Coffee Consumption and Cardiovascular Diseases: A Mendelian Randomization Study
Abstract
1. Introduction
2. Methods
2.1. Genetic Instrument Selection
2.2. Data Sources of Cardiovascular Diseases
2.3. Data Sources for BMI and Smoking Initiation
2.4. Statistical Methods
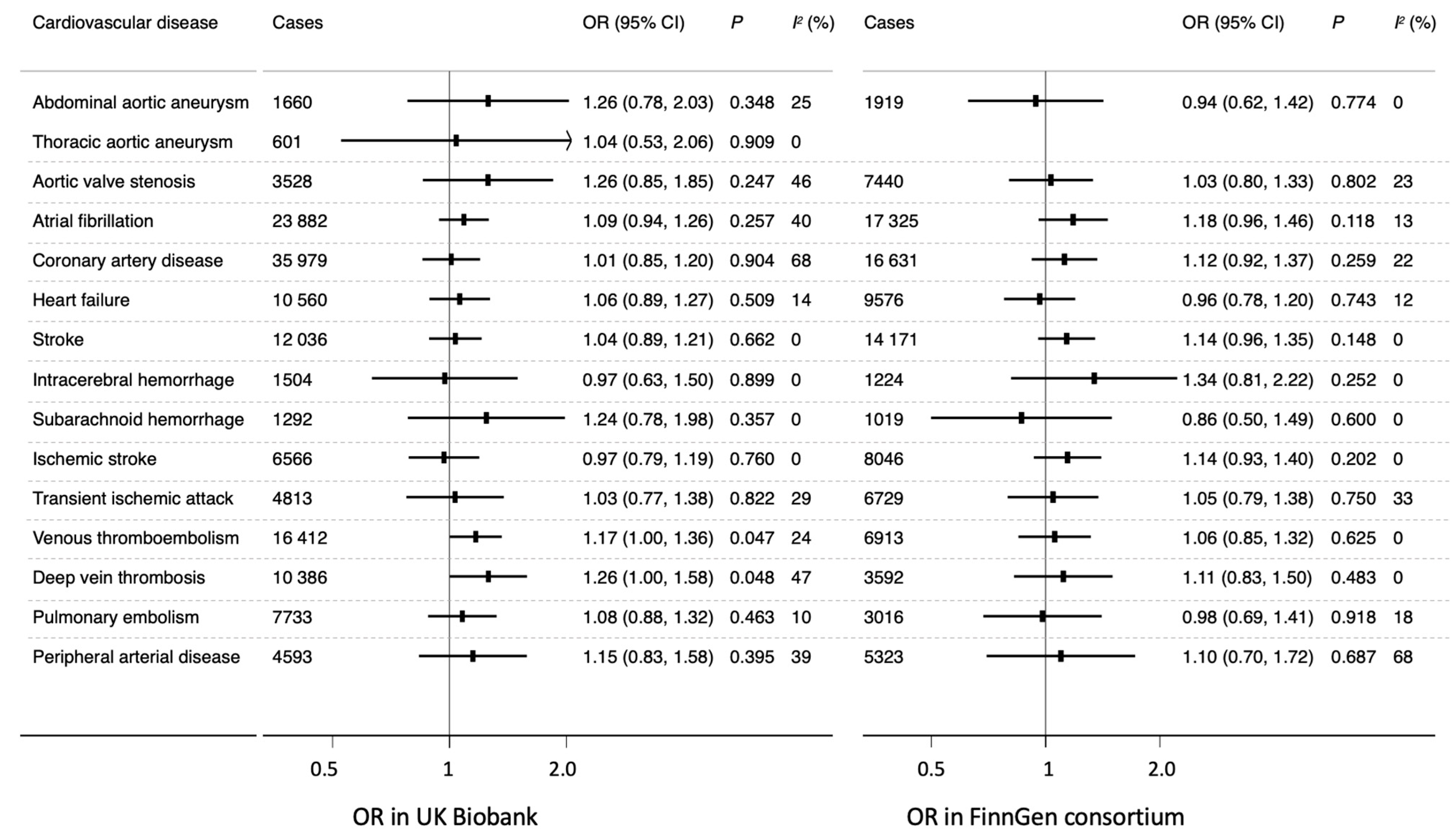
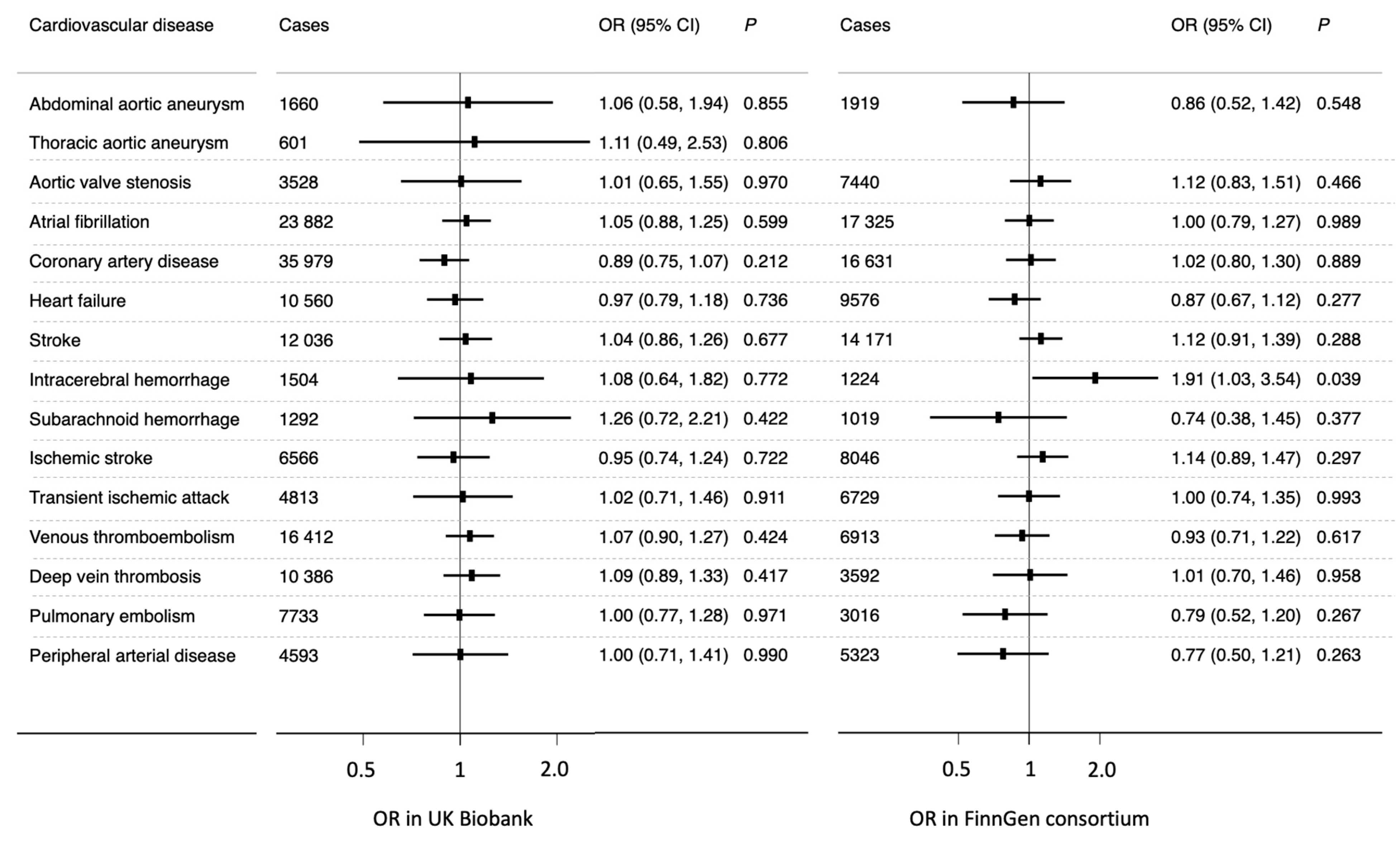
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rodríguez-Artalejo, F.; López-García, E. Coffee Consumption and Cardiovascular Disease: A Condensed Review of Epidemiological Evidence and Mechanisms. J. Agric. Food Chem. 2018, 66, 5257–5263. [Google Scholar] [CrossRef] [PubMed]
- Zulli, A.; Smith, R.M.; Kubatka, P.; Novak, J.; Uehara, Y.; Loftus, H.; Qaradakhi, T.; Pohanka, M.; Kobyliak, N.; Zagatina, A.; et al. Caffeine and cardiovascular diseases: Critical review of current research. Eur. J. Nutr. 2016, 55, 1331–1343. [Google Scholar] [CrossRef]
- Bøhn, S.K.; Ward, N.C.; Hodgson, J.M.; Croft, K.D. Effects of tea and coffee on cardiovascular disease risk. Food Funct. 2012, 3, 575–591. [Google Scholar] [CrossRef]
- Ding, M.; Bhupathiraju, S.N.; Satija, A.; van Dam, R.M.; Hu, F.B. Long-term coffee consumption and risk of cardiovascular disease: A systematic review and a dose-response meta-analysis of prospective cohort studies. Circulation 2014, 129, 643–659. [Google Scholar] [CrossRef]
- Poole, R.; Kennedy, O.J.; Roderick, P.; Fallowfield, J.A.; Hayes, P.C.; Parkes, J. Coffee consumption and health: Umbrella review of meta-analyses of multiple health outcomes. BMJ 2017, 359, j5024. [Google Scholar] [CrossRef]
- Wu, J.N.; Ho, S.C.; Zhou, C.; Ling, W.H.; Chen, W.Q.; Wang, C.L.; Chen, Y.M. Coffee consumption and risk of coronary heart diseases: A meta-analysis of 21 prospective cohort studies. Int. J. Cardiol. 2009, 137, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Garcia, E.; Rodriguez-Artalejo, F.; Rexrode, K.M.; Logroscino, G.; Hu, F.B.; van Dam, R.M. Coffee consumption and risk of stroke in women. Circulation 2009, 119, 1116–1123. [Google Scholar] [CrossRef] [PubMed]
- Mostofsky, E.; Rice, M.S.; Levitan, E.B.; Mittleman, M.A. Habitual coffee consumption and risk of heart failure: A dose-response meta-analysis. Circ. Heart Fail. 2012, 5, 401–405. [Google Scholar] [CrossRef] [PubMed]
- Stephen, B.; Thompson, S.G. Mendelian Randomization: Methods for Using Genetic Variants in Causal Estimation; Chapman and Hall/CRC: London, UK, 2015. [Google Scholar]
- Larsson, S.C. Mendelian randomization as a tool for causal inference in human nutrition and metabolism. Curr. Opin. Lipidol. 2020. [Google Scholar] [CrossRef]
- Qian, Y.; Ye, D.; Huang, H.; Wu, D.J.H.; Zhuang, Y.; Jiang, X.; Mao, Y. Coffee Consumption and Risk of Stroke: A Mendelian Randomization Study. Ann. Neurol. 2020, 87, 525–532. [Google Scholar] [CrossRef]
- Nordestgaard, A.T.; Nordestgaard, B.G. Coffee intake, cardiovascular disease and all-cause mortality: Observational and Mendelian randomization analyses in 95,000–223,000 individuals. Int. J. Epidemiol. 2016, 45, 1938–1952. [Google Scholar] [CrossRef]
- Yuan, S.; Larsson, S.C. No association between coffee consumption and risk of atrial fibrillation: A Mendelian randomization study. Nutr. Metab. Cardiovasc. Dis. 2019, 29, 1185–1188. [Google Scholar] [CrossRef]
- Van Oort, S.; Beulens, J.W.J.; van Ballegooijen, A.J.; Handoko, M.L.; Larsson, S.C. Modifiable lifestyle factors and heart failure: A Mendelian randomization study. Am. Heart J. 2020, 227, 64–73. [Google Scholar] [CrossRef]
- Kwok, M.K.; Leung, G.M.; Schooling, C.M. Habitual coffee consumption and risk of type 2 diabetes, ischemic heart disease, depression and Alzheimer’s disease: A Mendelian randomization study. Sci. Rep. 2016, 6, 36500. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, M.; Yuan, S.; Liu, X. Coffee consumption and risk of coronary artery disease. Eur. J. Prev. Cardiol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Cornelis, M.C.; Munafo, M.R. Mendelian Randomization Studies of Coffee and Caffeine Consumption. Nutrients 2018, 10, 1343. [Google Scholar] [CrossRef]
- Burgess, S.; Thompson, S.G. Multivariable Mendelian randomization: The use of pleiotropic genetic variants to estimate causal effects. Am. J. Epidemiol. 2015, 181, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Zhong, V.W.; Kuang, A.; Danning, R.D.; Kraft, P.; van Dam, R.M.; Chasman, D.I.; Cornelis, M.C. A genome-wide association study of bitter and sweet beverage consumption. Hum. Mol. Genet. 2019, 28, 2449–2457. [Google Scholar] [CrossRef] [PubMed]
- Clarke, L.; Zheng-Bradley, X.; Smith, R.; Kulesha, E.; Xiao, C.; Toneva, I.; Vaughan, B.; Preuss, D.; Leinonen, R.; Shumway, M.; et al. The 1000 Genomes Project: Data management and community access. Nat. Methods 2012, 9, 459–462. [Google Scholar] [CrossRef]
- Sudlow, C.; Gallacher, J.; Allen, N.; Beral, V.; Burton, P.; Danesh, J.; Downey, P.; Elliott, P.; Green, J.; Landray, M.; et al. UK biobank: An open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015, 12, e1001779. [Google Scholar] [CrossRef]
- The FinnGen Consortium. FinnGen Documentation of R4 Release. 2020. Available online: https://finngen.gitbook.io/documentation/ (accessed on 20 December 2020).
- Pulit, S.L.; Stoneman, C.; Morris, A.P.; Wood, A.R.; Glastonbury, C.A.; Tyrrell, J.; Yengo, L.; Ferreira, T.; Marouli, E.; Ji, Y.; et al. Meta-analysis of genome-wide association studies for body fat distribution in 694 649 individuals of European ancestry. Hum. Mol. Genet. 2019, 28, 166–174. [Google Scholar] [CrossRef]
- Liu, M.; Jiang, Y.; Wedow, R.; Li, Y.; Brazel, D.M.; Chen, F.; Datta, G.; Davila-Velderrain, J.; McGuire, D.; Tian, C.; et al. Association studies of up to 1.2 million individuals yield new insights into the genetic etiology of tobacco and alcohol use. Nat. Genet. 2019, 51, 237–244. [Google Scholar] [CrossRef]
- Burgess, S.; Bowden, J.; Fall, T.; Ingelsson, E.; Thompson, S.G. Sensitivity Analyses for Robust Causal Inference from Mendelian Randomization Analyses with Multiple Genetic Variants. Epidemiology 2017, 28, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Bowden, J.; Davey Smith, G.; Haycock, P.C.; Burgess, S. Consistent Estimation in Mendelian Randomization with Some Invalid Instruments Using a Weighted Median Estimator. Genet. Epidemiol. 2016, 40, 304–314. [Google Scholar] [CrossRef]
- Bowden, J.; Davey Smith, G.; Burgess, S. Mendelian randomization with invalid instruments: Effect estimation and bias detection through Egger regression. Int. J. Epidemiol. 2015, 44, 512–525. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- Burgess, S.; Davies, N.M.; Thompson, S.G. Bias due to participant overlap in two-sample Mendelian randomization. Genet. Epidemiol. 2016, 40, 597–608. [Google Scholar] [CrossRef] [PubMed]
- Spiller, W.; Davies, N.M.; Palmer, T.M. Software application profile: Mrrobust—A tool for performing two-sample summary Mendelian randomization analyses. Int. J. Epidemiol. 2018, 48, 684–690. [Google Scholar] [CrossRef]
- Hemani, G.; Zheng, J.; Elsworth, B.; Wade, K.H.; Haberland, V.; Baird, D.; Laurin, C.; Burgess, S.; Bowden, J.; Langdon, R.; et al. The MR-Base platform supports systematic causal inference across the human phenome. Elife 2018, 7, e34408. [Google Scholar] [CrossRef]
- Krittanawong, C.; Tunhasiriwet, A.; Wang, Z.; Farrell, A.M.; Chirapongsathorn, S.; Zhang, H.; Kitai, T.; Mehta, D. Is caffeine or coffee consumption a risk for new-onset atrial fibrillation? A systematic review and meta-analysis. Eur. J. Prev. Cardiol. 2020. [Google Scholar] [CrossRef]
- Larsson, S.C.; Drca, N.; Jensen-Urstad, M.; Wolk, A. Coffee consumption is not associated with increased risk of atrial fibrillation: Results from two prospective cohorts and a meta-analysis. BMC Med. 2015, 13, 207. [Google Scholar] [CrossRef] [PubMed]
- Nicolopoulos, K.; Mulugeta, A.; Zhou, A.; Hyppönen, E. Association between habitual coffee consumption and multiple disease outcomes: A Mendelian randomisation phenome-wide association study in the UK Biobank. Clin. Nutr. 2020, 39, 3467–3476. [Google Scholar] [CrossRef] [PubMed]
- Bodar, V.; Chen, J.; Gaziano, J.M.; Albert, C.; Djoussé, L. Coffee Consumption and Risk of Atrial Fibrillation in the Physicians’ Health Study. J. Am. Heart Assoc. 2019, 8, e011346. [Google Scholar] [CrossRef]
- Bazal, P.; Gea, A.; Navarro, A.M.; Salas-Salvadó, J.; Corella, D.; Alonso-Gómez, A.; Fitó, M.; Muñoz-Bravo, C.; Estruch, R.; Fiol, M.; et al. Caffeinated coffee consumption and risk of atrial fibrillation in two Spanish cohorts. Eur. J. Prev. Cardiol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S.C.; Männistö, S.; Virtanen, M.J.; Kontto, J.; Albanes, D.; Virtamo, J. Coffee and tea consumption and risk of stroke subtypes in male smokers. Stroke 2008, 39, 1681–1687. [Google Scholar] [CrossRef] [PubMed]
- Sakamaki, T.; Hara, M.; Kayaba, K.; Kotani, K.; Ishikawa, S. Coffee Consumption and Incidence of Subarachnoid Hemorrhage: The Jichi Medical School Cohort Study. J. Epidemiol. 2016, 26, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S.C.; Virtamo, J.; Wolk, A. Coffee consumption and risk of stroke in women. Stroke 2011, 42, 908–912. [Google Scholar] [CrossRef]
- Lee, S.M.; Choi, N.K.; Lee, B.C.; Cho, K.H.; Yoon, B.W.; Park, B.J. Caffeine-containing medicines increase the risk of hemorrhagic stroke. Stroke 2013, 44, 2139–2143. [Google Scholar] [CrossRef] [PubMed]
- Cano-Marquina, A.; Tarín, J.J.; Cano, A. The impact of coffee on health. Maturitas 2013, 75, 7–21. [Google Scholar] [CrossRef]
- Natella, F.; Nardini, M.; Belelli, F.; Pignatelli, P.; Di Santo, S.; Ghiselli, A.; Violi, F.; Scaccini, C. Effect of coffee drinking on platelets: Inhibition of aggregation and phenols incorporation. Br. J. Nutr. 2008, 100, 1276–1282. [Google Scholar] [CrossRef] [PubMed]
- Enga, K.F.; Braekkan, S.K.; Hansen-Krone, I.J.; Wilsgaard, T.; Hansen, J.B. Coffee consumption and the risk of venous thromboembolism: The Tromsø study. J. Thromb. Haemost. 2011, 9, 1334–1339. [Google Scholar] [CrossRef] [PubMed]
- Ogilvie, R.P.; Lutsey, P.L.; Heiss, G.; Folsom, A.R.; Steffen, L.M. Dietary intake and peripheral arterial disease incidence in middle-aged adults: The Atherosclerosis Risk in Communities (ARIC) Study. Am. J. Clin. Nutr. 2017, 105, 651–659. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S.C.; Wolk, A.; Håkansson, N.; Bäck, M. Coffee consumption and risk of aortic valve stenosis: A prospective study. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 803–807. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.E.; Davey, S.G.; Munafò, M.R. Associations of coffee genetic risk scores with consumption of coffee, tea and other beverages in the UK Biobank. Addiction 2018, 113, 148–157. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, S.; Carter, P.; Mason, A.M.; Burgess, S.; Larsson, S.C. Coffee Consumption and Cardiovascular Diseases: A Mendelian Randomization Study. Nutrients 2021, 13, 2218. https://doi.org/10.3390/nu13072218
Yuan S, Carter P, Mason AM, Burgess S, Larsson SC. Coffee Consumption and Cardiovascular Diseases: A Mendelian Randomization Study. Nutrients. 2021; 13(7):2218. https://doi.org/10.3390/nu13072218
Chicago/Turabian StyleYuan, Shuai, Paul Carter, Amy M. Mason, Stephen Burgess, and Susanna C. Larsson. 2021. "Coffee Consumption and Cardiovascular Diseases: A Mendelian Randomization Study" Nutrients 13, no. 7: 2218. https://doi.org/10.3390/nu13072218
APA StyleYuan, S., Carter, P., Mason, A. M., Burgess, S., & Larsson, S. C. (2021). Coffee Consumption and Cardiovascular Diseases: A Mendelian Randomization Study. Nutrients, 13(7), 2218. https://doi.org/10.3390/nu13072218






