Chronic Low or High Nutrient Intake and Myokine Levels
Abstract
1. Introduction
2. Material and Methods
2.1. Subjects
2.2. Cardiopulmonary Exercise Test
2.3. Blood Sampling
2.4. Determination of Myokines
2.5. Dietary Intake
2.6. Statistical Analyses
3. Results
3.1. General Characteristics
3.2. Dietary Intake
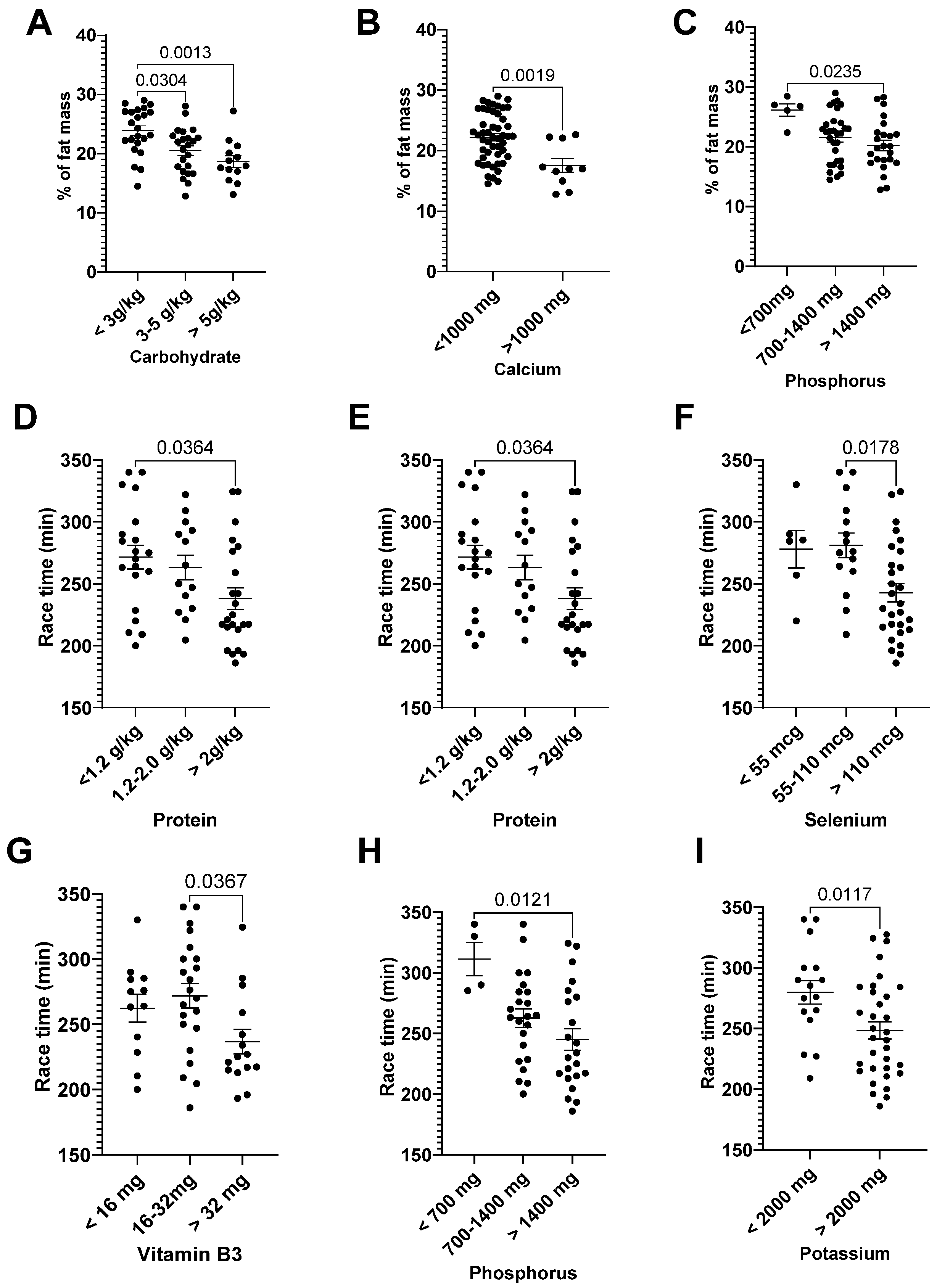
3.3. Correlation: Dietary Intake and General Characteristics
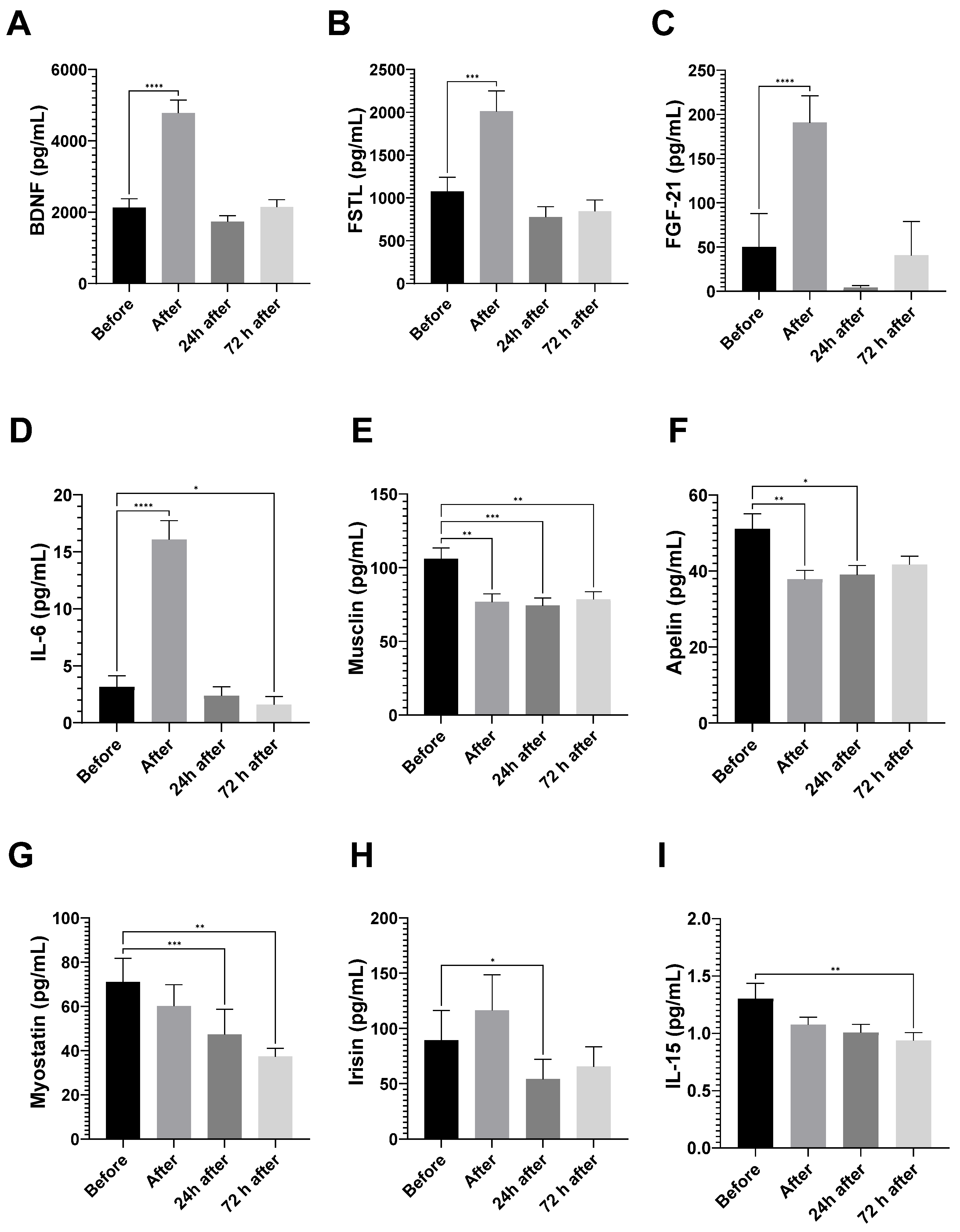
3.3.1. Myokine Analyses
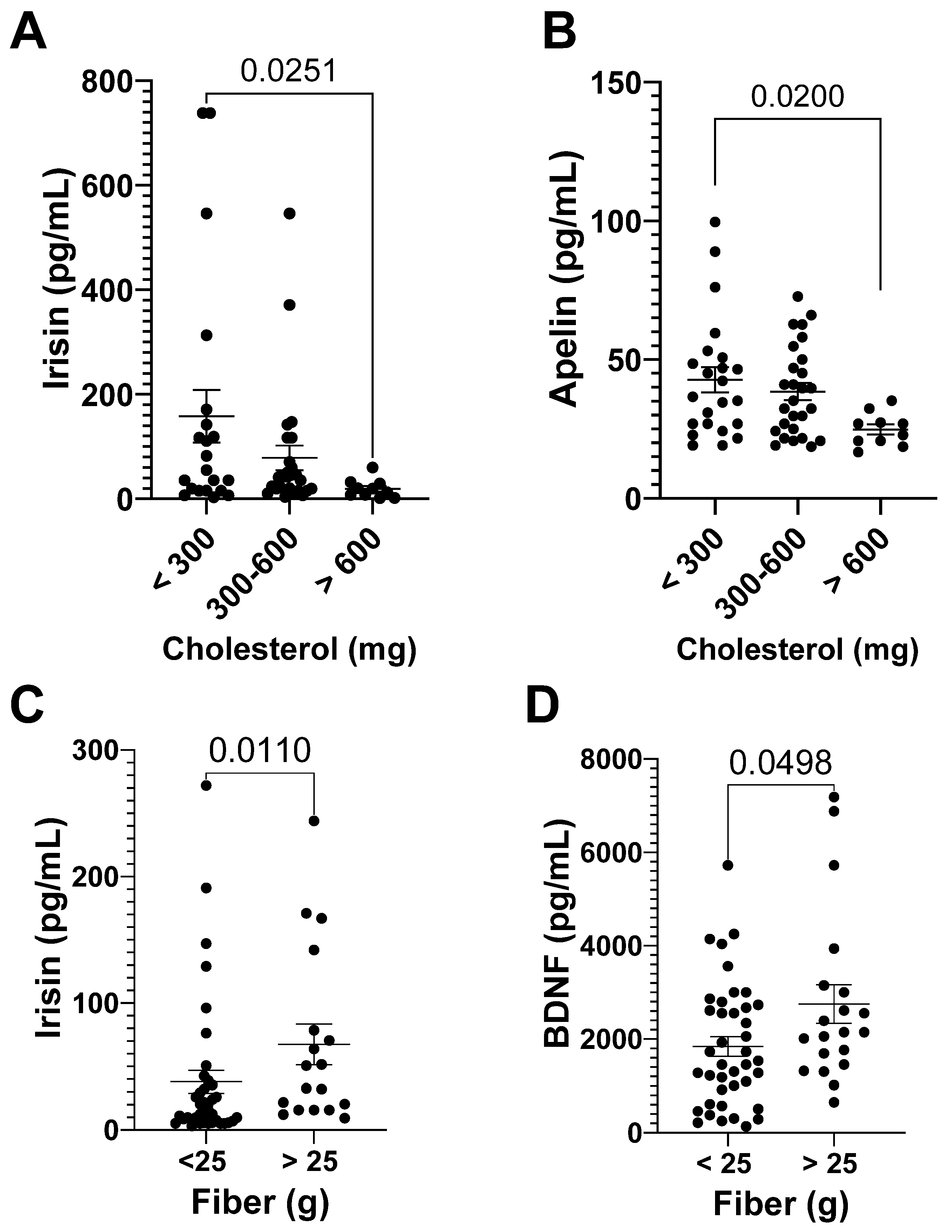
3.3.2. Myokines and DI
3.4. Myokines Induced by Exercise and Dietary Intake
4. Discussion
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hoffmann, C.; Weigert, C. Skeletal Muscle as an Endocrine Organ: The Role of Myokines in Exercise Adaptations. Cold Spring Harb. Perspect. Med. 2017, 7, a029793. [Google Scholar] [CrossRef] [PubMed]
- Laurens, C.; Bergouignan, A.; Moro, C. Exercise-Released Myokines in the Control of Energy Metabolism. Front. Physiol. 2020, 11, 91. [Google Scholar] [CrossRef] [PubMed]
- Piccirillo, R. Exercise-Induced Myokines with Therapeutic Potential for Muscle Wasting. Front. Physiol. 2019, 10, 287. [Google Scholar] [CrossRef]
- Sabaratnam, R.; Wojtaszewski, J.F.P.; Højlund, K. Factors mediating exercise-induced organ crosstalk. Acta Physiol. 2022, 234, e13766. [Google Scholar] [CrossRef]
- Chow, L.S.; Gerszten, R.E.; Taylor, J.M.; Pedersen, B.K.; van Praag, H.; Trappe, S.; Febbraio, M.A.; Galis, Z.S.; Gao, Y.; Haus, J.M.; et al. Exerkines in health, resilience and disease. Nat. Rev. Endocrinol. 2022, 18, 273–289. [Google Scholar] [CrossRef] [PubMed]
- de Sousa, C.A.Z.; Sierra, A.P.R.; Galán, B.S.M.; Maciel, J.F.D.S.; Manoel, R.; Barbeiro, H.V.; de Souza, H.P.; Cury-Boaventura, M.F. Time Course and Role of Exercise-Induced Cytokines in Muscle Damage and Repair After a Marathon Race. Front. Physiol. 2021, 12, 752144. [Google Scholar] [CrossRef]
- Bay, M.L.; Pedersen, B.K. Muscle-Organ Crosstalk: Focus on Immunometabolism. Front. Physiol. 2020, 11, 567881. [Google Scholar] [CrossRef]
- Lee, J.H.; Jun, H.-S. Role of Myokines in Regulating Skeletal Muscle Mass and Function. Front. Physiol. 2019, 10, 42. [Google Scholar] [CrossRef]
- Domin, R.; Dadej, D.; Pytka, M.; Zybek-Kocik, A.; Ruchała, M.; Guzik, P. Effect of Various Exercise Regimens on Selected Exercise-Induced Cytokines in Healthy People. Int. J. Environ. Res. Public Health 2021, 18, 1261. [Google Scholar] [CrossRef]
- Senesi, P.; Luzi, L.; Terruzzi, I. Adipokines, Myokines, and Cardiokines: The Role of Nutritional Interventions. Int. J. Mol. Sci. 2020, 21, 8372. [Google Scholar] [CrossRef]
- Hennigar, S.R.; McClung, J.P.; Pasiakos, S.M. Nutritional interventions and the IL-6 response to exercise. FASEB J. 2017, 31, 3719–3728. [Google Scholar] [CrossRef]
- Passos, B.N.; Lima, M.C.; Sierra, A.P.R.; Oliveira, R.A.; Maciel, J.F.S.; Manoel, R.; Rogante, J.I.; Pesquero, J.B.; Cury-Boaventura, M.F. Association of Daily Dietary Intake and Inflammation Induced by Marathon Race. Mediat. Inflamm. 2019, 2019, 1537274–1537278. [Google Scholar] [CrossRef] [PubMed]
- Casazza, G.A.; Tovar, A.P.; Richardson, C.E.; Cortez, A.N.; Davis, B.A. Energy Availability, Macronutrient Intake, and Nutritional Supplementation for Improving Exercise Performance in Endurance Athletes. Curr. Sports Med. Rep. 2018, 17, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Bronkowska, M.; Kosendiak, A.; Orzeł, D. Assessment of the frequency of intake of selected sources of dietary fibre among persons competing in marathons. Rocz. Panstw. Zakl. Hig. 2018, 69, 347–351. [Google Scholar] [CrossRef] [PubMed]
- Głąbska, D.; Jusińska, M. Analysis of the choice of food products and the energy value of diets of female middle- and long-distance runners depending on the self-assessment of their nutritional habits. Rocz. Panstw. Zakl. Hig. 2018, 69, 155–163. [Google Scholar] [PubMed]
- Franklin, B.A.; Thompson, P.D.; Al-Zaiti, S.S.; Albert, C.M.; Hivert, M.F.; Levine, B.D.; Lobelo, F.; Madan, K.; Sharrief, A.Z.; Eijsvogels, T.M.; et al. Exercise-Related Acute Cardiovascular Events and Potential Deleterious Adaptations Following Long-Term Exercise Training: Placing the Risks Into Perspective–An Update: A Scientific Statement From the American Heart Association. Circulation 2020, 141, e705–e736. [Google Scholar] [CrossRef]
- Thompson, P.D.; Arena, R.; Riebe, D.; Pescatello, L.S. ACSM’s New Preparticipation Health Screening Recommendations from ACSM’s Guidelines for Exercise Testing and Prescription, Ninth Edition. Curr. Sports Med. Rep. 2013, 12, 215–217. [Google Scholar] [CrossRef]
- Shirley, M.K.; Longman, D.P.; Elliott-Sale, K.J.; Hackney, A.C.; Sale, C.; Dolan, E. A Life History Perspective on Athletes with Low Energy Availability. Sports Med. 2022, 52, 1223–1234. [Google Scholar] [CrossRef]
- Mountjoy, M.; Sundgot-Borgen, J.; Burke, L.; Ackerman, K.E.; Blauwet, C.; Constantini, N.; Lebrun, C.; Lundy, B.; Melin, A.; Meyer, N.; et al. International Olympic Committee (IOC) Consensus Statement on Relative Energy Deficiency in Sport (RED-S): 2018 Update. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 316–331. [Google Scholar] [CrossRef]
- Mountjoy, M.L.; Burke, L.M.; Stellingwerff, T.; Sundgot-Borgen, J. Relative Energy Deficiency in Sport: The Tip of an Iceberg. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 313–315. [Google Scholar] [CrossRef]
- Hackney, A.C.; Elliott-Sale, K.J. Exercise Endocrinology: “What Comes Next?”. Endocrines 2021, 2, 167–170. [Google Scholar] [CrossRef] [PubMed]
- Elliott-Sale, K.J.; Tenforde, A.S.; Parziale, A.L.; Holtzman, B.; Ackerman, K.E. Endocrine Effects of Relative Energy Deficiency in Sport. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 335–349. [Google Scholar] [CrossRef] [PubMed]
- Karava, V.; Christoforidis, A.; Kondou, A.; Dotis, J.; Printza, N. Update on the Crosstalk Between Adipose Tissue and Mineral Balance in General Population and Chronic Kidney Disease. Front. Pediatr. 2021, 9, 696942. [Google Scholar] [CrossRef] [PubMed]
- Shan, T.; Liang, X.; Bi, P.; Kuang, S. Myostatin knockout drives browning of white adipose tissue through activating the AMPK-PGC1α-Fndc5 pathway in muscle. FASEB J. 2013, 27, 1981–1989. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.-F.; Ku, H.-C.; Lin, H. PGC-1α as a Pivotal Factor in Lipid and Metabolic Regulation. Int. J. Mol. Sci. 2018, 19, 3447. [Google Scholar] [CrossRef] [PubMed]
- Czaja, W.; Nakamura, Y.K.; Li, N.; Eldridge, J.A.; DeAvila, D.M.; Thompson, T.B.; Rodgers, B.D. Myostatin regulates pituitary development and hepatic IGF1. Am. J. Physiol. Endocrinol. 2019, 316, E1036–E1049. [Google Scholar] [CrossRef]
- Perry, C.A.; Van Guilder, G.P.; Butterick, T.A. Decreased myostatin in response to a controlled DASH diet is associated with improved body composition and cardiometabolic biomarkers in older adults: Results from a controlled-feeding diet intervention study. BMC Nutr. 2022, 8, 1–11. [Google Scholar] [CrossRef]
- Jeremic, N.; Chaturvedi, P.; Tyagi, S.C. Browning of White Fat: Novel Insight Into Factors, Mechanisms, and Therapeutics. J. Cell Physiol. 2017, 232, 61–68. [Google Scholar] [CrossRef]
- Subbotina, E.; Sierra, A.; Zhu, Z.; Gao, Z.; Koganti, S.R.K.; Reyes, S.; Stepniak, E.; Walsh, S.A.; Acevedo, M.R.; Perez-Terzic, C.M.; et al. Musclin is an activity-stimulated myokine that enhances physical endurance. Proc. Natl. Acad. Sci. USA 2015, 112, 16042–16047. [Google Scholar] [CrossRef]
- Liu, Y.; Huo, X.; Pang, X.F.; Zong, Z.H.; Meng, X.; Liu, G.L. Musclin Inhibits Insulin Activation of Akt/Protein Kinase B in Rat Skeletal Muscle. J. Int. Med. Res. 2008, 36, 496–504. [Google Scholar] [CrossRef]
- Rentería, I.; García-Suárez, P.C.; Fry, A.C.; Moncada-Jiménez, J.; Machado-Parra, J.P.; Antunes, B.M.; Jiménez-Maldonado, A. The Molecular Effects of BDNF Synthesis on Skeletal Muscle: A Mini-Review. Front. Physiol. 2022, 13, 934714. [Google Scholar] [CrossRef] [PubMed]
- Mughal, A.; O’Rourke, S.T. Vascular effects of apelin: Mechanisms and therapeutic potential. Pharmacol. Ther. 2018, 190, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Tanimura, Y.; Aoi, W.; Takanami, Y.; Kawai, Y.; Mizushima, K.; Naito, Y.; Yoshikawa, T. Acute exercise increases fibroblast growth factor 21 in metabolic organs and circulation. Physiol. Rep. 2016, 4, e12828. [Google Scholar] [CrossRef]
- Tezze, C.; Romanello, V.; Sandri, M. FGF21 as Modulator of Metabolism in Health and Disease. Front. Physiol. 2019, 10, 419. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, T.; Sugimoto, K.; Takahashi, T.; Yasunobe, Y.; Xie, K.; Tanaka, M.; Ohnishi, Y.; Yoshida, S.; Kurinami, H.; Akasaka, H.; et al. Overexpression of Interleukin-15 exhibits improved glucose tolerance and promotes GLUT4 translocation via AMP-Activated protein kinase pathway in skeletal muscle. Biochem. Biophys. Res. Commun. 2019, 509, 994–1000. [Google Scholar] [CrossRef]
- Akbari, H.; Hosseini-Bensenjan, M.; Salahi, S.; Moazzen, F.; Aria, H.; Manafi, A.; Hosseini, S.; Niknam, M.; Asadikaram, G. Apelin and its ratio to lipid factors are associated with cardiovascular diseases: A systematic review and meta-analysis. PLoS ONE 2022, 17, e0271899. [Google Scholar] [CrossRef]
- Waseem, R.; Shamsi, A.; Mohammad, T.; Hassan, M.I.; Kazim, S.N.; Chaudhary, A.A.; Rudayni, H.A.; Al-Zharani, M.; Ahmad, F.; Islam, A. FNDC5/Irisin: Physiology and Pathophysiology. Molecules 2022, 27, 1118. [Google Scholar] [CrossRef]
- Folino, A.; Accomasso, L.; Giachino, C.; Montarolo, P.G.; Losano, G.; Pagliaro, P.; Rastaldo, R. Apelin-induced cardioprotection against ischaemia/reperfusion injury: Roles of epidermal growth factor and Src. Acta Physiol. 2018, 222, e12924. [Google Scholar] [CrossRef]
- Esmaeili, S.; Bandarian, F.; Esmaeili, B.; Nasli-Esfahani, E. Apelin and stem cells: The role played in the cardiovascular system and energy metabolism. Cell Biol. Int. 2019, 43, 1332–1345. [Google Scholar] [CrossRef]
- Valenzuela, P.L.; Morales, J.S.; Emanuele, E.; Pareja-Galeano, H.; Lucia, A. Supplements with purported effects on muscle mass and strength. Eur. J. Nutr. 2019, 58, 2983–3008. [Google Scholar] [CrossRef] [PubMed]

| Lower | Adequate | Higher | Very Higher | |
|---|---|---|---|---|
| Sucrose (% of EI) | <10 | >10 | ||
| 40 | 20 | |||
| Carbohydrate (% of EI) | <45 | 45–65 | >65 | |
| 19 | 38 | 3 | ||
| Carbohydrate (g/kg/day) | <3 | 3–5 | >5 | |
| 23 | 23 | 14 | ||
| Protein (g/kg/day) | <1.2 | 1.2–2.0 | >2 | |
| 24 | 21 | 15 | ||
| Cholesterol (mg) | <300 | 300–600 | >600 | |
| 23 | 27 | 10 | ||
| Fiber (g) | <25 | >25 | ||
| 40 | 20 | |||
| Calcium (mg) | <1000 | >1000 | ||
| 50 | 10 | |||
| Sodium (mg) | <2300 | >2300 | ||
| 29 | 31 | |||
| Selenium (mcg) | <55 | 55–110 | >110 | |
| 7 | 20 | 33 | ||
| Vitamin B3 (mg) | <16 | 16–32 | >32 | |
| 16 | 29 | 15 | ||
| Phosphorus (mg) | <700 | 700–1400 | >1400 | |
| 5 | 30 | 25 | ||
| Potassium (mg) | <2000 | >2000 | ||
| 21 | 39 |
| Daily Intake | DV * | |
|---|---|---|
| Energy intake (kcal) | 2319 ± 117 | 2907 ± 36 |
| Energy availability (kcal/kg of FFM) | 40 ± 1.98 | >45 |
| Carbohydrate (g/kg) | 3.9 ± 0.3 | * 5–12 |
| Protein (g/kg) | 1.6 ± 0.1 | * 1.2–1.7 |
| Total fat (% of EI) | 29 ± 1 | <30% |
| Sucrose (% of EI) | 8 ± 1 | <10% |
| Cholesterol (mg) | 391 ± 28 | <300 |
| Fiber (g) | 22 ± 1 | >25 |
| Vitamins | Daily Intake | DV * | Minerals | Daily Intake | DV * |
|---|---|---|---|---|---|
| Vitamin A (mcg) | 994 ± 183 | 900 | Calcium (mg) | 715 ± 57 | 1000 |
| Vitamin B1 (mg) | 1.73 ± 0.14 | 1.2 | Iron (mg) | 15.7 ± 1.5 | 8 |
| Vitamin B2 (mg) | 1.94 ± 0.15 | 1.3 | Mn (mg) | 2.8 ± 0.30 | 2.3 |
| Vitamin B3 (mg) | 28 ± 3 | 16 | Se (mcg) | 163 ± 18 | 55 |
| Vitamin B6 (mg) | 2.3 ± 0.2 | 1.7 | Zinc (mg) | 12.7 ± 0.9 | 11 |
| Folic acid (mg) | 286 ± 27 | 400 | Mg (mg) | 289 ± 16 | 420 |
| Vitamin B12 (mcg) | 6.4 ± 1.6 | 2.4 | P (mg) | 1340 ± 73 | 700 |
| Vitamin C (mg) | 141 ± 28 | 90 | Potassium (g) | 2.6 ± 130 | 4.7 |
| Vitamin D (mcg) | 3.7 ± 0.53 | 15 | Sodium (g) | 2.5 ± 1.6 | 1.5 |
| Vitamin E (mg) | 12.34 ± 1.5 | 15 |
| % of Fat Mass | r | p | Race Time (min) | r | p |
|---|---|---|---|---|---|
| EI (kcal/kg of FFM) | −0.28 | 0.025 | Protein (g/kg) | −0.35 | 0.012 |
| % of adequate EI | −0.34 | 0.007 | Cholesterol (mg) | −0.40 | 0.043 |
| Carbohydrate (g/kg) | −0.41 | 0.0009 | Vitamin B3 (mg) | −0.30 | 0.033 |
| Protein (g/kg) | −0.32 | 0.013 | Se (mcg) | −0.30 | 0.032 |
| Sucrose (g) | −0.34 | 0.007 | Mg (mg) | −0.36 | 0.011 |
| Vitamin B2 (mg) | −0.27 | 0.033 | K (mg) | −0.29 | 0.043 |
| Calcium (mg) | −0.49 | <p.0001 | P (mg) | −0.38 | 0.006 |
| Mn (mg) | −0.31 | 0.016 | |||
| P (mg) | −0.35 | 0.007 |
| r | p | |
|---|---|---|
| IL-15 24 h after | 0.33 | 0.010 |
| Musclin after | 0.34 | 0.0075 |
| Musclin 24 h after | 0.27 | 0.038 |
| FSTL 24 h after | 0.26 | 0.049 |
| Myostatin after | 0.26 | 0.037 |
| Myostatin 24 h after | 0.27 | 0.033 |
| IL-6 24 h after | 0.30 | 0.019 |
| FGF-21 24 h after | 0.28 | 0.031 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sierra, A.P.R.; Fontes-Junior, A.A.; Paz, I.A.; de Sousa, C.A.Z.; Manoel, L.A.d.S.; Menezes, D.C.d.; Rocha, V.A.; Barbeiro, H.V.; Souza, H.P.d.; Cury-Boaventura, M.F. Chronic Low or High Nutrient Intake and Myokine Levels. Nutrients 2023, 15, 153. https://doi.org/10.3390/nu15010153
Sierra APR, Fontes-Junior AA, Paz IA, de Sousa CAZ, Manoel LAdS, Menezes DCd, Rocha VA, Barbeiro HV, Souza HPd, Cury-Boaventura MF. Chronic Low or High Nutrient Intake and Myokine Levels. Nutrients. 2023; 15(1):153. https://doi.org/10.3390/nu15010153
Chicago/Turabian StyleSierra, Ana Paula Renno, Antônio Alves Fontes-Junior, Inês Assis Paz, Cesar Augustus Zocoler de Sousa, Leticia Aparecida da Silva Manoel, Duane Cardoso de Menezes, Vinicius Alves Rocha, Hermes Vieira Barbeiro, Heraldo Possolo de Souza, and Maria Fernanda Cury-Boaventura. 2023. "Chronic Low or High Nutrient Intake and Myokine Levels" Nutrients 15, no. 1: 153. https://doi.org/10.3390/nu15010153
APA StyleSierra, A. P. R., Fontes-Junior, A. A., Paz, I. A., de Sousa, C. A. Z., Manoel, L. A. d. S., Menezes, D. C. d., Rocha, V. A., Barbeiro, H. V., Souza, H. P. d., & Cury-Boaventura, M. F. (2023). Chronic Low or High Nutrient Intake and Myokine Levels. Nutrients, 15(1), 153. https://doi.org/10.3390/nu15010153






