Abstract
Sleep is a crucial component of health, and insomnia is among the most common and vexing of life-habit-related disorders. While dietary sleep-support supplements can improve sleep, choosing an effective dietary supplement can be challenging for users due to the wide variety of options available and the varying effects experienced by different individuals. In this study, to identify new criteria for estimating the effects of dietary supplements, we examined the relationships among the dietary supplements, the pre-conditions (PCs; defined as the life habits and sleep conditions before supplementation), and the sleep problems of subjects before supplementation. An open, randomized, cross-over intervention trial enrolling 160 subjects was conducted to test the efficacy of each dietary supplement (Analysis 1) and the relationships among dietary supplements, the PCs, and sleep problems (Analysis 2). To this end, l-theanine (200 mg/day), γ-aminobutyric acid (GABA) (111.1 mg/day), Apocynum venetum leaf extract (AVLE) (50 mg/day), and l-serine (300 mg/day) were administered to subjects. Before the first intervention period, life habits and sleep conditions were surveyed to identify each subject’s PCs. For each combination of supplements and sleep problems, PCs were compared between subjects whose sleep problems were improved and subjects whose sleep problems were not improved via supplementation. All the tested supplements were found to ameliorate sleep problems significantly (Analysis 1). In Analysis 2, the PCs specific to improved subjects were found to differ depending on the dietary supplements and sleep problems. In addition, subjects who consumed dairy products often showed improvement in their sleep problems with all the tested supplements. This study suggests the possibility of personalizing sleep-support supplementation based on personal life habits, sleep conditions, and sleep problems, in addition to the known efficacy of dietary supplements.
1. Introduction
Insomnia is one of the most prevalent and vexing problems in modern society, with a worldwide prevalence of 20%, and it is known to cause lifestyle-related diseases such as hypertension, type 2 diabetes mellitus, and depression [1,2]. Insomnia is categorized according to the sleep problems that cause it, such as difficulty in falling asleep and interrupted sleep. Many solutions have been proposed to ease the various sleep problems, including pharmacotherapy, cognitive behavior therapy, etc. [2]. Pharmacotherapy is the most prevalent solution at present because of its good clinical effectiveness, but it often causes adverse effects such as dependency, memory loss, and behavioral problems [3].
In parallel with classical pharmacotherapy, many dietary sleep-support supplements have been introduced and proven to be effective to decrease sleep problems. For example, it has been reported that l-theanine is effective for refreshing postmenopausal women in the morning [4], γ-aminobutyric acid (GABA) shortens sleep latency, the Apocynum venetum leaf extract (AVLE) shortens sleep latency and induces deep sleep [5], and l-serine decreases the frequency of awakenings [6]. Some of these supplements are superior to pharmacotherapy in their side-effect profiles and accessibility. However, it can be difficult for users to select an effective dietary supplement due to the wide range of choices and the differential effects among users. Therefore, to select an effective dietary supplement for each user, practical criteria for estimating the effect of dietary supplements on each individual are necessary. Hence, this study focused on the life habits and sleep conditions of individual subjects before supplementation (pre-conditions: PCs); for example, the frequency of vegetable consumption per week, the frequency of the occurrence of interrupted sleep the day before supplementation, etc. Some previous reports also showed that specific dietary supplements were effective for subjects with specific PCs [6]. However, only limited PCs and dietary supplements were assessed, and there has been no comprehensive report researching the relationships among dietary supplements, sleep problems, and PCs. Therefore, this study aimed to clarify the wide-ranging relationships among dietary supplements, sleep problems, and PCs. To examine these, the PCs of the improved group were compared with those of the non-improved group for all 25 combinations of 5 sleep problems and 5 supplements (l-theanine, GABA, AVLE, l-serine, and placebo).
2. Materials and Methods
2.1. Clinical Study
Subjects and ethics. This study was performed according to the Declaration of Helsinki. Before engaging in this study, healthy full-time workers who did not smoke, have a habit of drinking, take antihistamines, or plan to stay outside their home during the pre-observation period and the intervention periods were assessed for eligibility with the Beck Depression Inventory (BDI) and the Pittsburgh Sleep Quality Index (PSQI) [7,8]. In order to select non-depressive subjects with sleep problems, the inclusion criteria were set to under 16 points for the BDI score and more than 5 points for the PSQI score. This study included 160 subjects (Figure 1A). All subjects were divided into 6 groups so that each group’s PSQI and BDI scores were almost the same. The study protocol was approved by the Shiba Palace Clinic Ethics Review Committee (145624-28996). All subjects were informed of the protocol and provided written informed consent prior to the investigation.
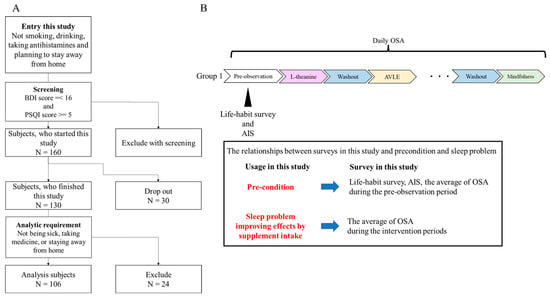
Figure 1.
Study design and flowchart of subject screening: (A) flowchart of subject screening; (B) study design and administration order. BDI is the Beck Depression Inventory, and PSQI is the Pittsburgh Sleep Quality Index.
Study design. An open, randomized, cross-over intervention trial was performed (12 May 2021–26 August 2021). The trial consisted of a pre-observation period of 9 days, followed by six intervention periods of 7 days each and five washout periods of at least 7 days (Figure 1B). The length of the intervention period was decided based on prior research [4,5,6]. In addition, they were limited to 7 days to minimize changes in sleep conditions depending on the day of the week. Intervention and washout periods were set to exclude the 2020 Tokyo Olympics (held in 2021) and Obon holidays (an annual Buddhist event, 6 August 2021–19 August 2021), which may have affected subjects’ sleep. During each of the six intervention periods, each subject took one of the four dietary supplements (l-theanine, GABA, AVLE, or l-serine) or a placebo, or they performed mindfulness. During the pre-observation period, the life-habit survey and the Athens Insomnia Scale (AIS) were administered [9]. During this trial, the Oguri–Shirakawa–Azumi (OSA) sleep questionnaire was administered every morning [10]. The OSA consists of a total score of five factors corresponding to five sleep problems: OSA Factor 1 (sleepiness on rising), OSA Factor 2 (initiation and maintenance of sleep), OSA Factor 3 (frequent dreaming), OSA Factor 4 (refreshing), and OSA Factor 5 (sleep length). We adopted the five OSA factor scores as indices of the corresponding sleep problems. The results of the life-habit survey and AIS and the average of the five OSA factor scores during the pre-observation period were considered as PCs. Subjects answered all assessments on the web.
Intervention. During each intervention period of 7 days, each subject was instructed to take either a dietary supplement or placebo or to perform mindfulness. The dietary supplements studied were l-theanine, GABA, AVLE, and l-serine, since these supplements are sold in Japan, reported to have different mechanisms of action and to ameliorate different sleep problems. The dose of each supplement and placebo was determined on the basis of previous reports [4,5,6]: 200 mg/day for l-theanine, 111.1 mg of barley lactic acid fermentation extract/day for GABA, 50 mg/day for AVLE, 300 mg/day for l-serine, and 3300 mg of maltodextrin/day as placebo. All supplements were bulked up to 3300 mg/day with maltodextrin so that the participants did not recognize the ingredient (for the blind intake). These supplements and placebo powders were taken with water within 30–60 min of bedtime. Mindfulness was performed by following a set of mindfulness instructions within 10 min of bedtime [11].
2.2. Data and Statistical Analysis
Analysis subjects and design. During the study, 30 subjects dropped out. During the intervention periods, 24 subjects were excluded from the data analysis because they experienced one or more of the following potentially confounding conditions for more than 4 days: illness, taking medicine, or staying away from home. A final total of 106 subjects were thus analyzed (Figure 1A and Table 1). We performed two analyses in this study. First, the ability of the individual supplements and mindfulness to improve sleep problems was analyzed (Analysis 1). Second, the relationships between and within supplements, sleep problems, and PCs were examined (Analysis 2).

Table 1.
Demographic information.
Analysis 1. To confirm the sleep-improving effects of each supplement and mindfulness, the OSA total score and scores for each of the 5 factors during the intervention periods were compared with those of the pre-observation period. It has been well established that sleep problems are often improved by placebo intake [12,13]. Our aim in this study was to extract data regarding PCs and sleep problems to predict the efficacy of dietary sleep-support supplements. Therefore, we compared the improvements in sleep problems before and after the use of the individual supplements, placebo, or mindfulness, and the sleep-improving efficacies were compared among these interventions, rather than comparing the efficacies of supplements to those of the placebo.
Placebo and mindfulness sessions were included as positive controls, in order to examine whether there are specific PCs that might be successfully treated using these interventions.
Analysis 2. The relationships between and within supplements, sleep problems, and PCs were examined (Figure 2). For testing 25 combinations (5 OSA factors × 5 supplements), 106 subjects were classified into either the improved group or the non-improved group based on the changes in their OSA score between the intervention and pre-observation period. Subjects whose scores for the 5 OSA factors during the intervention period were higher than those of the pre-observation period were considered to belong to the improved group, and the remaining participants were considered to belong to the non-improved group. For each of the 25 combinations, 31 PCs of the improved group were compared with those of the non-improved group (Table 2). In Analysis 2, placebo and mindfulness interventions were analyzed, but the results of mindfulness are not discussed, since we were unable to control the methods of mindfulness for all participants, and the effects may have varied depending on the methods used.
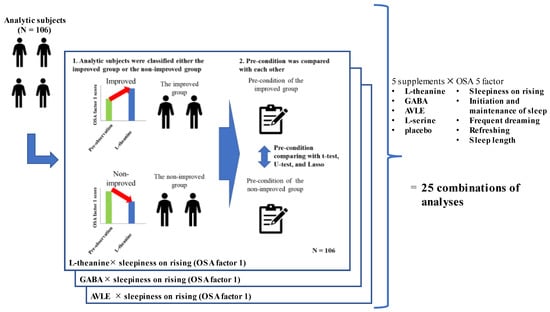
Figure 2.
Analysis design.

Table 2.
List of PCs.
Analyses. All data were calculated as means ± SEMs. In Analysis 1, a paired t-test was performed to examine the sleep-improving effects of dietary supplements and mindfulness. In Analysis 2, a univariate analysis (Mann–Whitney U test or Student’s t-test) and multivariate analysis (Lasso-regularized general linear model: Lasso) were performed to examine the relationships between and within supplements, sleep problems, and PCs. PCs with a p value < 0.05 in the univariate analysis and for which the estimated coefficient in Lasso was not zero were considered to be “significant PCs”. All analyses were performed with Python 3.9.5, scikit-learn 1.0, SciPy 1.8.1, and statsmodels 0.13.2.
3. Results
Analysis 1: Sleep-improving effects of supplements and mindfulness: Table 3 shows the overall sleep-improving effects of each supplement and mindfulness. Compared with the pre-observation period scores, all the tested supplements, mindfulness, and the placebo significantly improved the OSA total score and scores for OSA Factors 1 and 4. In addition, l-theanine, GABA, l-serine, mindfulness, and the placebo significantly improved the OSA Factor 2 score, and l-theanine significantly improved the OSA Factor 5 score.

Table 3.
Sleep-improving effects of supplements and mindfulness.
Analysis 2: The relationships between and within supplements, sleep problems, and PCs: PCs of the improved group were compared with those of the non-improved group for each of the 25 combinations of 5 OSA factors and 5 supplements. Table 4 shows the significant PCs of the improved group for each of the 25 combinations (5 OSA factors × 5 supplements). A summary of the significant PCs for each of the five supplements and placebo is shown in Table 5.

Table 4.
The significant PCs of the improved group.

Table 5.
Summary of significant PCs of supplements.
OSA Factor 1 (sleepiness on rising): In the l-theanine group, the frequency of dairy product intake, functioning capacity during the day, and OSA Factors 1 and 5 during the pre-observation period were significant PCs. In the GABA group, self-reported bad posture was a significant PC. In the AVLE group, OSA Factor 1 during the pre-observation period was a significant PC. There was no significant PC in the l-serine or placebo group (Table 4 and Supplemental Table S1).
In regard to OSA Factor 1, l-theanine was effective for the subjects who consumed dairy products frequently and had poor sleep quality (low functioning capacity during the day, sleepiness on rising, and short sleep length). For subjects who had bad posture, GABA was effective. For subjects with sleepiness upon rising, AVLE was effective (Table 5).
OSA Factor 2 (initiation and maintenance of sleep): In the l-theanine group, OSA Factor 2 during the pre-observation period was a significant PC. In the GABA group, OSA Factors 2 and 3 during the pre-observation period were significant PCs. In the AVLE group, the frequency of dairy product intake, and OSA Factors 2 and 3 during the pre-observation period were significant PCs. In the l-serine group, the frequency of bathing and dairy product intake and OSA Factor 2 during the pre-observation period were significant PCs. In the placebo group, the frequency of missed breakfast and OSA Factor 2 during the pre-observation period were significant PCs (Table 4 and Supplemental Table S2).
In regard to OSA Factor 2, l-theanine was effective for the subjects who had a hard time going to sleep and staying asleep. For the subjects who had a hard time going to sleep and staying asleep, and dreamed frequently, GABA was effective. For the subjects who consumed dairy products frequently, had a hard time going to sleep and staying asleep, and dreamed frequently, AVLE was effective. For the subjects who bathed frequently, consumed dairy products frequently, and had a hard time going to sleep and staying asleep, l-serine was effective (Table 5).
OSA Factor 3 (frequent dreaming): In the l-theanine group, the frequency of exercising during daylight and weekly physical activities, sleep quality, and OSA Factors 1 and 3 during the pre-observation period were significant PCs. In the GABA group, the frequency of dairy product intake and eating vegetables, and OSA Factor 3 during the pre-observation period were significant PCs. In the AVLE and l-serine groups, OSA Factor 3 during the pre-observation period was a significant PC. In the placebo group, the frequency of taking a bath within 90 min of bedtime and OSA Factor 3 during the pre-observation period were significant PCs (Table 4 and Supplemental Table S3).
With respect to OSA Factor 3, l-theanine was effective for the subjects who exercised frequently and had poor overall sleep quality, especially for those with sleepiness upon rising and who dreamed frequently. For the subjects who consumed dairy products frequently, ate vegetables frequently, and dreamed frequently, GABA was effective. For the subjects who dreamed frequently, AVLE and l-serine were effective (Table 5).
OSA Factor 4 (refreshing): In the l-theanine group, the frequency of smartphone or computer usage, bathing, and dairy product intake, and OSA Factors 2, 3, and 4 during the pre-observation period were significant PCs. In the GABA group, the frequency of smartphone usage before bedtime, early awakening, and OSA Factor 4 during the pre-observation period were significant PCs. In the AVLE, l-serine, and placebo groups, OSA Factor 4 during the pre-observation period was a significant PC (Table 4 and Supplemental Table S4).
In regard to OSA Factor 4, l-theanine was effective for the subjects who had good life habits (used a smartphone or computer infrequently, bathed frequently, and consumed dairy products frequently) and had poor sleep quality (had a hard time going to sleep and staying asleep, dreamed frequently, and were not refreshed). For the subjects who did not use a smartphone at night frequently, did not wake up early frequently, and were not refreshed, GABA was effective. For the subjects who were not refreshed, AVLE and l-serine were effective (Table 5).
OSA Factor 5 (sleep length): In the l-theanine group, the frequency of smartphone or computer usage and missing breakfast, and OSA Factor 5 during the pre-observation period were significant PCs. In the GABA group, the frequency of smartphone usage before bedtime and OSA Factor 5 during the pre-observation period were significant PCs. In the AVLE group, the frequency of having meals at irregular times, awakenings during the night, sleep quality, and OSA Factor 5 during the pre-observation period were significant PCs. In the l-serine and placebo groups, OSA Factor 5 during the pre-observation period was a significant PC (Table 4 and Supplemental Table S5).
In terms of OSA Factor 5, l-theanine was effective for the subjects who did not use a smartphone or computer frequently, did not eat breakfast every day, and slept for a short period of time. For the subjects who did not use a smartphone at night frequently and slept for a short period of time, GABA was effective. For the subjects who had meals at irregular times, had better overall sleep quality, and slept for a short period of time, AVLE was effective. For the subjects who slept for a short period of time, l-serine was effective (Table 5).
4. Discussion
In this study, Analyses 1 and 2 were performed to examine the relationships between and within dietary supplements, sleep problems, and PCs. In Analysis 1, compared with OSA scores in the pre-observation period, l-theanine, GABA, AVLE, l-serine, mindfulness, and the placebo all significantly improved the OSA total score and the scores of OSA Factors 1, 2, 4, and 5. In Analysis 2, the PCs of the improved group were compared with those of the non-improved group for each of the 25 combinations of the 5 OSA factors and 5 supplements (l-theanine, GABA, AVLE, l-serine, and placebo). As a result, for each combination, the significant PCs were different.
Dietary supplements may be superior to pharmacotherapy in their safety profiles and convenience to use, and many studies have been focused on assessing improvement in sleep problems through supplement intake compared with placebo intake [4,5,6]. However, these studies did not adequately account for the potential sleep-improving effects of placebo, or for the effects that the unique circumstances and conditions of subjects had on supplement’s efficacy. In particular, to the best of our knowledge, there has been only one study focused on individual differences in the effects of dietary supplements [6]. To overcome these limitations, we herein examined the improvement in sleep problems by comparing the OSA scores before and after various interventions, and the relationships between and within dietary supplements, sleep problems, and PCs.
Our results showed that each of the dietary supplements, the placebo, and mindfulness all significantly improved the OSA total score and the scores of OSA Factors 1, 2, 4, and 5. It was also notable that all the tested supplements had sleep-improving efficacies better or similar to those of the placebo and mindfulness. Most importantly, we found that PCs were more helpful for predicting the efficacy of dietary supplements than for predicting the efficacy of the placebo and mindfulness and that the dietary supplement with the greatest efficacy was dependent on a user’s PCs and sleep problems.
These results suggest the possibility of personalizing supplementation based on a user’s PCs, rather than merely selecting a dietary supplement based on the sleep-improving effect of supplement intake. Therefore, this study confirms the efficacy of dietary sleep-support supplements and further extends prior research by clarifying the significant PCs that impact personalized supplementation. As a result, the possibility of implementing personalized supplementation based on significant PCs was suggested. For example, a high frequency of eating vegetables was a PC that was only observed in the subjects whose OSA Factor 3 (frequent dreaming) was improved via GABA intake (see Table 5 and the OSA Factor 3 results in Analysis 2). This result suggests that GABA would be more effective for people who eat vegetables frequently. However, the mechanism underlying the relationship between the sleep-improving effects of GABA, the habit of eating vegetables, and the sleep problem of dreaming frequently is uncertain. Previous reports show that dietary habits can modify gene expressions and the microbiome through the consumption of certain functional ingredients. For example, it has been reported that the ethyl acetate extract from tomato juice inhibits CYP3A4 activity [14], and polyphenols, vitamins, minerals, and dietary fiber modulate the health-beneficial gut microbes [15,16]. The modification in genes and the microbiome may affect the ADME of each supplement, and therefore the sleep-improving effects of each supplement may not be uniform. In the case of GABA intake, some functional ingredients contained in some vegetables may affect the GABA-specific ADME and enhance the sleep-improving effect of GABA. As another example, we found that each of the supplements studied improved the sleep problems of individuals who consumed dairy products (Table 6). Previous studies have reported that sleep problems were improved by consuming certain functional ingredients in dairy products [17,18,19,20]. For example, α-lactalbumin reduces sleepiness by increasing plasma tryptophan [19]. In our study, in the case of placebo intake, there was no difference in the consumption frequency of dairy products between the improved group and the non-improved group. This may suggest that the intake of dairy products specifically enhances the sleep-improving effects of dietary supplements.

Table 6.
Effect of dairy products.
5. Prospects
This study suggests the possibility of life-habit recommendations to enhance the efficacy of dietary supplements based on supplements and sleep problems. For example, if l-serine is taken to improve going to and maintaining sleep (OSA Factor 2), bathing frequently can be recommended (as described in the OSA Factor 2 results for Analysis 2 and shown in Table 5). However, this study was an observational study for examining the amelioration of sleep problems via the combination of dietary supplements and life-habit interventions. Additional interventional studies will be needed to examine the degree to which life-habit recommendations can enhance the sleep-improving effects of dietary supplements.
6. Limitations
In this study, the relationships between and within the efficacy of dietary supplements, sleep problems, and PCs were explored. We succeeded in identifying the PCs and sleep problems that influence the efficacy of dietary sleep-support supplements. Individual patients should benefit from the use of our criteria to select dietary sleep-support supplements. However, little information is currently available in regard to how individual biological and genetic characteristics affect a supplement’s efficacy. It is therefore possible that more significant PCs will be identified in the future, and these will also be incorporated to personalize supplementation more precisely based on personal life habits, sleep conditions, sleep problems, and biological markers to support the sleep of specific individuals. In parallel, the mechanisms involved in the relationships between and within PCs, sleep problems, and the efficacy of dietary sleep-support supplements should be studied. However, it is not practical to reveal all mechanisms; therefore, to reveal the interesting interactions and mechanisms, in vitro and in vivo studies should be considered. In particular, enhancing supplement efficacy by taking dairy products is a novel approach with broad effects, so it is important to examine its mechanisms to evaluate the benefit and risks of this method.
7. Conclusions
In this research, the relationships between and within the PCs and estimations of improvement in sleep problems through dietary sleep-support supplements were revealed. The results suggest the possibility of implementing personalized sleep-improving supplementation based on personal life habits, sleep conditions, and sleep problems.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu15102377/s1. Table S1: Statistical analysis result of OSA Factor 1 (sleepiness on rising), Table S2: Statistical analysis result of OSA Factor 2 (initiation and maintenance of sleep), Table S3: Statistical analysis result of OSA Factor 3 (frequent dreaming), Table S4: Statistical analysis result of OSA Factor 4 (refreshing), Table S5: Statistical analysis result of OSA Factor 5 (sleep length).
Author Contributions
F.I.: data curation, formal analysis, investigation, visualization, writing—original draft preparation, and writing—review and editing, K.Y.: conceptualization, investigation, resources, and writing—review and editing, E.T.: conceptualization, investigation, resources, and writing—review and editing, R.A.: formal analysis and writing—review and editing, S.N.: conceptualization, writing—review and editing, and supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of the Shiba Palace Clinic (protocol code 145624-28996 in 18 March 2021).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data sharing is not applicable to this article.
Acknowledgments
Takeshita provided a critical review and helped edit the manuscript. Kawabata, Furuyashiki, and Ueda provided a critical review.
Conflicts of Interest
There are no conflict to declare.
References
- Doi, Y. Prevalence and health impacts of sleep disorders in Japan. J. Natl. Inst. Public Health 2012, 61, 3–10. [Google Scholar]
- Buysse, D.J. Insomnia. JAMA 2013, 309, 706–716. [Google Scholar] [CrossRef] [PubMed]
- Takano, A.; Ono, S.; Yamana, H.; Matsui, H.; Matsumoto, T.; Yasunaga, H.; Kawakami, N. Factors associated with long-term prescription of benzodiazepine: A retrospective cohort study using a health insurance database in Japan. BMJ Open 2019, 9, e029641. [Google Scholar] [CrossRef] [PubMed]
- Ozeki, M.; Juneja, L.R.; Shirakawa, S. The effects of L-theanine on automatic nervous system during sleep in postmenopausal women. Jpn. Soc. Physiol. Anthropol. 2008, 13, 17–24. [Google Scholar]
- Yamatsu, A.; Yamashita, Y.; Maru, I.; Yang, J.; Tatsuzaki, J.; Kim, M. The improvement of sleep by oral intake of GABA and Apocynum venetum leaf extract. J. Nutr. Sci. Vitaminol. 2015, 61, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Takahashi, S.; Shen, M.; Yamaguchi, K.; Satoh, M. Effects of L-serine ingestion on human sleep. SpringerPlus 2014, 3, 456. [Google Scholar] [CrossRef] [PubMed]
- Beck, A.T.; Ward, C.H.; Mendelson, M.; Mock, J.; Erbaugh, J. An inventory for measuring depression. Arch. Gen. Psychiatry 1961, 4, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Buysse, D.J.; Reynolds, C.F., 3rd; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef] [PubMed]
- Soldatos, C.R.; Dikeos, D.G.; Paparrigopoulos, T.J. Athens Insomnia Scale: Validation of an instrument based on ICD-10 criteria. J. Psychosom. Res. 2000, 48, 555–560. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Tanaka, H.; Takase, M.; Shirakawa, S. Standardization of revised version of OSA sleep inventory for middle age and aged. Brain Sci. Ment. Disord. 1999, 10, 401–409. [Google Scholar]
- Kaori, Y. [Mindfulness with Sleep] Mindfulness to End the Day Peacefully|5 Minutes [Video]. 2018. Available online: https://www.youtube.com/watch?v=CsuJoA_3zKM (accessed on 7 February 2023).
- Fratello, F.; Curcio, G.; Ferrara, M.; Marzano, C.; Couyoumdjian, A.; Petrillo, G.; Bertini, M.; De Gennaro, L. Can an inert sleeping pill affect sleep? Effects on polysomnographic, behavioral and subjective measures. Psychopharmacology 2005, 181, 761–770. [Google Scholar] [CrossRef] [PubMed]
- Winkler, A.; Rief, W. Effect of Placebo Conditions on Polysomnographic Parameters in Primary Insomnia: A Meta-Analysis. Sleep 2015, 38, 925–931. [Google Scholar] [CrossRef] [PubMed]
- Sunaga, K.; Ohkawa, K.; Nakamura, K.; Ohkubo, A.; Harada, S.; Tsuda, T. Mechanism-based inhibition of recombinant human cytochrome P450 3A4 by tomato juice extract. Biol. Pharm. Bull. 2012, 35, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Liang, Q.; Balakrishnan, B.; Belobrajdic, D.P.; Feng, Q.J.; Zhang, W. Role of dietary nutrients in the modulation of gut microbiota: A narrative review. Nutrients 2020, 12, 381. [Google Scholar] [CrossRef] [PubMed]
- Simpson, H.L.; Campbell, B.J. Review article: Dietary fibre-microbiota interactions. Aliment. Pharmacol. Ther. 2015, 42, 158–179. [Google Scholar] [CrossRef] [PubMed]
- Komada, Y.; Okajima, I.; Kuwata, T. The effects of milk and dairy products on sleep: A systematic review. Int. J. Environ. Res. Public Health 2020, 17, 9440. [Google Scholar] [CrossRef]
- Brezinová, V.; Oswald, I. Sleep after a bedtime beverage. Br. Med. J. 1972, 2, 431–433. [Google Scholar] [CrossRef]
- Markus, C.R.; Jonkman, L.M.; Lammers, J.H.; Deutz, N.E.; Messer, M.H.; Rigtering, N. Evening intake of alpha-lactalbumin increases plasma tryptophan availability and improves morning alertness and brain measures of attention. Am. J. Clin. Nutr. 2005, 81, 1026–1033. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, T.; Maruyama, K.; Suyama, K.; Nishijima, M.; Akamatsu, K.; Jogamoto, A.; Katakami, K.; Saito, I. Consumption of OLL1073R-1 yogurt improves psychological quality of life in women healthcare workers: Secondary analysis of a randomized controlled trial. BMC Gastroenterol. 2021, 21, 237. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).