The IHAT-GUT Iron Supplementation Trial in Rural Gambia: Barriers, Facilitators, and Benefits
Abstract
1. Introduction
1.1. Iron Deficiency and the IHAT-GUT Trial in the Gambia
1.2. Nutrition Interventions in Varying Contexts and the Need for Qualitative Data
1.3. Clinical Nutrition Trials in LMICs
2. Methods
2.1. IHAT-GUT
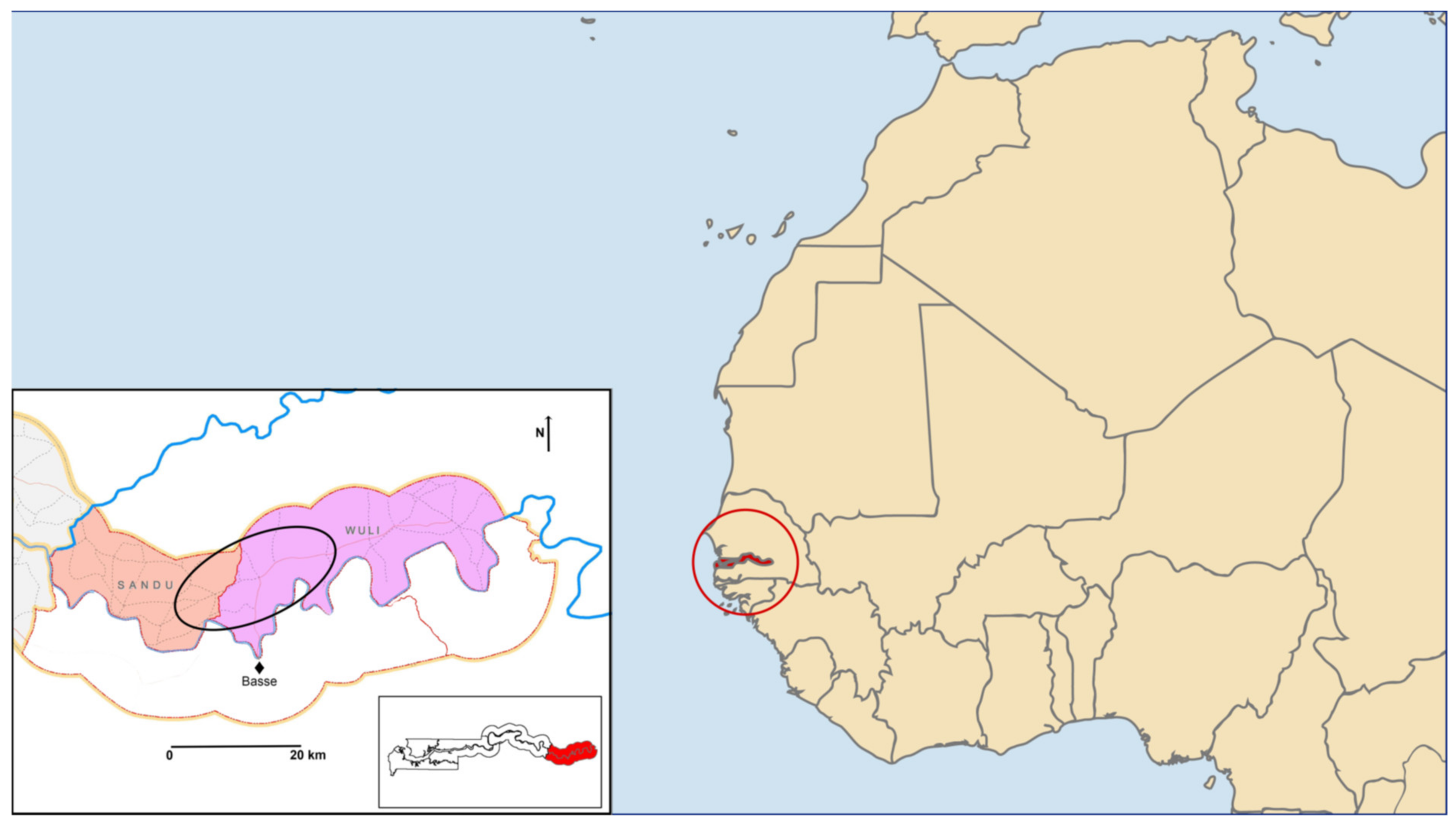
2.2. Study Setting
2.3. Study Participants
2.4. Data Collection
2.5. Data Analysis
2.6. Ethical Considerations
3. Results
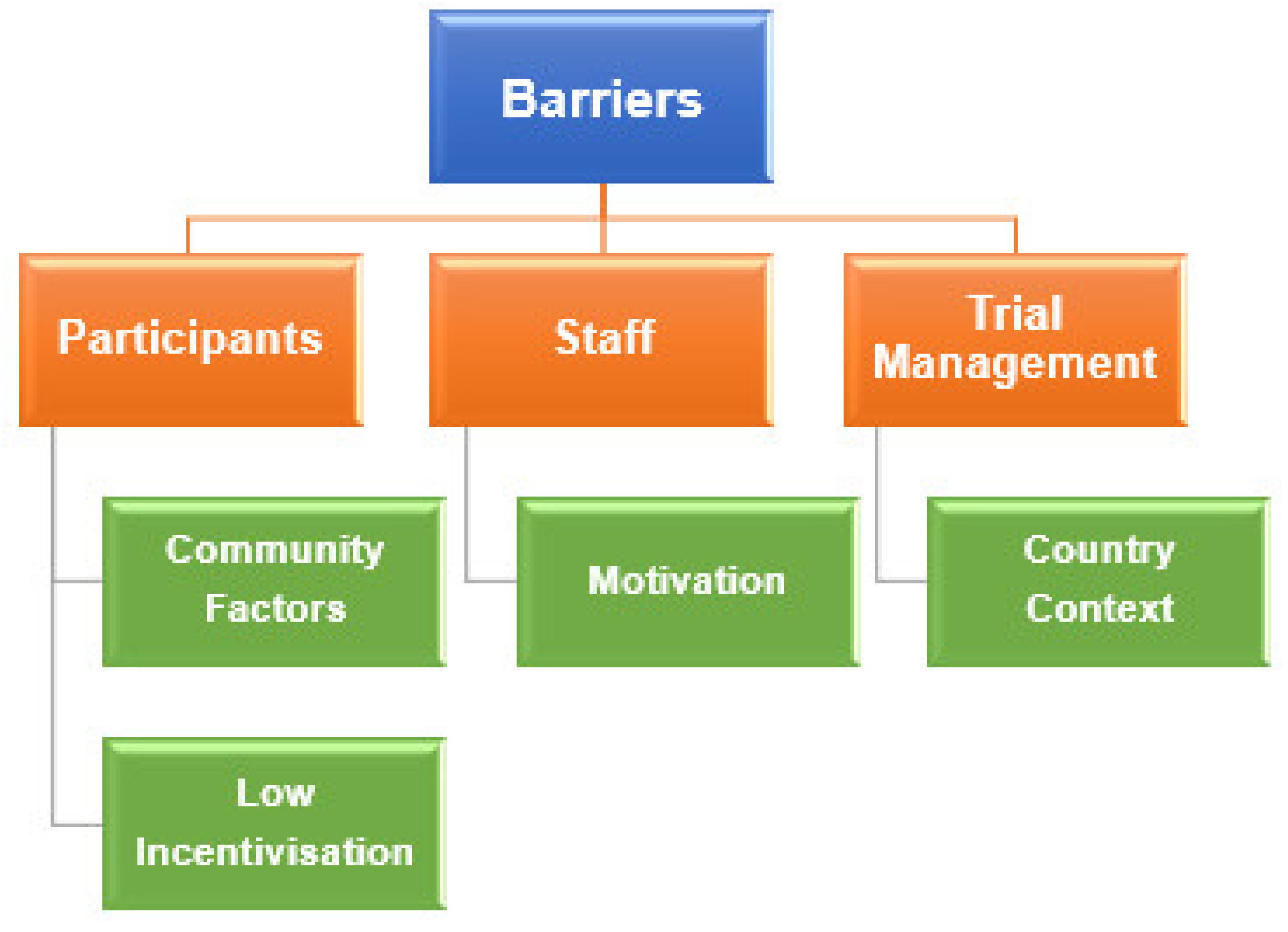
3.1. Barriers
3.1.1. Participants
Community Factors
Low Incentivisation
3.1.2. Staff
Motivation
3.1.3. Trial Management
Country Context
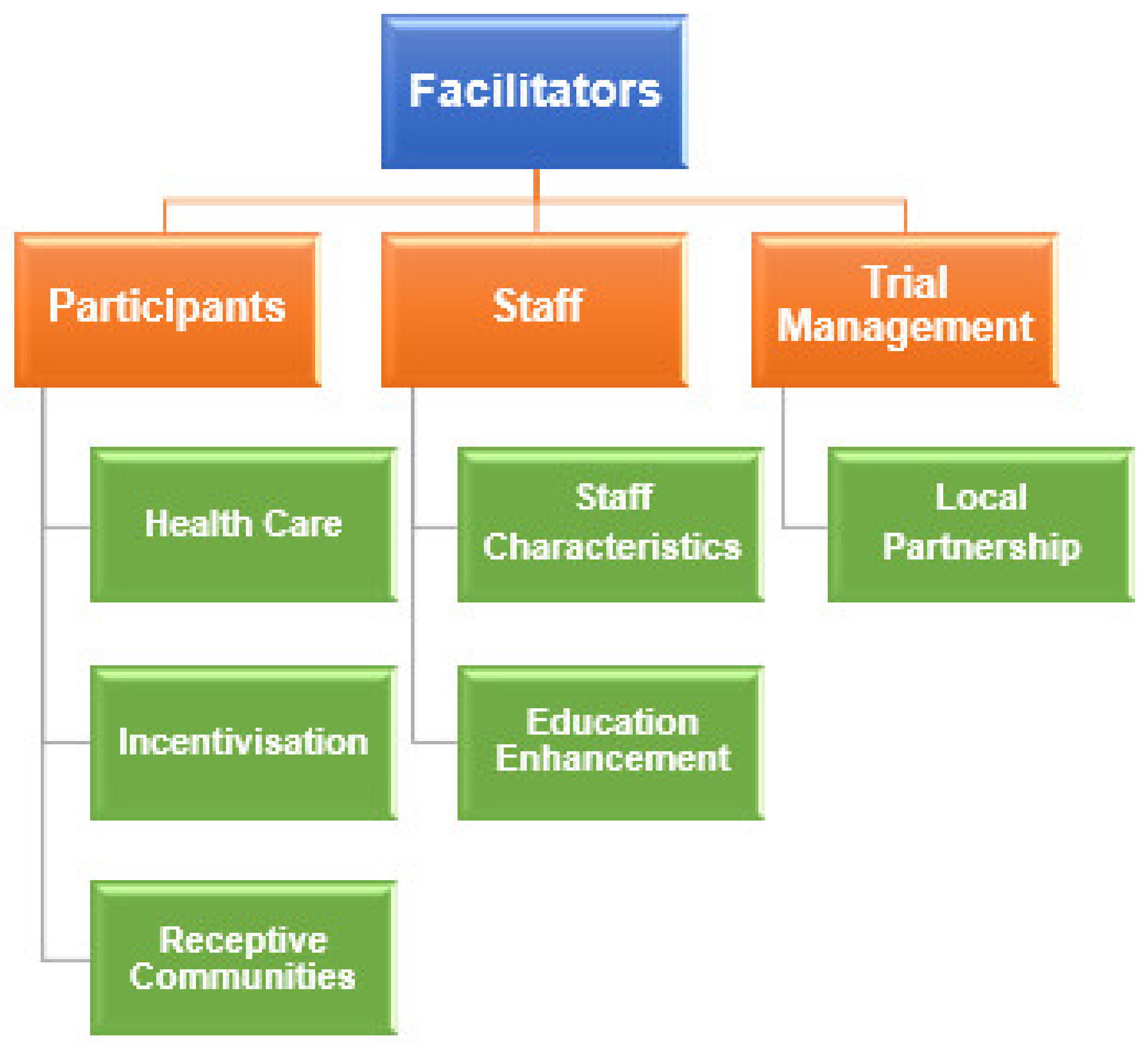
3.2. Facilitators & Benefits
3.2.1. Participants
Healthcare
Incentivisation
Receptive Communities
3.2.2. Staff
Staff Characteristics
Education Enhancement
3.2.3. Trial Management
Local Partnership
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Disclaimers
Abbreviations
| DNA | Deoxyribonucleic acid |
| GCP | Good Clinical Practice |
| Hb | Haemoglobin |
| ID | Iron deficiency |
| IDA | Iron deficiency anaemia |
| IHAT | Iron hydroxide adipate tartrate |
| LMIC | Low-middle income country |
| LSHTM | London School of Hygiene and Tropical Medicine |
| mL | Millilitre |
| MRC | Medical Research Council |
| MRCG | Medical Research Council Unit, the Gambia |
| MSc | Master of Science |
| PI | Principal investigator |
| SCC | Scientific Coordinating Committee |
| UK | United Kingdom |
| URR | Upper River Region |
| WHO | World Health Organisation |
References
- GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1211–1259. [Google Scholar] [CrossRef]
- Prentice, A.M.; Mendoza, Y.A.; Pereira, D.; Cerami, C.; Wegmuller, R.; Constable, A.; Spieldenner, J. Dietary strategies for improving iron status: Balancing safety and efficacy. Nutr. Rev. 2017, 75, 49–60. [Google Scholar] [CrossRef]
- Miller, J.L. Iron deficiency anemia: A common and curable disease. Cold Spring Harb. Perspect. Med. 2013, 3. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Worldwide Prevalence of Anaemia 1993–2005: WHO Global Database on Anaemia; De Benoist, B., McLean, E., Egli, I., McLean, E., Eds.; World Health Organization: Geneva, Switzerland, 2008. [Google Scholar]
- Stelle, I.; Kalea, A.Z.; Pereira, D.I.A. Iron deficiency anaemia: Experiences and challenges. Proc. Nutr. Soc. 2018, 1–8. [Google Scholar] [CrossRef]
- Hilty, F.M.; Arnold, M.; Hilbe, M.; Teleki, A.; Knijnenburg, J.T.N.; Ehrensperger, F.; Hurrell, R.F.; Pratsinis, S.E.; Langhans, W.; Zimmermann, M.B. Iron from nanocompounds containing iron and zinc is highly bioavailable in rats without tissue accumulation. Nat. Nanotechnol. 2010, 5, 374–380. [Google Scholar] [CrossRef]
- Pisani, A.; Riccio, E.; Sabbatini, M.; Andreucci, M.; Del Rio, A.; Visciano, B. Effect of oral liposomal iron versus intravenous iron for treatment of iron deficiency anaemia in CKD patients: A randomized trial. Nephrol. Dial. Transplant. 2014, 30, 645–652. [Google Scholar] [CrossRef]
- Powell, J.J.; Bruggraber, S.F.; Faria, N.; Poots, L.K.; Hondow, N.; Pennycook, T.J.; Latunde-Dada, G.O.; Simpson, R.J.; Brown, A.P.; Pereira, D.I. A nano-disperse ferritin-core mimetic that efficiently corrects anemia without luminal iron redox activity. Nanomedicine 2014, 10, 1529–1538. [Google Scholar] [CrossRef] [PubMed]
- Von Moos, L.M.; Schneider, M.; Hilty, F.M.; Hilbe, M.; Arnold, M.; Ziegler, N.; Mato, D.S.; Winkler, H.; Tarik, M.; Ludwig, C.; et al. Iron phosphate nanoparticles for food fortification: Biological effects in rats and human cell lines. Nanotoxicology 2017, 11, 496–506. [Google Scholar] [CrossRef] [PubMed]
- Pereira, D.I.; Bruggraber, S.F.; Faria, N.; Poots, L.K.; Tagmount, M.A.; Aslam, M.F.; Frazer, D.M.; Vulpe, C.D.; Anderson, G.J.; Powell, J.J. Nanoparticulate iron(III) oxo-hydroxide delivers safe iron that is well absorbed and utilised in humans. Nanomedicine 2014, 10, 1877–1886. [Google Scholar] [CrossRef] [PubMed]
- Pereira, D.I.; Mohammed, N.I.; Ofordile, O.; Camara, F.; Baldeh, B.; Mendy, T.; Sanyang, C.; Jallow, A.T.; Hossain, I.; Wason, J.; et al. A novel nano-iron supplement to safely combat iron deficiency and anaemia in young children: The IHAT-GUT double-blind, randomised, placebo-controlled trial protocol. Gates Open Res. 2018, 2, 48. [Google Scholar] [CrossRef]
- Salam, R.A.; Das, J.K.; Bhutta, Z.A. Integrating nutrition into health systems: What the evidence advocates. Matern. Child Nutr. 2019, 15, e12738. [Google Scholar] [CrossRef]
- Lang, T.; Siribaddana, S. Clinical Trials Have Gone Global: Is This a Good Thing? PLoS Med. 2012, 9, e1001228. [Google Scholar] [CrossRef] [PubMed]
- Hodge, S. The 10/90 report on health research 2000. J. R. Soc. Promo Health 2000, 120, 197. [Google Scholar]
- Moon, S.; Bermudez, J.; ’t Hoen, E. Innovation and Access to Medicines for Neglected Populations: Could a Treaty Address a Broken Pharmaceutical R&D System? PLoS Med. 2012, 9, e1001218. [Google Scholar] [CrossRef]
- Rottingen, J.-A.; Chamas, C.; Goyal, L.C.; Harb, H.; Lagrada, L.; Mayosi, B.M. Securing the public good of health research and development for developing countries. Bull. World Heal. Organ. 2012, 90, 398–400. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Boutron, I.; Dechartres, A.; Durieux, P.; Ravaud, P. Geographical Representativeness of Published and Ongoing Randomized Controlled Trials. The Example of: Tobacco Consumption and HIV Infection. PLoS ONE 2011, 6, e16878. [Google Scholar] [CrossRef]
- Perel, P.; Miranda, J.J.; Ortiz, Z.; Casas, J.P. Relation between the Global Burden of Disease and Randomized Clinical Trials Conducted in Latin America Published in the Five Leading Medical Journals. PLoS ONE 2008, 3, e1696. [Google Scholar] [CrossRef]
- Røttingen, J.-A.; Regmi, S.; Eide, M.; Young, A.J.; Viergever, R.F.; Årdal, C.; Guzman, J.; Edwards, D.; Matlin, S.A.; Terry, R.F. Mapping of available health research and development data: What’s there, what’s missing, and what role is there for a global observatory? Lancet 2013, 382, 1286–1307. [Google Scholar] [CrossRef]
- Chirac, P.; Torreele, E. Global framework on essential health R&D. Lancet 2006, 367, 1560–1561. [Google Scholar] [CrossRef]
- Mbuagbaw, L.; Thabane, L.; Ongolo-Zogo, P.; Lang, T. The challenges and opportunities of conducting a clinical trial in a low resource setting: The case of the Cameroon mobile phone SMS (CAMPS) trial, an investigator initiated trial. Trials 2011, 12, 145. [Google Scholar] [CrossRef]
- Devasenapathy, N.; Singh, K.; Prabhakaran, D. Conduct of clinical trials in developing countries: A perspective. Curr. Opin. Cardiol. 2009, 24, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Dierickx, S.; O’Neill, S.; Gryseels, C.; Anyango, E.I.; Bannister-Tyrrell, M.; Okebe, J.; Mwesigwa, J.; Jaiteh, F.; Gerrets, R.; Ravinetto, R.; et al. Community sensitization and decision-making for trial participation: A mixed-methods study from the Gambia. Dev. World Bioeth. 2017, 18, 406–419. [Google Scholar] [CrossRef]
- Costello, A. Moving to research partnerships in developing countries. BMJ 2000, 321, 827–829. [Google Scholar] [CrossRef] [PubMed]
- Eastwood, S.V.; Hill, P.C. A gender-focused qualitative study of barriers to accessing tuberculosis treatment in the Gambia, West Africa. Int. J. Tuberc. Lung Dis. 2004, 8, 70–75. [Google Scholar]
- O’Neill, S.; Dierickx, S.; Okebe, J.; Dabira, E.; Gryseels, C.; D’Alessandro, U.; Grietens, K.P. The Importance of Blood Is Infinite: Conceptions of Blood as Life Force, Rumours and Fear of Trial Participation in a Fulani Village in Rural Gambia. PLoS ONE 2016, 11, e0160464. [Google Scholar] [CrossRef] [PubMed]
- Franzen, S.R.P.; Chandler, C.; Enquselassie, F.; Siribaddana, S.; Atashili, J.; Angus, B.; Lang, T. Understanding the investigators: A qualitative study investigating the barriers and enablers to the implementation of local investigator-initiated clinical trials in Ethiopia. BMJ Open 2013, 3, e003616. [Google Scholar] [CrossRef]
- Alemayehu, C.; Mitchell, G.; Nikles, J. Barriers for conducting clinical trials in developing countries- a systematic review. Int. J. Equity Health 2018, 17, 1–11. [Google Scholar] [CrossRef]
- Dierickx, S.; Gryseels, C.; Mwesigwa, J.; O’Neill, S.; Bannister-Tyrell, M.; Ronse, M.; Jaiteh, F.; Gerrets, R.; D’Alessandro, U.; Grietens, K.P. Factors Associated with Non-Participation and Non-Adherence in Directly Observed Mass Drug Administration for Malaria in the Gambia. PLoS ONE 2016, 11, e0148627. [Google Scholar] [CrossRef] [PubMed]
- Bansal, N. The opportunities and challenges in conducting clinical trials globally. Clin. Res. Regul. Aff. 2012, 29, 9–14. [Google Scholar] [CrossRef]
- Bank, W. The World Bank in the Gambia 2019. Available online: https://www.worldbank.org/en/country/gambia/overview (accessed on 30 March 2020).
- Hennig, B.J.; Unger, S.A.; Dondeh, B.L.; Hassan, J.; Hawkesworth, S.; Jarjou, L.; Jones, K.S.; Moore, S.E.; Nabwera, H.M.; Ngum, M.; et al. Cohort Profile: The Kiang West Longitudinal Population Study (KWLPS)—A platform for integrated research and health care provision in rural Gambia. Int. J. Epidemiol. 2015, 46, e13. [Google Scholar] [CrossRef] [PubMed]
- Ross, S.; Grant, A.; Counsell, C.; Gillespie, W.; Russell, I.; Prescott, R. Barriers to participation in randomised controlled trials: A systematic review. J. Clin. Epidemiol. 1999, 52, 1143–1156. [Google Scholar] [CrossRef]
- Braun, V.; Clarke, V. Using thematic analysis in psychology. Qual. Res. Psychol. 2006, 3, 77–101. [Google Scholar] [CrossRef]
- Gambia Bureau of Statistics (GBOS); ICF International. The Gambia Demographic and Health Survey 2013; Gambia Bureau of Statistics (GBOS): Banjul, Republic of Gambia; ICF International: Rockville, MD, USA, 2014.
- Franzen, S.R.P.; Chandler, C.; Siribaddana, S.; Atashili, J.; Angus, B.; Lang, T. Strategies for developing sustainable health research capacity in low and middle-income countries: A prospective, qualitative study investigating the barriers and enablers to locally led clinical trial conduct in Ethiopia, Cameroon and Sri Lanka. BMJ Open 2017, 7, e017246. [Google Scholar] [CrossRef]
- McMichael, C.; Waters, M.E.; Volmink, B.J. Evidence-based public health: What does it offer developing countries? J. Public Health 2005, 27, 215–221. [Google Scholar] [CrossRef]
- Buekens, P.; Keusch, G.; Belizan, J.; Bhutta, Z.A. Evidence-based global health. JAMA 2004, 291, 2639–2641. [Google Scholar] [CrossRef] [PubMed]
- Volmink, J.; Siegfried, N.; Van Der Merwe, L.; Brocklehurst, P. Antiretrovirals for reducing the risk of mother-to-child transmission of HIV infection. Cochrane Database Syst. Rev. 2007, 2007, CD003510. [Google Scholar] [CrossRef]
- Broom, A.; Hand, K.; Tovey, P. The role of gender, environment and Individual biography in shaping qualitative interview data. Int. J. Soc. Res. Methodol. 2009, 12, 51–65. [Google Scholar] [CrossRef]
- Arendell, T. Reflections on the Researcher-Researched Relationship: A Woman Interviewing Men. Qual. Sociol. 1997, 20, 341–368. [Google Scholar] [CrossRef]
- Malham, P.B.; Saucier, G. The conceptual link between social desirability and cultural normativity. Int. J. Psychol. 2016, 51, 474–480. [Google Scholar] [CrossRef] [PubMed]

| Demographics | n | % |
|---|---|---|
| Ethnicity (Tribe) if applicable | ||
| Gambian (Fula: Mandinka: Wolof: Banbara: Manjago) | 12 (6:3:1:1:1) | 70 |
| Other African Countries | 4 | 24 |
| Other | 1 | 6 |
| Religion | ||
| Muslim | 16 | 94 |
| Christian | 1 | 6 |
| Sex | ||
| Female | 1 | 17 |
| Male | 16 | 83 |
| Age (in years) | ||
| 18–29 | 1 | 17 |
| 30–39 | 6 | 26 |
| 40–49 | 7 | 30 |
| 50–59 | 3 | 13 |
| Highest Level of Education | ||
| Secondary School | 5 | 29 |
| State Enrolled Nursing School | 3 | 18 |
| Bachelors | 1 | 5 |
| Medicine Degree | 3 | 18 |
| Masters | 3 | 18 |
| Doctorate a | 2 | 12 |
| Years Employed with MRCG b Projects | ||
| 0–5 | 3 | 18 |
| 6–10 | 6 | 35 |
| 11–15 | 3 | 18 |
| 16–20 | 4 | 23 |
| 21–25 | 0 | 0 |
| 25–30 | 1 | 6 |
| Phase | Description |
|---|---|
| 1. Data Familiarization | Transcribing, reading, and re-reading data |
| 2. Initial Codes | Coding interesting features systematically and collating the data to each code |
| 3. Theme Development | Collating codes into potential themes and adding relevant data to each |
| 4. Refining Themes | Ensuring themes work with the first (data familiarization) and second (initial codes) levels of analysis |
| 5. Naming Themes | Ongoing refinement, generating clear definitions and names for each theme |
| 6. The Report | Final analysis opportunity, extraction of compelling examples |
| Theme | Sub-Theme | Illustrative Quote |
|---|---|---|
| Participants | Community Factors | “There’s also a bit of cultural problem… because in Africa we believe the wife stays home to cook, clean. So, some husbands decide (to) have their wives stop going to the clinic visits.”—Data Manager |
| Low Incentivisation | “We take blood from these children, so we need to make life easy for them. Maybe they are on the drug that doesn’t do anything.”—State Enrolled Nurse 2 | |
| Staff | Motivation | “There will always be challenges. That you should expect. The biggest challenge is working with individuals and managing individuals. Everyone has negative qualities, I have them.”—Research Clinician |
| Trial Management | Country Context | “The major challenges in running these trials here is the start up. Making all the necessary arrangements. The necessary approvals from the Ministry, from the Medicine Control Agency, from the Ethics and SCC (Scientific Coordinating Committee), establishing the sites of the studies and so forth. And of course, it requires a lot of logistical support… especially if they are in remote areas. IHAT-GUT is running its study where no study has been done in the past at this scale…(and) during rainy season you have floods.”—Project Manager |
| Theme | Sub-Theme | Illustrative Quote |
|---|---|---|
| Participants | Health Care | “IHAT-GUT…helps the Gambian children and it’s the first one in the North Bank… We are working where they need it most… There was more anaemia, they had less hospitals. Medical care is lacking. Mothers say: ‘please come to our communities’.”—Senior Field Worker |
| Incentivisation | “You need to bring a social impact, so the participants feel valued (rather) than just coming to do what you want and not giving the mothers and children something.”—Data Manager | |
| Receptive Communities | “The Gambians, they are remarkable people. They are the most amazing, welcoming people. It’s a very friendly environment to work in. It’s a research-friendly country.”—Research Clinician | |
| Staff | Staff Characteristics | “In Europe, I don’t think it would be easy to conduct studies like this. In Africa, people don’t find it a problem that projects come in their communities. We are Gambian. When we go into our own communities, they are accepting.”—Senior Field Worker |
| Education Enhancement | “The Nutritional Course was great. It added value because it not only taught us about nutrition personally, but on the other hand, it’s great to do a team activity. It makes everyone feel appreciated… You want to develop the staff.”—Data Manager | |
| Trial Management | Local Partnership | “MRC has a great track record here in the Gambia, they have cordial relationships with the communities and with The Gambian government and its ministries.”—Nutrition Theme Administrator |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stelle, I.; McDonagh, L.K.; Hossain, I.; Kalea, A.Z.; Pereira, D.I.A. The IHAT-GUT Iron Supplementation Trial in Rural Gambia: Barriers, Facilitators, and Benefits. Nutrients 2021, 13, 1140. https://doi.org/10.3390/nu13041140
Stelle I, McDonagh LK, Hossain I, Kalea AZ, Pereira DIA. The IHAT-GUT Iron Supplementation Trial in Rural Gambia: Barriers, Facilitators, and Benefits. Nutrients. 2021; 13(4):1140. https://doi.org/10.3390/nu13041140
Chicago/Turabian StyleStelle, Isabella, Lorraine K. McDonagh, Ilias Hossain, Anastasia Z. Kalea, and Dora I. A. Pereira. 2021. "The IHAT-GUT Iron Supplementation Trial in Rural Gambia: Barriers, Facilitators, and Benefits" Nutrients 13, no. 4: 1140. https://doi.org/10.3390/nu13041140
APA StyleStelle, I., McDonagh, L. K., Hossain, I., Kalea, A. Z., & Pereira, D. I. A. (2021). The IHAT-GUT Iron Supplementation Trial in Rural Gambia: Barriers, Facilitators, and Benefits. Nutrients, 13(4), 1140. https://doi.org/10.3390/nu13041140







