Muscle Glycogen Assessment and Relationship with Body Hydration Status: A Narrative Review
Abstract
1. Background
2. Muscle Glycogen Measurement by 13C MRS
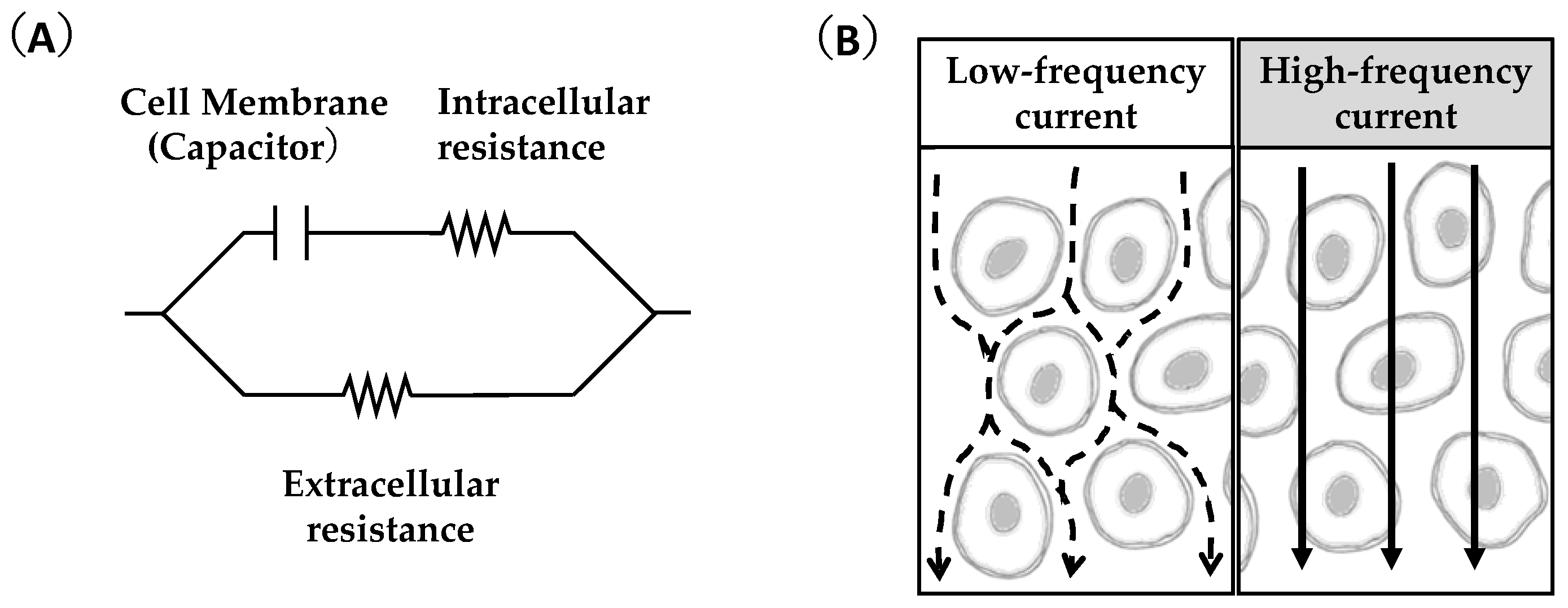
3. Feasibility of Ultrasound and Bioimpedance Outcomes as Indicators of Muscle Glycogen Levels
4. Muscle Glycogen and Body Hydration Status
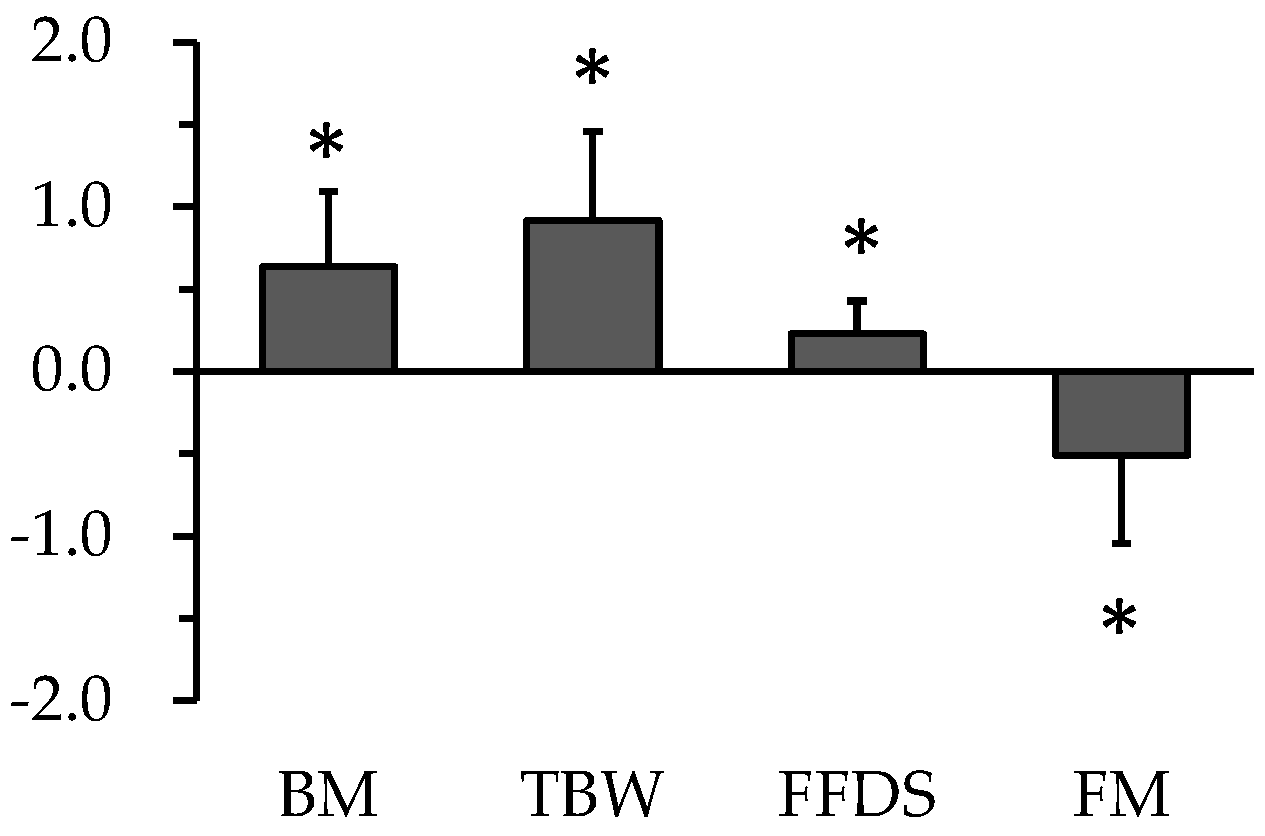
5. Change in Body Composition during Carbohydrate Loading
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chin, E.R.; Allen, D.G. Effects of reduced muscle glycogen concentration on force, Ca2+ release and contractile protein function in intact mouse skeletal muscle. J. Physiol. 1997, 498 Pt 1, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Ørtenblad, N.; Westerblad, H.; Nielsen, J. Muscle glycogen stores and fatigue. J. Physiol. 2013, 591, 4405–4413. [Google Scholar] [CrossRef] [PubMed]
- Bergström, J.; Hultman, E. Muscle glycogen synthesis after exercise: An enhancing factor localized to the muscle cells in man. Nature 1966, 210, 309–310. [Google Scholar] [CrossRef] [PubMed]
- Hansen, A.K.; Fischer, C.P.; Plomgaard, P.; Andersen, J.L.; Saltin, B.; Pedersen, B.K. Skeletal muscle adaptation: Training twice every second day vs. training once daily. J. Appl. Physiol. 2005, 98, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Burke, L.M. Fueling strategies to optimize performance: Training high or training low? Scand. J. Med. Sci. Sport. 2010, 20 (Suppl. S2), 48–58. [Google Scholar] [CrossRef] [PubMed]
- Impey, S.G.; Hearris, M.A.; Hammond, K.M.; Bartlett, J.D.; Louis, J.; Close, G.L.; Morton, J.P. Fuel for the work required: A theoretical framework for carbohydrate periodization and the glycogen threshold hypothesis. Sport. Med. 2018, 48, 1031–1048. [Google Scholar] [CrossRef]
- Hearris, M.; Hammond, K.; Fell, J.; Morton, J. Regulation of muscle glycogen metabolism during exercise: Implications for endurance performance and training adaptations. Nutrients 2018, 10, 298. [Google Scholar] [CrossRef]
- Bergström, J. Muscle electrolytes in man determined by neutron activation analysis on needle biopsy specimens. Scand. J. Clin. Lab. Investig. Engl. 1962, 14, 110. [Google Scholar]
- Takahashi, H.; Kamei, A.; Osawa, T.; Kawahara, T.; Takizawa, O.; Maruyama, K. 13C MRS reveals a small diurnal variation in the glycogen content of human thigh muscle. NMR Biomed. 2015, 28, 650–655. [Google Scholar] [CrossRef]
- Olsson, K.E.; Saltin, B. Variation in total body water with muscle glycogen changes in man. Acta Physiol. Scand. 1970, 80, 11–18. [Google Scholar] [CrossRef]
- Shiose, K.; Yamada, Y.; Motonaga, K.; Sagayama, H.; Higaki, Y.; Tanaka, H.; Takahashi, H. Segmental extracellular and intracellular water distribution and muscle glycogen after 72-h carbohydrate loading using spectroscopic techniques. J. Appl. Physiol. 2016, 121, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Iwayama, K.; Onishi, T.; Maruyama, K.; Takahashi, H. Diurnal variation in the glycogen content of the human liver using 13C MRS. NMR Biomed. 2020, 33, e4289. [Google Scholar] [CrossRef] [PubMed]
- Khowaja, A.; Choi, I.-Y.; Seaquist, E.R.; Öz, G. In vivo Magnetic Resonance Spectroscopy of cerebral glycogen metabolism in animals and humans. Metab. Brain Dis. 2015, 30, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, T.; Takahashi, H.; Nakamura, M.; Kanno, M.; Ogata, H.; Ishikawa, A.; Yamada, M.; Kamemoto, K.; Sakamaki-Sunaga, M. Influence of menstrual cycle on muscle glycogen utilization during high-intensity intermittent exercise until exhaustion in healthy women. Appl. Physiol. Nutr. Metab. 2022, 47, 671–680. [Google Scholar] [CrossRef]
- Ishibashi, A.; Kojima, C.; Tanabe, Y.; Iwayama, K.; Hiroyama, T.; Tsuji, T.; Kamei, A.; Goto, K.; Takahashi, H. Effect of low energy availability during three consecutive days of endurance training on iron metabolism in male long distance runners. Physiol. Rep. 2020, 8, e14494. [Google Scholar] [CrossRef]
- Kaggie, J.D.; Sapkota, N.; Thapa, B.; Jeong, K.; Shi, X.; Morrell, G.; Bangerter, N.K.; Jeong, E.-K. Synchronous radial 1H and 23Na dual-nuclear MRI on a clinical MRI system, equipped with a broadband transmit channel. Concepts Magn. Reson. Part B Magn. Reson. Eng. 2016, 46B, 191–201. [Google Scholar] [CrossRef]
- Taylor, R.; Price, T.B.; Rothman, D.L.; Shulman, R.G.; Shulman, G.I. Validation of 13C NMR measurement of human skeletal muscle glycogen by direct biochemical assay of needle biopsy samples. Magn. Reson. Med. 1992, 27, 13–20. [Google Scholar] [CrossRef]
- Price, T.B.; Taylor, R.; Mason, G.F.; Rothman, D.L.; Shulman, G.I.; Shulman, R.G. Turnover of human muscle glycogen with low-intensity exercise. Med. Sci. Sport. Exerc. 1994, 26, 983–991. [Google Scholar] [CrossRef]
- Buehler, T.; Bally, L.; Dokumaci, A.S.; Stettler, C.; Boesch, C. Methodological and physiological test–retest reliability of 13C-MRS glycogen measurements in liver and in skeletal muscle of patients with type 1 diabetes and matched healthy controls. NMR Biomed. 2016, 29, 796–805. [Google Scholar] [CrossRef]
- Tuthill, T.; Baggs, R.; Parker, K. Liver glycogen and water storage: Effect on ultrasound attenuation. Ultrasound Med. Biol. 1989, 15, 621–627. [Google Scholar] [CrossRef]
- Hill, J.C.; Millan, I.S. Validation of musculoskeletal ultrasound to assess and quantify muscle glycogen content. A novel approach. Physician Sportsmed. 2014, 42, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Nieman, D.C.; Shanely, R.A.; Zwetsloot, K.A.; Meaney, M.P.; Farris, G.E. Ultrasonic assessment of exercise-induced change in skeletal muscle glycogen content. BMC Sport. Sci. Med. Rehabil. 2015, 7, 9. [Google Scholar] [CrossRef] [PubMed]
- San-Millán, I.; Hill, J.C.; Calleja-González, J. Indirect assessment of skeletal muscle glycogen content in professional soccer players before and after a match through a non-invasive ultrasound technology. Nutrients 2020, 12, 971. [Google Scholar] [CrossRef] [PubMed]
- Routledge, H.E.; Bradley, W.J.; Shepherd, S.O.; Cocks, M.; Erskine, R.M.; Close, G.L.; Morton, J.P. Ultrasound does not detect acute changes in glycogen in vastus lateralis of man. Med. Sci. Sport. Exerc. 2019, 51, 2286–2293. [Google Scholar] [CrossRef]
- Bone, J.L.; Ross, M.L.; Tomcik, K.A.; Jeacocke, N.A.; McKay, A.K.A.; Burke, L.M. The validity of ultrasound technology in providing an indirect estimate of muscle glycogen concentrations is equivocal. Nutrients 2021, 13, 2371. [Google Scholar] [CrossRef]
- Ørtenblad, N.; Nielsen, J.; Gejl, K.; Routledge, H.; Morton, J.; Close, G.; Niemann, D.; Bone, J.; Burke, L. Comment on: “Indirect assessment of skeletal muscle glycogen content in professional soccer players before and after a match through a non-invasive ultrasound technology Technology Nutrients 2020, 12(4), 971”. Nutrients 2020, 12, 2070. [Google Scholar] [CrossRef]
- Kyle, U.G.; Bosaeus, I.; De Lorenzo, A.D.; Deurenberg, P.; Elia, M.; Gómez, J.M.; Heitmann, B.L.; Kent-Smith, L.; Melchior, J.-C.; Pirlich, M.; et al. Bioelectrical impedance analysis—Part I: Review of principles and methods. Clin. Nutr. 2004, 23, 1226–1243. [Google Scholar] [CrossRef]
- Kyle, U.G.; Bosaeus, I.; De Lorenzo, A.D.; Deurenberg, P.; Elia, M.; Gomez, J.M.; Heitmann, B.L.; Kent-Smith, L.; Melchior, J.C.; Pirlich, M.; et al. Bioelectrical impedance analysis—Part II: Utilization in clinical practice. Clin. Nutr. 2004, 23, 1430–1453. [Google Scholar] [CrossRef]
- Bone, J.L.; Ross, M.L.; Tomcik, K.A.; Jeacocke, N.A.; Hopkins, W.G.; Burke, L.M. Manipulation of Muscle Creatine and Glycogen Changes DXA Estimates of Body Composition. Med. Sci. Sport. Exerc. 2017, 49, 1029–1035. [Google Scholar] [CrossRef]
- Shiose, K.; Yamada, Y.; Motonaga, K.; Takahashi, H. Muscle glycogen depletion does not alter segmental extracellular and intracellular water distribution measured using bioimpedance spectroscopy. J. Appl. Physiol. 2018, 124, 1420–1425. [Google Scholar] [CrossRef]
- Raja, M.K.; Raymer, G.H.; Moran, G.; Marsh, G.; Thompson, R.T. Changes in tissue water content measured with multiple-frequency bioimpedance and metabolism measured with 31P-MRS during progressive forearm exercise. J. Appl. Physiol. 2006, 101, 1070–1075. [Google Scholar] [CrossRef] [PubMed]
- Buono, M.J.; Burke, S.; Endemann, S.; Graham, H.; Gressard, C.; Griswold, L.; Michalewicz, B. The effect of ambient air temperature on whole-body bioelectrical impedance. Physiol. Meas. 2004, 25, 119. [Google Scholar] [CrossRef] [PubMed]
- Shiose, K.; Yamada, Y.; Motonaga, K.; Takahashi, H. Circadian variation of extracellular and intracellular resistance of the leg, arm, and trunk in healthy humans: A segmental bioimpedance spectroscopy study. Biomed. Phys. Eng. Express 2017, 3, 065007. [Google Scholar] [CrossRef]
- Zuntz, N.; Müller, F.; Loewy, A.; Caspari, W. Höhenklima und Bergwanderungen in Ihrer Wirkung auf den Menschen, 1. aufl. ed.; Deuthches Verlagshaus Bong & Co.: Berlin, Germany, 1906; p. 114. [Google Scholar]
- Pavy, F.W. XXVI. Researches on sugar formation in the liver. Philos. Trans. R. Soc. Lond. 1860, 150, 595–609. [Google Scholar]
- Puckett, H.L.; Wiley, F.H. The relation of glycogen to water storage in the liver. J. Biol. Chem. 1932, 96, 367–371. [Google Scholar] [CrossRef]
- MacKay, E.M.; Bergman, H. The amount of water stored with glycogen in the liver. J. Biol. Chem. 1934, 105, 59–62. [Google Scholar] [CrossRef]
- Fenn, W.; Haege, L.F. The deposition of glycogen with water in the livers of cats. J. Biol. Chem. 1940, 136, 87–101. [Google Scholar] [CrossRef]
- McBride, J.; Guest, M.M.; Scott, E. The storage of the major liver components; emphasizing the relationship of glycogen to water in the liver and the hydration of glycogen. J. Biol. Chem. 1941, 139, 943–952. [Google Scholar] [CrossRef]
- Bridge, E.M.; Bridges, E. The relation of glycogen to water storage in the liver. J. Biol. Chem. 1931, 93, 181–187. [Google Scholar] [CrossRef]
- MacKay, E.M.; Bergman, H. The relation between glycogen and water storage in the liver. J. Biol. Chem. 1932, 96, 373–380. [Google Scholar] [CrossRef]
- Greisheimer, E.M.; Goldsworthy, E. Glycogen and Water Storage. Proc. Soc. Exp. Biol. Med. 1935, 33, 32–34. [Google Scholar] [CrossRef]
- Kaplan, A.; Chaikoff, I. The relation of glycogen, fat, and protein to water storage in the liver. J. Biol. Chem. 1936, 116, 663–683. [Google Scholar] [CrossRef]
- Fenn, W. The deposition of potassium and phosphate with glycogen in rat livers. J. Biol. Chem. 1939, 128, 297–308. [Google Scholar] [CrossRef]
- Sherman, W.; Plyley, M.; Sharp, R.; Van Handel, P.; McAllister, R.; Fink, W.; Costill, D. Muscle glycogen storage and its relationship with water. Int. J. Sport. Med. 1982, 3, 22–24. [Google Scholar] [CrossRef] [PubMed]
- Richter, E.A.; Hansen, S.; Hansen, B. Mechanisms limiting glycogen storage in muscle during prolonged insulin stimulation. Am. J. Physiol. Endocrinol. Metab. 1988, 255, E621–E628. [Google Scholar] [CrossRef] [PubMed]
- Nygren, A.T.; Karlsson, M.; Norman, B.; Kaijser, L. Effect of glycogen loading on skeletal muscle cross-sectional area and T2 relaxation time. Acta Physiol. Scand. 2001, 173, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Elías, V.E.; Ortega, J.F.; Nelson, R.K.; Mora-Rodriguez, R. Relationship between muscle water and glycogen recovery after prolonged exercise in the heat in humans. Eur. J. Appl. Physiol. 2015, 115, 1919–1926. [Google Scholar] [CrossRef] [PubMed]
- Shearer, J.; Graham, T.E. Novel aspects of skeletal muscle glycogen and its regulation during rest and exercise. Exerc. Sport Sci. Rev. 2004, 32, 120–126. [Google Scholar] [CrossRef]
- Jensen, R.; Ørtenblad, N.; Stausholm, M.L.H.; Skjærbæk, M.C.; Larsen, D.N.; Hansen, M.; Holmberg, H.C.; Plomgaard, P.; Nielsen, J. Glycogen supercompensation is due to increased number, not size, of glycogen particles in human skeletal muscle. Exp. Physiol. 2021, 106, 1272–1284. [Google Scholar] [CrossRef]
- Bergström, J.; Hermansen, L.; Hultman, E.; Saltin, B. Diet, muscle glycogen and physical performance. Acta Physiol. Scand. 1967, 71, 140–150. [Google Scholar] [CrossRef]
- Sherman, W.M.; Costill, D.L.; Fink, W.J.; Miller, J.M. Effect of exercise-diet manipulation on muscle glycogen and its subsequent utilization during performance. Int. J. Sport. Med. 1981, 2, 114–118. [Google Scholar] [CrossRef]
- Bussau, V.A.; Fairchild, T.J.; Rao, A.; Steele, P.; Fournier, P.A. Carbohydrate loading in human muscle: An improved 1 day protocol. Eur. J. Appl. Physiol. 2002, 87, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Burke, L.M.; van Loon, L.J.C.; Hawley, J.A. Postexercise muscle glycogen resynthesis in humans. J. Appl. Physiol. 2017, 122, 1055–1067. [Google Scholar] [CrossRef] [PubMed]
- Michalczyk, M.; Chycki, J.; Zajac, A.; Maszczyk, A.; Zydek, G.; Langfort, J. Anaerobic Performance after a Low-Carbohydrate Diet (LCD) Followed by 7 Days of Carbohydrate Loading in Male Basketball Players. Nutrients 2019, 11, 778. [Google Scholar] [CrossRef] [PubMed]
- Steen, S.N. Precontest strategies of a male bodybuilder. Int. J. Sport Nutr. Exerc. Metab. 1991, 1, 69–78. [Google Scholar] [CrossRef]
- Schoenfeld, B.J.; Alto, A.; Grgic, J.; Tinsley, G.; Haun, C.T.; Campbell, B.I.; Escalante, G.; Sonmez, G.T.; Cote, G.; Francis, A.; et al. Alterations in body composition, resting metabolic rate, muscular strength, and eating behavior in response to natural bodybuilding competition preparation: A case study. J. Strength Cond. Res. 2020, 34, 3124–3138. [Google Scholar] [CrossRef]
- Alves, R.C.; Prestes, J.; Enes, A.; de Moraes, W.M.; Trindade, T.B.; de Salles, B.F.; Aragon, A.A.; Souza-Junior, T.P. Training programs designed for muscle hypertrophy in bodybuilders: A narrative review. Sports 2020, 8, 149. [Google Scholar] [CrossRef]
- De Moraes, W.M.; de Almeida, F.N.; Dos Santos, L.E.; Cavalcante, K.D.; Santos, H.O.; Navalta, J.W.; Prestes, J. Carbohydrate loading practice in bodybuilders: Effects on muscle thickness, photo silhouette scores, mood states and gastrointestinal symptoms. J. Sport. Sci. Med. 2019, 18, 772–779. [Google Scholar]
- Sedlock, D.A. The latest on carbohydrate loading: A practical approach. Curr. Sport. Med. Rep. 2008, 7, 209–213. [Google Scholar] [CrossRef]
- Hackney, A.C. Human Performance Enhancement in Sports and Exercise: Nutritional Factors-“Carbohydrate Loading”. In Revista Universitaria de la Educación Física y el Deporte; Instituto Universitario Asociación Cristiana de Jóvenes; Dialnet: Montevideo, Uruguay, 2015; pp. 28–31. [Google Scholar]
- Madsen, K.; Pedersen, P.K.; Rose, P.; Richter, E.A. Carbohydrate supercompensation and muscle glycogen utilization during exhaustive running in highly trained athletes. Eur. J. Appl. Physiol. Occup. Physiol. 1990, 61, 467–472. [Google Scholar] [CrossRef]


| Study | Species | Glycogen Level | Glycogen Assessment | Body Water Assessment | Assessment Timing | Positive Relationship between Glycogen and Water | Estimated Glycogen: Water Ratio | ||
|---|---|---|---|---|---|---|---|---|---|
| Organ | Method | Organ | Method | ||||||
| Bridge and Bridges [40] | Rabbits | Low to high | Liver | Biochemical analysis | Liver | Dried and weighed | Post dietary manipulation | No | |
| MacKay and Bergman [41] | Rabbits | Low to high | (1) Liver (2) Muscle | Biochemical analysis | (1) Liver (2) Muscle | Dried and weighed | Post dietary manipulation | Liver; Yes Muscle; No | |
| Puckett and Wiley [36] | Rats | Low to high | Liver | Biochemical analysis | Liver | Dried and weighed | Post dietary manipulation | Yes | 1:2.4 |
| MacKay and Bergman [37] | Rats | Low to high | Liver | Biochemical analysis | Liver | Dried and weighed | Post dietary manipulation | Yes | 1:3.8 |
| Greisheimer and Goldsworthy [42] | Rats | Low to high | Liver | Biochemical analysis | Liver | Dried and weighed | Post dietary manipulation | Yes (Only in a condition where the rats were given a diet without priori fasting,) | |
| Kaplan and Chaikoff [43] | Dogs | Low to high | Liver | Biochemical analysis | Liver | Weighted total lipid and defatted dried tissues. | Post dietary manipulation | No | |
| Fenn [44] | Rats | Low, normal, high | Liver | Biochemical analysis | Liver | Dried and weighed | Post dietary manipulation | Yes | |
| Fenn and Haege [38] | Cats | Low to high | Liver | Biochemical analysis | Liver | Dried and weighed | Post dietary manipulation | Yes | 1: 1.63 |
| McBride et al. [39] | Rats | Low to high | Liver | Biochemical analysis | Liver | Dried and weighed | Post dietary manipulation | Yes | 1:2.7 |
| Olsson and Saltin [10] | 19 young healthy males | Low to high | Muscle (biopsy sampling) | Biochemical analysis | Whole-body | IDM | (1) Post-protein and fat diet (2) Post-carbohydrate and protein diet | Yes | 1:3–1:4 |
| Sherman et al. [45] | Rats | Low to high | Muscle | Biochemical analysis | Muscle | Dried and weighed | Post dietary manipulation | No | |
| Richter et al. [46] | Rats | Normal to high | Muscle | Biochemical analysis | Muscle | Dried and weighed | Post perfusion | No | |
| Nygren et al. [47] | 5 healthy males | Low to high | Muscle (biopsy sampling) | Biochemical analysis | Muscle | Magnetic resonance imaging | (1) Post carbohydrate-restricted diet (2) Post high-carbohydrate diet | Yes | |
| Fernández-Elías et al. [48] | 9 endurance-trained male cyclists | Low to high | Muscle | Biochemical analysis | Muscle | Dried and weighed | (1) Pre-exercise (2) Post-exercise (3) Post recovery | Yes | 1:3–1:17 |
| Shiose et al. [11] | 8 healthy males | Normal to high | Muscle | 13C-MRS | (1) Whole-body (2) Each body segment | (1) IDM (2) BIA | (1) Pre-exercise (2) Post high-carbohydrate diet | Yes | ≤1:4 |
| Bone et al. [29] | 18 well-trained male cyclists | Low to high | Muscle (biopsy sampling) | Biochemical analysis | Whole-body | BIA | (1) Pre exercise (2) Post exercise (3) Creatin loaded (4) Glycogen loaded (5) Creatin-glycogen loaded | Yes | |
| Shiose et al. [30] | 12 healthy males | Low to normal | Muscle | 13C-MRS | (1) Whole-body (2) Each body segment | (1) IDM (2) BIA | (1) Pre exercise (2) Post recovery | No | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shiose, K.; Takahashi, H.; Yamada, Y. Muscle Glycogen Assessment and Relationship with Body Hydration Status: A Narrative Review. Nutrients 2023, 15, 155. https://doi.org/10.3390/nu15010155
Shiose K, Takahashi H, Yamada Y. Muscle Glycogen Assessment and Relationship with Body Hydration Status: A Narrative Review. Nutrients. 2023; 15(1):155. https://doi.org/10.3390/nu15010155
Chicago/Turabian StyleShiose, Keisuke, Hideyuki Takahashi, and Yosuke Yamada. 2023. "Muscle Glycogen Assessment and Relationship with Body Hydration Status: A Narrative Review" Nutrients 15, no. 1: 155. https://doi.org/10.3390/nu15010155
APA StyleShiose, K., Takahashi, H., & Yamada, Y. (2023). Muscle Glycogen Assessment and Relationship with Body Hydration Status: A Narrative Review. Nutrients, 15(1), 155. https://doi.org/10.3390/nu15010155






