 2017, 169, 1276–1290.e1217. [Google Scholar] [CrossRef]
- Tu, H.J.; Su, C.J.; Peng, C.S.; Lin, T.E.; HuangFu, W.C.; Hsu, K.C.; Hwang, T.L.; Pan, S.L. Urolithin A exhibits a neuroprotective effect against Alzheimer’s disease by inhibiting DYRK1A activity. J. Food Drug Anal. 2023, 31, 358–370. [Google Scholar] [CrossRef]
- Frick, B.; Schroecksnadel, K.; Neurauter, G.; Leblhuber, F.; Fuchs, D. Increasing production of homocysteine and neopterin and degradation of tryptophan with older age. Clin. Biochem. 2004, 37, 684–687. [Google Scholar] [CrossRef]
- Platten, M.; Nollen, E.A.A.; Röhrig, U.F.; Fallarino, F.; Opitz, C.A. Tryptophan metabolism as a common therapeutic target in cancer, neurodegeneration and beyond. Nat. Rev. Drug Discov. 2019, 18, 379–401. [Google Scholar] [CrossRef]
- Roager, H.M.; Licht, T.R. Microbial tryptophan catabolites in health and disease. Nat. Commun. 2018, 9, 3294. [Google Scholar] [CrossRef]
- Rothhammer, V.; Mascanfroni, I.D.; Bunse, L.; Takenaka, M.C.; Kenison, J.E.; Mayo, L.; Chao, C.C.; Patel, B.; Yan, R.; Blain, M.; et al. Type I interferons and microbial metabolites of tryptophan modulate astrocyte activity and central nervous system inflammation via the aryl hydrocarbon receptor. Nat. Med. 2016, 22, 586–597. [Google Scholar] [CrossRef] [PubMed]
- Rothhammer, V.; Borucki, D.M.; Tjon, E.C.; Takenaka, M.C.; Chao, C.C.; Ardura-Fabregat, A.; de Lima, K.A.; Gutiérrez-Vázquez, C.; Hewson, P.; Staszewski, O.; et al. Microglial control of astrocytes in response to microbial metabolites. Nature 2018, 557, 724–728. [Google Scholar] [CrossRef]
- Yang, J.; Guo, Y.; Lee, R.; Henning, S.M.; Wang, J.; Pan, Y.; Qing, T.; Hsu, M.; Nguyen, A.; Prabha, S.; et al. Pomegranate Metabolites Impact Tryptophan Metabolism in Humans and Mice. Curr. Dev. Nutr. 2020, 4, nzaa165. [Google Scholar] [CrossRef] [PubMed]
- Livingston, S.; Mallick, S.; Lucas, D.A.; Sabir, M.S.; Sabir, Z.L.; Purdin, H.; Nidamanuri, S.; Haussler, C.A.; Haussler, M.R.; Jurutka, P.W. Pomegranate derivative urolithin A enhances vitamin D receptor signaling to amplify serotonin-related gene induction by 1,25-dihydroxyvitamin D. Biochem. Biophys. Rep. 2020, 24, 100825. [Google Scholar] [CrossRef] [PubMed]
- Green, P.S.; Simpkins, J.W. Neuroprotective effects of estrogens: Potential mechanisms of action. Int. J. Dev. Neurosci. 2000, 18, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Brann, D.W.; Dhandapani, K.; Wakade, C.; Mahesh, V.B.; Khan, M.M. Neurotrophic and neuroprotective actions of estrogen: Basic mechanisms and clinical implications. Steroids 2007, 72, 381–405. [Google Scholar] [CrossRef]
- Barroso, A.; Mahler, J.V.; Fonseca-Castro, P.H.; Quintana, F.J. The aryl hydrocarbon receptor and the gut-brain axis. Cell. Mol. Immunol. 2021, 18, 259–268. [Google Scholar] [CrossRef]
- Larrosa, M.; Gonzalez-Sarrias, A.; Garcia-Conesa, M.T.; Tomas-Barberan, F.A.; Espin, J.C. Urolithins, ellagic acid-derived metabolites produced by human colonic microflora, exhibit estrogenic and antiestrogenic activities. J. Agric. Food Chem. 2006, 54, 1611–1620. [Google Scholar] [CrossRef]
- Skledar, D.G.; Tomasic, T.; Dolenc, M.S.; Masic, L.P.; Zega, A. Evaluation of endocrine activities of ellagic acid and urolithins using reporter gene assays. Chemosphere 2019, 220, 706–713. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, J.-H.; Aguilera-Barrantes, I.; Shiau, C.-W.; Sheng, X.; Wang, L.-S.; Stoner, G.D.; Huang, Y.-W. Urolithin A suppresses the proliferation of endometrial cancer cells by mediating estrogen receptor-alpha-dependent gene expression. Mol. Nutr. Food Res. 2016, 60, 2387–2395. [Google Scholar] [CrossRef]
- Shen, P.-X.; Li, X.; Deng, S.-Y.; Zhao, L.; Zhang, Y.-Y.; Deng, X.; Han, B.; Yu, J.; Li, Y.; Wang, Z.-Z.; et al. Urolithin A ameliorates experimental autoimmune encephalomyelitis by targeting aryl hydrocarbon receptor. Ebiomedicine 2021, 64, 103227. [Google Scholar] [CrossRef] [PubMed]
- Muku, G.E.; Murray, I.A.; Espín, J.C.; Perdew, G.H. Urolithin A Is a Dietary Microbiota-Derived Human Aryl Hydrocarbon Receptor Antagonist. Metabolites 2018, 8, 86. [Google Scholar] [CrossRef] [PubMed]
- Gong, Q.-Y.; Cai, L.; Jing, Y.; Wang, W.; Yang, D.-X.; Chen, S.-W.; Tian, H.-L. Urolithin A alleviates blood-brain barrier disruption and attenuates neuronal apoptosis following traumatic brain injury in mice. Neural Regen. Res. 2022, 17, 2007–2013. [Google Scholar] [CrossRef]
- Shukur, K.T.; Ercetin, T.; Luise, C.; Sippl, W.; Sirkecioglu, O.; Ulgen, M.; Coskun, G.P.; Yarim, M.; Gazi, M.; Gulcan, H.O. Design, synthesis, and biological evaluation of new urolithin amides as multitarget agents against Alzheimer’s disease. Arch. Pharm. 2021, 354, e2000467. [Google Scholar] [CrossRef] [PubMed]
- Noshadi, B.; Ercetin, T.; Luise, C.; Yuksel, M.Y.; Sippl, W.; Sahin, M.F.; Gazi, M.; Gulcan, H.O. Synthesis, Characterization, Molecular Docking, and Biological Activities of Some Natural and Synthetic Urolithin Analogs. Chem. Biodivers. 2020, 17, e2000197. [Google Scholar] [CrossRef]
- Seeram, N.P.; Aronson, W.J.; Zhang, Y.; Henning, S.M.; Moro, A.; Lee, R.-P.; Sartippour, N.; Harris, D.M.; Rettig, M.; Suchard, M.A.; et al. Pomegranate ellagitannin-derived metabolites inhibit prostate cancer growth and localize to the mouse prostate gland. J. Agric. Food Chem. 2007, 55, 7732–7737. [Google Scholar] [CrossRef]
- Yao, X.; Kailin, L.; Haiyan, Z.; Yunlong, L.; Lin, H.; Hang, L.; Min, W. The profile of buckwheat tannins based on widely targeted metabolome analysis and pharmacokinetic study of ellagitannin metabolite urolithin A. LWT Food Sci. Technol. 2022, 156, 113069. [Google Scholar] [CrossRef]
- Kujawska, M.; Jourdes, M.; Witucki, Ł.; Karaźniewicz-Łada, M.; Szulc, M.; Górska, A.; Mikołajczak, P.; Teissedre, P.L.; Jodynis-Liebert, J. Pomegranate Juice Ameliorates Dopamine Release and Behavioral Deficits in a Rat Model of Parkinson’s Disease. Brain Sci. 2021, 11, 1127. [Google Scholar] [CrossRef]
- Aguilera-Carbo, A.; Augur, C.; Prado-Barragan, L.A.; Favela-Torres, E.; Aguilar, C.N. Microbial production of ellagic acid and biodegradation of ellagitannins. Appl. Microbiol. Biotechnol. 2008, 78, 189–199. [Google Scholar] [CrossRef]
| Sample | Superoxide Radical 1 | DPPH Radical | Peroxyl Radicals 2 | ABTS Radical | Hydroxyl Radical |
|---|---|---|---|---|---|
| UroA | 5.01 ± 5.01 μM | 152.66 ± 35.01 μM | 13.1 μM | --- | --- |
| Gallic acid | 0.26 ± 0.21 μM | 3.10 ± 3.11 μM | --- | --- | --- |
| Ascorbic acid | --- | 14.81 ± 14.90 μM | --- | --- | --- |
| Pomegranate extracts | --- | --- | 0.49 μM | --- | --- |
| UroB | 495.32 ± 3.28 mM | 295.41 ± 2.36 mM | --- | 316.18 ± 1.85 mM | 306.28 ± 4.61 mM |
| Ascorbic acid | 874.39 ± 1.48 mM | 446.25 ± 1.78 mM | --- | 526.24 ± 3.18 mM | 540.16 ± 2.52 mM |
| Animals | Route of Administration | Treatment | Brain Tissue | Identified Metabolites in Brain | Plasma Concentratio | Refs |
|---|---|---|---|---|---|---|
| male C57BL/6 mice (7 months, 25–30 g) | i.g. | UroA 0.3 mg/mouse, single administration | brain tissues | mUroA: 8 ng/g | --- | [147] |
| male C57BL/6 mice (6 weeks) | i.g. | UroA 200 mg/kg b.w., single administration | cortex | UroA: 28 ng/g | 15 ng/mL | [148] |
| hippocampus | UroA: 35 ng/g | |||||
| male rats (12 weeks, 288 ± 20 g) | i.v. | Polyphenol metabolites (12.5 μg UroA + 5.3 μgUroB) 2.7 µmol/rat/day for 2 days | brain tissues | UroA: 2.2 ng/g | --- | [41] |
| UroB: 0.5 ng/g | ||||||
| male albino Wistar rats (6 weeks, 250–300 g) | i.g. | Pomegranate juice 500 mg/kg b.w./day for 10 days | brain tissues | UroA: 1.68 ± 0.25 ng/g | 18.75 ± 3.21 ng/mL | [36] |
| male albino Wistar rats (6 weeks, 250–300 g) | i.g. | Pomegranate juice 500 mg/kg b.w./day for 45 days | brain tissues | UroA: 2.068 ± 0.274 ng/g | --- | [149] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
An, L.; Lu, Q.; Wang, K.; Wang, Y. Urolithins: A Prospective Alternative against Brain Aging. Nutrients 2023, 15, 3884. https://doi.org/10.3390/nu15183884
An L, Lu Q, Wang K, Wang Y. Urolithins: A Prospective Alternative against Brain Aging. Nutrients. 2023; 15(18):3884. https://doi.org/10.3390/nu15183884
Chicago/Turabian StyleAn, Lei, Qiu Lu, Ke Wang, and Yousheng Wang. 2023. "Urolithins: A Prospective Alternative against Brain Aging" Nutrients 15, no. 18: 3884. https://doi.org/10.3390/nu15183884
APA StyleAn, L., Lu, Q., Wang, K., & Wang, Y. (2023). Urolithins: A Prospective Alternative against Brain Aging. Nutrients, 15(18), 3884. https://doi.org/10.3390/nu15183884





