A Low-FODMAP Diet Provides Benefits for Functional Gastrointestinal Symptoms but Not for Improving Stool Consistency and Mucosal Inflammation in IBD: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Methods
2.1. Literature and Search Strategy
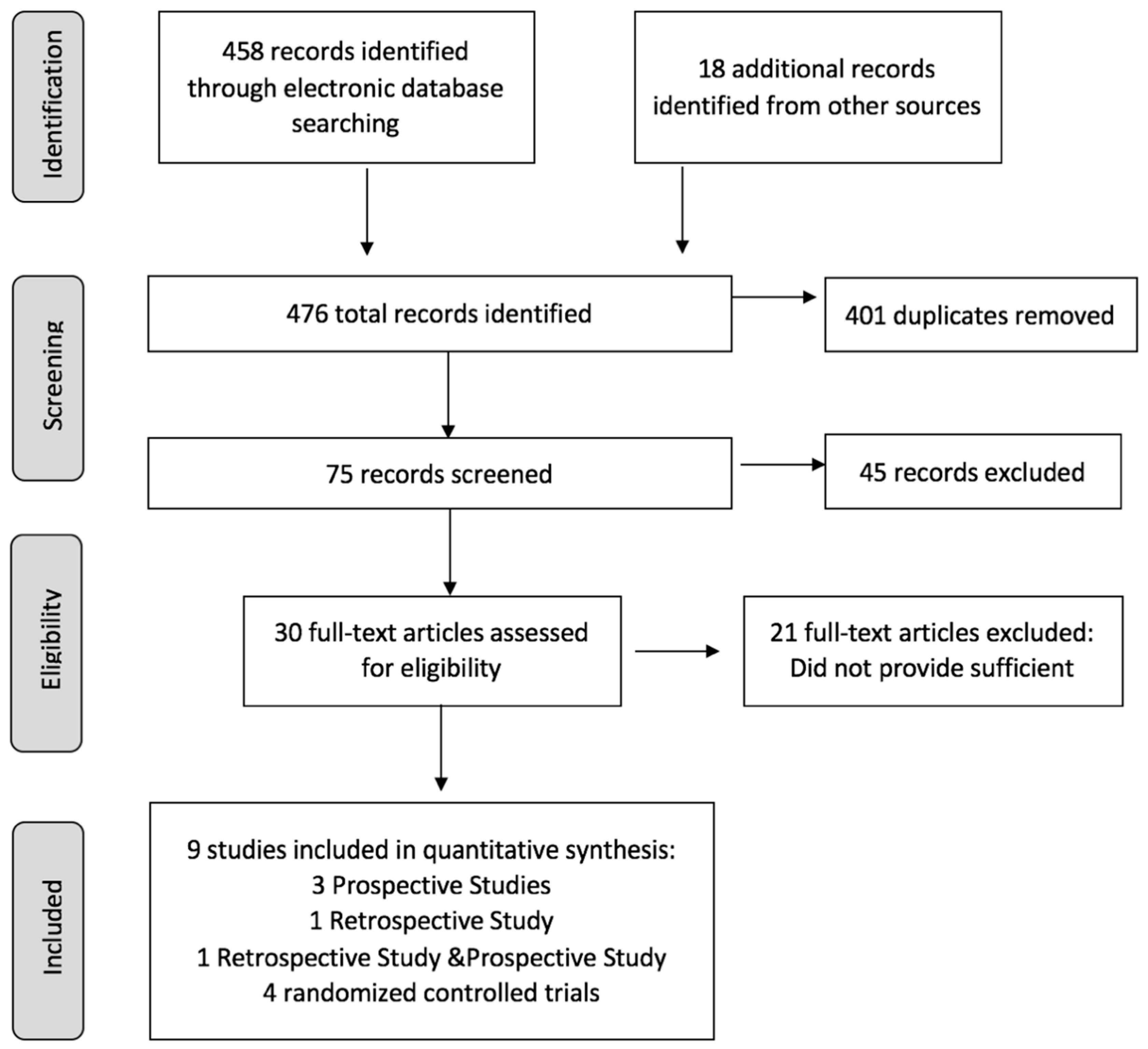
2.2. Literature Screening
2.3. Data Extraction
2.4. Assessment of Risk of Bias
2.5. Statistical Analysis
3. Results
3.1. Characteristics of Eligible Studies
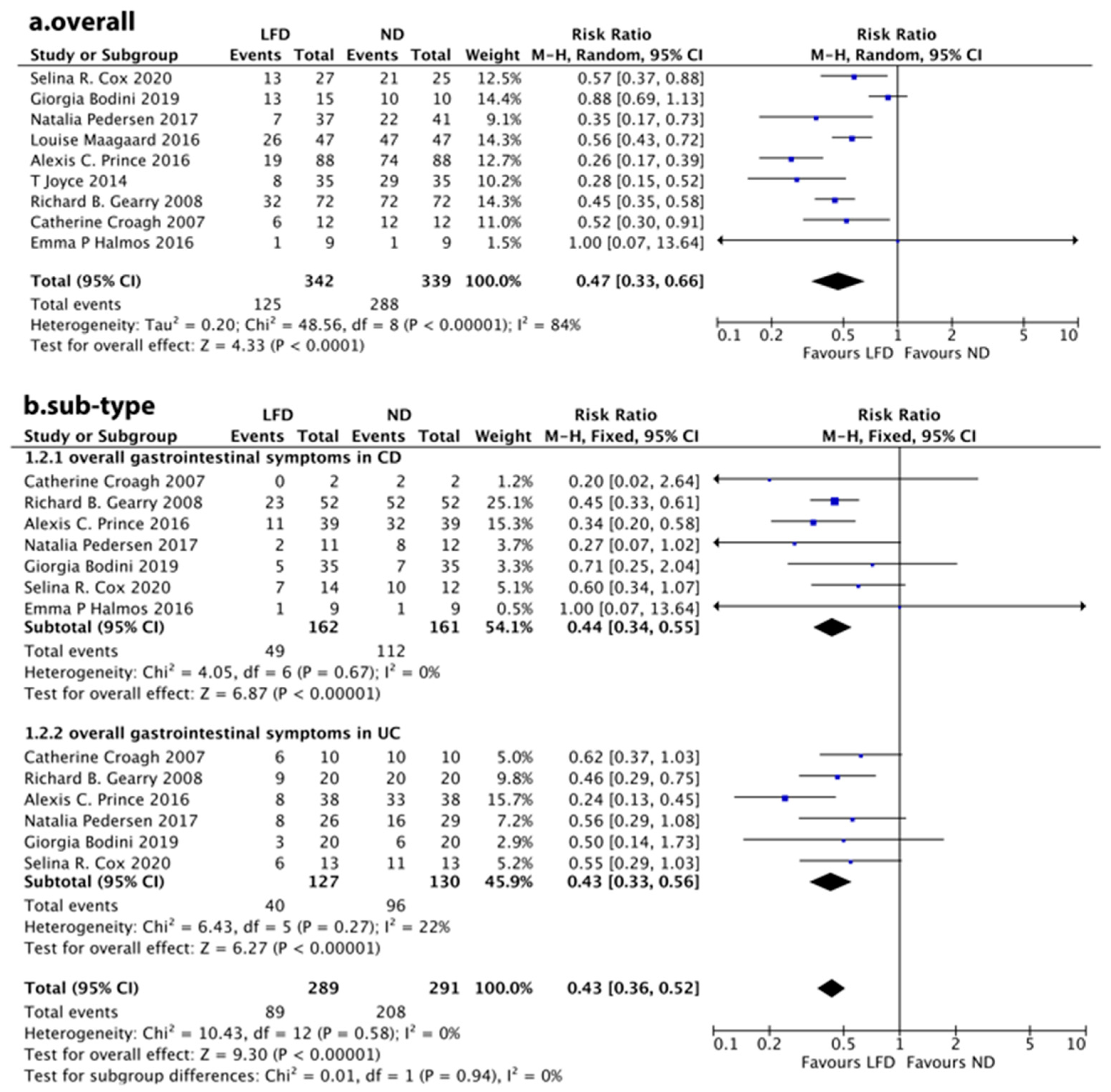
3.2. Overall Symptom Response
3.3. Individual Symptom Response
3.4. Degrees of Change in FGSs
3.5. QoL Score
3.6. Stool Consistency
3.7. Disease Activity
3.8. FC
3.9. Quality of the Included Studies
3.10. Publication Bias
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gracie, D.J.; Williams, C.; Sood, R.; Mumtaz, S.; Bholah, M.H.; Hamlin, P.J.; Ford, A. Negative Effects on Psychological Health and Quality of Life of Genuine Irritable Bowel Syndrome–type Symptoms in Patients with Inflammatory Bowel Disease. Clin. Gastroenterol. Hepatol. 2016, 15, 376–384.e5. [Google Scholar] [CrossRef] [PubMed]
- Tomita, T.; Kato, Y.; Takimoto, M.; Yamasaki, T.; Kondo, T.; Kono, T.; Tozawa, K.; Yokoyama, Y.; Ikehara, H.; Ohda, Y.; et al. Prevalence of Irritable Bowel Syndrome-like Symptoms in Japanese Patients with Inactive Inflammatory Bowel Disease. J. Neurogastroenterol. Motil. 2016, 22, 661–669. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Abdalla, M.I.; Sandler, R.S.; Kappelman, M.D.; Martin, C.F.; Chen, W.; Anton, K.; Long, M.D. Prevalence and Impact of Inflammatory Bowel Disease–Irritable Bowel Syndrome on Patient-reported Outcomes in CCFA Partners. Inflamm. Bowel Dis. 2017, 23, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Quigley, E.M.M. Overlapping irritable bowel syndrome and inflammatory bowel disease: Less to this than meets the eye? Ther. Adv. Gastroenterol. 2016, 9, 199–212. [Google Scholar] [CrossRef] [PubMed]
- Moayyedi, P.; Simrén, M.; Bercik, P. Evidence-based and mechanistic insights into exclusion diets for IBS. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Kalantar-Zadeh, K.; Berean, K.J.; Burgell, R.E.; Muir, J.G.; Gibson, P.R. Intestinal gases: Influence on gut disorders and the role of dietary manipulations. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 733–747. [Google Scholar] [CrossRef]
- Moayyedi, P.; Quigley, E.M.M.; Lacy, B.E.; Lembo, A.J.; Saito, Y.A.; Schiller, L.R.; Soffer, E.E.; Spiegel, B.M.R.; Ford, A. The Effect of Fiber Supplementation on Irritable Bowel Syndrome: A Systematic Review and Meta-analysis. Am. J. Gastroenterol. 2014, 109, 1367–1374. [Google Scholar] [CrossRef]
- Herfarth, H.H.; Martin, C.F.; Sandler, R.S.; Kappelman, M.D.; Long, M.D. Prevalence of a Gluten-free Diet and Improvement of Clinical Symptoms in Patients with Inflammatory Bowel Diseases. Inflamm. Bowel Dis. 2014, 20, 1194–1197. [Google Scholar] [CrossRef]
- Major, G.; Pritchard, S.; Murray, K.; Alappadan, J.P.; Hoad, C.; Marciani, L.; Gowland, P.; Spiller, R. Colon Hypersensitivity to Distension, Rather Than Excessive Gas Production, Produces Carbohydrate-Related Symptoms in Individuals with Irritable Bowel Syndrome. Gastroenterology 2017, 152, 124–133.e2. [Google Scholar] [CrossRef]
- Barrett, J.S.; Gearry, R.; Muir, J.G.; Irving, P.M.; Rose, R.; Rosella, O.; Haines, M.L.; Shepherd, S.J.; Gibson, P.R. Dietary poorly absorbed, short-chain carbohydrates increase delivery of water and fermentable substrates to the proximal colon. Aliment. Pharmacol. Ther. 2010, 31, 874–882. [Google Scholar] [CrossRef]
- Barrett, J.S. How to institute the low-FODMAP diet. J. Gastroenterol. Hepatol. 2017, 32, 8–10. [Google Scholar] [CrossRef] [PubMed]
- Frame, L.A.; Costa, E.; Jackson, S.A. Current explorations of nutrition and the gut microbiome: A comprehensive evaluation of the review literature. Nutr. Rev. 2020, 78, 798–812. [Google Scholar] [CrossRef] [PubMed]
- Casén, C.; Vebø, H.C.; Sekelja, M.; Hegge, F.T.; Karlsson, M.K.; Ciemniejewska, E.; Dzankovic, S.; Frøyland, C.J.; Nestestog, R.; Engstrand, L.; et al. Deviations in human gut microbiota: A novel diagnostic test for determining dysbiosis in patients with IBS or IBD. Aliment. Pharmacol. Ther. 2015, 42, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Grammatikopoulou, M.G.; Goulis, D.G.; Gkiouras, K.; Nigdelis, M.P.; Papageorgiou, S.T.; Papamitsou, T.; Forbes, A.; Bogdanos, D.P. Low FODMAP Diet for Functional Gastrointestinal Symptoms in Quiescent Inflammatory Bowel Disease: A Systematic Review of Randomized Controlled Trials. Nutrients 2020, 12, 3648. [Google Scholar] [CrossRef] [PubMed]
- Colombel, J.-F.; Shin, A.; Gibson, P.R. AGA Clinical Practice Update on Functional Gastrointestinal Symptoms in Patients with Inflammatory Bowel Disease: Expert Review. Clin. Gastroenterol. Hepatol. 2019, 17, 380–390. [Google Scholar] [CrossRef]
- Levine, A.; Boneh, R.S.; Wine, E. Evolving role of diet in the pathogenesis and treatment of inflammatory bowel diseases. Gut 2018, 67, 1726–1738. [Google Scholar] [CrossRef]
- Schreiner, P.; Yilmaz, B.; Rossel, J.B.; Franc, Y.; Misselwitz, B.; Scharl, M.; Swiss IBD Cohort Study Group. Vegetarian or gluten-free diets in patients with inflammatory bowel disease are associated with lower psychological well-being and a different gut microbiota, but no beneficial effects on the course of the disease. United Eur. Gastroenterol. J. 2019, 7, 767–781. [Google Scholar] [CrossRef]
- Chiba, M.; Abe, T.; Tsuda, H.; Sugawara, T.; Tsuda, S.; Tozawa, H.; Imai, H. Lifestyle-related disease in Crohn’s disease: Relapse prevention by a semi-vegetarian diet. World J. Gastroenterol. 2010, 16, 2484–2495. [Google Scholar] [CrossRef]
- Fritsch, J.; Garces, L.; Quintero, M.A.; Pignac-Kobinger, J.; Santander, A.M.; Fernández, I.; Abreu, M.T. Low-Fat, High-Fiber Diet Reduces Markers of Inflammation and Dysbiosis and Improves Quality of Life in Patients with Ulcerative Colitis. Clin. Gastroenterol. Hepatol. 2021, 19, 1189–1199. [Google Scholar] [CrossRef]
- Prince, A.C.; Myers, C.E.; Joyce, T.; Irving, P.; Lomer, M.; Whelan, K. Fermentable Carbohydrate Restriction (Low FODMAP Diet) in Clinical Practice Improves Functional Gastrointestinal Symptoms in Patients with Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2016, 22, 1129–1136. [Google Scholar] [CrossRef]
- Gearry, R.; Irving, P.; Barrett, J.S.; Nathan, D.M.; Shepherd, S.J.; Gibson, P.R. Reduction of dietary poorly absorbed short-chain carbohydrates (FODMAPs) improves abdominal symptoms in patients with inflammatory bowel disease—A pilot study. J. Crohn’s Colitis 2009, 3, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Joyce, T.; Staudacher, H.; Whelan, K.; Irving, P.; Lomer, M. PWE-092 Symptom Response Following Advice On A Diet Low In Short-chain Fermentable Carbohydrates (fodmaps) For Functional Bowel Symptoms In Patients with Ibd. Gut 2014, 63 (Suppl. S1), A164. [Google Scholar] [CrossRef]
- Maagaard, L.; Ankersen, D.V.; Végh, Z.; Burisch, J.; Jensen, L.; Pedersen, N.; Munkholm, P. Follow-up of patients with functional bowel symptoms treated with a low FODMAP diet. World J. Gastroenterol. 2016, 22, 4009–4019. [Google Scholar] [CrossRef] [PubMed]
- Croagh, C.; Shepherd, S.J.; Berryman, M.; Muir, J.G.; Gibson, P.R. Pilot study on the effect of reducing dietary FODMAP intake on bowel function in patients without a colon. Inflamm. Bowel Dis. 2007, 13, 1522–1528. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, N.; Ankersen, D.V.; Felding, M.; Wachmann, H.; Végh, Z.; Molzen, L.; Burisch, J.; Andersen, J.R.; Munkholm, P. Low-FODMAP diet reduces irritable bowel symptoms in patients with inflammatory bowel disease. World J. Gastroenterol. 2017, 23, 3356–3366. [Google Scholar] [CrossRef]
- Bodini, G.; Zanella, C.; Crespi, M.; Pumo, S.L.; Demarzo, M.G.; Savarino, E.; Savarino, V.; Giannini, E.G. A randomized, 6-wk trial of a low FODMAP diet in patients with inflammatory bowel disease. Nutrition 2019, 67-68, 110542. [Google Scholar] [CrossRef]
- Cox, S.R.; Lindsay, J.O.; Fromentin, S.; Stagg, A.J.; McCarthy, N.E.; Galleron, N.; Ibraim, S.B.; Roume, H.; Levenez, F.; Pons, N.; et al. Effects of Low FODMAP Diet on Symptoms, Fecal Microbiome, and Markers of Inflammation in Patients with Quiescent Inflammatory Bowel Disease in a Randomized Trial. Gastroenterology 2020, 158, 176–188. [Google Scholar] [CrossRef]
- Halmos, E.P.; Christophersen, C.T.; Bird, A.R.; Shepherd, S.J.; Muir, J.G.; Gibson, P.R. Consistent Prebiotic Effect on Gut Microbiota with Altered FODMAP Intake in Patients with Crohn’s Disease: A Randomised, Controlled Cross-Over Trial of Well-Defined Diets. Clin. Transl. Gastroenterol. 2016, 7, e164. [Google Scholar] [CrossRef]
- Zhan, Y.-l.; Zhan, Y.-A.; Dai, S.-X. Is a low FODMAP diet beneficial for patients with inflammatory bowel disease? A meta-analysis and systematic review. Clin. Nutr. 2018, 37, 123–129. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann. Intern. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef]
- Higgins, J.P.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed]
- Dent, O. Methodological index for non-randomized studies. ANZ J. Surg. 2003, 73, 675–676. [Google Scholar] [CrossRef] [PubMed]
- Longstreth, G.F.; Thompson, W.G.; Chey, W.D.; Houghton, L.A.; Mearin, F.; Spiller, R.C. Functional bowel disorders. Gastroenterology 2006, 130, 1480–1491. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, L.J.; Virjee, J.; Heaton, K.W. Detection of pseudodiarrhoea by simple clinical assessment of intestinal transit rate. BMJ 1990, 300, 439–440. [Google Scholar] [CrossRef]
- Staudacher, H.M.; Whelan, K. The low FODMAP diet: Recent advances in understanding its mechanisms and efficacy in IBS. Gut 2017, 66, 1517–1527. [Google Scholar] [CrossRef]
- Mehtab, W.; Agarwal, A.; Singh, N.; Malhotra, A.; Makharia, G.K. All that a physician should know about FODMAPs. Indian J. Gastroenterol. 2019, 38, 378–390. [Google Scholar] [CrossRef]
- Halmos, E.P.; Power, V.A.; Shepherd, S.J.; Gibson, P.R.; Muir, J.G. A Diet Low in FODMAPs Reduces Symptoms of Irritable Bowel Syndrome. Gastroenterology 2014, 146, 67–75.e5. [Google Scholar] [CrossRef]
- Staudacher, H.M.; Lomer, M.C.; Anderson, J.L.; Barrett, J.S.; Muir, J.G.; Irving, P.M.; Whelan, K. Fermentable Carbohydrate Restriction Reduces Luminal Bifidobacteria and Gastrointestinal Symptoms in Patients with Irritable Bowel Syndrome. J. Nutr. 2012, 142, 1510–1518. [Google Scholar] [CrossRef]
- Daveson, A.J.M.; Tye-Din, J.A.; Goel, G.; Goldstein, K.E.; Hand, H.L.; Neff, K.M.; Williams, L.J.; Truitt, K.E.; Anderson, R.P. The RESET CeD Study Group Masked bolus gluten challenge low in FODMAPs implicates nausea and vomiting as key symptoms associated with immune activation in treated coeliac disease. Aliment. Pharmacol. Ther. 2019, 51, 244–252. [Google Scholar] [CrossRef]
- Barello, S.; Guida, E.; Leone, S.; Previtali, E.; Graffigna, G. Does patient engagement affect IBD patients’ health-related quality of life? Findings from a cross-sectional study among people with inflammatory bowel diseases. Health Qual. Life Outcomes 2021, 19, 1–9. [Google Scholar] [CrossRef]
- McCormick, J.B.; Hammer, R.R.; Farrell, R.M.; Geller, G.; James, K.M.; Loftus, E.V.; Mercer, M.B.; Tilburt, J.C.; Sharp, R.R. Experiences of patients with chronic gastrointestinal conditions: In their own words. Health Qual. Life Outcomes 2012, 10, 25. [Google Scholar] [CrossRef] [PubMed]
- Ciccocioppo, R.; Klersy, C.; Russo, M.L.; Valli, M.; Boccaccio, V.; Imbesi, V.; Corazza, G.R. Validation of the Italian translation of the Inflammatory Bowel Disease Questionnaire. Dig. Liver Dis. 2011, 43, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Boye, B.; Lundin, K.E.; Jantschek, G.; Leganger, S.; Mokleby, K.; Tangen, T.; Jahnsen, J. INSPIRE study: Does stress management improve the course of inflammatory bowel disease and disease-specific quality of life in distressed patients with ulcerative colitis or Crohn’s disease? A randomized controlled trial. Inflamm. Bowel Dis. 2011, 17, 1863–1873. [Google Scholar] [CrossRef]
- van der Eijk, I.; Vlachonikolis, I.G.; Munkholm, P.; Nijman, J.; Bernklev, T.; Politi, P.; EC-IBD Study Group. The role of quality of care in health-related quality of life in patients with IBD. Inflamm. Bowel Dis. 2004, 10, 392–398. [Google Scholar] [CrossRef] [PubMed]
- Sajadinejad, M.S.; Asgari, K.; Molavi, H.; Kalantari, M.; Adibi, P. Psychological Issues in Inflammatory Bowel Disease: An Overview. Gastroenterol. Res. Pract. 2012, 2012, 106502. [Google Scholar] [CrossRef]
- Perera, L.P.; Radigan, M.; Guilday, C.; Banerjee, I.; Eastwood, D.; Babygirija, R.; Massey, B.T. Presence of Irritable Bowel Syndrome Symptoms in Quiescent Inflammatory Bowel Disease Is Associated with High Rate of Anxiety and Depression. Am. J. Dig. Dis. 2019, 64, 1923–1928. [Google Scholar] [CrossRef] [PubMed]
- Wenzl, H.H.; Fine, K.D.; Schiller, L.R.; Fordtran, J.S. Determinants of decreased fecal consistency in patients with diarrhea. Gastroenterology 1995, 108, 1729–1738. [Google Scholar] [CrossRef]
- Prati, M.; Molteni, M.; Pomati, F.; Rossetti, C.; Bernardini, G. Biological effect of the Planktothrix sp. FP1 cyanobacterial extract. Toxicon 2002, 40, 267–272. [Google Scholar] [CrossRef]
- Drossman, D.; Morris, C.B.; Hu, Y.; Toner, B.B.; Diamant, N.; Whitehead, W.E.; Dalton, C.B.; Leserman, J.; Patrick, N.L.; Bangdiwala, S.I. Characterization of Health Related Quality of Life (HRQOL) for Patients with Functional Bowel Disorder (FBD) and Its Response to Treatment. Am. J. Gastroenterol. 2007, 102, 1442–1453. [Google Scholar] [CrossRef]
- Wenzl, H.H. Diarrhea in chronic inflammatory bowel diseases. Gastroenterol. Clin. N. Am. 2012, 41, 651–675. [Google Scholar] [CrossRef]
- Anbazhagan, A.N.; Priyamvada, S.; Alrefai, W.A.; Dudeja, P.K. Pathophysiology of IBD associated diarrhea. Tissue Barriers 2018, 6, e1463897. [Google Scholar] [CrossRef] [PubMed]
- Falvey, J.D.; Hoskin, T.; Meijer, B.; Ashcroft, A.; Walmsley, R.; Day, A.S.; Gearry, R.B. Disease activity assessment in IBD: Clinical indices and biomarkers fail to predict endoscopic remission. Inflamm. Bowel Dis. 2015, 21, 824–831. [Google Scholar] [CrossRef] [PubMed]
- Gomes, P.; du Boulay, C.; Smith, C.L.; Holdstock, G. Relationship between disease activity indices and colonoscopic findings in patients with colonic inflammatory bowel disease. Gut 1986, 27, 92–95. [Google Scholar] [CrossRef] [PubMed]
- Sandborn, W.J.; Feagan, B.G.; Hanauer, S.B.; Lochs, H.; Löfberg, R.; Modigliani, R.; Sutherland, L.R. A review of activity indices and efficacy endpoints for clinical trials of medical therapy in adults with Crohn’s disease. Gastroenterology 2002, 122, 512–530. [Google Scholar] [CrossRef]
- Baars, J.E.; Nuij, V.J.; Oldenburg, B.; Kuipers, E.J.; Van Der Woude, C.J. Majority of patients with inflammatory bowel disease in clinical remission have mucosal inflammation. Inflamm. Bowel Dis. 2012, 18, 1634–1640. [Google Scholar] [CrossRef]
- Zittan, E.; Kabakchiev, B.; Kelly, O.B.; Milgrom, R.; Nguyen, G.C.; Croitoru, K.; Steinhart, A.H.; Silverberg, M.S. Development of the Harvey-Bradshaw Index-pro (HBI-PRO) Score to Assess Endoscopic Disease Activity in Crohn’s Disease. J. Crohn’s Colitis 2016, 11, 543–548. [Google Scholar] [CrossRef]
- Kostas, A.; I Siakavellas, S.; Kosmidis, C.; Takou, A.; Nikou, J.; Maropoulos, G.; Vlachogiannakos, J.; Papatheodoridis, G.V.; Papaconstantinou, I.; Bamias, G. Fecal calprotectin measurement is a marker of short-term clinical outcome and presence of mucosal healing in patients with inflammatory bowel disease. World J. Gastroenterol. 2017, 23, 7387–7396. [Google Scholar] [CrossRef]
- Mooiweer, E.; Severs, M.; Schipper, M.E.; Fidder, H.H.; Siersema, P.D.; Laheij, R.J.; Oldenburg, B. Low Fecal Calprotectin Predicts Sustained Clinical Remission in Inflammatory Bowel Disease Patients: A Plea for Deep Remission. J. Crohn’s Colitis 2014, 9, 50–55. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. J. Clin. Epidemiol. 2009, 62, e1–e34. [Google Scholar] [CrossRef]
- Gibson, P.R. Use of the low-FODMAP diet in inflammatory bowel disease. J. Gastroenterol. Hepatol. 2017, 32, 40–42. [Google Scholar] [CrossRef]
- Damas, O.M.; Garces, L.; Abreu, M.T. Diet as Adjunctive Treatment for Inflammatory Bowel Disease: Review and Update of the Latest Literature. Curr. Treat. Options Gastroenterol. 2019, 17, 313–325. [Google Scholar] [CrossRef] [PubMed]





| Study | Design | Total Case (Controls or Cohort Size) | Age Range or Mean Age (SD)/Year | Male/Female | Type of IBD (CD/UC/IBD-u) | MINORS Scores |
|---|---|---|---|---|---|---|
| Alexis C. Prince et al. | Non-RCT | 88 | 20–80 | 26/62 | 39/38/11 | 14 |
| Richard B. Gearry et al. | Non-RCT | 72 | 18–72 | 39/33 | 52/20/0 | 14 |
| T. Joyce et al. | Non-RCT | 35 | 39(NA) | 13/22 | 17/17/1 | 12 |
| Louise Maagaar et al. | Non-RCT | 49 | 19–70 | 9/40 | 32/12/5 | 14 |
| Catherine Croagh et al. | Non-RCT | 12 | 35–74 | 5/7 | 2/10/0 | 14 |
| Natalia Pedersen et al. | RCT | 78(LFD:37 ND:41) | LFD:20–70 ND:24–69 | LFD:12/32 ND:10/35 * | LFD:14/30/0 ND:14/31/0 * | - |
| Giorgia Bodini et al. | RCT | 51(LFD:26 ND:29) | LFD:34–48 ND:44–57 | LFD:7/19 ND:12/17 | LFD:18/8/0 ND:17/12/0 | - |
| Selina R. Cox et al. | RCT | 52(LFD:27 ND:25) | LFD:33(11) ND:40(13) | LFD:10/17 ND:13/12 | LFD:14/13/0 ND:12/13/0 * | - |
| Emma P. Halmos et al. # | RCT | 9(LFD:9 ND:9) | 29–41 | 3/6 | 9/0/0 | - |
| Author | Year | Country | Participants | Intervention | Duration of Therapy | Outcome Evaluated FGS |
|---|---|---|---|---|---|---|
| Prospective Study | ||||||
| Alexis C. Prince et al. | 2016 | United Kingdom | IBD patients with persistent FGS | Low FODMAP | 6 Weeks | Primary outcome was assessment of satisfactory relief of FGS measured using GSQ. Individual symptoms were assessed using the GSRS. |
| Richard B. Gearry et al. | 2008 | Australia | IBD patients with persistent abdominal symptoms | Low FODMAP | 3 Months | An arbitrary improvement of 5 or more on a custom gastrointestinal symptoms scale was used as a measure of unequivocal improvement for each symptom. |
| T. Joyce et al. | 2014 | United Kingdom | Patients with inactive IBD and FBD | Low FODMAP | 6 Weeks | Symptoms were measured using the GSQ and the GSRS. |
| Retrospective Study | ||||||
| Louise Maagaard et al. | 2016 | Denmark | Consecutive patients with IBD | Low FODMAP | 6–8 Weeks | Patient-reported effectiveness of the low-FODMAP diet. Effectiveness was categorized as full, partial, or no effect. |
| Retrospective Study and Prospective Study | ||||||
| Catherine Croagh et al. | 2007 | Australia | IBD with colectomy and ileal pouch formation or ileorectal anastomosis | Low FODMAP | 6 Weeks | Patient-reported effectiveness of diet on symptoms. Effectiveness was categorized as improved, no change, or worse. |
| Randomized Controlled Trial | ||||||
| Natalia Pedersen et al. | 2017 | Denmark | IBD patients with a baseline IBS-SSS of at least 75 points | Low-FODMAP or normal habitual diet | 6 Weeks | Primary outcome was the number of patients achieving a 50-point reduction in IBS-SSS. |
| Giorgia Bodini et al. | 2019 | Italy | IBD patients in the remission phase or mild disease activity | Low-FODMAP or standard diet | 6 Weeks | Patients with a total IBD-Q score >170 were assessed as being in symptomatic remission. |
| Selina R. Cox et al. | 2020 | United Kingdom | Adult quiescent IBD patients with ongoing gut symptoms | Low-FODMAP or placebo sham diet | 4 Weeks | The global symptom question was used to assess adequate relief of FGS at end of trial. |
| Emma P. Halmos et al. | 2016 | Australia | Quiescent CD patients with stable therapy | Low or typical (Australian) FODMAP diets | 21 Days | The visual analog scale score was used to measure overall gastrointestinal symptoms. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, Z.; Yi, J.; Liu, X. A Low-FODMAP Diet Provides Benefits for Functional Gastrointestinal Symptoms but Not for Improving Stool Consistency and Mucosal Inflammation in IBD: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 2072. https://doi.org/10.3390/nu14102072
Peng Z, Yi J, Liu X. A Low-FODMAP Diet Provides Benefits for Functional Gastrointestinal Symptoms but Not for Improving Stool Consistency and Mucosal Inflammation in IBD: A Systematic Review and Meta-Analysis. Nutrients. 2022; 14(10):2072. https://doi.org/10.3390/nu14102072
Chicago/Turabian StylePeng, Ziheng, Jun Yi, and Xiaowei Liu. 2022. "A Low-FODMAP Diet Provides Benefits for Functional Gastrointestinal Symptoms but Not for Improving Stool Consistency and Mucosal Inflammation in IBD: A Systematic Review and Meta-Analysis" Nutrients 14, no. 10: 2072. https://doi.org/10.3390/nu14102072
APA StylePeng, Z., Yi, J., & Liu, X. (2022). A Low-FODMAP Diet Provides Benefits for Functional Gastrointestinal Symptoms but Not for Improving Stool Consistency and Mucosal Inflammation in IBD: A Systematic Review and Meta-Analysis. Nutrients, 14(10), 2072. https://doi.org/10.3390/nu14102072






