Abstract
Dietary counselling has been identified as one of the nutritional strategies to alleviate cardiometabolic health conditions. Its effectiveness however may vary due to factors such as intensity level and provider while this has not been comprehensively studied. This systematic review and meta-analysis aimed to assess the effects of dietary counselling on the cardiometabolic health in middle-aged and older adults and the sub-group analyses with dietary counselling intensity and the provider were also assessed. Four databases including PubMed, CINAHL Plus with Full Text, Cochrane Library and EMBASE were systematically searched. Data from 22 randomised controlled trials (RCTs) were compiled and those from 9 RCTs were utilised for meta-analysis. Dietary counselling lowered total cholesterol (TC) and fasting blood sugar (FBS) but had no impact on triglycerides (TG) and low-density lipoprotein (LDL). Sub-group analysis revealed significant lowering effect of high intensity dietary counselling for TG (weighted mean difference (WMD): −0.24 mmol/L, 95% confidence intervals (CIs): −0.40 to −0.09), TC (WMD: −0.31 mmol/L, 95% CIs: −0.49 to −0.13), LDL (WMD: −0.39 mmol/L, 95% CIs: −0.61 to −0.16) and FBS (WMD: −0.69 mmol/L, 95% CIs: −0.99 to −0.40) while medium or low intensity dietary counselling did not show favouring effects. Counselling provider showed differential responses on cardiometabolic health between dietitian and all other groups. The findings from this systematic review and meta-analysis suggest that dietary counselling is a beneficial dietary strategy to improve cardiometabolic health in middle-aged and older adults with the emphasis on the counselling intensity.
1. Introduction
Aging has become a worldwide phenomenon and according to the United Nations, the projected number of older people over the next thirty years will double, reaching over 1.5 billion persons [1]. The largest increase is anticipated to occur in Eastern and South-Eastern Asia [1]. Aging can be associated with increased morbidity including cardiometabolic risks [2]. A longitudinal cohort study reported an increase in cardiometabolic multimorbidity with age from 5.2% and 11.6% in the population aged ≥40 and ≥60 respectively [3].
Some studies have advanced the research in examining the relationship between overall diet quality and cardiometabolic disorders. A wide array of evidence has shown that a lower dietary quality in older age is associated with an increased risk of cardiovascular disease (CVD) and type 2 diabetes mellitus [4,5]. In a recent study conducted to assess the prospective association between a 6-year change in diet quality and the risk for incident CVD, a higher dietary quality was strongly associated with lower CVD risk within the middle-aged community [6]. These findings indicate that diet quality may have a pivotal role in influencing the development and progression of cardiovascular disorders.
Two primary strategies for the management of cardiometabolic risk has been identified such as the modification of underlying risk factors and an isolated treatment for each underlying risk factor [7]. Some of the interventions involved lifestyle change such as altering dietary habit or exercise training and medical procedures such as pharmacologic therapy or weight loss surgical procedure have shown improvements in the management of cardiometabolic risk factors [8,9,10]. However, pharmacological and surgical management may not provide a long-term solution as many other cardiometabolic risk factors may co-occur leading to polypharmacy [11]. Therefore, the introduction of lifestyle changes such as dietary modification and exercise training may be able to provide a long-term and less invasive solution. An example of dietary modification can be achieved through the provision of dietary counselling. Dietary counselling is defined as a two-way interaction between a patient or a group and a member of the medical team through nutritional assessment to identify any nutritional problems, needs, and goals [12]. This is also in accordance with the American Dietetic Association (ADA)’s definition of nutrition counselling as “advising and assisting individuals and groups on appropriate nutrition intake by integrating information from the nutrition assessment with information on food and other sources of nutrients and meal preparation consistent with cultural background and socioeconomic status” [13]. Dietary counselling is suggested to be an effective nutritional strategy to improve the dietary quality in older adults and may prevent the onset of various aged-related health conditions or diseases [14,15]. A recent systematic review and meta-analysis analysing the effectiveness of dietary counselling for lowering blood lipid levels in high-risk individuals observed a significant reduction in triglycerides (TG) levels [16]. Another meta-analysis which assessed the impact of nutrition counselling in diabetic patients observed significant reductions in fasting blood sugar (FBS), total cholesterol (TC) and systolic blood pressure (SBP) [17]. However, a randomised controlled trial (RCT) carried out in Hong Kong which sought to investigate the impact of Dietary Approach to Stop Hypertension (DASH)-based dietary counselling on grade 1 hypertensive patients found no significant reduction in blood pressure over a period of 12 months [18].
Collectively, mixed results of dietary counselling on cardiometabolic health have been observed. Different studies presented different methodological combination of dietary counselling such as the number of times the counselling sessions were provided, how long each session was held and the personnel who conducted the counselling session. A review study conducted by Lin et al. [19] discovered that high intensity dietary counselling trials have shown greater dietary compliance as compared to low and medium intensity trials. Hence, the intensity of dietary counselling has become an important subject for discussion. Moreover, at present, no systematic review has been conducted to examine the effect and the extensivity of dietary counselling on the cardiometabolic health of middle-aged and older adults. Therefore, the aim of this systematic review and meta-analysis is to assess the effects of dietary counselling on cardiometabolic health in the middle-aged and older population. Further subgroup analysis will be performed to elucidate the effect of specific components of dietary counselling (e.g., intensity of dietary counselling and counselling provider) on the cardiometabolic health).
2. Materials and Methods
2.1. Registration
This systematic review was completed in adherence to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [20]. The PICOS (participant, intervention, comparison, outcome, and study design) criteria and the research question is described in Table 1. The review is registered with PROSPERO International prospective register of systematic reviews (CRD4202015379).

Table 1.
PICOS criteria for the inclusion of studies.
2.2. Search Strategy and Inclusion Criteria
The article search was conducted by the primary (JHML) and secondary (DWKT) reviewers. A total of four online databases including PubMed, CINAHL Plus with Full Text, Cochrane Library and EMBASE were searched from inception until February 2020 and the search was updated as of April 2021. The key search string used was common across all databases and included (‘diet counseling’ OR ‘dietary counseling’ OR ‘nutritional counseling’ OR ‘nutrition counseling’) AND (‘blood glucose’ OR ‘insulin’ OR ‘blood pressure’ OR ‘lipoprotein, hdl’ OR ‘lipoprotein, ldl’, OR ‘triglycerides’ OR ‘cholesterol’). Human and English language were selected as limiters and medical subject headings (MeSH) used when applicable. Reference lists of relevant reviews were also hand-searched to identify additional articles.
The articles were accepted based on the following search criteria: (1) RCT study design; (2) subject’s mean age ≥50 years old; (3) reporting of cardiometabolic health related classical outcomes [i.e., TC, low density lipoprotein cholesterol (LDL), high density lipoprotein cholesterol (HDL), TG, SBP, diastolic blood pressure (DBP), FBG, and insulin]. Articles that included other life-style interventions such as exercises or the co-consumption of supplementation were excluded. Studies with no intervention control groups or standard care groups were included.
2.3. Articles Selection and Data Extraction
The titles and abstracts of all articles were first screened by the primary and secondary reviewers and disagreements between reviewers were resolved by consensus with a third reviewer (JEK). Articles were rejected after full text screening based on 1 of the 3 reasons: (1) study design was not an RCT, or the intervention included exercises or co-consumption of other supplementation or medication; (2) population was not human or had a mean age of <50 years; (3) outcomes of interest were not analysed. A hand search of relevant reviews from relevant reviews and research articles was also conducted. The following data were extracted from selected articles onto an electronic form: first author name; publication year; population size; drop-out rate; study design and duration; population mean age, body mass index and gender ratio; intervention specifics, including the method of dietary counselling, who conducted the counselling and whether or not additional dietary changes were introduced; method of diet administration; compliance assessment; intensity of dietary counselling; mean and standard deviations (SDs) of the pre-intervention, post-intervention, and change values for selected outcomes variables; and key conclusions. Units for the lipid-lipoproteins were standardized to mmol/L (TC, LDL-C, and HDL-C—mg/dL × 0.02586; TG—mg/dL × 0.01129; FBG—mg/dL × 0.0555). Upon circumstances where clarification was required to obtain unpublished useful data, corresponding authors were contacted via email. Studies that included more than one relevant intervention and control arm were considered as a distinct group for multiple pairwise comparisons to account for within-study variations [21].
2.4. Risk of Bias Assessment
The Cochrane Collaboration modified tool for assessing risk of bias for studies was used to determine the quality of the studies selected [22]. A judgement level (high, low, or unclear) was assigned to each article to determine any prevalence of selection bias (random sequence generation, allocation concealment), reporting bias (selective reporting), performance bias (blinding of participants and investigator), detection bias (blinding of outcome assessor) and attrition bias (incomplete outcome data) and other sources of bias.
2.5. Calculation and Statistical Analysis
If the SDs of the change values for both the control and intervention groups of each study were unavailable, the values were calculated using a correlation factor representative of the change-value SDs that were available from the other studies [23]. In addition, the post values, the reported and/or calculated change values and their ranges for each outcome were collected.
Obtained data were analysed using STATA (Version 13, StataCorp LP, College Station, TX, USA) for the meta-analysis. The metan function was utilized for the determination of pooled outcome effects. The overall effect sizes for all outcomes were determined using the weighted mean difference (WMD) of the change values between the dietary counselling and no dietary counselling groups with 95% confidence intervals (CIs). Random-effect model was utilized to account for the heterogeneity since variation of dietary counselling within the intervention groups was evident. Positive effect sizes did not favour dietary counselling while negative effect sizes favoured dietary counselling. Heterogeneity was quantified through the Cochran’s Q and I2 statistic, which was derived from chi-square statistic, with a value of more than 50% and a p-value < 0.05 indicative of significant heterogeneity [24].
Sub-group analyses were conducted based on the intensity of the dietary counselling and the provider of dietary counselling. With regards to the classification of the dietary counselling intensity, a rating of ‘low’, ‘medium’ or ‘high’ was allocated for each available intervention arm. This was derived based on the number and length of counselling contacts. Low intensity interventions involved 1 contact lasting less than 30 min, high intensity interventions involved contacts greater than 6, each lasting at least 30 min. Dietary counselling intensities in between belonged to medium intensity interventions [25]. For the sub-group analysis according to the provider, it was spilt into dietitian involvement and all others. Any studies that employed the usage of dietitian was grouped together under ‘Dietitian Involvement’ and the rest was classified under ‘All Others’. All others included nutritionist, physician, research staff and nurses. The sensitivity of the result was tested by repeating the meta-analysis with the removal of single pairwise comparisons.
3. Results
3.1. Study Selection and Subject Characteristics
A total of 824 articles were obtained through the search and all the identified articles were exported to EndNote X7 (Thomas Research Software, Carlsbad, CA, USA) for article management and the exclusion of duplicates (n = 274). Articles were rejected after full text screening based on one of the three reasons: (1) study design was not an RCT, or the intervention included exercises or co-consumption of other supplementation or medication; (2) population was not human or had a mean age of <50 years; (3) outcomes of interest were not analysed. A hand search of relevant reviews and article references yielded one additional article. Bibliographies from relevant reviews and research articles identified were further inspected to obtain 1 additional article for a complete study listing. In total, 22 articles were selected for qualitative systematic review and 9 articles were eligible for quantitative analysis as the remaining 13 articles did not provide sufficient details required for the analysis (Figure 1). All the 22 studies were eligible for cardiometabolic health outcomes [23,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46] and the study features and subject characteristics of the selected articles are summarized in Table 2. A parallel study design was performed in all 22 studies. Amongst the 22 studies, only one of the studies featured generally healthy subjects [43], the remaining of the studies featured subjects with various disease conditions such as type 2 diabetes, hypercholesterolemia, hypertension, metabolic syndrome, certain types of cancer and renal disease.
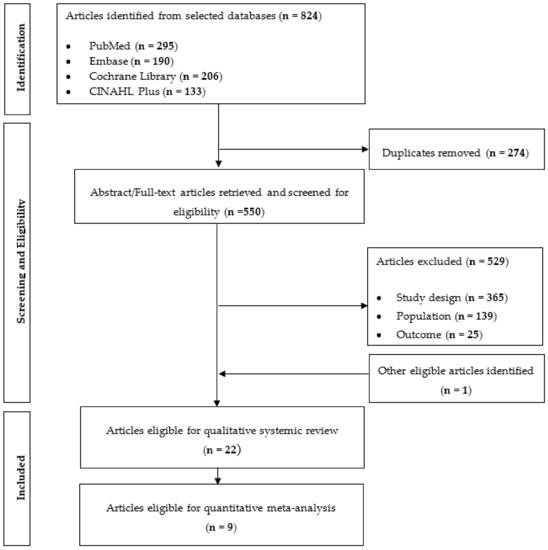
Figure 1.
Flow diagram of article search and screening process showing the number of studies assessed for eligibility and included in the review for cardiometabolic health related clinical trials.

Table 2.
Characteristics of the 22 included RCTs for systematic review and meta-analysis.
3.2. Risk of Bias Assessment
The risk of bias assessment results are summarized in Supplementary Table S1. Selection bias (random sequence allocation and allocation concealment) was marked ‘low’ for about one-third of the articles, while most were labelled under the unclear category due to the lack of clear description of how the randomization or concealment was conducted. A ‘high’ score was allocated to Gans et al. [36] for random sequence allocation. According to Gans et al. [36], the study site was randomised however the subjects were all exposed to the same intervention. Other sources of bias were marked ‘unclear’ for all the articles. Performance bias and detection bias were marked “unclear” for some of the articles, with a few scored ‘low’ as evident blinding for the respective components were mentioned. Performance bias was marked high for Henkin et al. [37], Muchiri et al. [40], Noda et al. [41], Takahashi et al. [43], Tan et al. [47], Pimentel et al. [45], Klein et al. [29], Cheng et al. [30], Samuelsson et al. [32], Wu et al. [33], and Holland et al. [48]. This was due to the inevitable nature of dietary counselling; subjects were unable to be blinded when undergoing the intervention and hence resulting in only single blinding. Blinding of assessor was marked high for Muchiri et al. [40], Britton et al. [49], Cheng et al. [30], Samuelsson et al. [32], Wu et al. [33], and Holland et al. [48]. Attrition bias was deemed ‘unclear’ for most articles due to the lack of more explicit stating, though some articles were clear in their presentation and hence necessitated a “low” rating.
3.3. Results of Systematic Review
Table 3 provides a summary of the post and change values in lipid-lipoproteins, blood pressure, glucose, and insulin for all the 22 studies. Amongst all the studies included in Table 3, some studies had more than one intervention arm while some studies only had control group. Although no major differences in post values were observed between the control and intervention groups, change values indicate an increment in the HDL concentration coupled with a reduction in blood pressure and glucose concentration after dietary counselling.

Table 3.
Summary of post and change values in lipid-lipoproteins, blood pressure, glucose, and insulin of all the 22 studies.
3.4. Results of Meta-Analysis
Meta-analysis results reveal that there were no significant differences in the change values of TG (WMD: −0.08 mmol/L, 95% CIs: −0.21 to 0.05) (Figure 2) and LDL (WMD: −0.03 mmol/L, 95% CIs: −0.12 to 0.05) concentrations between the control and intervention groups (Figure 3). However, dietary counselling provided favourable effect in TC (WMD: −0.16 mmol/L, 95% CIs: −0.31 to −0.01) (Figure 4) and FBS (WMD: −0.29 mmol/L, 95% CIs: −0.49 to −0.10) concentrations (Figure 5). For the analyses, the observed trends were robust and mainly stable to sensitivity analysis (Supplementary Table S2).
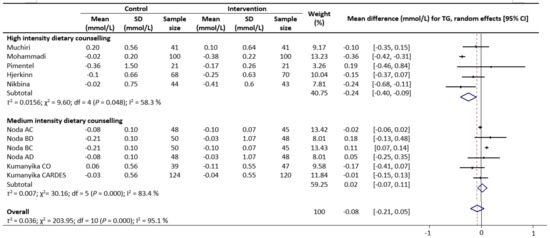
Figure 2.
Random-effects model meta-analysis for comparing the changes in TG of RCTs providing dietary counselling compared to not providing dietary counselling and subcategory analysis in accordance with the intensity level for dietary counselling. Each study is identified by author and year. The horizontal line represents the effect size for each study and the whiskers extending on both sides represent the study effect’s 95% CI. The diamond indicates the overall effect size. Abbreviations: AC, group A and group C; BD, group B and group D; BC, group B and group D; AD, group A and group D; CO, counselling only; CARDES, components of cardiovascular dietary education system.
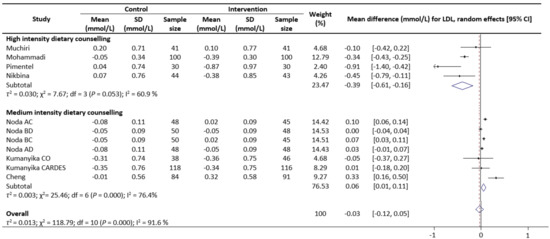
Figure 3.
Random-effects model meta-analysis for comparing the changes in LDL of RCTs providing dietary counselling compared to not providing dietary counselling and subcategory analysis in accordance with the intensity level for dietary counselling. Each study is identified by author and year. The horizontal line represents the effect size for each study and the whiskers extending on both sides represent the study effect’s 95% CI. The diamond indicates the overall effect size. Abbreviations: AC, group A and group C; BD, group B and group D; BC, group B and group D; AD, group A and group D; CO, counselling only; CARDES, components of cardiovascular dietary education system.
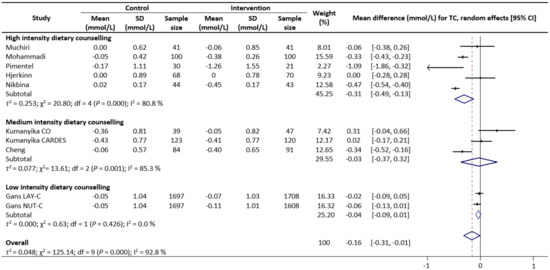
Figure 4.
Random-effects model meta-analysis for comparing the changes in TC of RCTs providing dietary counselling compared to not providing dietary counselling and subcategory analysis in accordance with the intensity level for dietary counselling. Each study is identified by author and year. The horizontal line represents the effect size for each study and the whiskers extending on both sides represent the study effect’s 95% CI. The diamond indicates the overall effect size. Abbreviations: AC, group A and group C; BD, group B and group D; BC, group B and group D; AD, group A and group D; CO, counselling only; CARDES, components of cardiovascular dietary education system; LAY-C, lay person counselling; NUT-C, nutritionist counselling.
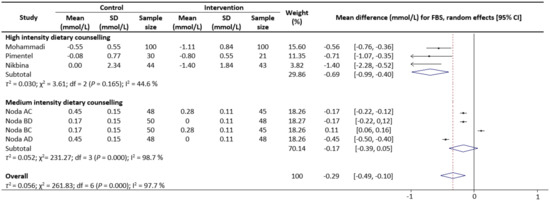
Figure 5.
Random-effects model meta-analysis for comparing the changes in FBS of RCTs providing dietary counselling compared to not providing dietary counselling and subcategory analysis in accordance with the intensity level for dietary counselling. Each study is identified by author and year. The horizontal line represents the effect size for each study and the whiskers extending on both sides represent the study effect’s 95% CI. The diamond indicates the overall effect size. Abbreviations: AC, group A and group C; BD, group B and group D; BC, group B and group D; AD, group A and group D.
Sub-group analyses were conducted for TG, TC, LDL and FBS outcomes based on the intensity of the dietary counselling and the provider of dietary counselling. For the intensity sub-group analysis, TC had three categories (low, medium, and high) whilst the remaining had two categories (medium and high). High intensity of dietary counselling showed a decrease in TG (WMD: −0.24 mmol/L, 95% CIs: −0.40 to −0.09) (Figure 2), LDL (WMD: −0.39 mmol/L, 95% CIs: −0.61 to −0.16) (Figure 3), TC (WMD: −0.31 mmol/L, 95% CIs: −0.49 to −0.13) (Figure 4) and FBS (WMD: −0.69 mmol/L, 95% CIs: −0.99 to −0.40) (Figure 5) concentrations however the same impact was not reflected in the medium intensity for TG (WMD: 0.02 mmol/L, 95% CIs: −0.07 to 0.11), LDL (WMD: 0.06 mmol/L, 95% CIs: 0.01 to 0.11), TC (WMD: −0.03 mmol/L, 95% CIs: −0.37 to 0.32)and FBS (WMD: −0.17 mmol/L, 95% CIs: −0.39 to 0.05). The low intensity dietary counselling has also showed no impact on the TC (WMD: −0.04 mmol/L, 95% CIs: −0.09 to 0.01). Additionally, differences between high intensity and medium intensity in TG, LDL, and FBS were statistically significant with the non-overlap of 95% CIs. There was also no overlap of 95% CIs observed between the high and low intensity in TC.
According to the analysis for the provider of dietary counselling, involvement of dietitian showed no effects for TG (Supplementary Figure S1) and FBS (Supplementary Figure S2) concentrations while an unfavourable effect for LDL concentrations (Supplementary Figure S3). Results were similar for counselling that employed all other providers for TG and LDL concentrations, however, a significant and favourable response was observed for FBS concentrations.
4. Discussion
Dietary counselling has been identified as one of the beneficial nutritional strategies that improves cardiometabolic health, but its effectiveness has not been systematically studied. The findings from this systematic review and meta-analysis support evidence that dietary counselling can provide an improvement in cardiometabolic health parameters in middle-aged and older adults. Moreover, this improvement was found to be more pronounced with the provision of high intensity dietary counselling.
Due to the lack of data, meta-analysis for SBP and DBP were not able to be carried out while systematic review result demonstrated an improvement in blood pressure with dietary counselling in middle-aged and older adults. Included studies which reported blood pressure lowering responses featured a similar set of dietary counselling guidelines such as the reduction of dietary saturated fat intake and increment of dietary fibre intake. Decreasing saturated fat intake and increasing dietary fibre is in accordance with guidelines for a DASH diet and a Mediterranean diet [51,52]. With reference to a previous meta-analysis of RCTs conducted by Ndanuka et al. [53], adherence to a DASH diet and a Mediterranean diet favourably lowered systolic and diastolic blood pressure. Although the potential underlying mechanisms of dietary fibre on lowering blood pressure have not been fully elucidated, one possibility is the modulation of insulin metabolism [54,55]. Insulin may contribute to blood pressure regulation and insulin resistance and compensatory hyperinsulinemia is suggested to be a major risk factor for the development of hypertension [56]. Previous studies found increased insulin sensitivity and improved vascular endothelial function with dietary fibre consumption [57,58]. Additionally, the consumption of dietary fibre rich foods such as fruits and vegetables may contribute to the overall blood pressure lowering effect as they contain substantial amount of nitrates which increase the plasma levels of nitrate and nitrite. Those are important substrate for producing nitric oxide which is a known as vasodilator and can regulate blood pressure [59,60]. Furthermore, a positive association between saturated fat intake and blood pressure has also been reported [61] and a previous RCT reported that consumption of a meal with decrease in saturated fatty acid while increase in monounsaturated fatty acid led to a decrease in DBP [62].
Meta-analysis results revealed that when middle-aged or older adults participated in dietary counselling, they showed lowered TC and FBG concentration. A study conducted by Rijnaarts et al. showed an increase in fibre consumption after the provision of tailored dietary counselling [63]. Dietary fibre plays a pivotal role in the management of lipid metabolism and glycaemic control [64,65]. This is also supported by a meta-analysis evaluating the impact of increased fibre intake on the FBG in patients with type 2 diabetes mellitus [66]. Furthermore, previous studies have also reported a significantly lower FBG concentration when the intervention group consumed higher dietary fibre [67,68]. Several studies within our meta-analysis also revealed an increase in fibre intake and decrease in saturated fat intake after the provision of dietary counselling [23,45]. A systematic review and meta-analysis conducted to review trials of physical activity or dietary counselling to prevent cardiovascular disease found out that in high intensity counselling trials, intervention group participants showed greater increases in fruits and vegetables consumption [19]. This review also reported that high intensity trials collectively resulted in moderate to large decrease in total fat and saturated fat intake in participants of the intervention group while low and medium intensity intervention resulted in smaller reductions in fat intake which is in accordance to our findings [19]. According to the observation of an animal study, Wistar rats fed with palm (saturated) oil resulted in a lower rate of insulin stimulated glucose metabolism and insulin binding to cells when compared to sunflower (polyunsaturated) oil [69]. This result is in accordance to another animal study that a highly saturated diet decreased the rate of insulin stimulated glucose transport [70]. Therefore, the decrease in saturated fat intake may be a contributing source to the lowered FBG. The dietary counselling content may also play a role in the subsequent outcome, especially for the case in FBS where selected studies were focusing on patients with type 2 diabetes mellitus and tailored the counselling to the improvement of glycemic control in type 2 diabetes mellitus patients.
The sub-group analysis results for favourable effects on TG, TC, LDL and FBS with high intensity dietary counselling. In relevance to our study, a high intensity dietary counselling consists of six or more contacts during the intervention period and each lasting more than 30 min. Consistent with our findings, a systematic review and meta-analysis conducted by Rees et al. [71], showed that high intensity dietary intervention involving more than three scheduled personal contact is associated with reduction in TG than low intensity dietary intervention. This further supported our findings on the importance of intervention intensity and that higher intensity counselling may provide more time for adaptation to a healthy diet in order for beneficial changes to be observed consistently. The impact of duration of the study was also one of the considerations for this review, however the duration for each study varies greatly. In our review, studies that we collected results for the cardiometabolic outcomes have various length of study duration ranging from 12 weeks to 144 weeks [23,35,39,40,45]. Amongst the studies, a study conducted by Mohammadi et al. [23] with a trial length of 12 weeks has shown favourable response to TG level as compared to a study conducted by Kumanyika et al. [42] with a trial length of 48 weeks. Between the studies, Mohammadi et al. [23] reported an increase in diabetic nutrition knowledge score pre and post intervention with an indicated compliance in dietary counselling component after the provision of eight counselling sessions while Kumanyika et al. [42] reported no statistically significant difference between groups in their adherence and usage of the counselling material after the provision of four counselling sessions. This finding indicates that as compared to the duration of the study, the intensity of the dietary counselling which accounts for the length and frequency of the dietary counselling given during the duration of the study may provide a greater influence on the subject’s compliance to the program.
In contrast, the sub-group analysis results for TG, LDL and FBS did not favour counselling conducted by dietitians. This observation is in accordance to a RCT conducted by Wong et al. [18] that a dietary counselling conducted by dietitian conferred no beneficial results on blood lipids profile and blood pressure. In this RCT, counselling was only provided once in the beginning of the intervention. Under the dietitian led sub-group analysis, it consisted of two studies, Muchiri et al. [40] and Noda et al. [41], and the level of dietary counselling intensity for Muchiri et al. [40] is high while medium for and Noda et al. [41]. Whereas, for majority of the studies under All Others provided high intensity dietary counselling. A study conducted to investigate the effect of structured dietary advice provided by dietitian compared to standard advice provided by physician on dietary changes to lower the blood LDL found out that dietitians were more effective when the intensity of counselling was high with 6 counselling sessions at 30 min per session whereas the physician group only had one face-to-face consultation [72]. The study observed sustainable dietary changes and greater dietary adherence and this may be explained by the higher intensity of the dietary counselling provided which allowed for better understanding of the counselling material and also the dietitians were all trained to conduct the session in accordance to national and Mediterranean dietary guidelines [72]. Collectively, the intensity of dietary counselling appears to be one of the key factors that may determine the impact of dietary counselling on the cardiometabolic outcomes regardless of the counselling provider. Several studies [30,41,42] did not discuss the compliancy of the study subjects to the dietary counselling intervention. Whereas other studies [35,40,45] investigated and discussed the compliance of the study subjects to the intervention. Under the dietitian led group, Muchiri et al. [40] showed no significant changes in the macronutrient, fruits and vegetables intakes. Whereas for studies under All Others, Pimentel et al. [45] observed a decrease in energy, total fat, saturated fat and cholesterol intake in subjects post intervention and in case of Mohammadi et al. [23], compliance to the intervention was not studied but the knowledge scores on diabetes were significantly increased at post intervention. Compliance in nutrition education studies has an overt importance when it comes to dietetic control [73].
The key strength of this systematic review and meta-analysis is compiled data from dietary intervention studies and findings can provide causality between dietary counselling and its impact on cardiometabolic outcomes in middle-aged and older adults who show an increased risk of cardiometabolic diseases [74]. Another strength lies in its wide inclusion criteria, which shortlist all studies that provided dietary counselling without restrictions in terms of subject conditions and counselling settings. This allowed us to have a comprehensive overview of the impact of dietary counselling as a whole as compared to previous systematic review and meta-analysis which were done with reference to subjects with particular diseases, settings or specific key dietary components [75,76,77]. Exclusion of other lifestyle interventions allowed us to solely examine the impact of dietary counselling on the cardiometabolic outcomes. Lastly, this study investigated the contributing components of dietary counselling that may potentially explain the effect of dietary counselling on the cardiometabolic health via the application of subgroup analysis.
However, a few limitations of this review warrant a discussion. Assessment of adherence to the dietary counselling is important to evaluate the impact of dietary counselling, while the majority of the studies did not examine the adherence of dietary intake, or the adherence was studied via self-reported dietary intake. The quality of the studies also necessitates further discussion, only one third of the articles obtained a ‘low-risk’ bias due to the inevitable nature of dietary counselling. Subjects undergoing intervention were unable to be blinded and hence resulting in only single blinding. Additionally, the characteristic information regarding the studies which may reveal significant and meaningful insights were unreported. The meta-analytic portion of this review was limited by the significant high heterogeneity observed across studies due to the small number of studies available where the sub-group analyses utilised results from a single study with multiple arms. Besides the limited number of studies available, variation between the studies may also lead to high heterogeneity such as the wide range of dietary counselling content for each intervention and disease state of the participants. Lastly, all the trials involved in this review assessed only the classical cardiometabolic health parameters such as blood pressure and blood lipids panel without the inclusion of some other risk factors such as the body mass index, anthropometry measurement and inflammatory markers. The untenable importance of classical risk factors for cardiometabolic health are largely illustrated, however, the incorporation of other novel or clinical parameters may be helpful to improve risk prediction models and provide more insights on the cardiometabolic health status [78].
5. Conclusions
In conclusion, the findings from this systematic review and meta-analysis suggest that dietary counselling is a beneficial dietary strategy to improve cardiometabolic health in middle-aged and older adults with the emphasis on the counselling intensity. In particular, these findings can provide insights for dietary counselling providers to establish well-designed counselling sessions with greater consideration of the dietary counselling intensity to improve cardiometabolic health.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/nu13092936/s1, Figure S1: Random-effects model meta-analysis for comparing the changes in TG of RCTs providing dietary counselling compared to not providing dietary counselling and subcategory analysis in accordance with the provider of dietary counselling. Each study is identified by author and year. The horizontal line represents the effect size for each study and the whiskers extending on both sides represent the study effect’s 95% CI. The diamond indicates the overall effect size. Abbreviations: AC, group A and group C; BD, group B and group D; BC, group B and group D; AD, group A and group D; CO, counselling only; CARDES, components of cardiovascular dietary education system. Figure S2: Random-effects model meta-analysis for comparing the changes in FBS of RCTs providing dietary counselling compared to not providing dietary counselling and subcategory analysis in accordance with the provider of dietary counselling. Each study is identified by author and year. The horizontal line represents the effect size for each study and the whiskers extending on both sides represent the study effect’s 95% CI. The diamond indicates the overall effect size. Abbreviations: AC, group A and group C; BD, group B and group D; BC, group B and group D; AD, group A and group D. Figure S3: Random-effects model meta-analysis for comparing the changes in LDL of RCTs providing dietary counselling compared to not providing dietary counselling and subcategory analysis in accordance with the provider of dietary counselling. Each study is identified by author and year. The horizontal line represents the effect size for each study and the whiskers extending on both sides represent the study effect’s 95% CI. The diamond indicates the overall effect size. Abbreviations: AC, group A and group C; BD, group B and group D; BC, group B and group D; AD, group A and group D; CO, counselling only; CARDES, components of cardiovascular dietary education system. Table S1: Risk of bias assessment using Cochrane’s Modified Tool, Table S2: Sensitivity analysis following the removal of single groups or randomized controlled trials to assess the robustness of meta-analyses results studying the impact of dietary counselling fruit on cardiovascular disease risk factors in older adults.
Author Contributions
The authors’ responsibilities were as follows—J.H.M.L. and J.E.K. designed the research; J.H.M.L. and M.T.T.N. worked on the relevant keyword researching; J.H.M.L. and D.W.K.T. conducted data extraction, statistical analysis, and data interpretation; J.H.M.L. wrote the manuscript under the supervision of J.F., E.H.K. and J.E.K.; J.H.M.L. and J.E.K. had primary responsibility for the final content. All authors have read and agreed to the published version of the manuscript.
Funding
This work is supported by the National University of Singapore, Singapore, and the National University of Singapore—Mind Science Centre.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study will be made available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- United Nations. World Population Ageing 2019; United Nations: New York, NY, USA, 2019. [Google Scholar]
- Machado-Oliveira, G.; Ramos, C.; Marques, A.R.A.; Vieira, O.V. Cell Senescence, Multiple Organelle Dysfunction and Atherosclerosis. Cells 2020, 9, 2146. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Tang, X.; Shen, P.; Si, Y.; Liu, X.; Xu, Z.; Wu, J.; Zhang, J.; Lu, P.; Lin, H.; et al. Multimorbidity of cardiometabolic diseases: Prevalence and risk for mortality from one million Chinese adults in a longitudinal cohort study. BMJ Open 2019, 9, e024476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reedy, J.; Krebs-Smith, S.M.; Miller, P.E.; Liese, A.D.; Kahle, L.L.; Park, Y.; Subar, A.F. Higher Diet Quality Is Associated with Decreased Risk of All-Cause, Cardiovascular Disease, and Cancer Mortality among Older Adults. J. Nutr. 2014, 144, 881–889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwingshackl, L.; Hoffmann, G. Diet Quality as Assessed by the Healthy Eating Index, the Alternate Healthy Eating Index, the Dietary Approaches to Stop Hypertension Score, and Health Outcomes: A Systematic Review and Meta-Analysis of Cohort Studies. J. Acad. Nutr. Diet. 2015, 115, 780–800. [Google Scholar] [CrossRef]
- Xu, Z.; Steffen, L.M.; Selvin, E.; Rebholz, C.M. Diet quality, change in diet quality and risk of incident CVD and diabetes. Publ. Health Nutr. 2020, 23, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Repas, T.B. Challenges and strategies in managing cardiometabolic risk. J. Am. Osteopath. Assoc. 2007, 107, 4–11. [Google Scholar]
- Esposito, K.; Pontillo, A.; Di Palo, C.; Giugliano, G.; Masella, M.; Marfella, R.; Giugliano, D. Effect of Weight Loss and Lifestyle Changes on Vascular Inflammatory Markers in Obese Women: A Randomized Trial. J. Am. Med. Assoc. 2003, 289, 1799–1804. [Google Scholar] [CrossRef] [Green Version]
- Yesilbursa, D.; Serdar, A.; Heper, Y.; Sarac, M.; Coskun, S.; Kazazoglu, A.R.; Cordan, J. The effect of orlistat-induced weight loss on interleukin-6 and C-reactive protein levels in obese subjects. Acta Cardiol. 2005, 60, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Monzillo, L.U.; Hamdy, O.; Horton, E.S.; Ledbury, S.; Mullooly, C.; Jarema, C.; Porter, S.; Ovalle, K.; Moussa, A.; Mantzoros, C.S. Effect of lifestyle modification on adipokine levels in obese subjects with insulin resistance. Obes. Res. 2003, 11, 1048–1054. [Google Scholar] [CrossRef] [Green Version]
- Grundy, S.M. Drug therapy of the metabolic syndrome: Minimizing the emerging crisis in polypharmacy. Nat. Rev. Drug Discov. 2006, 5, 295–309. [Google Scholar] [CrossRef]
- Vasiloglou, M.F.; Fletcher, J.; Poulia, K.A. Challenges and Perspectives in Nutritional Counselling and Nursing: A Narrative Review. J. Clin. Med. 2019, 8, 1489. [Google Scholar] [CrossRef] [Green Version]
- ADA Policy statement on licensure. J. Am. Diet. Assoc. 1991, 91, 985.
- Talib, R.; Ali, O.; Arshad, F.; Kadir, K.A. The effectiveness of group dietary counselling among non insulin dependent diabetes mellitus (NIDDM) patients in resettlement scheme areas in Malaysia. Asia Pac. J. Clin. Nutr. 1997, 6, 84–87. [Google Scholar]
- Nguyen, H.T.; Pavey, T.G.; Collins, P.F.; Nguyen, N.V.; Pham, T.D.; Gallegos, D. Effectiveness of Tailored Dietary Counseling in Treating Malnourished Outpatients with Chronic Obstructive Pulmonary Disease: A Randomized Controlled Trial. J. Acad. Nutr. Diet. 2020, 120, 778–791. [Google Scholar] [CrossRef]
- Ross, L.J.; Barnes, K.A.; Ball, L.E.; Mitchell, L.J.; Sladdin, I.; Lee, P.; Williams, L.T. Effectiveness of dietetic consultation for lowering blood lipid levels in the management of cardiovascular disease risk: A systematic review and meta-analysis of randomised controlled trials. Nutr. Diet. 2019, 76, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Razaz, J.M.; Rahmani, J.; Varkaneh, H.K.; Thompson, J.; Clark, C.; Abdulazeem, H.M. The health effects of medical nutrition therapy by dietitians in patients with diabetes: A systematic review and meta-analysis: Nutrition therapy and diabetes. Prim. Care Diabetes 2019, 13, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.C.S.; Wang, H.H.X.; Kwan, M.W.M.; Fong, B.C.Y.; Chan, W.M.; Zhang, D.X.; Li, S.T.S.; Yan, B.P.; Coats, A.J.S.; Griffiths, S.M. Dietary counselling has no effect on cardiovascular risk factors among Chinese Grade 1 hypertensive patients: A randomized controlled trial. Eur. Heart J. 2015, 36, 2598–2607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, J.S.; O’Connor, E.; Whitlock, E.P.; Beil, T.L. Behavioral counseling to promote physical activity and a healthful diet to prevent cardiovascular disease in adults: A systematic review for the U.S. Preventive Services Task Force. Ann. Intern. Med. 2010, 153, 736–750. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int. J. Surg. 2010, 8, 336–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Decoster, J.; Hall, G.P. Meta-Analysis Notes; University of Alabama: Tuscaloosa, AL, USA, 2004. [Google Scholar]
- Higgins, J.P.T.; Altman, D.G. Assessing Risk of Bias in Included Studies. Cochrane Handb. Syst. Rev. Interv. 2008, 187–241. [Google Scholar] [CrossRef]
- Mohammadi, S.; Karim, N.A.; Talib, R.A.; Amani, R. The impact of self-efficacy education based on the health belief model in Iranian patients with type 2 diabetes: A randomised controlled intervention study. Asia Pac. J. Clin. Nutr. 2018, 27, 546–555. [Google Scholar] [CrossRef]
- Deeks, J.J.; Higgins, J.P.; Altman, D.G. Analysing Data and Undertaking Meta-Analyses. Cochrane Handb. Syst. Rev. Interv. Cochrane B Ser. 2008, 243–296. [Google Scholar] [CrossRef]
- Ammerman, A.; Pignone, M.; Fernandez, L.; Lohr, K.; Jacobs, A.D.; Nester, C.; Orleans, T.; Pender, N.; Woolf, S.; Sutton, S.F.; et al. Counseling to Promote a Healthy Diet. Agency Healthc. Res. Qual. 2002, 18, 40–55. [Google Scholar]
- Lindgarde, F. The effect of orlistat body weight and coronary heart disease risk profile in obese patients: The swedish multimorbidity study. J. Intern. Med. 2000, 248, 245–254. [Google Scholar] [CrossRef]
- Al-Shookri, A.; Khor, G.L.; Chan, Y.M.; Loke, S.C.; Al-Maskari, M. Effectiveness of medical nutrition treatment delivered by dietitians on glycaemic outcomes and lipid profiles of Arab, Omani patients with Type 2 diabetes. Diabet. Med. 2012, 29, 236–244. [Google Scholar] [CrossRef]
- Thomson, C.A.; Stopeck, A.T.; Bea, J.W.; Cussler, E.; Nardi, E.; Frey, G.; Thompson, P.A. Changes in body weight and metabolic indexes in overweight breast cancer survivors enrolled in a randomized trial of low-fat vs. reduced carbohydrate diets. Nutr. Cancer 2010, 62, 1142–1152. [Google Scholar] [CrossRef] [PubMed]
- Klein, G.A.; Stefanuto, A.; Boaventura, B.C.B.; De Morais, E.C.; Da Cavalcante, L.S.; De Andrade, F.; Wazlawik, E.; Di Pietro, P.F.; Maraschin, M.; Da Silva, E.L. Mate Tea (Ilex paraguariensis) Improves Glycemic and Lipid Profiles of Type 2 Diabetes and Pre-Diabetes Individuals: A Pilot Study. J. Am. Coll. Nutr. 2011, 30, 320–332. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Graziani, C.; Diamond, J.J. Cholesterol-lowering effect of the Food for Heart Nutrition Education Program. J. Am. Diet. Assoc. 2004, 104, 1868–1872. [Google Scholar] [CrossRef] [PubMed]
- Kaliora, A.C.; Kokkinos, A.; Diolintzi, A.; Stoupaki, M.; Gioxari, A.; Kanellos, P.T.; Dedoussis, G.V.Z.; Vlachogiannakos, J.; Revenas, C.; Ladas, S.D.; et al. The effect of minimal dietary changes with raisins in NAFLD patients with non-significant fibrosis: A randomized controlled intervention. Food Funct. 2016, 7, 4533–4544. [Google Scholar] [CrossRef]
- Samuelsson, O.; Attman, P.O.; Knight-Gibson, C.; Kron, B.; Larsson, R.; Mulec, H.; Weiss, L.; Alaupovic, P. Effect of gemfibrozil on lipoprotein abnormalities in chronic renal insufficiency: A controlled study in human chronic renal disease. Nephron 1997, 75, 286–294. [Google Scholar] [CrossRef]
- Wu, H.L.; Sung, J.M.; Kao, M.D.; Wang, M.C.; Tseng, C.C.; Chen, S.T. Nonprotein Calorie Supplement Improves Adherence to Low-Protein Diet and Exerts Beneficial Responses on Renal Function in Chronic Kidney Disease. J. Ren. Nutr. 2013, 23, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, Z.; Liu, Y.; Lv, X.; Yang, W. Effects of pistachios on body weight in Chinese subjects with metabolic syndrome. Nutr. J. 2012, 11, 20. [Google Scholar] [CrossRef] [Green Version]
- Hjerkinn, E.M.; Abdelnoor, M.; Breivik, L.; Bergengen, L.; Ellingsen, I.; Seljeflot, I.; Aase, O.; Klemsdal, T.O.; Hjermann, I.; Arnesen, H. Effect of diet or very long chain ω-3 fatty acids on progression of atherosclerosis, evaluated by carotid plaques, intima-media thickness and by pulse wave propagation in elderly men with hypercholesterolaemia. Eur. J. Prev. Cardiol. 2006, 13, 325–333. [Google Scholar] [CrossRef]
- Gans, K.M.; Burkholder, G.J.; Risica, P.M.; Harrow, B.; Lasater, T.M. Cost-effectiveness of minimal contact education strategies for cholesterol change. Ethn. Dis. 2006, 16, 443–451. [Google Scholar] [PubMed]
- Henkin, Y.; Shai, I.; Zuk, R.; Brickner, D.; Zuilli, I.; Neumann, L.; Shany, S. Dietary treatment of hypercholesterolemia: Do dietitians do it better? A randomized, controlled trial. Am. J. Med. 2000, 109, 549–555. [Google Scholar] [CrossRef]
- Meuleman, Y.; Hoekstra, T.; Dekker, F.W.; Navis, G.; Vogt, L.; van der Boog, P.J.M.; Bos, W.J.W.; van Montfrans, G.A.; van Dijk, S.; Boeschoten, E.W.; et al. Sodium Restriction in Patients With CKD: A Randomized Controlled Trial of Self-management Support. Am. J. Kidney Dis. 2017, 69, 576–586. [Google Scholar] [CrossRef] [Green Version]
- Nikbina, M.; Mameneh, M.; Bakaeian, M.; Dehcheshmeh, N.F. Effectiveness of nutrition education and counseling on metabolic control parameters of diabetes mellitus type 2 patients in primary health care centers. Clin. Diabetol. 2020, 9, 293–299. [Google Scholar] [CrossRef]
- Muchiri, J.W.; Gericke, G.J.; Rheeder, P. Effect of a nutrition education programme on clinical status and dietary behaviours of adults with type 2 diabetes in a resource-limited setting in South Africa: A randomised controlled trial. Public Health Nutr. 2016, 19, 142–155. [Google Scholar] [CrossRef] [Green Version]
- Noda, K.; Zhang, B.; Iwata, A.; Nishikawa, H.; Ogawa, M.; Nomiyama, T.; Miura, S.-I.; Sako, H.; Matsuo, K.; Yahiro, E.; et al. Lifestyle changes through the use of delivered meals and dietary counseling in a single-blind study-the STYLIST study. Circ. J. 2012, 76, 1335–1344. [Google Scholar] [CrossRef] [Green Version]
- Kumanyika, S.K.; Adams-Campbell, L.; Van Horn, B.; Ten Have, T.R.; Treu, J.A.; Askov, E.; Williams, J.; Achterberg, C.; Zaghloul, S.; Monsegu, D.; et al. Outcomes of a cardiovascular nutrition counseling program in African- Americans with elevated blood pressure or cholesterol level. J. Am. Diet. Assoc. 1999, 99, 1380–1388. [Google Scholar] [CrossRef]
- Takahashi, Y.; Sasaki, S.; Okubo, S.; Hayashi, M.; Tsugane, S. Blood pressure change in a free-living population-based dietary modification study in Japan. J. Hypertens. 2006, 24, 451–458. [Google Scholar] [CrossRef]
- Schwab, U.; Louheranta, A.; Törrönen, A.; Uusitupa, M. Impact of sugar beet pectin and polydextrose on fasting and postprandial glycemia and fasting concentrations of serum total and lipoprotein lipids in middle-aged subjects with abnormal glucose metabolism. Eur. J. Clin. Nutr. 2006, 60, 1073–1080. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, G.D.; Portero-Mclellan, K.C.; Oliveira, É.P.; Spada, A.P.M.; Oshiiwa, M.; Zemdegs, J.C.S.; Barbalho, S.M. Long-term nutrition education reduces several risk factors for type 2 diabetes mellitus in Brazilians with impaired glucose tolerance. Nutr. Res. 2010, 30, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Morán, M.; Guerrero-Romero, F.; Lazcano-Burciaga, G. Lipid-and glucose-lowering efficacy of Plantago Psyllium in type II diabetes. J. Diabetes Complicat. 1998, 12, 273–278. [Google Scholar] [CrossRef]
- Tan, X.; Alén, M.; Wang, K.; Tenhunen, J.; Wiklund, P.; Partinen, M.; Cheng, S. Effect of six-month diet intervention on sleep among overweight and obese men with chronic insomnia symptoms: A randomized controlled trial. Nutrients 2016, 8, 751. [Google Scholar] [CrossRef]
- Holland-Carter, L.; Tuerk, P.W.; Wadden, T.A.; Fujioka, K.N.; Becker, L.E.; Miller-Kovach, K.; Hollander, P.L.; Garvey, W.T.; Weiss, D.; Rubino, D.M.; et al. Impact on psychosocial outcomes of a nationally available weight management program tailored for individuals with type 2 diabetes: Results of a randomized controlled trial. J. Diabetes Complicat. 2017, 31, 891–897. [Google Scholar] [CrossRef] [PubMed]
- Britton, B.; Baker, A.L.; Wolfenden, L.; Wratten, C.; Bauer, J.; Beck, A.K.; McCarter, K.; Harrowfield, J.; Isenring, E.; Tang, C.; et al. Eating As Treatment (EAT): A Stepped-Wedge, Randomized Controlled Trial of a Health Behavior Change Intervention Provided by Dietitians to Improve Nutrition in Patients With Head and Neck Cancer Undergoing Radiation Therapy (TROG 12.03). Int. J. Radiat. Oncol. Biol. Phys. 2019, 103, 353–362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munk, T.; Tolstrup, U.; Beck, A.M.; Holst, M.; Rasmussen, H.H.; Hovhannisyan, K.; Thomsen, T. Individualised dietary counselling for nutritionally at-risk older patients following discharge from acute hospital to home: A systematic review and meta-analysis. J. Hum. Nutr. Diet. 2016, 29, 196–208. [Google Scholar] [CrossRef]
- Tosti, V.; Bertozzi, B.; Fontana, L. Health Benefits of the Mediterranean Diet: Metabolic and Molecular Mechanisms. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2018, 73, 318–326. [Google Scholar] [CrossRef] [Green Version]
- Tyson, C.C.; Nwankwo, C.; Lin, P.H.; Svetkey, L.P. The dietary approaches to stop hypertension (dash) eating pattern in special populations. Curr. Hypertens. Rep. 2012, 14, 388–396. [Google Scholar] [CrossRef] [Green Version]
- Ndanuko, R.N.; Tapsell, L.C.; Charlton, K.E.; Neale, E.P.; Batterham, M.J. Dietary patterns and blood pressure in adults: A systematic review and meta-analysis of randomized controlled trials. Adv. Nutr. 2016, 7, 76–89. [Google Scholar] [CrossRef] [PubMed]
- Fukagawa, N.K.; Anderson, J.W.; Hageman, G.; Young, V.R.; Minaker, K.L. High-carbohydrate, high-fiber diets increase peripheral insulin sensitivity in healthy young and old adults. Am. J. Clin. Nutr. 1990, 52, 524–528. [Google Scholar] [CrossRef] [PubMed]
- Aleixandre, A.; Miguel, M. Dietary fiber and blood pressure control. Food Funct. 2016, 7, 1864–1871. [Google Scholar] [CrossRef] [PubMed]
- Tarray, R.; Saleem, S.; Afroze, D.; Yousuf, I.; Gulnar, A.; Laway, B.; Verma, S. Role of insulin resistance in essential hypertension. Cardiovasc. Endocrinol. 2014, 3, 129–133. [Google Scholar] [CrossRef]
- Cleland, S.J.; Petrie, J.R.; Ueda, S.; Elliott, H.L.; Connell, J.M. Insulin as a vascular hormone: Implications for the pathophysiology of cardiovascular disease. Clin. Exp. Pharmacol. Physiol. 1998, 25, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Bessesen, D.H. The role of carbohydrates in insulin resistance. J. Nutr. 2001, 131, 2782S–2786S. [Google Scholar] [CrossRef]
- Brock, D.W.; Davis, C.K.; Irving, B.A.; Rodriguez, J.; Barrett, E.J.; Weltman, A.; Taylor, A.G.; Gaesser, G.A. A high-carbohydrate, high-fiber meal improves endothelial function in adults with the metabolic syndrome. Diabetes Care 2006, 29, 2313–2315. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, J.; Ohtake, K.; Uchida, H. No-rich diet for lifestyle-related diseases. Nutrients 2015, 7, 4911–4937. [Google Scholar] [CrossRef] [Green Version]
- Micha, R.; Mozaffarian, D. Saturated fat and cardiometabolic risk factors, coronary heart disease, stroke, and diabetes: A fresh look at the evidence. Lipids 2010, 45, 893–905. [Google Scholar] [CrossRef] [Green Version]
- Rasmussen, B.M.; Vessby, B.; Uusitupa, M.; Berglund, L.; Pedersen, E.; Riccardi, G.; Rivellese, A.A.; Tapsell, L.; Hermansen, K. Effects of dietary saturated, monounsaturated, and n-3 fatty acids on blood pressure in healthy subjects. Am. J. Clin. Nutr. 2006, 83, 221–226. [Google Scholar] [CrossRef]
- Rijnaarts, I.; De Roos, N.M.; Wang, T.; Zoetendal, E.G.; Top, J.; Timmer, M.; Bouwman, E.P.; Hogenelst, K.; Witteman, B.; De Wit, N. Increasing dietary fibre intake in healthy adults using personalised dietary advice compared with general advice: A single-blind randomised controlled trial. Public Health Nutr. 2021, 24, 1117–1128. [Google Scholar] [CrossRef]
- Brennan, C.S. Dietary fibre, glycaemic response, and diabetes. Mol. Nutr. Food Res. 2005, 49, 560–570. [Google Scholar] [CrossRef] [PubMed]
- Kritchevsky, D. Dietary fibre and lipid metabolism. Int. J. Obes. 1987, 11 (Suppl. 1), 33–43. [Google Scholar] [PubMed]
- Post, R.E.; Mainous, A.G.; King, D.E.; Simpson, K.N. Dietary fiber for the treatment of type 2 diabetes mellitus: A meta-analysis. J. Am. Board Fam. Med. 2012, 25, 16–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.; Zeng, Y.; Xu, J.; Zheng, H.; Liu, J.; Fan, R.; Zhu, W.; Yuan, L.; Qin, Y.; Chen, S.; et al. Therapeutic effects of soluble dietary fiber consumption on type 2 diabetes mellitus. Exp. Ther. Med. 2016, 12, 1232–1242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaizu, S.; Kishimoto, H.; Iwase, M.; Fujii, H.; Ohkuma, T.; Ide, H.; Jodai, T.; Kikuchi, Y.; Idewaki, Y.; Hirakawa, Y.; et al. Impact of leisure-time physical activity on glycemic control and cardiovascular risk factors in Japanese patients with type 2 diabetes mellitus: The Fukuoka Diabetes Registry. PLoS ONE 2014, 9, e98768. [Google Scholar] [CrossRef] [Green Version]
- Van Amelsvoort, J.M.M.; Van Der Beek, A.; Stam, J.J. Effects of the type of dietary fatty acid on the insulin receptor function in rat epididymal fat cells. Ann. Nutr. Metab. 1986, 30, 273–280. [Google Scholar] [CrossRef]
- Field, C.J.; Ryan, E.A.; Thomson, A.B.; Clandinin, M.T. Diet fat composition alters membrane phospholipid composition, insulin binding, and glucose metabolism in adipocytes from control and diabetic animals. J. Biol. Chem. 1990, 265, 11143–11150. [Google Scholar] [CrossRef]
- Rees, K.; Dyakova, M.; Wilson, N.; Ward, K.; Thorogood, M.; Brunner, E. Dietary advice for reducing cardiovascular risk. Cochrane Database Syst. Rev. 2013, 2013. [Google Scholar] [CrossRef] [Green Version]
- Sialvera, T.E.; Papadopoulou, A.; Efstathiou, S.P.; Trautwein, E.A.; Ras, R.T.; Kollia, N.; Farajian, P.; Goumas, G.; Dimakopoulos, I.; Papavasiliou, K.; et al. Structured advice provided by a dietitian increases adherence of consumers to diet and lifestyle changes and lowers blood low-density lipoprotein (LDL)-cholesterol: The Increasing Adherence of Consumers to Diet & Lifestyle Changes to Lower (LDL) Cholester. J. Hum. Nutr. Diet. 2018, 31, 197–208. [Google Scholar] [CrossRef]
- Weickert, M.; Möhlig, M.; Schöfl, C.; Arafat, A.; Otto, B.; Viehoff, H.; Koebnick, C.; Kohl, A.; Spranger, J.; Pfeiffer, A. Cereal fiber improves whole-body insulin. Diabetes Care 2006, 29, 773–780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodgers, J.L.; Jones, J.; Bolleddu, S.I.; Vanthenapalli, S.; Rodgers, L.E.; Shah, K.; Karia, K.; Panguluri, S.K. Cardiovascular Risks Associated with Gender and Aging. J. Cardiovasc. Dev. Dis. 2019, 6, 19. [Google Scholar] [CrossRef] [Green Version]
- Houston, K.A. Systematic Review of Literature on the Effectiveness of Behavioral Weight Loss Programs to Achieve Weight Reduction. J. Obes. Weight Loss Ther. 2012, 3, 2. [Google Scholar] [CrossRef]
- Baldwin, C.; Weekes, C.E. Dietary counselling with or without oral nutritional supplements in the management of malnourished patients: A systematic review and meta-analysis of randomised controlled trials. J. Hum. Nutr. Diet. 2012, 25, 411–426. [Google Scholar] [CrossRef]
- Bauer, J.; Isenring, E.; Ferguson, M. Dietary counseling: Evidence in chemotherapy patients. J. Support. Oncol. 2008, 6, 354–355, author reply 355. [Google Scholar] [PubMed]
- Folsom, A.R. Classical and Novel Biomarkers for Cardiovascular Risk Prediction in the United States. J. Epidemiol. 2013, 23, 158–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).