Abstract
Advanced glycated end products (AGEs) accumulate systemically and cause diabetes complications. However, whether noninvasive measurable AGEs are associated with diabetes status and physical functions remains unclear. One hundred and ten patients with cardiovascular disease (CVD) who underwent outpatient cardiac rehabilitation were included. AGEs scores, using AGEs sensors, were evaluated concomitantly with a physical evaluation, including testing the isometric knee extension strength (IKES) and 6 min walking distance (6MWD). Thirty-three (30%) patients had a history of diabetes mellitus (DM). The AGEs score was not different in the presence of DM history (0.52 ± 0.09 vs. 0.51 ± 0.09, p = 0.768) and was not correlated with blood glucose (r = 0.001, p = 0.995). The AGEs score was positively correlated with hemoglobin A1c (HbA1c, r = 0.288, p = 0.004) and negatively correlated with physical functions (IKES, r = −0.243, p = 0.011; 6MWD, r = −0.298, p = 0.002). The multivariate analysis demonstrated that 6MWD was independently associated with a high AGEs score (>0.52). The AGEs score was associated with HbA1c, IKES, and 6MWD in patients with CVD. The AGEs score might be a useful indicator for evaluating not only glycemic control but also physical functions.
1. Introduction
Diabetes mellitus (DM) affects all-cause mortality in patients with cardiovascular disease (CVD) [1]. DM is an important factor in the secondary prevention and management of CVD. When hyperglycemia continues, serum advanced glycation end-products (AGEs) are produced during glucose metabolism [2]. Serum AGEs induce oxidative stress, which causes nitric oxide inactivation, inflammatory responses, thrombus formation, and progression of arteriosclerosis [3,4]. The accumulation of AGEs leads to the development of CVD. AGEs were evaluated by using a noninvasive physical method that uses the forearm and as a blood sampling test [5]. Skin autofluorescence (sAF) measured using the forearm was strongly correlated with AGEs from skin biopsy [6], although sAF did not match either serum or urine AGEs [7]. This sAF was associated with the duration of DM [8,9,10] and hemoglobin A1c [6,8,11]. Moreover, sAF was associated not only with DM but also with physical functions, including muscle strength and exercise capacity [11]. Furthermore, skin AGEs in patients undergoing cardiac rehabilitation are predictive of all-cause mortality and hospitalization for heart failure [12]. A meta-analysis reported that sAF levels measured by the forearm might be useful in assessing mortality risk in patients with CVD [13]. The most important aspect of CVD management is the noninvasive assessment of AGEs. Yamanaka et al. developed an AGEs sensor that can easily and quickly evaluate sAF by using a fingertip [14]. Several studies that used this new device showed that the AGEs score measured by the AGEs sensor was associated with serum AGEs [14] levels and glycation stress [15]. However, the relationship between AGEs score and clinical characteristics, including DM, has not been completely evaluated. Furthermore, the relationship between AGEs score and physical functions remains unclear. Thus, the purpose of this study was to (1) investigate the relationship between AGEs score and DM, (2) evaluate the relationship between AGEs score and glycemic control, and (3) assess the relationship between AGEs score and physical functions in patients with CVD.
2. Materials and Methods
2.1. Study Population
We conducted a single-center retrospective observational study between August 2020 and November 2021 at the cardiac rehabilitation center of Kitasato University Kitasato Institute Hospital. We enrolled 149 patients with CVD who underwent cardiac rehabilitation. CVD diagnosis included ischemic heart disease (myocardial infarction, angina pectoris, and vasospastic angina), heart failure, valvular heart disease, and atrial fibrillation. After excluding patients aged < 65 years (n = 33) and those who had difficulty measuring physical functions due to a decline in cognitive function or orthopedic disease (n = 6), 110 patients were finally included in the study (Figure 1). The Kitasato Institute Hospital Research Ethics Committee approved the study protocol (clinical trial registration number 21028).
Figure 1.
Study flowchart.
2.2. Assessment of AGEs Score
To estimate the AGEs score, cardiac rehabilitation measurements of sAF levels were performed by using an AGEs sensor (SHARP, Kobe, Japan). AGEs have the property of emitting fluorescence when the specific excitation light irradiates them. The AGEs sensor irradiates the fingertips with excitation light, acquires percutaneous fluorescence of the fingertips, and measures skin autofluorescence [4]. The sAF levels were measured by using the middle finger of the left hand, in which the least amount of skin melanin was present [14]. We performed sAF measurements twice before cardiac rehabilitation and used the mean values for the analysis. The measured AGEs were expressed as the AGEs score in arbitrary units with an upper limit of 10.0 and a lower limit of 0.0. According to a recent manufacturer survey, 0.5 is an arbitrary unit that approximately corresponds to the average score of healthy Japanese patients aged 50 years. The AGEs sensor displayed a value when the coefficient of variation was less than 1%. A previous study has demonstrated that AGEs sensor is useful for the noninvasive assessment of glycation stress [15].
2.3. Assessment of Physical Functions
We evaluated handgrip strength (HGS), isometric knee extension strength (IKES), and 6 min walking distance (6MWD) as physical functions and used a dynamometer to measure HGS (TKK 5401; Takei, Tokyo, Japan). The patients performed two maximal isometric voluntary contractions of both hands for 3 s each while seated on a bench with the elbow flexed at 90°. The width of the dynamometer handle was adjusted for each patient to match their hand size. The highest strength measurement (kg) was used for the analysis. IKES was measured by using a handheld dynamometer to determine leg strength (μ-Tas; ANIMA, Tokyo, Japan). Briefly, with the patient seated in a chair with a non-extensible strap connecting the ankle to a strain gauge, 5 s of maximal isometric voluntary contractions of the quadriceps was collected twice for both legs, with the hip joint at approximately 90° flexion. Consecutive measurements were obtained for the right and left quadriceps muscles. The highest strength values on the right or left side were expressed as absolute values (kg) and relative to the body mass (%BM). The 6MWD was determined according to the guidelines of the American Thoracic Society, under the supervision of technicians. The patients were instructed to walk at their own pace along a straight, flat hallway from chair to chair, and the distance (in meters) was recorded after six minutes.
2.4. Definition
Hypertension was defined as an arterial blood pressure of ≥140/90 mmHg or the use of antihypertensive medication. Dyslipidemia (DL) was defined as low-density lipoprotein cholesterol ≥ 140 mg/dL, triglyceride ≥ 150 mg/dL, or the use of medication for DL. DM was defined as symptoms of diabetes plus random plasma glucose concentration ≥ 200 mg/dL, fasting plasma glucose concentration ≥ 126 mg/dL, or use of medication for DM. AGEs score, physical function, laboratory data, and clinical information were obtained within 2 weeks.
2.5. Statistical Analysis
Continuous variables with normal distribution were expressed as mean ± standard deviation (SD), whereas the median value with interquartile range was reported when the data were not normally distributed. The basic characteristics of patients with or without DM were compared. We analyzed the correlation between the AGEs score and patient characteristics. Continuous variables were analyzed by using a t-test. Categorical variables were reported as counts (%) and analyzed by using the chi-squared test. Multivariate regression analysis was performed to identify the factors associated with the presence of high AGEs score among variables with p < 0.050 in the univariate logistic regression analysis. Statistical significance was defined at p < 0.050. SPSS 27 version (IBM Corporation, Armonk, NY, USA) was used to perform all statistical analyses. Since related studies were limited, the cutoff value of the AGEs score is unknown. In this study, the AGEs score was normally distributed (Figure 2). We defined values above the median high as high AGEs score and below the median low as low AGEs score. Thus, the median AGEs score (0.52) was classified into two groups (high- and low-AGE patients) and subsequently analyzed.
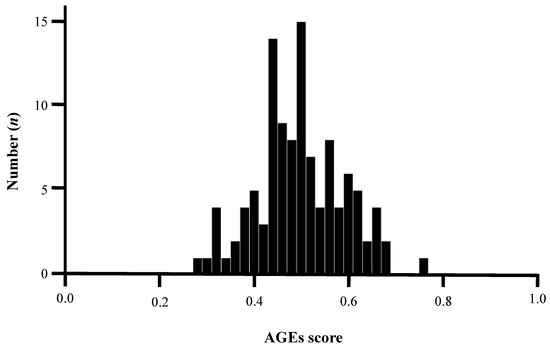
Figure 2.
Normality test of AGEs score by Kolmogorov–Smirnov test. The average AGEs score was 0.51, and the median value was 0.52, indicating a normal distribution (p = 0.124). AGEs, advanced glycated end products.
3. Results
3.1. Clinical Characteristics between DM (+) and DM (−)
Among one hundred and ten older patients with cardiac rehabilitation, the number of patients with DM (+) and DM (−) were thirty-three (30%) and seventy-seven (70%), respectively. Table 1 describes the baseline clinical characteristics according to DM status. All clinical characteristics were similar between the two groups, except for male sex (79% vs. 58%, p = 0.041), DL (55% vs. 25%, p = 0.002), dipeptidyl peptidase-4 inhibitors (42% vs. 0%, p = 0.001), insulin (9% vs. 0%, p = 0.002), metformin (33% vs. 0%, p = 0.001), sodium-glucose cotransporter 2 inhibitors (61% vs. 12%, p = 0.001), blood glucose (139.6 ± 46.8 vs. 109.7 ± 22.4 mg/dL, p = 0.001), and HbA1c (7.0 ± 0.7 vs. 5.9 ± 0.5%, p = 0.001). There was no significant difference in the AGEs scores between DM (+) and DM (−) (0.52 ± 0.09 vs. 0.51 ± 0.09, p = 0.768).

Table 1.
Clinical characteristics of patients with and without DM.
3.2. Correlation between AGEs Score and Clinical Characteristics
Table 2 describes the correlation between AGEs score and clinical characteristics. The AGEs score was not correlated with DM history (r = 0.038, p = 0.690), diabetic retinopathy (r = 0.133, p = 0.165), diabetic nephropathy (r = 0.109, p = 0.257), diabetic complications (r = 0.130, p = 0.175), or blood glucose (r = 0.001, p = 0.995). In the DM(−) population, there was no correlation between HbA1c and AGEs score (r = 0.299, p = 0.102). AGEs score was positively correlated with HbA1c (r = 0.286, p = 0.004) (Figure 3a) and negatively correlated with IKES (r = −0.248, p = 0.010) (Figure 3b) and 6MWD (r = −0.298, p = 0.002) (Figure 3c). The AGEs score was not correlated with other clinical characteristics in this study (Supplementary Figure S1).

Table 2.
Correlation between AGEs score and clinical characteristics.
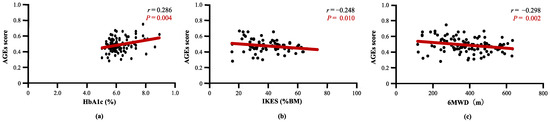
Figure 3.
Correlation between AGEs score and clinical characteristics. (a) AGEs score was significantly correlated with HbA1c, (b) AGEs score was significantly correlated with Isometric knee extension strength, and (c) AGEs score was significantly correlated with 6MWD. AGEs, advanced glycated end products; HbA1c, hemoglobin-A1c; IKES, isometric knee extension strength; 6MWD, 6 min walking distance.
3.3. Comparison of Clinical Characteristics between High and Low AGEs Score
The number of patients with a high and low AGEs score was fifty-three (48%) and fifty-seven (52%), respectively. Table 3 shows the comparison of clinical characteristics between the high AGE and low AGE score. All the clinical characteristics were similar between the two groups, except for HbA1c (6.4 ± 0.8 vs. 6.1 ± 0.7%, p = 0.044), HGS (21.1 ± 7.6 vs. 24.8 ± 8.8 kg, p = 0.023), IKES (36.5 ± 12.0 vs. 42.8 ± 13.5%BM, p = 0.013), and 6MWD (345 ± 132 vs. 410 ± 112 m, p = 0.010).

Table 3.
Comparison of baseline characteristics of patents with high AGEs and low AGEs score.
3.4. Physical Function and Presence of High AGEs Score
The univariate analysis showed that 6MWD (odds ratio (OR) 0.996; 95% confidence interval (CI) 0.993–0.999; p = 0.015) was significantly associated with the presence of a high AGEs score (>0.52) (Table 4). The multivariate analysis demonstrated that the 6MWD was independently associated with a high AGEs score (>0.52) (Table 5). Compared to the factors previously associated with AGEs [16,17,18], 6MWD was independently associated with a high AGEs score (Table 6 and Table 7).

Table 4.
Univariate analysis for the presence of high AGEs score (>0.52).

Table 5.
Multivariate analysis for high AGEs score (>0.52).

Table 6.
Multivariate analysis for high AGEs score (>0.52).

Table 7.
Multivariate analysis for high AGEs score (>0.52).
4. Discussion
The main findings of this study were as follows: (1) there was no significant difference in AGEs score of patients with or without DM; (2) the AGEs score was significantly correlated with HbA1c but not with blood glucose; and (3) the AGEs score was also significantly correlated with physical functions, including IKES and 6MWD. In particular, the 6MWD was independently associated with a high AGEs score (>0.52).
4.1. AGEs Score and DM
In this study, AGEs score was not associated with a history of DM. Our results corroborated the findings of a previous study, which reported that AGEs measured by the forearm were not associated with a history of DM [12]. AGEs are metabolites of blood glucose and may not directly reflect the history of DM. AGEs include a variety of substances, such as pentosidine, carboxymethyl lysine, and pyrraline, which are produced by metabolites of blood glucose [19]. AGEs measured in the forearm have been shown to correlate with pentosidine levels [8]. AGEs measured at the fingertip were correlated with methylglyoxal 5-hydro-5-methylimidazolones [15]. Both are metabolites of blood glucose and may reflect glucose metabolism, which is not directly related to the history of DM. Furthermore, AGEs are not necessarily related to blood glucose because they are produced not only from glucose but also from fructose and aldehydes [20,21]. In contrast, it has also been reported that patients with DM have higher serum AGEs than those without DM [22]. These differences might be caused by variations in glucose metabolism, DM treatment, and subsequent DM status. Thus, the relationship between the AGEs score and a history of DM remains controversial. This small number of studies indicates that further data accumulation is needed in the future.
4.2. AGEs Score and HbA1c
The AGEs score was significantly correlated with HbA1c and was not correlated with blood glucose levels in this study. Previously, the serum AGEs were also strongly associated with HbA1c [22] but were not associated with blood glucose levels [11]. This is because AGEs are metabolized relatively slowly over weeks to months [23], reflecting the status of glycemic control over the medium-to-long-term from the vein to the skin [24]. Furthermore, HbA1c is an Amadori rearrangement substance produced by the same process as that of AGEs [25]. Therefore, AGEs are thought to reflect mid-to-long-term glycemic control rather than current glycemic control. Our study showed that the AGEs score may be useful as an indicator of noninvasive glycemic control in patients with CVD.
4.3. AGEs Score and Physical Functions
In this study, the AGEs score was also significantly correlated with physical functions, including IKES and 6MWD. The 6MWD was independently associated with a high AGEs score (>0.52). Previous studies have shown that forearm AGEs are significantly associated with reduced exercise tolerance [11] and physical functions, such as handgrip strength [26] and walking speed [27]. Accumulated AGEs increase muscle stiffness, reduce the viscoelastic properties of muscles, and impair muscle function [28]. In endothelial cells, AGEs affect endothelial dysfunction and loss of muscle mass and strength [29]. Clinically, serum AGEs are associated with evaluated endothelial function by brachial flow-mediated vasodilation [30]. Similarly, AGEs decrease exercise tolerance in the myocardium by inducing myocardial stiffness and diastolic dysfunction [3,31]. Therefore, patients with high AGEs have weaker HGS, IKES, and 6MWD than those with lower AGEs score. Furthermore, high physical function reduces the accumulation of AGEs. Since muscles consume glucose, AGEs accumulate less because of high muscle strength and exercise tolerance [32]. Several studies have clarified the relationship between physical activity and AGEs [33,34]. It has been reported that patients with higher physical activity have lower forearm AGEs [33]. Furthermore, AGEs are influenced by lifestyle habits such as physical activity, sleeping time, and cognitive function [35]. Individuals with low physical activity have been reported to accumulate more AGEs than those with high physical activity [34]. As mentioned above, the accumulation of AGEs affects muscle strength and exercise tolerance, whereas decreased physical activity accelerates the accumulation of AGEs. However, the causal relationship between AGEs and physical function remains unclear. A recent study suggests that long-term exercise may be reduced AGEs [36]. Further longitudinal studies are required to elucidate the causal relationship between AGEs accumulation and physical functions.
4.4. Limitations
This study had several limitations. First, this was a retrospective and observational study conducted at a single center with a limited number of patients. Second, there was no control group without CVD, because we focused on patients with CVD. Therefore, the results should be interpreted cautiously. Third, there was no complete removal of potential confounding factors that might affect the AGEs score (e.g., temperature, time, and season). Fourth, the AGEs score index may vary among the CVD types. Fifth, the results might have changed if a different cutoff AGEs score was applied. Sixth, there were no data on the diagnosis of DM in these patients. Seventh, this study did not consider the effects of dietary guidance or lifestyle. Eighth, three were no data of blood, plasma, or serum AGEs concentration. Thus, the interpretation needs caution about the association between the AGEs score and the presence of DM history. Ninth, this study included limited patients with chronic heart failure (52%), ischemia heart disease who underwent percutaneous coronary intervention (28%), valvular disease (6%), and atrial fibrillation (30%) who were outpatients for cardiac rehabilitation. The other cardiovascular disease could not speculate the AGEs score by sAF. Lastly, the findings of this observational study did not clarify the causal relationship between AGEs score and DM.
5. Conclusions
The AGEs score was associated with HbA1c level and physical functions in patients with CVD. The AGEs score might be a useful indicator for evaluating not only glycemic control but also physical functions.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu14153032/s1, Figure S1: Correlation between AGEs score and clinical characteristics.
Author Contributions
Conceptualization, T.H. and K.F.; methodology, T.H. and K.F.; software, T.H.; validation, T.H.; formal analysis, T.H.; investigation, T.H., K.F., T.M., J.K. and K.S.; resources, T.T.; data curation, T.H.; writing—original draft preparation, T.H., K.F., S.Y. and M.Y.-T.; writing—review and editing, T.H., K.F., S.Y., T.M., J.K., K.I., M.I., M.Y.-T., T.I., K.S. and T.T.; visualization, T.H. and K.F.; supervision, T.H. and K.F.; project administration, T.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of The Kitasato Institute Hospital Research Ethics Committee, protocol code 21028.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to thank all patients and hospital staff involved in this study. The authors would like to thank the Kitasato University Kitasato Institute Hospital for providing the facilities to perform this study.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Kavanagh, T.; Mertens, D.J.; Hamm, L.F.; Beyene, J.; Kennedy, J.; Corey, P.; Shephard, R.J. Prediction of long-term prognosis in 12,169 men referred for cardiac rehabilitation. Circulation 2002, 106, 666–671. [Google Scholar] [CrossRef] [PubMed]
- Brownlee, M. Lilly Lecture 1993 Glycation and Diabetic Complications. Diabetes 1994, 43, 836–841. [Google Scholar] [CrossRef] [PubMed]
- Kovacic, J.C.; Castellano, J.M.; Farkouh, M.E.; Fuster, V. The relationships between cardiovascular disease and diabetes: Focus on pathogenesis. Endocrinol. Metab. Clin. 2014, 43, 41–57. [Google Scholar] [CrossRef] [PubMed]
- Yamagishi, S.; Matsui, T.; Nakamura, K.; Takeuchi, M.; Imaizumi, T. Pigment epithelium-derived factor (PEDF) prevents diabetes- or advanced glycation end products (AGE)-elicited retinal leukostasis. Microvasc. Res. 2006, 72, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.H.; Craig, M.E.; Januszewski, A.S.; Aguirre, P.B.; Hing, S.; Jenkins, A.J.; Donaghue, K.C. Higher skin autofluorescence in young people with Type 1 diabetes and microvascular complications. Diabet. Med. 2017, 34, 543–550. [Google Scholar] [CrossRef]
- Meerwaldt, R.; Links, T.; Graff, R.; Thorpe, S.R.; Baynes, J.W.; Hartog, J.; Gans, R.; Smit, A. Simple noninvasive measurement of skin autofluorescence. Ann. N. Y. Acad. Sci. 2005, 1043, 290–298. [Google Scholar] [CrossRef]
- Mera, K.; Nagai, M.; Brock, W.C.; Fujiwara, Y.; Murata, T.; Maruyama, T.; Baynes, J.W.; Otagiri, M.; Nagai, R. Glutaraldehyde is an effective cross-linker for production of antibodies against advanced glycation end-products. J. Immunol. Methods 2008, 334, 82–90. [Google Scholar] [CrossRef][Green Version]
- Meerwaldt, R.; Graaff, R.; Oomen, P.H.N.; Links, T.P.; Jager, J.J.; Alderson, N.L.; Thorpe, S.R.; Baynes, J.W.; Gans, R.O.B.; Smit, A.J. Simple non-invasive assessment of advanced glycation endproduct accumulation. Diabetologia 2004, 47, 1324–1330. [Google Scholar] [CrossRef]
- Ali, S.; Rao, N.L. Correlation of serum fluorescence of advanced glycation end products with diabetes duration and glycemic control in type 2 diabetic patients. Biomed. Res. Ther. 2020, 7, 1002–1007. [Google Scholar] [CrossRef]
- Rajaobelina, K.; Gregoire, A.C.; Delcourt, C.; Gin, H.; Gateau, P.B.; Rigalleau, V. Autofluorescence of skin advanced glycation end products: Marker of metabolic memory in elderly population. J. Gerontol. Ser. A: Biomed. Sci. Med. Sci. 2014, 70, 841–846. [Google Scholar] [CrossRef]
- Kunimoto, M.; Shimada, K.; Yokoyama, M.; Matsubara, T.; Aikawa, T.; Ouchi, S.; Shimizu, M.; Fukao, K.; Miyazaki, T.; Kadoguchi, T.; et al. Association between the tissue accumulation of advanced glycation end products and exercise capacity in cardiac rehabilitation patients. BMC Cardiovasc. Disord. 2020, 20, 195. [Google Scholar] [CrossRef]
- Kunimoto, M.; Yokoyama, M.; Shimada, K.; Matsubara, T.; Aikawa, T.; Ouchi, S.; Fukao, K.; Miyazaki, T.; Fujiwara, K.; Abulimiti, A.; et al. Relationship between skin autofluorescence levels and clinical events in patients with heart failure undergoing cardiac rehabilitation. Cardiovasc. Diabetol. 2021, 20, 208. [Google Scholar] [CrossRef]
- Redondo, I.C.; Cano, A.S.; Bueno, C.A.; Cunha, P.G.; Hortelano, J.A.M.; Miguel, M.G.; Macias, C.B.; Vizcaino, V.M. Skin Autofluorescence-Indicated Advanced Glycation End Products as Predictors of Cardiovascular and All-Cause Mortality in High-Risk Subjects: A Systematic Review and Meta-analysis. J. Am. Heart Assoc. 2018, 18, e009833. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, M.; Matsumura, T.; Ohno, R.; Fujiwara, Y.; Shinagawa, M.; Sugawa, H.; Hatano, K.; Shinkawa, J.; Kinoshita, H.; Ito, K.; et al. Nonninvasive measurement of skin autofluorescence to evaluate diabetic complications. J. Clin. Biochem. Nutr. 2016, 58, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Morita, Y.; Yagi, M.; Ishizaki, K.; Takabe, W.; Komatsu, T.; Nakazawa, M.; Matsushima, M.; Urata, T.; Yohei, Y. Evaluation of the glycative stress by non-invansive skin AGEs measurement devices. Glycative Stress Res. 2019, 6, 92–102. [Google Scholar] [CrossRef]
- Hangai, M.; Takebe, N.; Honma, H.; Sasaki, A.; Chiba, A.; Nakano, R.; Togashi, H.; Nakagawa, R.; Oda, T.; Matsui, M.; et al. Association of advanced glycation end products with coronary artery calcification in Japanese subjects with type 2 diabetes as assessed by skin autofluorescence. J. Atheroscler. Thromb. 2016, 23, 1178–1187. [Google Scholar] [CrossRef] [PubMed]
- Dyer, D.G.; Dunnm, J.A.; Thorpe, S.R.; Bailie, K.E.; Lyons, T.J.; McCance, D.R.; Baynes, J.W. Accumulation of Maillard Reaction Products in Skin Collagen in Diabetes and Aging. J. Clin. Investig. 1993, 91, 2463–2469. [Google Scholar] [CrossRef]
- Fukushima, Y.; Daida, H.; Morimoto, T.; Kasai, T.; Miyauchi, K.; Yamaguchi, S.; Takeuchi, M.; Hiro, T.; Kimura, T.; Nakagawa, Y.; et al. Relationship between Advanced Glycation End Products and Plaque Progression in Patients with Acute Coronary Syndrome: The JAPAN-ACS Sub-study. Cardiovasc. Diabetol. 2013, 12, 5. [Google Scholar] [CrossRef]
- Nagai, R.; Mori, T.; Yamamoto, Y.; Kaji, Y.; Yonei, Y. Significance of Advanced Glycation End Products in Aging-Related Disease. Anti-Aging Med. 2010, 7, 112–119. [Google Scholar] [CrossRef]
- Delbridge, L.M.D.; Benson, V.L.; Ritchie, R.H.; Mellor, K.M. Diabetic cardiomyopathy: The case for a role of fructose in disease etiology. Diabetes 2016, 65, 3521–3528. [Google Scholar] [CrossRef]
- Vasdev, S.; Gill, V.; Singal, P.K. Beneficial effect of low ethanol intake on the cardiovascular system: Possible biochemical mechanisms. Vasc. Health Risk Manag. 2006, 2, 263–276. [Google Scholar] [CrossRef]
- Jakuš, V.; Šándorová, E.; Kalninová, J.; Krahulec, B. Monitoring of glycation, oxidative stress and inflammation in relation to the occurrence of vascular complications in patients with type 2 diabetes mellitus. Physiol. Res. 2014, 63, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Brownlee, M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001, 414, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Yamagishi, S. Potential clinical utility of advanced glycation end product cross-link breakers in age- and diabetes-associated disorders. Rejuvenation Res. 2012, 15, 564–572. [Google Scholar] [CrossRef] [PubMed]
- Meerwaldt, R.; Links, T.; Zeebregts, C.; Tio, R.; Hillebrands, J.L.; Smit, A. The clinical relevance of assessing advanced glycation endproducts accumulation in diabetes. Cardiovasc. Diabetol. 2008, 7, 29. [Google Scholar] [CrossRef] [PubMed]
- Dalal, M.; Ferrucci, L.; Sun, K.; Beck, J.; Fried, L.P.; Semba, R.D. Elevated serum advanced glycation end products and poor grip strength in older community-dwelling women. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2009, 64, 132–137. [Google Scholar] [CrossRef]
- Semba, R.D.; Bandinelli, S.; Sun, K.; Guralnik, J.M.; Ferrucci, L. Relationship of an advanced glycation end product, plasma carboxymethyl-lysine, with slow walking speed in older adults: The InCHIANTI study. Eur. J. Appl. Physiol. 2010, 108, 191–195. [Google Scholar] [CrossRef]
- Haus, J.M.; Carrithers, J.A.; Trappe, S.W.; Trappe, T.A. Collagen, cross-linking, and advanced glycation end products in aging human skeletal muscle. J. Appl. Physiol. 2007, 103, 2068–2076. [Google Scholar] [CrossRef]
- Payne, G.W. Effect of Inflammation on the Aging Microcirculation: Impact on Skeletal Muscle Blood Flow Control. Microcirclation 2006, 13, 343–352. [Google Scholar] [CrossRef]
- Ares, S.C.; Cardelo, M.P.; Mariscal, M.G.; Pena, J.D.T.; Rios, A.G.; Katsuki, N.; Malagon, M.M.; Miranda, J.L.; Martinez, P.P.; Serrano, E.M.Y. Endothelial dysfunction and advanced glycation end products in patients with newly diagnosed versus established diabetes: From the CORDIOPREV study. Nutrients 2020, 12, 238. [Google Scholar] [CrossRef]
- Hegab, Z.; Gibbons, S.; Neyses, L.; Mamas, M.A. Role of advanced glycation end products in cardiovascular disease. World J. Cardiol. 2012, 4, 90–102. [Google Scholar] [CrossRef] [PubMed]
- Kawamoto, T.; Takabe, W.; Ogura, M.; Yagi, M.; Watanabe, K.; Yonei, Y. Effect of continuous walking exercise program on the glycative stress marker in the elderly. Glycative Stress Res. 2017, 4, 144–157. [Google Scholar]
- Drenth, H.; Zuidema, S.U.; Krijnen, W.P.; Bautmans, I.; Smit, A.J.; Schans, C.; Hobbelen, H. Advanced glycation end products are associated with physical activity and physical functioning in the older population. J. Gerontol. A Biol. Sci. Med. Sci. 2018, 73, 1545–1551. [Google Scholar] [CrossRef] [PubMed]
- Isami, F.; West, B.J.; Nakajima, S.; Yamagishi, I. Association of advanced glycation end products, evaluated by skin autofluorescence, with lifestyle habits in a general Japanese population. J. Int. Med. 2018, 46, 1043–1051. [Google Scholar] [CrossRef]
- D’cunha, N.M.; Sergi, D.; Lane, M.M.; Naumovski, N.; Gamage, E.; Rajendran, A.; Kouvari, M.; Gauci, S.; Dissanayka, T.; Mrax, W.; et al. The Effects of Dietary Advanced Glycation End-Products on Neurocognitive and Mental Disorders. Nutrients 2022, 14, 2421. [Google Scholar] [CrossRef]
- Hjerrild, J.N.; Wobbe, A.; Stausholm, M.B.; Larsen, A.E.; Josefsen, C.O.; Clausen, N.M.M.; Dela, F.; Kjaer, M.; Magnusson, S.P.; Hansen, M.; et al. Effects of Long-Term Physical Activity and Diet on Skin Glycation and Achilles Tendon Structure. Nutrients 2019, 22, 1409. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).