Intra-Amniotic Administration of Cashew Nut (Anacardium occidentale L.) Soluble Extract Improved Gut Functionality and Morphology In Vivo (Gallus gallus)
Abstract
1. Introduction
2. Materials and Methods
2.1. Proteins and Dietary Fiber in Cashew Nut Flour and Cashew Nut Soluble Extract (CNSE)
2.2. Extract Preparation
2.3. Animals and Study Design
2.4. Gene Expression Analysis
2.4.1. Extraction of Total RNA from Duodenum Tissue
2.4.2. Real-Time Polymerase Chain Reaction (RT-PCR)
2.4.3. Primer Design
2.4.4. Real-Time qPCR Design
2.5. Collection of Microbial Samples and Intestinal Contents DNA Extraction
2.6. Primers Design and PCR Amplification of Bacterial 16S rDNA Analysis
2.7. Morphological Examination of Duodenal Tissue
2.8. Statistical Analysis
3. Results
3.1. Concentration of Proteins, Total Dietary Fiber, and Fractions
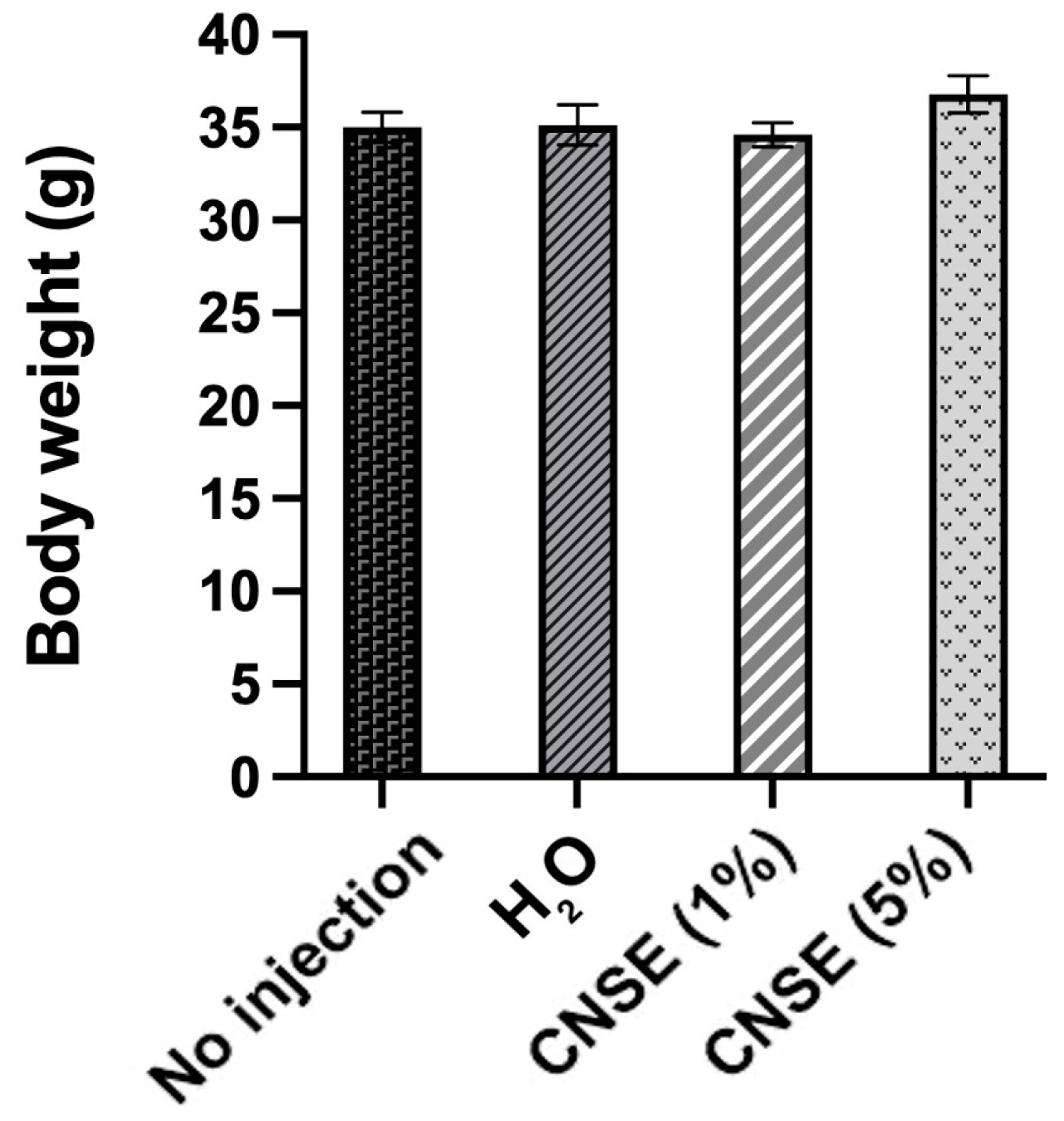
3.2. Body Weight
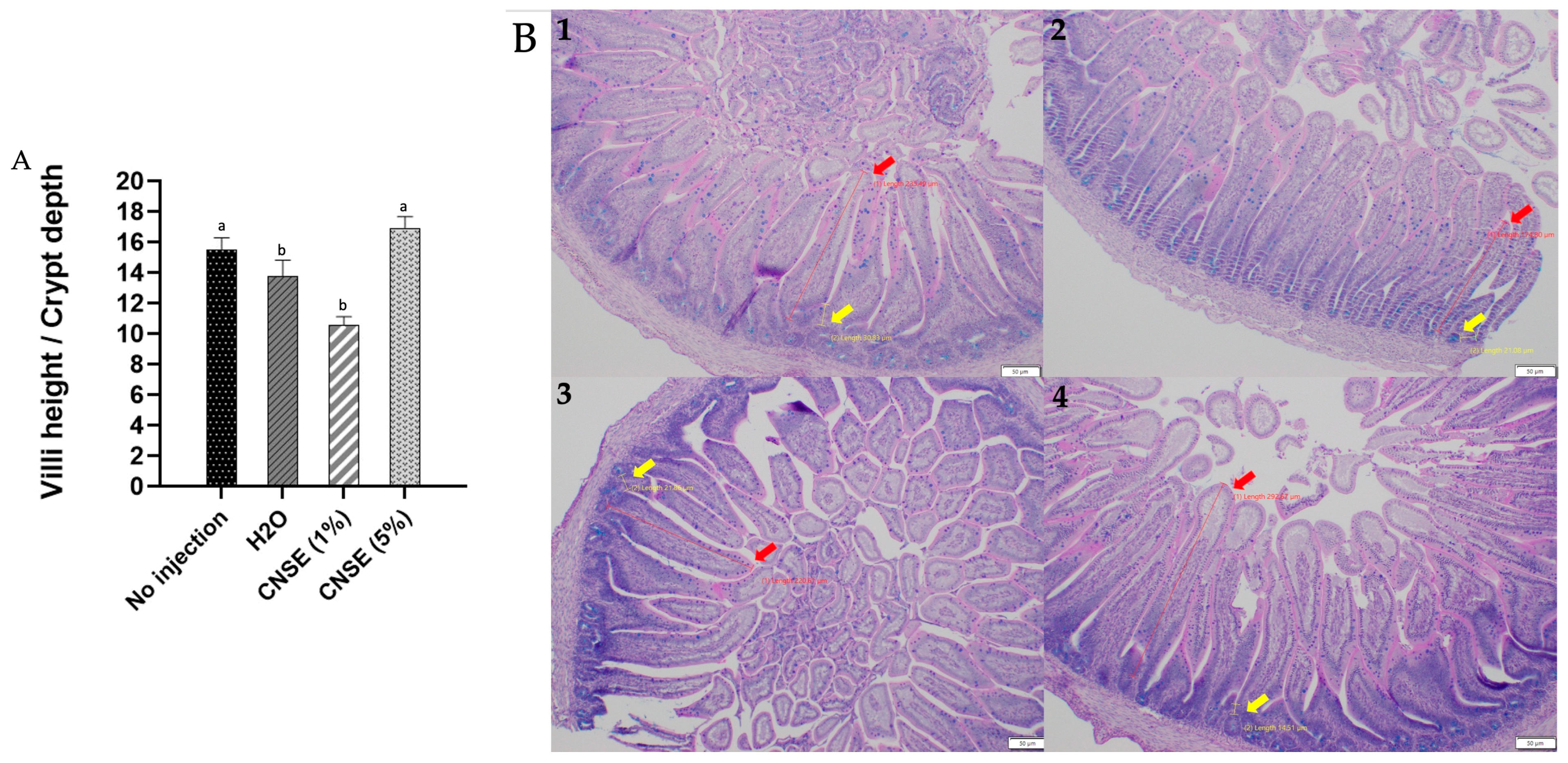
3.3. Effect of Cashew Nut Soluble Extract (CNSE) on Duodenal Morphological Parameters
3.4. Effect of Cashew Nut Soluble Extract on the Abundance of Intestinal Bacterial Populations
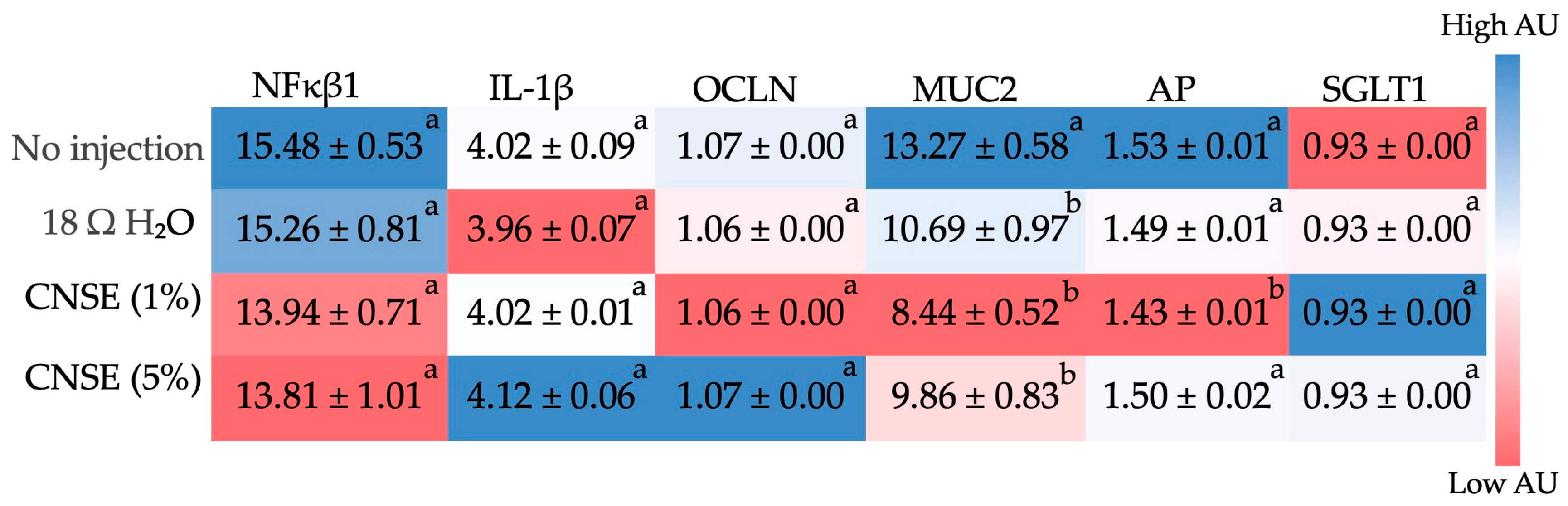
3.5. Effect of Cashew Nut Soluble Extract on Gene Expression of Intestinal Barrier Proteins and Inflammatory Biomarkers
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Neale, E.P.; Tapsell, L.C.; Guan, V.; Batterham, M.J. The Effect of Nut Consumption on Markers of Inflammation and Endothelial Function: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. BMJ Open 2017, 7, e016863. [Google Scholar] [CrossRef] [PubMed]
- Mayhew, A.J.; de Souza, R.J.; Meyre, D.; Anand, S.S.; Mente, A. A Systematic Review and Meta-Analysis of Nut Consumption and Incident Risk of CVD and All-Cause Mortality. Br. J. Nutr. 2016, 115, 212–225. [Google Scholar] [CrossRef] [PubMed]
- Tindall, A.M.; Johnston, E.A.; Kris-Etherton, P.M.; Petersen, K.S. The Effect of Nuts on Markers of Glycemic Control: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Am. J. Clin. Nutr. 2019, 109, 297–314. [Google Scholar] [CrossRef]
- Xia, J.Y.; Yu, J.H.; Xu, D.F.; Yang, C.; Xia, H.; Sun, G.J. The Effects of Peanuts and Tree Nuts on Lipid Profile in Type 2 Diabetic Patients: A Systematic Review and Meta-Analysis of Randomized, Controlled-Feeding Clinical Studies. Front. Nutr. 2021, 8, 765571. [Google Scholar] [CrossRef] [PubMed]
- Eslami, O.; Khorramrouz, F.; Sohouli, M.; Bagheri, N.; Shidfar, F.; Fernandez, M.L. Effect of Nuts on Components of Metabolic Syndrome in Healthy Adults with Overweight/Obesity: A Systematic Review and Meta-Analysis. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 2459–2469. [Google Scholar] [CrossRef] [PubMed]
- Asbaghi, O.; Moodi, V.; Hadi, A.; Eslampour, E.; Shirinbakhshmasoleh, M.; Ghaedi, E.; Miraghajani, M. The Effect of Almond Intake on Lipid Profile: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Food Funct. 2021, 12, 1882–1896. [Google Scholar] [CrossRef]
- Godos, J.; Giampieri, F.; Micek, A.; Battino, M.; Forbes-Hernández, T.Y.; Quiles, J.L.; Paladino, N.; Falzone, L.; Grosso, G. Effect of Brazil Nuts on Selenium Status, Blood Lipids, and Biomarkers of Oxidative Stress and Inflammation: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Antioxidants 2022, 11, 403. [Google Scholar] [CrossRef]
- Baer, D.J.; Gebauer, S.K.; Novotny, J.A. Walnuts Consumed by Healthy Adults Provide Less Available Energy than Predicted by the Atwater Factors. J. Nutr. 2016, 146, 9–13. [Google Scholar] [CrossRef]
- Ley, R.E.; Bäckhed, F.; Turnbaugh, P.; Lozupone, C.A.; Knight, R.D.; Gordon, J.I. Obesity Alters Gut Microbial Ecology. Proc. Natl. Acad. Sci. USA 2005, 102, 11070–11075. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Qin, J.; Li, Y.; Cai, Z.; Li, S.; Zhu, J.; Zhang, F.; Liang, S.; Zhang, W.; Guan, Y.; et al. A Metagenome-Wide Association Study of Gut Microbiota in Type 2 Diabetes. Nature 2012, 490, 55–60. [Google Scholar] [CrossRef]
- Wang, Z.; Klipfell, E.; Bennett, B.J.; Koeth, R.; Levison, B.S.; Dugar, B.; Feldstein, A.E.; Britt, E.B.; Fu, X.; Chung, Y.M.; et al. Gut Flora Metabolism of Phosphatidylcholine Promotes Cardiovascular Disease. Nature 2011, 472, 57–65. [Google Scholar] [CrossRef]
- Tindall, A.M.; Petersen, K.S.; Kris-Etherton, P.M. Dietary Patterns Affect the Gut Microbiome-the Link to Risk of Cardiometabolic Diseases. J. Nutr. 2018, 148, 1402–1407. [Google Scholar] [CrossRef] [PubMed]
- Creedon, A.C.; Hung, E.S.; Berry, S.E.; Whelan, K. Nuts and Their Effect on Gut Microbiota, Gut Function and Symptoms in Adults: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Nutrients 2020, 12, 2347. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, E.; Lambert, K.; Stanford, J.; Neale, E.P. The Effect of Nut Consumption (Tree Nuts and Peanuts) on the Gut Microbiota of Humans: A Systematic Review. Br. J. Nutr. 2021, 125, 508–520. [Google Scholar] [CrossRef] [PubMed]
- Ukhanova, M.; Wang, X.; Baer, D.J.; Novotny, J.A.; Fredborg, M.; Mai, V. Effects of Almond and Pistachio Consumption on Gut Microbiota Composition in a Randomised Cross-over Human Feeding Study. Br. J. Nutr. 2014, 111, 2146–2152. [Google Scholar] [CrossRef] [PubMed]
- Salas-Salvadó, J.; Bulló, M.; Pérez-Heras, A.; Ros, E. Dietary Fibre, Nuts and Cardiovascular Diseases. Br. J. Nutr. 2006, 96, S45–S51. [Google Scholar] [CrossRef]
- Soliman, G.A. Dietary Fiber, Atherosclerosis, and Cardiovascular Disease. Nutrients 2019, 11, 1155. [Google Scholar] [CrossRef] [PubMed]
- Lamuel-Raventos, R.M.; Onge, M.P.S. Prebiotic Nut Compounds and Human Microbiota. Crit. Rev. Food Sci. Nutr. 2017, 57, 3154–3163. [Google Scholar] [CrossRef]
- INC. Nuts & Dried Fruits Statistical Yearbook 2019/2020; INC: Reus, Spain, 2021. [Google Scholar]
- Mazzetto, S.E.; Lomonaco, D.; Mele, G. Cashew Nut Oil: Opportunities and Challenges in the Context of Sustainable Industrial Development. Quim. Nova 2009, 32, 732–741. [Google Scholar] [CrossRef]
- Rico, R.; Bulló, M.; Salas-Salvadó, J. Nutritional Composition of Raw Fresh Cashew (Anacardium occidentale L.) Kernels from Different Origin. Food Sci. Nutr. 2016, 4, 329–338. [Google Scholar] [CrossRef]
- Asogwa, E.U. Ndubuaku Integrated Production and Protection Practices of Cashew (Anacardium occidentale) in Nigeria. Afr. J. Biotechnol. 2008, 7, 4868–4873. [Google Scholar]
- INC. Cashew Technical Information; INC: Reus, Spain, 2015. [Google Scholar]
- Kornsteiner-Krenn, M.; Wagner, K.; Elmadfa, I. Phytosterol Content and Fatty Acid Pattern of Ten Different Nut Types. Int. J. Vitam. Nutr. Res 2013, 83, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Tan, Z. BioactiveCompounds from Cashew Nut and Its Coproducts. In Bioactive Compounds of Cashew Nuts; Alasalvar, C., Shahidi, F., Eds.; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Guo, T.; Song, D.; Cheng, L.; Zhang, X. Interactions of Tea Catechins with Intestinal Microbiota and Their Implication for Human Health. Food Sci. Biotechnol. 2019, 28, 1617–1625. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.L.U.; Sena-Evangelista, K.C.M.; de Azevedo, E.P.; Pinheiro, F.I.; Cobucci, R.N.; Pedrosa, L.F.C. Selenium in Human Health and Gut Microflora: Bioavailability of Selenocompounds and Relationship with Diseases. Front. Nutr. 2021, 8, 685317. [Google Scholar] [CrossRef]
- Hui, J.; Li, L.; Li, R.; Wu, M.; Yang, Y.; Wang, J.; Fan, Y.; Zheng, X. Effects of Supplementation with β-Carotene on the Growth Performance and Intestinal Mucosal Barriers in Layer-Type Cockerels. Anim. Sci. J. 2020, 91, e13344. [Google Scholar] [CrossRef] [PubMed]
- Honarbakhsh, M.; Malta, K.; Ericsson, A.; Holloway, C.; Kim, Y.K.; Hammerling, U.; Quadro, L. β-Carotene Improves Fecal Dysbiosis and Intestinal Dysfunctions in a Mouse Model of Vitamin A Deficiency. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2022, 1867, 159122. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, Y.; Ding, H.; Hu, S.; Wu, X.; Ma, A.; Ma, Y. Lutein Prevents Liver Injury and Intestinal Barrier Dysfunction in Rats Subjected to Chronic Alcohol Intake. Nutrients 2023, 15, 1229. [Google Scholar] [CrossRef]
- Pei, R.; Liu, X.; Bolling, B. Flavonoids and Gut Health. Curr. Opin. Biotechnol. 2020, 61, 153–159. [Google Scholar] [CrossRef]
- Sisconeto Bisinotto, M.; da Silva, D.C.; de Carvalho Fino, L.; Moreira Simabuco, F.; Neves Bezerra, R.M.; Costa Antunes, A.E.; Bertoldo Pacheco, M.T. Bioaccessibility of Cashew Nut Kernel Flour Compounds Released after Simulated in Vitro Human Gastrointestinal Digestion. Food Res. Int. 2021, 139, 109906. [Google Scholar] [CrossRef]
- Hadi, A.; Alizadeh, K.; Hajianfar, H.; Mohammadi, H.; Miraghajani, M. Efficacy of Synbiotic Supplementation in Obesity Treatment: A Systematic Review and Meta-Analysis of Clinical Trials. Crit. Rev. Food Sci. Nutr. 2020, 60, 584–596. [Google Scholar] [CrossRef]
- Li, H.; Li, X.; Yuan, S.; Jin, Y.; Lu, J. Nut Consumption and Risk of Metabolic Syndrome and Overweight/Obesity: A Meta-Analysis of Prospective Cohort Studies and Randomized Trials. Nutr. Metab. 2018, 15, 46. [Google Scholar] [CrossRef] [PubMed]
- Barczynska, R.; Bandurska, K.; Slizewska, K.; Litwin, M.; Szalecki, M.; Libudzisz, Z.; Kapusniak, J. Intestinal Microbiota, Obesity and Prebiotics. Pol. J. Microbiol. 2015, 64, 93100. [Google Scholar] [CrossRef]
- Ellis, P.R.; Kendall, C.W.; Ren, Y.; Parker, C.; Pacy, J.F.; Waldron, K.W.; Jenkins, D.J. Role of Cell Walls in the Bioaccessibility of Lipids in Almond Seeds. Am. J. Clin. Nutr. 2004, 80, 604–613. [Google Scholar] [CrossRef]
- Traoret, C.J.; Lokko, P.; Cruz, A.C.R.F.; Oliveira, C.G.; Costa, N.M.B.; Bressan, J.; Alfenas, R.C.G.; Mattes, R.D. Peanut Digestion and Energy Balance. Int. J. Obes. 2008, 32, 322–328. [Google Scholar] [CrossRef]
- Davis, L.; Stonehouse, W.; Loots, D.T.; Mukuddem-Petersen, J.; van der Westhuizen, F.H.; Hanekom, S.M.; Jerling, J.C. The Effects of High Walnut and Cashew Nut Diets on the Antioxidant Status of Subjects with Metabolic Syndrome. Eur. J. Nutr. 2007, 46, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Damavandi, R.D.; Mousavi, S.N.; Shidfar, F.; Mohammadi, V.; Rajab, A.; Hosseini, S.; Heshmati, J. Effects of Daily Consumption of Cashews on Oxidative Stress and Atherogenic Indices in Patients with Type 2 Diabetes: A Randomized, Controlled-Feeding Trial. Int. J. Endocrinol. Metab. 2019, 17, e70744. [Google Scholar] [CrossRef]
- Jalali, M.; Karamizadeh, M.; Ferns, G.A.; Zare, M.; Moosavian, S.P.; Akbarzadeh, M. The Effects of Cashew Nut Intake on Lipid Profile and Blood Pressure: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Complement. Ther. Med. 2020, 50, 102387. [Google Scholar] [CrossRef]
- Mohan, V.; Gayathri, R.; Jaacks, L.M.; Lakshmipriya, N.; Anjana, R.M.; Spiegelman, D.; Jeevan, R.G.; Balasubramaniam, K.K.; Shobana, S.; Jayanthan, M.; et al. Cashew Nut Consumption Increases HDL Cholesterol and Reduces Systolic Blood Pressure in Asian Indians with Type 2 Diabetes: A 12-Week Randomized Controlled Trial. J. Nutr. 2018, 148, 63–69. [Google Scholar] [CrossRef]
- Morvaridzadeh, M.; Sepidarkish, M.; Farsi, F.; Akbari, A.; Mostafai, R.; Omidi, A.; Potter, E.; Heshmati, J. Effect of Cashew Nut on Lipid Profile: A Systematic Review and Meta-Analysis. Complement. Med. Res. 2020, 27, 348–356. [Google Scholar] [CrossRef]
- Mukuddem-Petersen, J.; Stonehouse Oosthuizen, W.; Jerling, J.C.; Hanekom, S.M.; White, Z. Effects of a High Walnut and High Cashew Nut Diet on Selected Markers of the Metabolic Syndrome: A Controlled Feeding Trial. Br. J. Nutr. 2007, 97, 1144–1153. [Google Scholar] [CrossRef]
- Cordaro, M.; Fusco, R.; D’amico, R.; Siracusa, R.; Peritore, A.F.; Gugliandolo, E.; Genovese, T.; Crupi, R.; Mandalari, G.; Cuzzocrea, S.; et al. Cashew (Anacardium occidentale L.) Nuts Modulate the Nrf2 and Nlrp3 Pathways in Pancreas and Lung after Induction of Acute Pancreatitis by Cerulein. Antioxidants 2020, 9, 992. [Google Scholar] [CrossRef] [PubMed]
- Fusco, R.; Cordaro, M.; Siracusa, R.; Peritore, A.F.; Gugliandolo, E.; Genovese, T.; D’Amico, R.; Crupi, R.; Smeriglio, A.; Mandalari, G.; et al. Consumption of Anacardium occidentale L. (Cashew Nuts) Inhibits Oxidative Stress through Modulation of the Nrf2/HO−1 and NF-KB Pathways. Molecules 2020, 25, 4426. [Google Scholar] [CrossRef] [PubMed]
- Dias, C.C.Q.; Madruga, M.S.; Pintado, M.M.E.; Almeida, G.H.O.; Alves, A.P.V.; Dantas, F.A.; Bezerra, J.K.G.; de Melo, M.F.F.T.; Viera, V.B.; Soares, J.K.B. Cashew Nuts (Anacardium occidentale L.) Decrease Visceral Fat, yet Augment Glucose in Dyslipidemic Rats. PLoS ONE 2019, 14, e0225736. [Google Scholar] [CrossRef] [PubMed]
- Yegani, M.; Korver, D.R. Factors Affecting Intestinal Health in Poultry. Poult. Sci. 2008, 87, 2052–2063. [Google Scholar] [CrossRef] [PubMed]
- Tako, E.; Rutzke, M.A.; Glahn, R.P. Using the Domestic Chicken (Gallus gallus) as an in Vivo Model for Iron Bioavailability. Poult. Sci. 2010, 89, 514–521. [Google Scholar] [CrossRef]
- Hou, T.; Tako, E. The in Ovo Feeding Administration (Gallus gallus)—An Emerging in Vivo Approach to Assess Bioactive Compounds with Potential Nutritional Benefits. Nutrients 2018, 10, 418. [Google Scholar] [CrossRef]
- Tako, E.; Glahn, R.P.; Knez, M.; Stangoulis, J.C. The Effect of Wheat Prebiotics on the Gut Bacterial Population and Iron Status of Iron Deficient Broiler Chickens. Nutr. J. 2014, 13, 58. [Google Scholar] [CrossRef]
- Agarwal, N.; Kolba, N.; Jung, Y.; Cheng, J.; Tako, E. Saffron (Crocus sativus L.) Flower Water Extract Disrupts the Cecal Microbiome, Brush Border Membrane Functionality, and Morphology In Vivo (Gallus gallus). Nutrients 2022, 14, 220. [Google Scholar] [CrossRef]
- Dias, D.M.; Kolba, N.; Hart, J.J.; Ma, M.; Sha, S.T.; Lakshmanan, N.; Nutti, M.R.; Martino, H.S.D.; Glahn, R.P.; Tako, E. Soluble Extracts from Carioca Beans (Phaseolus vulgaris L.) Affect the Gut Microbiota and Iron Related Brush Border Membrane Protein Expression in Vivo (Gallus gallus). Food Res. Int. 2019, 123, 172–180. [Google Scholar] [CrossRef]
- Hillier, L.W.; Miller, W.; Birney, E.; Groenen, M.A.M.; Crooijmans, R.P.M.A.; Aerts, J.; van der Poel, J.J. Sequence and Comparative Analysis of the Chicken Genome Provide Unique Perspectives on Vertebrate Evolution. Nature 2004, 432, 695–716. [Google Scholar] [CrossRef]
- Flores-Santin, J.; Burggren, W.W. Beyond the Chicken: Alternative Avian Models for Developmental Physiological Research. Front. Physiol. 2021, 12, 712633. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Methods of Analysis; AOAC: Rockville, MD, USA, 2012. [Google Scholar]
- Hou, T.; Kolba, N.; Glahn, R.P.; Tako, E. Intra-Amniotic Administration (Gallus gallus) of Cicer Arietinum and Lens Culinaris Prebiotics Extracts and Duck Egg White Peptides Affects Calcium Status and Intestinal Functionality. Nutrients 2017, 9, 785. [Google Scholar] [CrossRef] [PubMed]
- Agrizzi Verediano, T.; Stampini Duarte Martino, H.; Kolba, N.; Fu, Y.; Cristina Dias Paes, M.; Tako, E. Black Corn (Zea mays L.) Soluble Extract Showed Anti-Inflammatory Effects and Improved the Intestinal Barrier Integrity in Vivo (Gallus gallus). Food Res. Int. 2022, 157, 111227. [Google Scholar] [CrossRef] [PubMed]
- Tako, E.; Glahn, R.P.; Welch, R.M.; Lei, X.; Yasuda, K.; Miller, D.D. Dietary Inulin Affects the Expression of Intestinal Enterocyte Iron Transporters, Receptors and Storage Protein and Alters the Microbiota in the Pig Intestine. Br. J. Nutr. 2008, 99, 472–480. [Google Scholar] [CrossRef]
- Tako, E.; Ferket, P.R.; Uni, Z. Changes in Chicken Intestinal Zinc Exporter MRNA Expression and Small Intestinal Functionality Following Intra-Amniotic Zinc-Methionine Administration. J. Nutr. Biochem. 2005, 16, 339–346. [Google Scholar] [CrossRef]
- Kolba, N.; Zarei, A.; Cheng, J.; Agarwal, N.; Dadmohammadi, Y.; Khazdooz, L.; Abbaspourrad, A.; Tako, E. Alterations in Intestinal Brush Border Membrane Functionality and Bacterial Populations Following Intra-Amniotic Administration (Gallus gallus) of Catechin and Its Derivatives. Nutrients 2022, 14, 3924. [Google Scholar] [CrossRef]
- Cheng, J.; Kolba, N.; García-Rodríguez, A.; Marques, C.N.H.; Mahler, G.J.; Tako, E. Food-Grade Metal Oxide Nanoparticles Exposure Alters Intestinal Microbial Populations, Brush Border Membrane Functionality and Morphology, In Vivo (Gallus gallus). Antioxidants 2023, 12, 431. [Google Scholar] [CrossRef]
- Carboni, J.; Reed, S.; Kolba, N.; Eshel, A.; Koren, O.; Tako, E. Alterations in the Intestinal Morphology, Gut Microbiota, and Trace Mineral Status Following Intra-Amniotic Administration (Gallus gallus) of Teff (Eragrostis tef) Seed Extracts. Nutrients 2020, 12, 3020. [Google Scholar] [CrossRef]
- Makki, K.; Deehan, E.C.; Walter, J.; Bäckhed, F. The Impact of Dietary Fiber on Gut Microbiota in Host Health and Disease. Cell Host Microbe 2018, 23, 705–715. [Google Scholar] [CrossRef]
- Bintsis, T. Lactic Acid Bacteria as Starter Cultures: An Update in Their Metabolism and Genetics. AIMS Microbiol. 2018, 4, 665–684. [Google Scholar] [CrossRef]
- Da Silva, B.P.; Kolba, N.; Martino, H.S.D.; Hart, J.; Tako, E. Soluble Extracts from Chia Seed (Salvia hispanica L.) Affect Brush Border Membrane Functionality, Morphology and Intestinal Bacterial Populations in Vivo (Gallus gallus). Nutrients 2019, 11, 2457. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, B.P.; Dias, D.M.; de Castro Moreira, M.E.; Toledo, R.C.L.; da Matta, S.L.P.; Lucia, C.M.D.; Martino, H.S.D.; Pinheiro-Sant’Ana, H.M. Chia Seed Shows Good Protein Quality, Hypoglycemic Effect and Improves the Lipid Profile and Liver and Intestinal Morphology of Wistar Rats. Plant Foods Hum. Nutr. 2016, 71, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; McGee, R.; Vandemark, G.; Brick, M.; Thompson, H.J. Dietary Fiber Analysis of Four Pulses Using AOAC 2011.25: Implications for Human Health. Nutrients 2016, 8, 829. [Google Scholar] [CrossRef]
- Aoe, S.; Nakamura, F.; Fujiwara, S. Effect of Wheat Bran on Fecal Butyrate-Producing Bacteria and Wheat Bran Combined with Barley on Bacteroides Abundance in Japanese Healthy Adults. Nutrients 2018, 10, 1980. [Google Scholar] [CrossRef]
- Wang, X.; Kolba, N.; Liang, J.; Tako, E. Alterations in Gut Microflora Populations and Brush Border Functionality Following Intra-Amniotic Administration (Gallus gallus) of Wheat Bran Prebiotic Extracts. Food Funct. 2019, 10, 4834–4843. [Google Scholar] [CrossRef]
- Bengmark, S. Immunonutrition: Role of Biosurfactants, Fiber, and Probiotic Bacteria. Nutrition 1998, 14, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Gomes, M.J.C.; Martino, H.S.D.; Kolba, N.; Cheng, J.; Agarwal, N.; de Moura Rocha, M.; Tako, E. Zinc Biofortified Cowpea (Vigna unguiculata L. Walp.) Soluble Extracts Modulate Assessed Cecal Bacterial Populations and Gut Morphology In Vivo (Gallus gallus). Front. Biosci. Landmark 2022, 27, 140. [Google Scholar] [CrossRef]
- Zou, J.; Chassaing, B.; Singh, V.; Pellizzon, M.; Ricci, M.; Fythe, M.D.; Kumar, M.V.; Gewirtz, A.T. Fiber-Mediated Nourishment of Gut Microbiota Protects against Diet-Induced Obesity by Restoring IL-22-Mediated Colonic Health. Cell Host Microbe 2018, 23, 41–53.e4. [Google Scholar] [CrossRef]
- Wu, S.; Bhat, Z.F.; Gounder, R.S.; Ahmed, I.A.M.; Al-Juhaimi, F.Y.; Ding, Y.; Bekhit, A.E.D.A. Effect of Dietary Protein and Processing on Gut Microbiota—A Systematic Review. Nutrients 2022, 14, 453. [Google Scholar] [CrossRef]
- Qi, H.; Xiang, Z.; Han, G.; Yu, B.; Huang, Z.; Chen, D. Effects of Different Dietary Protein Sources on Cecal Microflora in Rats. Afr. J. Biotechnol. 2011, 10, 3704–3708. [Google Scholar] [CrossRef]
- Zhu, Y.; Lin, X.; Zhao, F.; Shi, X.; Li, H.; Li, Y.; Zhu, W.; Xu, X.; Lu, C.; Zhou, G. Meat, Dairy and Plant Proteins Alter Bacterial Composition of Rat Gut Bacteria. Sci. Rep. 2015, 5, 15220. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Lin, X.; Li, H.; Li, Y.; Shi, X.; Zhao, F.; Xu, X.; Li, C.; Zhou, G. Intake of Meat Proteins Substantially Increased the Relative Abundance of Genus Lactobacillus in Rat Feces. PLoS ONE 2016, 11, e0152678. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Shi, X.; Lin, X.; Ye, K.; Xu, X.; Li, C.; Zhou, G. Beef, Chicken, and Soy Proteins in Diets Induce Different Gut Microbiota and Metabolites in Rats. Front. Microbiol. 2017, 8, 1395. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Nishio, H.; Tanigawa, T.; Yamagami, H.; Okazaki, H.; Watanabe, K.; Tominaga, K.; Fujiwara, Y.; Oshitani, N.; Asahara, T.; et al. Probiotic Lactobacillus casei Strain Shirota Prevents Indomethacin-Induced Small Intestinal Injury: Involvement of Lactic Acid. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 297, 506–513. [Google Scholar] [CrossRef]
- Rivière, A.; Selak, M.; Lantin, D.; Leroy, F.; de Vuyst, L. Bifidobacteria and Butyrate-Producing Colon Bacteria: Importance and Strategies for Their Stimulation in the Human Gut. Front. Microbiol. 2016, 7, 979. [Google Scholar] [CrossRef] [PubMed]
- Chao, L.; Levin, B.R. Structured Habitats and the Evolution of Anticompetitor Toxins in Bacteria (Allelopathy/Interference Competition/Altruism/Frequency-Dependent Selection/Colicin). Proc. Natl. Acad. Sci. USA 1981, 78, 6324–6328. [Google Scholar] [CrossRef]
- Smirnov, A.; Perez, R.; Amit-Romach, E.; Sklan, D.; Uni, Z. Mucin Dynamics and Microbial Populations in Chicken Small Intestine Are Changed by Dietary Probiotic and Antibiotic Growth Promoter Supplementation. J. Nutr. 2005, 135, 187–192. [Google Scholar] [CrossRef]
- Lueschow, S.R.; McElroy, S.J. The Paneth Cell: The Curator and Defender of the Immature Small Intestine. Front. Immunol. 2020, 11, 587. [Google Scholar] [CrossRef]
- Stylianou, E.; Saklatvala, J. Interleukin-1. Int. J. Biochem. Cell Biol. 1998, 30, 1075–1079. [Google Scholar] [CrossRef]
- Yao, D.; Dai, W.; Dong, M.; Dai, C.; Wu, S. MUC2 and Related Bacterial Factors: Therapeutic Targets for Ulcerative Colitis. eBioMedicine 2021, 74, 103751. [Google Scholar] [CrossRef]
- Ensari, A.; Marsh, M.N. Exploring the Villus Introduction—Why “Villus”? Gastroenterol. Hepatol. Bed Bench 2018, 11, 181–190. [Google Scholar]
- Gustafsson, J.K.; Johansson, M.E.V. The Role of Goblet Cells and Mucus in Intestinal Homeostasis. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 785–803. [Google Scholar] [CrossRef] [PubMed]
- Duangnumsawang, Y.; Zentek, J.; Goodarzi Boroojeni, F. Development and Functional Properties of Intestinal Mucus Layer in Poultry. Front. Immunol. 2021, 12, 756849. [Google Scholar] [CrossRef] [PubMed]
- Mack, D.R.; Michail, S.; Wei, S.; Mcdougall, L.; Hollingsworth, M.A.; Mc-Dougall, L.; Hollingsworth Probiotics, M.A. Probiotics Inhibit Enteropathogenic E. coli Adherence In Vitro by Inducing Intestinal Mucin Gene Expression. Am. J. Physiol. Gastrointest. Liver Physiol. 1999, 276, G941–G950. [Google Scholar] [CrossRef] [PubMed]
- Szentkuti, L.; Riedesel, H.; Enss, M.-L.; Gaertner, K.; von Engelhardt, W. Pre-Epithelial Mucus Layer in the Colon of Conventional and Germ-Free Rats. Histochem. J. 1990, 22, 491–497. [Google Scholar] [CrossRef]
- Kandori, H.; Hirayama, K.; Takeda, M.; Doi, K. Histochemical, Lectin-Histochemical and Morphometrical Characteristics of Intestinal Goblet Cells of Germfree and Conventional Mice. Exp. Anim. 1996, 45, 155–160. [Google Scholar] [CrossRef]
- Fan, W.; Ren, H.; Cao, Y.; Wang, Y.; Huo, G. Low Dietary Protein and High Carbohydrate Infant Formula Affects the Microbial Ecology of the Large Intestine in Neonatal Rats. Can. J. Microbiol. 2017, 63, 951–960. [Google Scholar] [CrossRef]
- Kaminski, N.A.; Wong, E.A. Differential MRNA Expression of Nutrient Transporters in Male and Female Chickens. Poult. Sci. 2018, 97, 313–318. [Google Scholar] [CrossRef]
- Feldman, G.J.; Mullin, J.M.; Ryan, M.P. Occludin: Structure, Function and Regulation. Adv. Drug Deliv. Rev. 2005, 57, 883–917. [Google Scholar] [CrossRef]
- Hartono, K.; Reed, S.; Ankrah, N.A.; Glahn, R.P.; Tako, E. Alterations in Gut Microflora Populations and Brush Border Functionality Following Intra-Amniotic Daidzein Administration. RSC Adv. 2015, 5, 6407–6412. [Google Scholar] [CrossRef]
- Kolba, N.; Zarei, A.; Cheng, J.; Agarwal, N.; Dadmohammadi, Y.; Khazdooz, L.; Abbaspourrad, A.; Tako, E. Alterations in Intestinal Brush Border Membrane Functionality and Bacterial Populations Following Intra-Amniotic Administration (Gallus gallus) of Nicotinamide Riboside and Its Derivatives. Nutrients 2022, 14, 3130. [Google Scholar] [CrossRef]
- Uni, Z.; Noy, Y.; Sklan, D. Posthatch Development of Small Intestinal Function in the Poult. Poult. Sci. 1999, 78, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Sobolewska, A.; Elminowska-Wenda, G.; Bogucka, J.; Dankowiakowska, A.; Kułakowska, A.; Szczerba, A.; Stadnicka, K.; Szpinda, M.; Bednarczyk, M. The Influence of in Ovo Injection with the Prebiotic DiNovo® on the Development of Histomorphological Parameters of the Duodenum, Body Mass and Productivity in Large-Scale Poultry Production Conditions. J. Anim. Sci. Biotechnol. 2017, 8, 45. [Google Scholar] [CrossRef] [PubMed]
- Lan, A.; Andriamihaja, M.; Blouin, J.M.; Liu, X.; Descatoire, V.; Desclée de Maredsous, C.; Davila, A.M.; Walker, F.; Tomé, D.; Blachier, F. High-Protein Diet Differently Modifies Intestinal Goblet Cell Characteristics and Mucosal Cytokine Expression in Ileum and Colon. J. Nutr. Biochem. 2015, 26, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Wu, G. Intestinal Mucosal Amino Acid Catabolism. Recent Adv. Nutr. Sci. 1998, 128, 1249–1252. [Google Scholar] [CrossRef]
- Obianwuna, U.E.; Qiu, K.; Chang, X.Y.; Zhang, H.J.; Wang, J.; Qi, G.H.; Sun, T.H.; Su, Y.B.; Wu, S.G. Enhancing Egg Production and Quality by the Supplementation of Probiotic Strains (Clostridium and Brevibacillus) via Improved Amino Acid Digestibility, Intestinal Health, Immune Response, and Antioxidant Activity. Front. Microbiol. 2022, 13, 987241. [Google Scholar] [CrossRef] [PubMed]
- Shamoto, K.; Yamauchi, K. Recovery Responses of Chick Intestinal Villus Morphology to Different Refeeding Procedures. Poult. Sci. 2000, 79, 718–723. [Google Scholar] [CrossRef] [PubMed]
- Paone, P.; Cani, P.D. Mucus Barrier, Mucins and Gut Microbiota: The Expected Slimy Partners? Gut 2020, 69, 2232–2243. [Google Scholar] [CrossRef]
- Meslin, J.-C.; Fontaine, N.; Andrieux, C. Variation of Mucin Distribution in the Rat Intestine, Caecum and Colon: Effect of the Bacterial Flora. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 1999, 123, 235–239. [Google Scholar] [CrossRef]

| Analyte | Forward Primer (5′→3′) | Reverse Primer (5′→3′) | Base Pair | GI Identifier |
|---|---|---|---|---|
| Intestinal Barrier | ||||
| AP | CGTCAGCCAGTTTGACTATGTA | CTCTCAAAGAAGCTGAGGATGG | 138 | 45,382,360 |
| SGLT1 | GCATCCTTACTCTGTGGTACTG | TATCCGCACATCACACATCC | 106 | 8,346,783 |
| MUC2 | CCTGCTGCAAGGAAGTAGAA | GGAAGATCAGAGTGGTGCATAG | 155 | 423,101 |
| OCLN | GTCTGTGGGTTCCTCATCGT | GTTCTTCACCCACTCCTCCA | 124 | 396,026 |
| Inflammatory Response | ||||
| NF-κβ | CACAGCTGGAGGGAAGTAAAT | TTGAGTAAGGAAGTGAGGTTGAG | 100 | 2,130,627 |
| IL-1β | TCATCCATCCCAAGTTCATTCA | GACACACTTCTCTGCCATCTT | 105 | 395,872 |
| Proteins | Fibers | |||
|---|---|---|---|---|
| Total | Insoluble | Soluble | ||
| Cashew nut flour (g/100 g) | 21.50 ± 0.51 | 22.36 ± 0.05 | 21.33 ± 0.40 | 1.03 ± 0.45 |
| Cashew nut soluble extract (g/100 g) | 41.65 ± 0.18 | 9.82 ± 0.42 | 9.29 ± 0.47 | 0.53 ± 0.05 |
| Groups | Paneth Cell | |
|---|---|---|
| Number | Diameter (μM) | |
| No injection | 1.94 ± 0.08 bc | 1.45 ± 0.04 a |
| 18 Ω H2O injection | 1.68 ± 0.06 b | 1.40 ± 0.04 a |
| CNSE (1%) | 2.43 ± 0.11 a | 1.42 ± 0.03 a |
| CNSE (5%) | 2.39 ± 0.12 ac | 1.34 ± 0.04 a |
| Groups | Crypt | ||
| GC Number | GC Diameter (μM) | Depth (μM) | |
| No injection | 12.67 ± 0.55 a | 2.92 ± 0.05 b | 14.02 ± 0.50 b |
| 18 Ω H2O injection | 10.95 ± 0.62 b | 3.13 ± 0.05 a | 16.00 ± 0.65 ab |
| CNSE (1%) | 9.72 ± 0.41 b | 3.12 ± 0.06 ab | 15.80 ± 0.56 ab |
| CNSE (5%) | 9.39 ± 0.42 b | 3.23 ± 0.06 a | 17.10 ± 0.69 a |
| Villi | |||
| GC number | GC Diameter (μM) | Surface Area (mm²) | |
| No injection | 24.68 ± 0.74 c | 2.46 ± 0.06 b | 11668.81 ± 446.06 b |
| 18 Ω H2O injection | 38.38 ± 0.91 a | 2.20 ± 0.05 c | 8248.42 ± 364.05 c |
| CNSE (1%) | 33.41 ± 0.81 b | 2.81 ± 0.07 a | 8341.78 ± 342.77 c |
| CNSE (5%) | 25.59 ± 0.82 c | 2.79 ± 0.10 a | 14491.08 ± 505.97 a |
| Groups | GC per Villi | GC per Crypt | ||||
|---|---|---|---|---|---|---|
| Acid | Neutral | Mixed | Acid | Neutral | Mixed | |
| No injection | 15.28 ± 0.71 b | 0.79 ± 0.13 a | 8.68 ± 0.57 c | 8.53 ± 0.46 a | 0.41 ± 0.06 ab | 3.73 ± 0.27 a |
| 18 Ω H2O injection | 26.71 ± 1.12 a | 0.10 ± 0.04 b | 11.57 ± 0.66 b | 7.88 ± 0.51 a | 0.50 ± 0.07 a | 2.58 ± 0.21 bc |
| CNSE (1%) | 12.13 ± 0.54 c | 0.24 ± 0.09 b | 21.03 ± 0.75 a | 6.93 ± 0.30 a | 0.29 ± 0.06 ab | 2.63 ± 0.17 ab |
| CNSE (5%) | 10.87 ± 0.66 c | 0.57 ± 0.14 b | 14.16 ± 0.72 b | 7.34 ± 0.35 a | 0.30 ± 0.07 b | 1.76 ± 0.14 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meneguelli, T.S.; Kolba, N.; Misra, A.; Dionísio, A.P.; Pelissari Kravchychyn, A.C.; Da Silva, B.P.; Stampini Duarte Martino, H.; Hermsdorff, H.H.M.; Tako, E. Intra-Amniotic Administration of Cashew Nut (Anacardium occidentale L.) Soluble Extract Improved Gut Functionality and Morphology In Vivo (Gallus gallus). Nutrients 2023, 15, 2378. https://doi.org/10.3390/nu15102378
Meneguelli TS, Kolba N, Misra A, Dionísio AP, Pelissari Kravchychyn AC, Da Silva BP, Stampini Duarte Martino H, Hermsdorff HHM, Tako E. Intra-Amniotic Administration of Cashew Nut (Anacardium occidentale L.) Soluble Extract Improved Gut Functionality and Morphology In Vivo (Gallus gallus). Nutrients. 2023; 15(10):2378. https://doi.org/10.3390/nu15102378
Chicago/Turabian StyleMeneguelli, Talitha Silva, Nikolai Kolba, Arundhati Misra, Ana Paula Dionísio, Ana Claudia Pelissari Kravchychyn, Bárbara Pereira Da Silva, Hercia Stampini Duarte Martino, Helen Hermana Miranda Hermsdorff, and Elad Tako. 2023. "Intra-Amniotic Administration of Cashew Nut (Anacardium occidentale L.) Soluble Extract Improved Gut Functionality and Morphology In Vivo (Gallus gallus)" Nutrients 15, no. 10: 2378. https://doi.org/10.3390/nu15102378
APA StyleMeneguelli, T. S., Kolba, N., Misra, A., Dionísio, A. P., Pelissari Kravchychyn, A. C., Da Silva, B. P., Stampini Duarte Martino, H., Hermsdorff, H. H. M., & Tako, E. (2023). Intra-Amniotic Administration of Cashew Nut (Anacardium occidentale L.) Soluble Extract Improved Gut Functionality and Morphology In Vivo (Gallus gallus). Nutrients, 15(10), 2378. https://doi.org/10.3390/nu15102378







