Efficacy of Polyphenols in the Management of Dyslipidemia: A Focus on Clinical Studies
Abstract
1. Introduction
2. Dyslipidemia
2.1. Definition of Dyslipidemia and Related Biomarkers
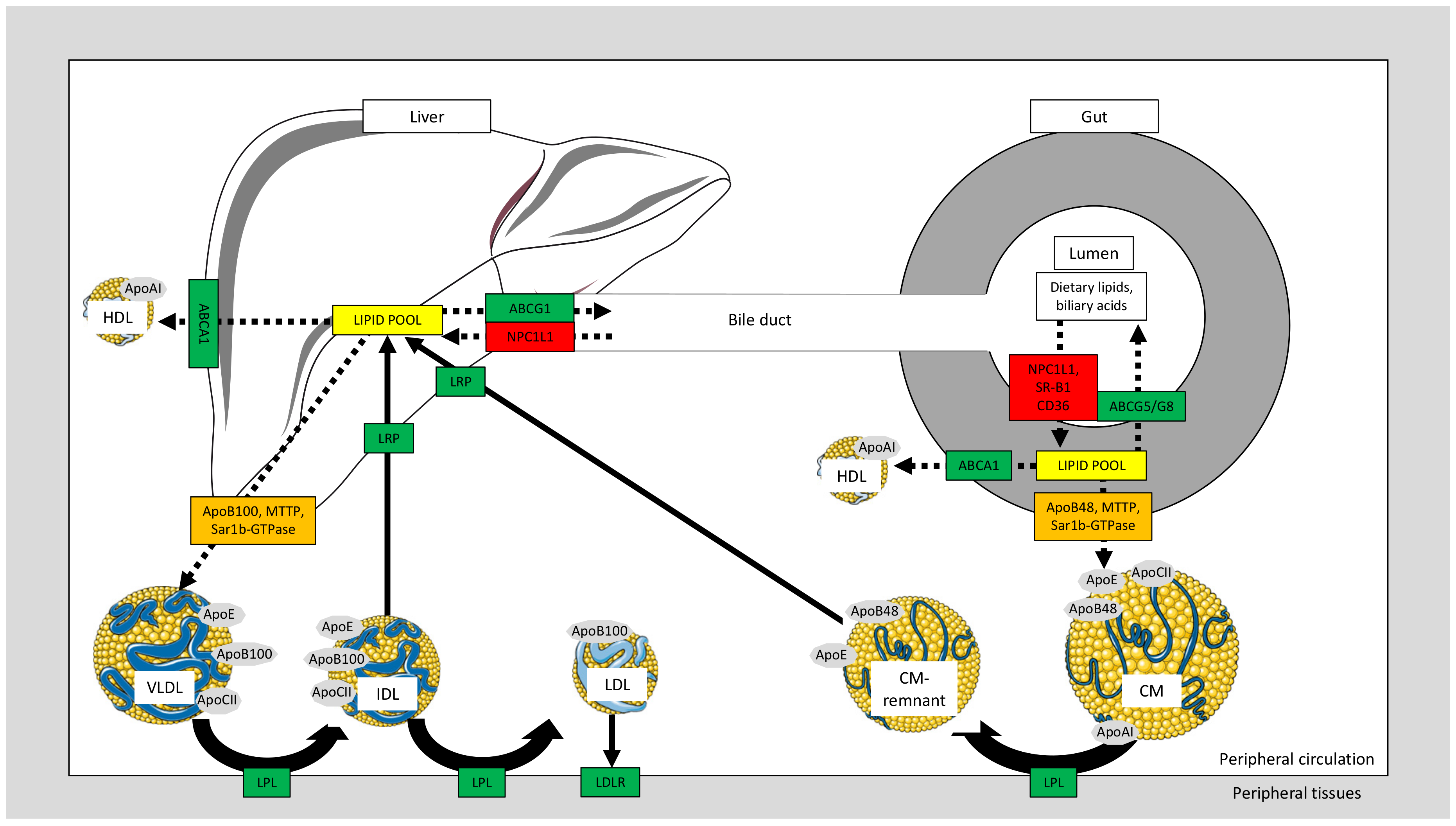
2.2. Chylomicron Formation and Postprandial Dyslipidemia
2.3. Intestinal Cholesterol Transporters and Relation to Dyslipidemia
2.4. VLDL Metabolism and Relation to Dyslipidemia
2.5. Additional Congenital Types of Primary Hyperlipoproteinemia
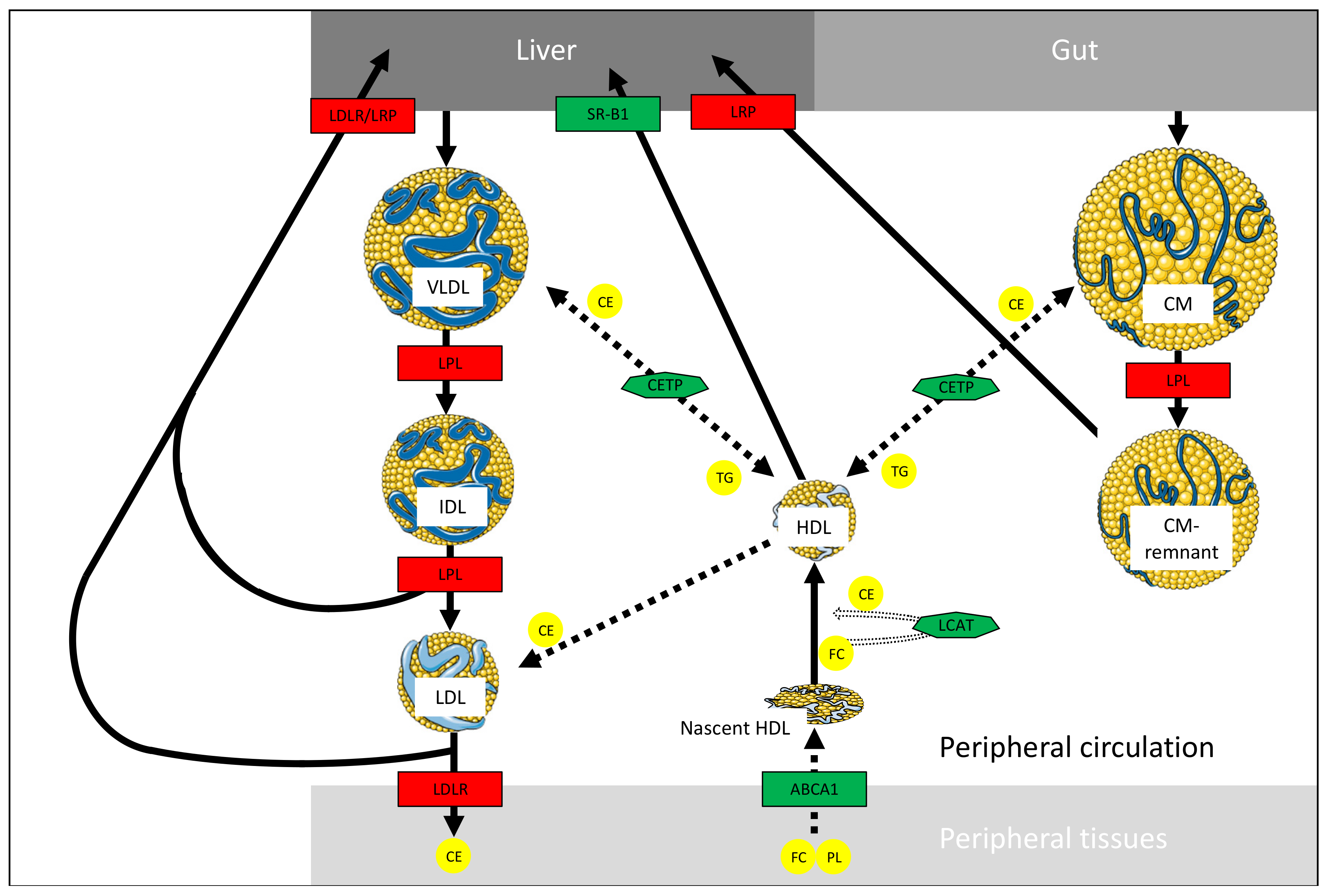
2.6. HDL Metabolism
2.6.1. HDL Synthesis and Functions
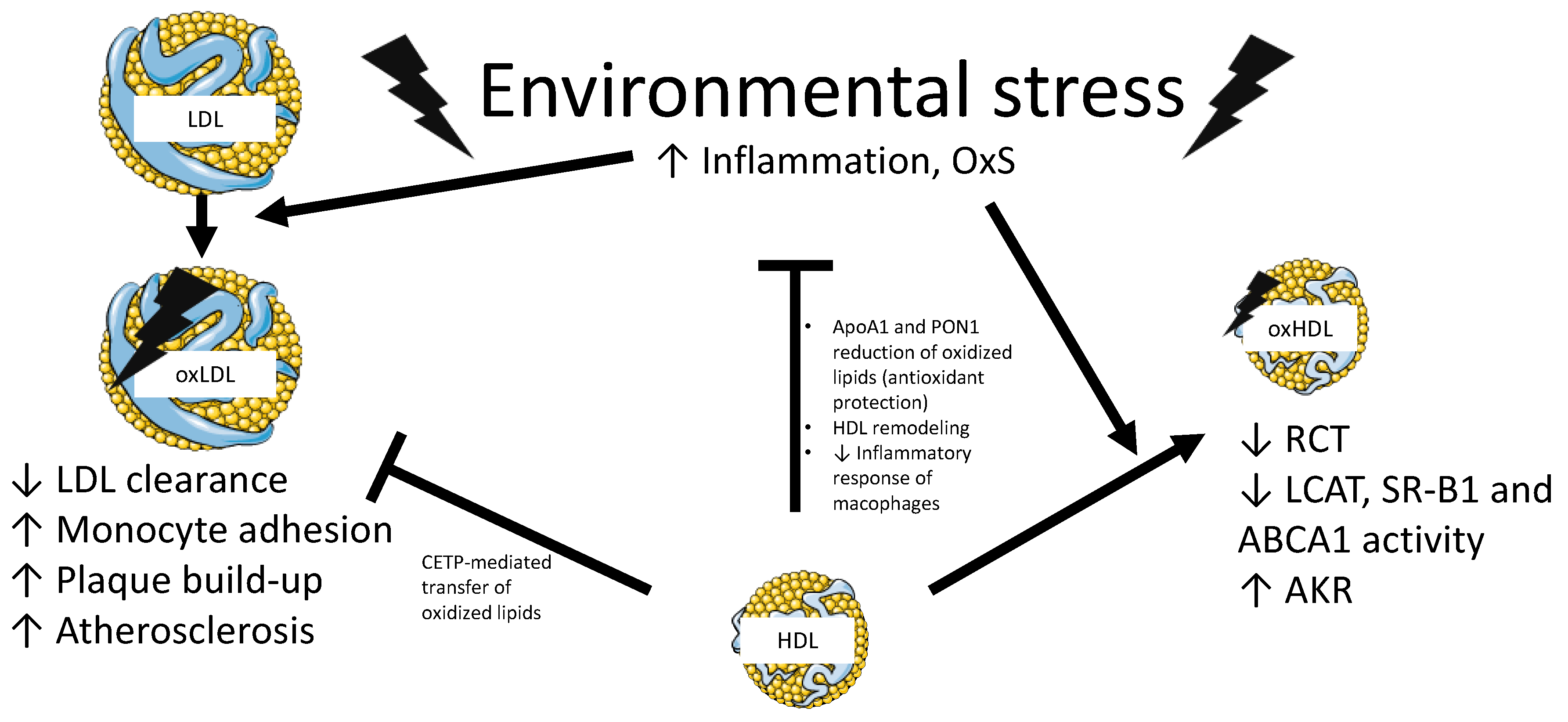
2.6.2. HDL-Related Disorders
2.7. Treatment of Dyslipidemia
| Treatment | Underlying Mechanism | Lipid Profile Variation (%) | Indication | CI Possible Adverse Effects | Reference | |||
|---|---|---|---|---|---|---|---|---|
| TG | Non-HDL-C | LDL-C | HDL-C | |||||
| Pharmacological therapies | ||||||||
| Statins | HMG-CoA-R inhibitors | ↓7–30 | ↓15–51 | ↓18–55 | ↑5–15 | First line treatment | CI: Possible drug-drug interaction (3A4 inhibitors), pre-existent hepatic disease, end-stage kidney failure, heart failure (>class I on NYHA scale), pregnancy and/or breast-feeding. PAE: hepatic toxicity, myopathy, rhabdomyolysis, acute renal failure. | [9,10,64,65,66] |
| Bile acid sequestrants | Cholesterol chelation in gut’s lumen | ↑0–10 | ↓4–16 | ↓15–30 | ↑3–5 | Adjunct with statins or first line treatment if statins not recommended | PAE: GI symptoms, reduced effectiveness of other medications, increase in TG | [9,10,66] |
| Fibrates | PPARαagonist (VLDL secretion inhibition, LPL induction) | ↓20–50 | ↓5–19 | ↓5–↑20 | ↑10–20 | HyperTG | CI: Not recommended with statins | [9,10,66,67] |
| NPC1L1 inhibitors | Cholesterol absorption inhibitor | ↓5–11 | ↓14–19 | ↓13–20 | ↑3–5 | Adjunct with statins or first line treatment if statins not recommended | CI: Presence of an underlying hepatic disease | [9,10,66,67] |
| PCSK9 inhibitors | Inhibition of LDLR degradation | ↓50 | Adjunct with statins or first line treatment if statins intolerance | Injection site reactions | [10,66,71] | |||
| Mipomersen | Inhibition of Apo B synthesis | ↓25 | HyperTG related to acute pancreatitis | PAE: Hepatic steatosis | [64,65] | |||
| Lomitapide | Inhibition of MTTP in liver and gut | ↓50 | HyperTG related to acute pancreatitis | PAE: GI symptoms, hepatic steatosis | [64,65] | |||
| Non-pharmacological therapies | ||||||||
| Niacine | Increased expression/ activity of adipose LPL | ↓20–50 | ↓8–23 | ↓5–25 | ↑15–35 | HyperTG | CI: Not recommended with statins | [9,10,66,67] |
| Omega-3 fatty acids | PPARα agonist | ↓19–44 | ↓5–14 | ↓6–↑25 1 | ↓5–↑7 | HyperTG | CI: Fish allergy | [9,66,67] |
| Dietary fibers | Delayed/ reduced cholesterol absorption | ↓3–5 | Primary prevention | PAE: GI symptoms | [72,73] | |||
| Monacolin | HMG-CoA-R inhibitor | ↓0–20 | Primary prevention | PAE: safety issues regarding presence of contaminants | [73] | |||
| Phytosterols | Cholesterol absorption inhibitor | ↓4–9 | ↓7–10 | Primary prevention | [66,74,75] | |||
3. Polyphenols and Metabolic Benefits
3.1. Polyphenol Background
3.2. Regulation of Oxidative Stress, a Component Affecting Metabolic Syndrome, by Polyphenols
3.3. Regulation of Inflammation in Cardiometabolic Disorders by Polyphenols
3.4. Polyphenols Counteract Cardiometabolic Complications by Regulating the Gut Microbiota
4. Methods
5. Polyphenol Supplementation in Humans—Intervention Trials
5.1. Chronic Intake Interventions
5.1.1. Impact of Polyphenols on Healthy Participants
5.1.2. Impact of Polyphenols on Dyslipidemia
Impact of Polyphenols on Patients with a Single Dyslipidemia Component
Impact of Polyphenols on Patients with Two Dyslipidemia Components
Impact of Polyphenols on Patients with Three Dyslipidemia Components
| Polyphenols | Protocol | Participants | Variation of Lipid Profile 1 | Reference | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dietary Source (Main PLPs) 2 | Dosage (mg/Day) | Matrix | Intake Repartition | Length S.D. (Days) | n (Female) | Age 3 (Years) | D.-O. (%) | ↑LDL- C | ↑TG | ↓HDL- C | Obesity | IR | ↑SBP | FRS (%) | TG | TC | LDL- C | HDL- C | |
| Coffee (hydroxycinnamic acids, methylxanthines) | 510.6 | Diet (drink) | Tid | 56 CO | 27 (17) | 33.7 ± 1.8 | 4 | √ | 0.4 | ↓20% * | N/A | N/A | ↓1% | [149] | |||||
| Virgin olive oil (lignans) | 2.9 | Diet | Die | 21 CO | 33 (14) | 55.2 ± 1.8 | 15 | √ | 9.5 | ↓6% | ↓5% | ↑1% | NV | [156] | |||||
| Enriched virgin olive oil (hydroxytyrosol derivatives, lignans, flavonoids) | 12.1 | Diet | Die | 21 CO | 33 (14) | 55.2 ± 1.8 | 15 | √ | 9.5 | ↑3% | ↓4% | NV | ↑2% | [156] | |||||
| Enriched virgin olive oil (hydroxytyrosol derivatives, lignans) | 12.6 | Diet | Die | 21 CO | 33 (14) | 55.2 ± 1.8 | 15 | √ | 9.5 | ↑3% | ↓4% | ↓8% * | ↓1% | [156] | |||||
| Olive oil (not specified) | 0.05 | Diet | Die | 21 CO | 182 (0) | 33.3 ± 0.8 | 8 | √ | 1.9 | ↓6% | NV | ↑1% | ↑2% * | [157] | |||||
| Olive Oil (not specified) | 3.6 | Diet | Die | 21 CO | 184 (0) | 33.3 ± 0.8 | 8 | √ | 1.9 | ↓4% | NV | ↑1% | ↑3% * | [157] | |||||
| Olive Oil (not specified) | 8.1 | Diet | die | 21 CO | 183 (0) | 33.3 ± 0.8 | 8 | √ | 1.9 | ↓5% | NV | ↑2% | ↑4% * | [157] | |||||
| Pine Bark (flavonoids) | 150 | Capsule | Die | 42 CO | 25 (15) | 30.0 ± 1.5 | 0 | √ | 1.7 | ↑2% | ↓2% | ↓7% * | ↑11% * | [158] | |||||
| Cocoa (epicatechin, catechin, procyanidin) | 282 | Diet (drink) | Bid | 28 P | 37 (21) | 49.9 ± 1.3 | 0 | √ | 5.5 | ↓7% | ↓3% | ↓5% * | ↑9% * | [159] | |||||
| Cocoa (epicatechin, catechin, procyanidin) | 211 | Diet (drink) | Bid | 28 P | 32 (18) | 49.9 ± 1.3 | 0 | √ | 5.4 | ↓2% | ↓2% | ↓4% * | ↑7% * | [159] | |||||
| Cocoa (epicatechin, catechin, procyanidin) | 141 | Diet (drink) | Bid | 28 P | 31 (18) | 49.9 ± 1.3 | 0 | √ | 5.5 | NV | ↓3% * | ↓5% * | ↑5% * | [159] | |||||
| Chocolate (flavanol, epicatechin) + fibers | 45.3 | Diet (drink) | Bid | 28 CO | 20 (11) | 30.0 ± 6.2 | 12 | √ | 2.0 | ↑1% | ↑2% | NV | ↑12% * | [146] | |||||
| Dark chocolate (not specified) | 2148 | Diet | Die | 15 CO | 92 (34) | 45.0 ± 1.1 | 40 | √ | 5.9 | ↓8% | ↑2% | ↑4% | ↑5% * | [160] | |||||
| Dealcoholized red wine (not specified) | 1000 | Diet (drink) | Die | 42 P | 15 (15) | 57.6 ± 1.3 | 0 | √ | 6.3 | ↓2% | ↓1% | NV | ↓5% | [86] | |||||
| Mate Tea (chlorogenic acid) | 107 | Diet (drink) | Die | 15 CO | 92 (34) | 45.0 ± 1.1 | 40 | √ | 5.9 | ↓3% | ↑1% | ↑3% | ↑1% * | [160] | |||||
| Polyphenols | Protocol | Participants | Variation of Lipid Profile 1 | Reference | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dietary Source (Main PLPs) 2 | Dosage (mg/Day) | Matrix | Intake Repartition | Length S.D. (Days) | n (Female) | Age 3 (Years) | D.-O. (%) | ↑LDL- C | ↑TG | ↓HDL-C | Obesity | IR | ↑SBP | FRS (%) | TG | TC | LDL- C | HDL-C | |
| Carob (not specified) +7.2 g insoluble fibers | 40 | Capsule | Bid | 30 P | 43 (22) | 42.9 ± 9.5 | 9 | √ | √ | 6.6 | ↓23% * | ↓18% * | ↓23% * | ↑6% * | [161] | ||||
| Red grape (anthocyanidins, quercetin, myricetin) | 640 | Juice | Bid | 14 P | 26 (13) | 62.0 ± 3.4 | 10 | √ | √ | 12.8 | ↓8% | ↓11% * | ↓18% * | ↑13% * | [89] | ||||
| Red wine (not specified) | 1000 | Diet (drink) | Die | 42 P | 14 (14) | 58.4 ± 1.3 | 0 | √ | √ | 7.3 | ↑17% | NV | ↓8% * | ↑17% * | [86] | ||||
| Catechins, theaflavins | 224.4 | Capsule | Die | 77 P | 31 (11) | 50.1 ± 0.5 | 0 | √ | √ | 7.0 | ↓13% | ↓1% * | ↓2% * | ↑3% | [162] | ||||
| Theaflavins | 77.5 | Capsule | Die | 77 P | 34 (12) | 47.5 ± 1.0 | 0 | √ | √ | 7.0 | ↑7% | ↓3% * | ↓7% * | ↑2% | [162] | ||||
| Resveratrol | 1500 | Capsule | Bid | 14 CO | 8 (0) | 45.8 ± 3.1 | 0 | √ | √ | 6.7 | ↓20% | N/A | N/A | N/A | [163] | ||||
| Resveratrol | 150 | Capsule | Die | 30 CO | 18 (11) | 50.4 ± 2.0 | 18 | √ | √ | 5.4 | ↓8% | ↓4% | ↑1% | ↑3% | [85] | ||||
| Cranberry (proanthocyanidins, anthocyanidins) | 346 | Diet (drink) | Bid | 56 P | 29 (15) | 76.6 ± 1.6 | 12 | √ | √ | 4.7 | ↓8% | NV | ↑1% | ↓3% | [164] | ||||
| Polyphenols | Protocol | Participants | Variation of Lipid Profile 1 | Reference | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dietary Source (Main PLPs) 2 | Dosage (mg/Day) | Matrix | Intake Repartition | Length S.D. (Days) | n (Female) | Age 3 (Years) | D.-O. (%) | ↑LDL-C | ↑TG | ↓HDL-C | Obesity | IR | ↑SBP | FRS (%) | TG | TC | LDL-C | HDL- C | |
| Bergamot PLP (neoeriocitrin, naringin, neohesperidin) (+ statin) | 1000 | Capsule | Die | 30 P | 15 (N/A) | N/A | 0 | √ | √ | √ | >20 | ↓36% * | ↓38% * | ↓53% * | ↑37% * | [165] | |||
| Bergamot PLP (neoeriocitrin, naringin, neohesperidin) | 1000 | Capsule | Die | 30 P | 15 (N/A) | N/A | 0 | √ | √ | √ | >20 | ↓31% * | ↓31% * | ↓41% * | ↑18% * | [165] | |||
| Amla (Indian gooseberry) (not specified) | 350 | Capsule | Bid | 84 P | 49 (27) | 40.7 ± 1.6 | 0 | √ | √ | √ | 5.5 | ↓34% * | ↓24% * | ↓20% * | ↓10% * | [166] | |||
| Chokeberry (anthocyanidins) | 772 | Diet (drink) | Die | 28 P | 23 (11) | 47.5 ± 1.5 | 0 | √ | √ | √ | 6.7 | ↓19% * | ↓4% | ↓7% | ↓1% | [167] | |||
| Yerba mate tea (green or roasted) (cholorogenic acid, 4,5-dicaffeolquinic acid, gallocatechin) | 3589 | Diet (drink) | Tid | 20 P | 57 (34) | 45.8 ± 1.6 | 12 | √ | √ | √ | 3.9 | ↓3% | ↓3% | ↓8% * | ↑4% * | [144] | |||
| Yerba mate tea (green or roasted) (cholorogenic acid, 4,5-dicaffeolquinic acid, gallocatechin) | 3589 | Diet (drink) | Tid | 40 P | 57 (34) | 45.8 ± 1.6 | 12 | √ | √ | √ | 3.9 | ↓3% | ↓5% * | ↓9% * | ↑3% | [144] | |||
| Whole red grape (not specified) + 2.7 g of fibers | 63 | Diet | 5x/day | 56 P | 22 (18) | 50.5 ± 1.5 | 0 | √ | √ | √ | 9.5 | ↓1% | ↓9% * | ↓15% * | ↓6% | [168] | |||
| Whole white grape (not specified) + 5.3 g of fibers | 58 | Diet | 5x/day | 56 P | 24 (18) | 50.6 ± 1.3 | 0 | √ | √ | √ | 7.1 | ↓4% | ↓8% * | ↓10% * | ↓7% | [168] | |||
| Resveratrol | 500 | Capsule | Bid | 30 P | 24 (12) | 58.5 ± 3.4 | 0 | √ | √ | √ | 12.8 | ↑20% | ↑5% * | ↑5% | ↓2% | [169] | |||
| Resveratrol | 3000 | Capsule | Bid | 56 P | 10 (0) | 48.8 ± 1.7 | 0 | √ | √ | √ | 18.4 | ↑31% | ↑2% | ↑9% | NV | [170] | |||
5.1.3. Impact of Polyphenols on Metabolic Syndrome and Type 2 Diabetes
| Polyphenols | Protocol | Participants | Variation of Lipid Profile 2 | Reference | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dietary Source (Main PLPs) 3 | Dosage (mg/Day) | Matrix | Intake Repartition | Length S.D. (Days) | n (Female) | Age 4 (Years) | D.-O. (%) | ↑LDL-C | ↑TG | ↓HDL-C | Obesity | IR | ↑SBP | FRS (%) | TG | TC | LDL- C | HDL- C | |
| Eckonia cava (not specified) | 144 | Diet (drink) | Bid | 84 P | 32 (21) | 40.2 ± 10.1 | 0 | √ | √ | √ | √ | 3.8 | ↓8% | ↓9% * | ↓14% * | ↑13% * | [178] | ||
| Eckonia cava (not specified) | 72 | Diet (drink) | Bid | 84 P | 33 (22) | 40.6 ± 9.3 | 0 | √ | √ | √ | √ | 3.8 | ↓3% | ↓7% * | ↓10% * | ↑9% | [178] | ||
| Grape (flavanols, anthocyanidins) | 195 | Diet (drink) | Bid | 28 CO | 20 (8) | 53.5 ± 1.4 | 0 | √ | √ | √ | √ | 9.6 | ↓22% * | ↓4% | NV | ↓1% | [179] | ||
| PLP (various) | 2776 | Diet | Tid | 56 P | 20 (11) | 53.0 ± 1.2 | 9 | √ | √ | √ | 7.7 | ↓15% * | ↓5% | ↓6% | ↓6% * | [180] | |||
| PLP (various) + omega-3 | 2667 | Diet | Tid | 56 P | 19 (11) | 55.0 ± 1.2 | 9 | √ | √ | √ | 5.4 | ↓12% * | ↓1% | ↓5% | ↓8% * | [180] | |||
| Cranberry (proanthocyanidins, anthocyanidins) | 458 | Diet (drink) | Bid | 56 P | 15 (15) | 52.0 ± 1.1 | 3 | √ | √ | √ | 8.6 | ↑4% | ↓3% | ↓4% | ↓3% | [181] | |||
| Cranberry and strawberries (phenolic acids, pro-anthocyanidins) | 333 | Liquid supplement | Die | 42 P | 20 (11) | 57.0 ± 1.0 | 9 | √ | √ | √ | √ | 9.1 | ↓10% | ↓2% | NV | ↑1% | [182] | ||
| Red wine (catechin, epicatechin, gallic acid) | 798 | Diet (drink) | Die | 28 CO | 67 (0) | 60.0 ± 1.0 | 8 | √ | √ | √ | √ | >30 | ↑2% | ↓1% | ↓4% | ↑7% * | [183] | ||
| Dealcoholized wine (catechin, epicatechin, gallic acid) | 733 | Diet (drink) | Die | 28 CO | 67 (0) | 60.0 ± 1.0 | 8 | √ | √ | √ | √ | >30 | ↓2% | ↓4% | ↓2% | NV | [183] | ||
| Pomegranate (not specified) | 119.1 | Diet (drink) | die | 365 P | 66 (29) | 65.9 ± 1.4 | 34 | √ | √ | √ | 24.5 | ↓9% * | ↑1% | ↑6% | ↑11% * | [184] | |||
| Onion (quercetin) | 162 | Capsule | Tid | 21 CO | 68 (34) | 47.4 ± 1.5 | 3 | √ | √ | √ | √ | 9.3 | ↑1% | ↓1% | ↓1% | ↓2% | [185] | ||
| Quercetin | 150 | Capsule | Tid | 56 CO | 19 (0) | 59.5 ± 1.4 | 0 | √ | √ | √ | 13.3 | ↑31% | ↑5% * | ↑3% | ↓2% * | [186] | |||
| Quercetin | 150 | Capsule | Tid | 56 CO | 30 (0) | 59.4 ± 0.9 | 0 | √ | √ | √ | √ | 15.6 | ↑4% | ↑2% | ↑2% | ↓1% * | [186] | ||
| Resveratrol | 150 | Capsule | Die | 30 CO | 11 (0) | 52.5 ± 2.1 | 0 | √ | √ | 11.2 | ↑13% * | N/A | N/A | N/A | [187] | ||||
| Polyphenols | Protocol | Participants | Variation of Lipid Profile 2 | Reference | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dietary Source (Main PLP) 3 | Dosage (mg/Day) | Matrix | Intake Repartition | Length S.D (Days) | n (Female) | Age 4 (Years) | D.-O. (%) | TG | TC | LDL- C | HDL-C | |
| Black soybean (proanthocyanidin, isoflavone) (+120 mg fenofibrate) (+70 mg fibers) | 538 | Capsule | Die | 56 P | 7 (3) | 57.4 ± 4.3 | N/A | ↓42% * | ↓6% | ↓15% * | ↑11% | [188] |
| Black soybean (proanthocyanidin, isoflavone) (+70 mg fibers) | 538 | Capsule | Die | 56 P | 18 (6) | 56.7 ± 2.7 | N/A | ↓13% | ↑2% | ↓1% | ↑2% | [188] |
| Chlorogenic acid | 1200 | Capsule | Tid | 84 P | 14 (14) | 43.0 ± 1.7 | 13 | ↓19% * | ↓4% * | ↓17% * | ↑6% | [189] |
| Curcuminoid | 70 | Capsule | Die | 56 P | 37 (20) | 46.4 ± 1.7 | 7 | ↓13% * | ↓12% * | ↓11% | ↑5% | [190] |
| Grapefruit, green tea, black carrot and guarana seed extract (no information provided) | 370 | Capsule | Bid | 84 P | 8 (4) | 40.7 ± 0.7 | 0 | ↓14% * | ↓9% * | ↓10% * | ↑9% * | [191] |
| Resveratrol | 40 | Capsule | Die | 183 P | 59 (25) | 64.9 ± 1.1 | 7 | ↑1% | ↑5% | ↑7% | ↑1% | [177] |
| Resveratrol | 500 | Capsule | Die | 183 P | 62 (23) | 65.0 ± 0.9 | 7 | ↑21% * | ↑6% * | ↑6% | NV | [177] |
5.2. Postprandial Interventions
5.3. Matrix and Methods of Delivery
5.4. Dosage
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Apo | Apolipoprotein |
| ABCA1 | ATP-binding cassette transporter A1 |
| CVD | Cardiovascular disease |
| CHOL | Cholesterol |
| CM | Chylomicron |
| CD-36 | Cluster of Differentiation-36 |
| DLP | Dyslipidemia |
| FH | Familial hypercholesterolemia |
| FA | Fatty acid |
| HDL-C | High-density lipoprotein cholesterol |
| HLP | Hyperlipoproteinemia |
| IR | Insulin resistance |
| LCAT | lecithin cholesteryl ester transfer protein |
| LDL-C | Low-density lipoprotein cholesterol |
| LDLR | LDL receptor |
| LPL | Lipoprotein lipase |
| MetS | Metabolic syndrome |
| MTTP | Microsomal triglyceride transport protein |
| NPC1L1 | Niemann-Pick-C1-like-1 |
| OxS | Oxidative stress |
| PLP | Polyphenol |
| PCSK9 | Proprotein convertase subtilisin/kexin type 9 |
| ROS | Reactive oxygen species |
| SR-B1 | Scavenger receptor B-1 |
| TC | Total cholesterol |
| TG | Triglycerides |
| T2D | Type 2 diabetes |
| VLDL | Very-low-density lipoprotein |
References
- Roth, G.A.; Johnson, C.; Abajobir, A.; Abd-Allah, F.; Abera, S.F.; Abyu, G.; Ahmed, M.; Aksut, B.; Alam, T.; Alam, K.; et al. Global, Regional, and National Burden of Cardiovascular Diseases for 10 Causes, 1990 to 2015. J. Am. Coll. Cardiol. 2017, 70, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Fryar, C.D.; Chen, T.C.; Li, X. Prevalence of uncontrolled risk factors for cardiovascular disease: United States, 1999–2010. NCHS Data Brief 2012, 1–8. [Google Scholar]
- Reiner, Z.; Catapano, A.L.; De Backer, G.; Graham, I.; Taskinen, M.R.; Wiklund, O.; Agewall, S.; Alegria, E.; Chapman, M.J.; Durrington, P.; et al. ESC/EAS Guidelines for the management of dyslipidaemias: The Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS). Eur. Heart J. 2011, 32, 1769–1818. [Google Scholar]
- Rees, K.; Hartley, L.; Flowers, N.; Clarke, A.; Hooper, L.; Thorogood, M.; Stranges, S. ‘Mediterranean’ dietary pattern for the primary prevention of cardiovascular disease. Cochrane Database Syst. Rev. 2013. [Google Scholar] [CrossRef]
- Kastorini, C.M.; Milionis, H.J.; Esposito, K.; Giugliano, D.; Goudevenos, J.A.; Panagiotakos, D.B. The effect of Mediterranean diet on metabolic syndrome and its components: A meta-analysis of 50 studies and 534,906 individuals. J. Am. Coll. Cardiol. 2011, 57, 1299–1313. [Google Scholar] [CrossRef] [PubMed]
- Mehmood, A.; Usman, M.; Patil, P.; Zhao, L.; Wang, C. A review on management of cardiovascular diseases by olive polyphenols. Food Sci. Nutr. 2020, 8, 4639–4655. [Google Scholar] [CrossRef]
- Hussain, M.M. Intestinal lipid absorption and lipoprotein formation. Curr. Opin. Lipidol. 2014, 25, 200–206. [Google Scholar] [CrossRef]
- Semenkovich, C.F. Disorders of Lipid Metabolism. In Goldman’s Cecil Medicine, 24th ed.; Goldman, L., Schafer, A.I., Eds.; Saunders: Philadelphia, PA, USA, 2012; pp. 1346–1354. [Google Scholar]
- Jacobson, T.A.; Ito, M.K.; Maki, K.C.; Orringer, C.E.; Bays, H.E.; Jones, P.H.; McKenney, J.M.; Grundy, S.M.; Gill, E.A.; Wild, R.A.; et al. National lipid association recommendations for patient-centered management of dyslipidemia: Part 1--full report. J. Clin. Lipidol. 2015, 9, 129–169. [Google Scholar] [CrossRef] [PubMed]
- Anderson, T.J.; Grégoire, J.; Pearson, G.J.; Barry, A.R.; Couture, P.; Dawes, M.; Francis, G.A.; Genest, J., Jr.; Grover, S.; Gupta, M.; et al. 2016 Canadian Cardiovascular Society Guidelines for the Management of Dyslipidemia for the Prevention of Cardiovascular Disease in the Adult. Can. J. Cardiol. 2016, 32, 1263–1282. [Google Scholar] [CrossRef] [PubMed]
- Hansson, G.K.; Hamsten, A. Atherosclerosis, Thrombosis, and Vascular Biology. In Goldman’s Cecil Medicine, 24th ed.; Goldman, L., Schafer, A.I., Eds.; Saunders: Philadelphia, PA, USA, 2012; pp. 409–412. [Google Scholar]
- Levy, E.; Poinsot, P.; Spahis, S. Chylomicron retention disease: Genetics, biochemistry, and clinical spectrum. Curr. Opin. Lipidol. 2019, 30, 134–139. [Google Scholar] [CrossRef]
- Levy, E. Insights from human congenital disorders of intestinal lipid metabolism. J. Lipid Res. 2015, 56, 945–962. [Google Scholar] [CrossRef] [PubMed]
- Ko, C.W.; Qu, J.; Black, D.D.; Tso, P. Regulation of intestinal lipid metabolism: Current concepts and relevance to disease. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 169–183. [Google Scholar] [CrossRef]
- Warnakula, S.; Hsieh, J.; Adeli, K.; Hussain, M.M.; Tso, P.; Proctor, S.D. New insights into how the intestine can regulate lipid homeostasis and impact vascular disease: Frontiers for new pharmaceutical therapies to lower cardiovascular disease risk. Can. J. Cardiol. 2011, 27, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, J.; Hayashi, A.A.; Webb, J.; Adeli, K. Postprandial dyslipidemia in insulin resistance: Mechanisms and role of intestinal insulin sensitivity. Atheroscler. Suppl. 2008, 9, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Roy, C.C.; Levy, E.; Green, P.H.; Sniderman, A.; Letarte, J.; Buts, J.P.; Orquin, J.; Brochu, P.; Weber, A.M.; Morin, C.L.; et al. Malabsorption, hypocholesterolemia, and fat-filled enterocytes with increased intestinal apoprotein B. Chylomicron retention disease. Gastroenterology 1987, 92, 390–399. [Google Scholar] [CrossRef]
- Jones, B.; Jones, E.L.; Bonney, S.A.; Patel, H.N.; Mensenkamp, A.R.; Eichenbaum-Voline, S.; Rudling, M.; Myrdal, U.; Annesi, G.; Naik, S.; et al. Mutations in a Sar1 GTPase of COPII vesicles are associated with lipid absorption disorders. Nat. Genet. 2003, 34, 29–31. [Google Scholar] [CrossRef]
- Abumrad, N.A.; Davidson, N.O. Role of the gut in lipid homeostasis. Physiol. Rev. 2012, 92, 1061–1085. [Google Scholar] [CrossRef]
- Levy, E.; Roy, C.C.; Thibault, L.; Bonin, A.; Brochu, P.; Seidman, E.G. Variable expression of familial heterozygous hypobetalipoproteinemia: Transient malabsorption during infancy. J. Lipid Res. 1994, 35, 2170–2177. [Google Scholar] [CrossRef]
- Young, S.G.; Hubl, S.T.; Smith, R.S.; Snyder, S.M.; Terdiman, J.F. Familial hypobetalipoproteinemia caused by a mutation in the apolipoprotein B gene that results in a truncated species of apolipoprotein B (B-31). A unique mutation that helps to define the portion of the apolipoprotein B molecule required for the formation of buoyant, triglyceride-rich lipoproteins. J. Clin. Investig. 1990, 85, 933–942. [Google Scholar]
- Lee, S.J.; Grosskopf, I.; Choi, S.Y.; Cooper, A.D. Chylomicron remnant uptake in the livers of mice expressing human apolipoproteins E3, E2 (Arg158-->Cys), and E3-Leiden. J. Lipid Res. 2004, 45, 2199–2210. [Google Scholar] [CrossRef] [PubMed]
- Morgantini, C.; Xiao, C.; Dash, S.; Lewis, G.F. Dietary carbohydrates and intestinal lipoprotein production. Curr. Opin. Clin. Nutr. Metab. Care 2014, 17, 355–359. [Google Scholar] [CrossRef]
- Levy, E.; Spahis, S.; Ziv, E.; Marette, A.; Elchebly, M.; Lambert, M.; Delvin, E. Overproduction of intestinal lipoprotein containing apolipoprotein B-48 in Psammomys obesus: Impact of dietary n-3 fatty acids. Diabetologia 2006, 49, 1937–1945. [Google Scholar] [CrossRef]
- Zoltowska, M.; Ziv, E.; Delvin, E.; Sinnett, D.; Kalman, R.; Garofalo, C.; Seidman, E.; Levy, E. Cellular aspects of intestinal lipoprotein assembly in Psammomys obesus: A model of insulin resistance and type 2 diabetes. Diabetes 2003, 52, 2539–2545. [Google Scholar] [CrossRef] [PubMed]
- Haidari, M.; Leung, N.; Mahbub, F.; Uffelman, K.D.; Kohen-Avramoglu, R.; Lewis, G.F.; Adeli, K. Fasting and postprandial overproduction of intestinally derived lipoproteins in an animal model of insulin resistance. Evidence that chronic fructose feeding in the hamster is accompanied by enhanced intestinal de novo lipogenesis and ApoB48-containing lipoprotein overproduction. J. Biol. Chem. 2002, 277, 31646–31655. [Google Scholar]
- Xiao, C.; Dash, S.; Morgantini, C.; Lewis, G.F. New and emerging regulators of intestinal lipoprotein secretion. Atherosclerosis 2014, 233, 608–615. [Google Scholar] [CrossRef] [PubMed]
- Cox, R.A.; Garcia-Palmieri, M.R. Cholesterol, Triglycerides, and Associated Lipoproteins. In Clinical Methods: The History, Physical, and Laboratory Examinations, 3rd ed.; Walker, H.K., Hall, W.D., Hurst, J.W., Eds.; Butterworths: Boston, MA, USA, 1990. [Google Scholar]
- Gomez-Delgado, F.; Alcala-Diaz, J.F.; Leon-Acuña, A.; Lopez-Moreno, J.; Delgado-Lista, J.; Gomez-Marin, B.; Roncero-Ramos, I.; Yubero-Serrano, E.M.; Rangel-Zuñiga, O.A.; Vals-Delgado, C.; et al. Apolipoprotein E genetic variants interact with Mediterranean diet to modulate postprandial hypertriglyceridemia in coronary heart disease patients: CORDIOPREV study. Eur. J. Clin. Invest. 2019, 49, e13146. [Google Scholar] [CrossRef]
- Durst, R.; Ibe, U.K.; Shpitzen, S.; Schurr, D.; Eliav, O.; Futema, M.; Whittall, R.; Szalat, A.; Meiner, V.; Knobler, H.; et al. Molecular genetics of familial hypercholesterolemia in Israel-revisited. Atherosclerosis 2017, 257, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Dron, J.S.; Hegele, R.A. Genetics of Triglycerides and the Risk of Atherosclerosis. Curr. Atheroscler. Rep. 2017, 19, 31. [Google Scholar] [CrossRef]
- Bjorn, L.; Trond, P.L.; Ose, L.; Hamsten, A.; Karpe, F. A functional polymorphism in the promoter region of the microsomal triglyceride transfer protein (MTP -493G/T) influences lipoprotein phenotype in familial hypercholesterolemia. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1784–1788. [Google Scholar] [CrossRef] [PubMed]
- St-Pierre, J.; Lemieux, I.; Miller-Felix, I.; Prud’homme, D.; Bergeron, J.; Gaudet, D.; Nadeau, A.; Despres, J.P.; Vohl, M.C. Visceral obesity and hyperinsulinemia modulate the impact of the microsomal triglyceride transfer protein -493G/T polymorphism on plasma lipoprotein levels in men. Atherosclerosis 2002, 160, 317–324. [Google Scholar] [CrossRef]
- Levy, E.; Spahis, S.; Garofalo, C.; Marcil, V.; Montoudis, A.; Sinnet, D.; Sanchez, R.; Peretti, N.; Beaulieu, J.F.; Sane, A. Sar1b transgenic male mice are more susceptible to high-fat diet-induced obesity, insulin insensitivity and intestinal chylomicron overproduction. J. Nutr. Biochem. 2014, 25, 540–548. [Google Scholar] [CrossRef]
- Levy, E.; Spahis, S.; Sinnett, D.; Peretti, N.; Maupas-Schwalm, F.; Delvin, E.; Lambert, M.; Lavoie, M.A. Intestinal cholesterol transport proteins: An update and beyond. Curr. Opin. Lipidol. 2007, 18, 310–318. [Google Scholar] [CrossRef]
- Sane, A.T.; Sinnett, D.; Delvin, E.; Bendayan, M.; Marcil, V.; Ménard, D.; Beaulieu, J.F.; Levy, E. Localization and role of NPC1L1 in cholesterol absorption in human intestine. J. Lipid Res. 2006, 47, 2112–2120. [Google Scholar] [CrossRef]
- Levy, E.; Ménard, D.; Suc, I.; Delvin, E.; Marcil, V.; Brissette, L.; Thibault, L.; Bendayan, M. Ontogeny, immunolocalisation, distribution and function of SR-BI in the human intestine. J. Cell Sci. 2004, 117, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Suc, I.; Brunet, S.; Mitchell, G.; Rivard, G.E.; Levy, E. Oxidative tyrosylation of high density lipoproteins impairs cholesterol efflux from mouse J774 macrophages: Role of scavenger receptors, classes A and B. J. Cell Sci. 2003, 116, 89–99. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ulug, E.; Nergiz-Unal, R. Dietary Fatty Acids and CD36 Mediated Cholesterol Homeostasis: Potential Mechanisms. Nutr. Res. Rev. 2020, 1–44. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, H.; Yamanashi, Y.; Takada, T.; Mu, S.; Tanaka, Y.; Komine, T.; Suzuki, H. Hepatic Expression of Niemann-Pick C1-Like 1, a Cholesterol Reabsorber from Bile, Exacerbates Western Diet-Induced Atherosclerosis in LDL Receptor Mutant Mice. Mol. Pharmacol. 2019, 96, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Tomkin, G.H. Dyslipidaemia--hepatic and intestinal cross-talk. Atheroscler. Suppl. 2010, 11, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Levy, E.; Ben Djoudi Ouadda, A.; Spahis, S.; Sane, A.T.; Garofalo, C.; Grenier, É.; Emonnot, L.; Yara, S.; Couture, P.; Beaulieu, J.-F.; et al. PCSK9 plays a significant role in cholesterol homeostasis and lipid transport in intestinal epithelial cells. Atherosclerosis 2013, 227, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Temel, R.E.; Brown, J.M. A new model of reverse cholesterol transport: EnTICEing strategies to stimulate intestinal cholesterol excretion. Trends Pharmacol. Sci. 2015, 36, 440–451. [Google Scholar] [CrossRef]
- Xie, P.; Zhu, H.; Jia, L.; Ma, Y.; Tang, W.; Wang, Y.; Xue, B.; Shi, H.; Yu, L. Genetic demonstration of intestinal NPC1L1 as a major determinant of hepatic cholesterol and blood atherogenic lipoprotein levels. Atherosclerosis 2014, 237, 609–617. [Google Scholar] [CrossRef]
- Yu, X.H.; Zhang, D.W.; Zheng, X.L.; Tang, C.K. Cholesterol transport system: An integrated cholesterol transport model involved in atherosclerosis. Prog. Lipid Res. 2019, 73, 65–91. [Google Scholar] [CrossRef]
- Adeli, K.; Farr, J.; Xiao, S.; Lewis, C.; Gary, F. Diabetic Dyslipidaemia. In Biochemistry of Lipids, Lipoproteins and Membranes, 6th ed.; Ridgway, N.D., McLeod, R.S., Eds.; Elsevier: Boston, MA, USA, 2016; pp. 549–573. [Google Scholar]
- Rust, S.; Rosier, M.; Funke, H.; Real, J.; Amoura, Z.; Piette, J.C.; Deleuze, J.F.; Brewer, H.B.; Duverger, N.; Denèfle, P.; et al. Tangier disease is caused by mutations in the gene encoding ATP-binding cassette transporter 1. Nat. Genet. 1999, 22, 352–355. [Google Scholar] [CrossRef] [PubMed]
- Wellington, C.L.; Brunham, L.R.; Zhou, S.; Singaraja, R.R.; Visscher, H.; Gelfer, A.; Ross, C.; James, E.; Liu, G.; Huber, M.T.; et al. Alterations of plasma lipids in mice via adenoviral-mediated hepatic overexpression of human ABCA1. J. Lipid Res. 2003, 44, 1470–1480. [Google Scholar] [CrossRef]
- Basso, F.; Freeman, L.; Knapper, C.L.; Remaley, A.; Stonik, J.; Neufeld, E.B.; Tansey, T.; Amar, M.J.; Fruchart-Najib, J.; Duverger, N.; et al. Role of the hepatic ABCA1 transporter in modulating intrahepatic cholesterol and plasma HDL cholesterol concentrations. J. Lipid Res. 2003, 44, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, O.; Fukamachi, I.; Mori, A.; Hashimoto, H.; Kawashiri, M.A.; Nohara, A.; Noguchi, T.; Inazu, A.; Yamagishi, M.; Mabuchi, H.; et al. Formation of prebeta1-HDL during lipolysis of triglyceride-rich lipoprotein. Biochem. Biophys. Res. Commun. 2009, 379, 55–59. [Google Scholar] [CrossRef]
- Barter, P.J.; Brewer, H.B., Jr.; Chapman, M.J.; Hennekens, C.H.; Rader, D.J.; Tall, A.R. Cholesteryl ester transfer protein: A novel target for raising HDL and inhibiting atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 160–170. [Google Scholar] [CrossRef]
- Yokoyama, S. Assembly of high-density lipoprotein. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 20–27. [Google Scholar] [CrossRef]
- Oram, J.F. HDL apolipoproteins and ABCA1: Partners in the removal of excess cellular cholesterol. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 720–727. [Google Scholar] [CrossRef] [PubMed]
- Dobiasova, M. Atherogenic impact of lecithin-cholesterol acyltransferase and its relation to cholesterol esterification rate in HDL (FER(HDL)) and AIP [log(TG/HDL-C)] biomarkers: The butterfly effect? Physiol. Res. 2017, 66, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Herscovitz, H.; Ronen, I.; Bilu, S.; Tietz, A. Bile acid synthesis from HDL cholesterol and cholesterol ester by cultured chick embryo hepatocytes. Biochim. Biophys. Acta 1986, 878, 426–434. [Google Scholar] [CrossRef]
- Wanon, J.; Guertin, F.; Brunet, S.; Delvin, E.; Gavino, V.; Bouthillier, D.; Lairon, D.; Yotov, W.; Levy, E. The effects of cholesterol uptake from high-density lipoprotein subfractions on biliary sterol secretion in rats with essential fatty-acid deficiency. Hepatology 1998, 27, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Varban, M.L.; Rinninger, F.; Wang, N.; Fairchild-Huntress, V.; Dunmore, J.H.; Fang, Q.; Gosselin, M.L.; Dixon, K.L.; Deeds, J.D.; Acton, S.L.; et al. Targeted mutation reveals a central role for SR-BI in hepatic selective uptake of high density lipoprotein cholesterol. Proc. Natl. Acad. Sci. USA 1998, 95, 4619–4624. [Google Scholar] [CrossRef]
- Fournier, M.; Bonneil, E.; Garofalo, C.; Grimard, G.; Laverdière, C.; Krajinovic, M.; Drouin, S.; Sinnett, D.; Marcil, V.; Levy, E. Altered proteome of high-density lipoproteins from paediatric acute lymphoblastic leukemia survivors. Sci. Rep. 2019, 9, 4268. [Google Scholar] [CrossRef] [PubMed]
- Tall, A.R. Cholesterol efflux pathways and other potential mechanisms involved in the athero-protective effect of high density lipoproteins. J. Intern. Med. 2008, 263, 256–273. [Google Scholar] [CrossRef] [PubMed]
- Tall, A.R. Plasma high density lipoproteins: Therapeutic targeting and links to atherogenic inflammation. Atherosclerosis 2018, 276, 39–43. [Google Scholar] [CrossRef]
- Xepapadaki, E.; Zvintzou, E.; Kalogeropoulou, C.; Filou, S.; Kypreos, K.E. Tauhe Antioxidant Function of HDL in Atherosclerosis. Angiology 2020, 71, 112–121. [Google Scholar] [CrossRef]
- Schmitz, G.; Drobnik, W. ABCA1 Defects. In Encyclopedia of Endocrine Diseases, 1st ed.; Martini, L., Ed.; Elsevier: New York, NY, USA, 2004; pp. 1–5. [Google Scholar]
- Inazu, A.; Brown, M.L.; Hesler, C.B.; Agellon, L.B.; Koizumi, J.; Takata, K.; Maruhama, Y.; Mabuchi, H.; Tall, A.R. Increased high-density lipoprotein levels caused by a common cholesteryl-ester transfer protein gene mutation. N. Engl. J. Med. 1990, 323, 1234–1238. [Google Scholar] [CrossRef]
- Kopin, L.; Lowenstein, C. Dyslipidemia. Ann. Intern. Med. 2017, 167, ITC81–ITC96. [Google Scholar] [CrossRef]
- Kockx, M.; Kritharides, L. Triglyceride-Rich Lipoproteins. Cardiol. Clin. 2018, 36, 265–275. [Google Scholar] [CrossRef]
- Sizar, O.; Khare, S.; Jamil, R.T.; Talati, R. Statin Medications; StatPearls: Treasure Island, CA, USA, 2020. [Google Scholar]
- Goldberg, A.C.; Hopkins, P.N.; Toth, P.P.; Ballantyne, C.M.; Rader, D.J.; Robinson, J.G.; Daniels, S.R.; Gidding, S.S.; de Ferranti, S.D.; Ito, M.K.; et al. Familial hypercholesterolemia: Screening, diagnosis and management of pediatric and adult patients: Clinical guidance from the National Lipid Association Expert Panel on Familial Hypercholesterolemia. J. Clin. Lipidol. 2011, 5, S1–S8. [Google Scholar] [CrossRef] [PubMed]
- Bambauer, R.; Bambauer, C.; Lehmann, B.; Latza, R.; Schiel, R. LDL-apheresis: Technical and clinical aspects. Sci. World J. 2012, 2012, 314283. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Orringer, C.E.; Jacobson, T.A.; Saseen, J.J.; Brown, A.S.; Gotto, A.M.; Ross, J.L.; Underberg, J.A. Update on the use of PCSK9 inhibitors in adults: Recommendations from an Expert Panel of the National Lipid Association. J. Clin. Lipidol. 2017, 11, 880–890. [Google Scholar] [CrossRef]
- Cory, H.; Passarelli, S.; Szeto, J.; Tamez, M.; Mattei, J. The Role of Polyphenols in Human Health and Food Systems: A Mini-Review. Front. Nutr. 2018, 5, 87. [Google Scholar] [CrossRef]
- EFSA Panel on Food Additives and Nutrients Sources Added to Food. Risk assessment for peri- and post-menopausal women taking food supplements containing isolated isoflavones. EFSA J. 2015, 13, 4246. [Google Scholar] [CrossRef]
- Trautwein, E.A.; McKay, S. The Role of Specific Components of a Plant-Based Diet in Management of Dyslipidemia and the Impact on Cardiovascular Risk. Nutrients 2020, 12, 2671. [Google Scholar] [CrossRef]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef]
- Jaffe, R.; Mani, J. Polyphenolics Evoke Healing Responses: Clinical Evidence and Role of Predictive Biomarkers. In Polyphenols in Human Health and Disease; Watson, R.R., Preedy, V.R., Zibadi, S., Eds.; Academic Press: San Diego, CA, USA, 2014; pp. 695–705. [Google Scholar]
- Cires, M.J.; Wong, X.; Carrasco-Pozo, C.; Gotteland, M. The Gastrointestinal Tract as a Key Target Organ for the Health-Promoting Effects of Dietary Proanthocyanidins. Front. Nutr. 2016, 3, 57. [Google Scholar] [CrossRef] [PubMed]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.; Tognolini, M.; Borgesm, G.; Crozierm, A. Dietary (poly)phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid. Redox Signal. 2013, 18, 1818–1892. [Google Scholar] [CrossRef] [PubMed]
- Koch, W. Dietary Polyphenols-Important Non-Nutrients in the Prevention of Chronic Noncommunicable Diseases. A Systematic Review. Nutrients 2019, 11, 1039. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Xia, M. Anthocyanins and Diabetes Regulation. In Polyphenols: Mechanisms of Action in Human Health and Disease, 2nd ed.; Watson, R.R., Preedy, V.R., Zibadi, S., Eds.; Academic Press: San Diego, CA, USA, 2014; pp. 135–145. [Google Scholar]
- Christensen, L.P.; Christensen, K.B. The Role of Direct and Indirect Polyphenolic Antioxidants in Protection Against Oxidative Stress. In Polyphenols in Human Health and Disease; Watson, R.R., Preedy, V.R., Zibadi, S., Eds.; Academic Press: San Diego, CA, USA, 2014; pp. 289–309. [Google Scholar]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative Stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef]
- Toyokuni, S. Mysterious link between iron overload and CDKN2A/2B. J. Clin. Biochem. Nutr. 2011, 48, 46–49. [Google Scholar] [CrossRef]
- Bast, A.; Haenen, G.R. Ten misconceptions about antioxidants. Trends Pharmacol. Sci. 2013, 34, 430–436. [Google Scholar] [CrossRef]
- Koudoufio, M.; Desjardins, Y.; Feldman, F.; Spahis, S.; Delvin, E.; Levy, E. Insight into Polyphenol and Gut Microbiota Crosstalk: Are Their Metabolites the Key to Understand Protective Effects against Metabolic Disorders? Antioxidants 2020, 9, 982. [Google Scholar] [CrossRef]
- Apostolidou, C.; Adamopoulos, K.; Iliadis, S.; Kourtidou-Papadeli, C. Alterations of antioxidant status in asymptomatic hypercholesterolemic individuals after resveratrol intake. Int. J. Food Sci. Nutr. 2015, 67, 541–552. [Google Scholar] [CrossRef] [PubMed]
- Naissides, M.; Mamo, J.C.; James, A.P.; Pal, S. The effect of chronic consumption of red wine on cardiovascular disease risk factors in postmenopausal women. Atherosclerosis 2006, 185, 438–445. [Google Scholar] [CrossRef]
- Kroon, P.A.; Clifford, M.N.; Crozier, A.; Day, A.J.; Donovan, J.L.; Manach, C.; Williamson, G. How should we assess the effects of exposure to dietary polyphenols in vitro? Am. J. Clin. Nutr. 2004, 80, 15–21. [Google Scholar] [CrossRef]
- Tsao, R. Chemistry and biochemistry of dietary polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef] [PubMed]
- Castilla, P.; Echarri, R.; Dávalos, A.; Cerrato, F.; Ortega, H.; Teruel, J.L.; Lucas, M.F.; Gómez-Coronado, D.; Ortuño, J.; Lasunción, M.A. Concentrated red grape juice exerts antioxidant, hypolipidemic, and antiinflammatory effects in both hemodialysis patients and healthy subjects. Am. J. Clin. Nutr. 2006, 84, 252–262. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, S.; Fujita, T.; Shimabukuro, M.; Iwaki, M.; Yamada, Y.; Nakajima, Y.; Nakayama, O.; Makishima, M.; Matsuda, M.; Shimomura, I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Investig. 2004, 114, 1752–1761. [Google Scholar] [CrossRef] [PubMed]
- Herranz-Lopez, M.; Fernández-Arroyo, S.; Pérez-Sanchez, A.; Barrajón-Catalán, E.; Beltrán-Debón, R.; Menéndez, J.A.; Alonso-Villaverde, C.; Segura-Carretero, A.; Joven, J.; Micol, V. Synergism of plant-derived polyphenols in adipogenesis: Perspectives and implications. Phytomedicine 2012, 19, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Yeop Han, C.; Kargi, A.Y.; Omer, M.; Chan, C.K.; Wabitsch, M.; O’Brien, K.D.; Wight, T.N.; Chait, A. Differential effect of saturated and unsaturated free fatty acids on the generation of monocyte adhesion and chemotactic factors by adipocytes: Dissociation of adipocyte hypertrophy from inflammation. Diabetes 2010, 59, 386–396. [Google Scholar] [PubMed]
- Chung, M.Y.; Park, H.J.; Manautou, J.E.; Koo, S.I.; Bruno, R.S. Green tea extract protects against nonalcoholic steatohepatitis in ob/ob mice by decreasing oxidative and nitrative stress responses induced by proinflammatory enzymes. J. Nutr. Biochem. 2012, 23, 361–367. [Google Scholar] [CrossRef]
- Park, H.J.; DiNatale, D.A.; Chung, M.Y.; Park, Y.K.; Lee, J.Y.; Koo, S.I.; O’Connor, M.; Manautou, J.E.; Bruno, R.S. Green tea extract attenuates hepatic steatosis by decreasing adipose lipogenesis and enhancing hepatic antioxidant defenses in ob/ob mice. J. Nutr. Biochem. 2011, 22, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Zhu, W.; Shen, C.L.; Gao, W. Green tea polyphenols reduce body weight in rats by modulating obesity-related genes. PLoS ONE 2012, 7, e38332. [Google Scholar] [CrossRef]
- Franco, J.G.; Lisboa, P.C.; Lima, N.S.; Amaral, T.A.; Peixoto-Silva, N.; Resende, A.C.; Oliveira, E.; Passos, M.C.; Moura, E.G. Resveratrol attenuates oxidative stress and prevents steatosis and hypertension in obese rats programmed by early weaning. J. Nutr. Biochem. 2013, 24, 960–966. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Zorita, S.; Fernández-Quintela, A.; Macarulla, M.T.; Aguirre, L.; Hijona, E.; Bujanda, L.; Milagro, F.; Martínez, J.A.; Portillo, M.P. Resveratrol attenuates steatosis in obese Zucker rats by decreasing fatty acid availability and reducing oxidative stress. Br. J. Nutr. 2012, 107, 202–210. [Google Scholar] [CrossRef]
- Roberts, C.K.; Sindhu, K.K. Oxidative stress and metabolic syndrome. Life Sci. 2009, 84, 705–712. [Google Scholar] [CrossRef]
- Otani, H. Oxidative stress as pathogenesis of cardiovascular risk associated with metabolic syndrome. Antioxid. Redox. Signal. 2011, 15, 1911–1926. [Google Scholar] [CrossRef]
- Bujanda, L.; Hijona, E.; Larzabal, M.; Beraza, M.; Aldazabal, P.; García-Urkia, N.; Sarasqueta, C.; Cosme, A.; Irastorza, B.; González, A.; et al. Resveratrol inhibits nonalcoholic fatty liver disease in rats. BMC Gastroenterol. 2008, 8, 40. [Google Scholar] [CrossRef]
- Li, H.; Xia, N.; Forstermann, U. Cardiovascular effects and molecular targets of resveratrol. Nitric Oxide 2012, 26, 102–110. [Google Scholar] [CrossRef]
- Li, X.N.; Ma, L.Y.; Ji, H.; Qin, Y.H.; Jin, S.S.; Xu, L.X. Resveratrol protects against oxidative stress by activating the Keap-1/Nrf2 antioxidant defense system in obese-asthmatic rats. Exp. Ther. Med. 2018, 16, 4339–4348. [Google Scholar] [CrossRef]
- Ding, H.; Heng, B.; He, W.; Shi, L.; Lai, C.; Xiao, L.; Ren, H.; Mo, S.; Su, Z. Chronic reactive oxygen species exposure inhibits glucose uptake and causes insulin resistance in C2C12 myotubes. Biochem. Biophys. Res. Commun. 2016, 478, 798–803. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Guo, S.; Zou, Z. Resveratrol ameliorates metabolic disorders and insulin resistance in high-fat diet-fed mice. Life Sci. 2020, 242, 117212. [Google Scholar] [CrossRef] [PubMed]
- Brasnyo, P.; Molnár, G.A.; Mohás, M.; Markó, L.; Laczy, B.; Cseh, J.; Mikolás, E.; Szijártó, I.A.; Mérei, A.; Halmai, R.; et al. Resveratrol improves insulin sensitivity, reduces oxidative stress and activates the Akt pathway in type 2 diabetic patients. Br. J. Nutr. 2011, 106, 383–389. [Google Scholar] [CrossRef]
- Do, G.M.; Jung, U.J.; Park, H.J.; Kwon, E.Y.; Jeon, S.M.; McGregor, R.A.; Choi, M.S. Resveratrol ameliorates diabetes-related metabolic changes via activation of AMP-activated protein kinase and its downstream targets in db/db mice. Mol. Nutr. Food Res. 2012, 56, 1282–1291. [Google Scholar] [CrossRef]
- Su, H.C.; Hung, L.M.; Chen, J.K. Resveratrol, a red wine antioxidant, possesses an insulin-like effect in streptozotocin-induced diabetic rats. Am. J. Physiol. Endocrinol. Metab. 2006, 290, E1339–E1346. [Google Scholar] [CrossRef]
- Bhatt, S.R.; Lokhandwala, M.F.; Banday, A.A. Resveratrol prevents endothelial nitric oxide synthase uncoupling and attenuates development of hypertension in spontaneously hypertensive rats. Eur. J. Pharmacol. 2011, 667, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Dai, Y.; Yan, S.; Shi, Y.; Li, J.; Liu, J.; Cha, L.; Mu, J. Resveratrol lowers blood pressure in spontaneously hypertensive rats via calcium-dependent endothelial NO production. Clin. Exp. Hypertens. 2016, 38, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Pereira, R.; Tatsch, E.; Bochi, G.V.; Kober, H.; Duarte, T.; dos Santos Montagner, G.F.; da Silva, J.E.; Duarte, M.M.; da Cruz, I.B.; Moresco, R.N. Assessment of oxidative, inflammatory, and fibrinolytic biomarkers and DNA strand breakage in hypercholesterolemia. Inflammation 2013, 36, 869–877. [Google Scholar] [CrossRef]
- Nourooz-Zadeh, J.; Smith, C.C.T.; Betteridge, D.J. Measures of oxidative stress in heterozygous familial hypercholesterolaemia. Atherosclerosis 2001, 156, 435–441. [Google Scholar] [CrossRef]
- Tangvarasittichai, S. Oxidative stress, insulin resistance, dyslipidemia and type 2 diabetes mellitus. World J. Diabetes 2015, 6, 456–480. [Google Scholar] [CrossRef]
- Harangi, M.; Remenyik, E.E.; Seres, I.; Varga, Z.; Katona, E.; Paragh, G. Determination of DNA damage induced by oxidative stress in hyperlipidemic patients. Mutat. Res. 2002, 513, 17–25. [Google Scholar] [CrossRef]
- Khatana, C.; Saini, N.K.; Chakrabarti, S.; Saini, V.; Sharma, A.; Saini, R.V.; Saini, A.K. Mechanistic Insights into the Oxidized Low-Density Lipoprotein-Induced Atherosclerosis. Oxid. Med. Cell. Longev. 2020, 2020, 5245308. [Google Scholar] [CrossRef] [PubMed]
- Hauck, A.K.; Bernlohr, D.A. Oxidative stress and lipotoxicity. J. Lipid Res. 2016, 57, 1976–1986. [Google Scholar] [CrossRef]
- Narverud, I.; Halvorsen, B.; Nenseter, M.S.; Retterstøl, K.; Yndestad, A.; Dahl, T.B.; Ulven, S.M.; Olstad, O.K.; Ose, L.; Holven, K.B.; et al. Oxidized LDL level is related to gene expression of tumour necrosis factor super family members in children and young adults with familial hypercholesterolaemia. J. Intern. Med. 2013, 273, 69–78. [Google Scholar] [CrossRef]
- Holvoet, P.; Mertens, A.; Verhamme, P.; Bogaerts, K.; Beyens, G.; Verhaeghe, R.; Collen, D.; Muls, E.; Van de Werf, F. Circulating oxidized LDL is a useful marker for identifying patients with coronary artery disease. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 844–848. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.S.; Su, Y.F.; Yang, H.W.; Lee, Y.H.; Chou, J.I.; Ueng, K.C. Lipid-lowering effects of curcumin in patients with metabolic syndrome: A randomized, double-blind, placebo-controlled trial. Phytother. Res. 2014, 28, 1770–1777. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Wo, X.; Qian, Y.; Yin, J.; Gao, L. Effect of curcumin on the expression of LDL receptor in mouse macrophages. J. Ethnopharmacol. 2006, 105, 251–254. [Google Scholar] [CrossRef]
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef]
- Strowig, T.; Henao-Mejia, J.; Elinav, E.; Flavell, R. Inflammasomes in health and disease. Nature 2012, 481, 278–286. [Google Scholar] [CrossRef]
- Curti, M.L.; Jacob, P.; Borges, M.C.; Rogero, M.M.; Ferreira, S.R. Studies of gene variants related to inflammation, oxidative stress, dyslipidemia, and obesity: Implications for a nutrigenetic approach. J. Obes. 2011, 2011, 497401. [Google Scholar] [CrossRef]
- Haybar, H.; Shahrabi, S.; Rezaeeyan, H.; Shirzad, R.; Saki, N. Endothelial Cells: From Dysfunction Mechanism to Pharmacological Effect in Cardiovascular Disease. Cardiovasc. Toxicol. 2019, 19, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Steven, S.; Frenis, K.; Oelze, M.; Kalinovic, S.; Kuntic, M.; Bayo Jimenez, M.T.; Vujacic-Mirski, K.; Helmstaedter, J.; Kroeller-Schoen, S.; Munzel, T.; et al. Vascular Inflammation and Oxidative Stress: Major Triggers for Cardiovascular Disease. Oxid. Med. Cell. Longev. 2019, 2019, 7092151. [Google Scholar] [CrossRef] [PubMed]
- Yuan, T.; Yang, T.; Chen, H.; Fu, D.; Hu, Y.; Wang, J.; Yuan, Q.; Yu, H.; Xu, W.; Xie, X. New insights into oxidative stress and inflammation during diabetes mellitus-accelerated atherosclerosis. Redox Biol. 2019, 20, 247–260. [Google Scholar] [CrossRef] [PubMed]
- Bondia-Pons, I.; Ryan, L.; Martinez, J.A. Oxidative stress and inflammation interactions in human obesity. J. Physiol. Biochem. 2012, 68, 701–711. [Google Scholar] [CrossRef]
- Staels, B. Cardiovascular biology: A cholesterol tether. Nature 2002, 417, 699–701. [Google Scholar] [CrossRef]
- Ansell, B.J.; Fonarow, G.C.; Fogelman, A.M. The paradox of dysfunctional high-density lipoprotein. Curr. Opin. Lipidol. 2007, 18, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Navab, M.; Hama, S.Y.; Hough, G.P.; Subbanagounder, G.; Reddy, S.T.; Fogelman, A.M. A cell-free assay for detecting HDL that is dysfunctional in preventing the formation of or inactivating oxidized phospholipids. J. Lipid Res. 2001, 42, 1308–1317. [Google Scholar] [CrossRef]
- Mitjavila, M.T.; Moreno, J.J. The effects of polyphenols on oxidative stress and the arachidonic acid cascade. Implications for the prevention/treatment of high prevalence diseases. Biochem. Pharmacol. 2012, 84, 1113–1122. [Google Scholar] [CrossRef] [PubMed]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.; Rahu, N. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxid. Med. Cell. Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef] [PubMed]
- Vane, J.R.; Botting, R.M. Mechanism of Action of Nonsteroidal Anti-inflammatory Drugs. Am. J. Med. 1998, 104, 2S–8S. [Google Scholar] [CrossRef]
- Tremaroli, V.; Backhed, F. Functional interactions between the gut microbiota and host metabolism. Nature 2012, 489, 242–249. [Google Scholar] [CrossRef]
- De Angelis, M.; Garruti, G.; Minervini, F.; Bonfrate, L.; Portincasa, P.; Gobbetti, M. The Food-gut Human Axis: The Effects of Diet on Gut Microbiota and Metabolome. Curr. Med. Chem. 2019, 26, 3567–3583. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Tang, R.; Yang, S.; Lu, Y.; Luo, J.; Liu, Z. Rutin and Its Combination With Inulin Attenuate Gut Dysbiosis, the Inflammatory Status and Endoplasmic Reticulum Stress in Paneth Cells of Obese Mice Induced by High-Fat Diet. Front. Microbiol. 2018, 9, 2651. [Google Scholar] [CrossRef]
- González-Sarrías, A.; Combet, E.; Pinto, P.; Mena, P.; Dall’Asta, M.; Garcia-Aloy, M.; Rodríguez-Mateos, A.; Gibney, E.R.; Dumont, J.; Massaro, M.; et al. A Systematic Review and Meta-Analysis of the Effects of Flavanol-Containing Tea, Cocoa and Apple Products on Body Composition and Blood Lipids: Exploring the Factors Responsible for Variability in Their Efficacy. Nutrients 2017, 9, 746. [Google Scholar]
- Tsang, C.; Smail, N.F.; Almoosawi, S.; McDougall, G.J.M.; Al-Dujaili, E.A.S. Antioxidant Rich Potato Improves Arterial Stiffness in Healthy Adults. Plant Foods Hum. Nutr. 2018, 73, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Xu, J.; Guo, T.L. Exposure to Polyphenolic Compounds Modulates Type 1 Diabetes: The Case of Genistein. In Polyphenols: Mechanisms of Action in Human Health and Disease, 2nd ed.; Watson, R.R., Preedy, V.R., Zibadi, S., Eds.; Academic Press: London, UK, 2018; pp. 193–203. [Google Scholar]
- Pathak, S.; Kesavan, P.; Banerjee, A.; Banerjee, A.; Sagdicoglu Celep, G.; Bissi, L.; Marotta, F. Metabolism of Dietary Polyphenols by Human Gut Microbiota and Their Health Benefits. In Polyphenols: Mechanisms of Action in Human Health and Disease, 2nd ed.; Watson, R.R., Preedy, V.R., Zibadi, S., Eds.; Academic Press: London, UK, 2018; pp. 347–359. [Google Scholar]
- Zhang, L.; Carmody, R.N.; Kalariya, H.M.; Duran, R.M.; Moskal, K.; Poulev, A.; Kuhn, P.; Tveter, K.M.; Turnbaugh, P.J.; Raskin, I.; et al. Grape proanthocyanidin-induced intestinal bloom of Akkermansia muciniphila is dependent on its baseline abundance and precedes activation of host genes related to metabolic health. J. Nutr. Biochem. 2018, 56, 142–151. [Google Scholar]
- Roopchand, D.E.; Carmody, R.N.; Kuhn, P.; Moskal, K.; Rojas-Silva, P.; Turnbaugh, P.J.; Raskin, I. Dietary Polyphenols Promote Growth of the Gut Bacterium Akkermansia muciniphila and Attenuate High-Fat Diet-Induced Metabolic Syndrome. Diabetes 2015, 64, 2847–2858. [Google Scholar] [CrossRef]
- Neyrinck, A.M.; Van Hée, V.F.; Bindels, L.B.; De Backer, F.; Cani, P.D.; Delzenne, N.M. Polyphenol-rich extract of pomegranate peel alleviates tissue inflammation and hypercholesterolaemia in high-fat diet-induced obese mice: Potential implication of the gut microbiota. Br. J. Nutr. 2013, 109, 802–809. [Google Scholar] [CrossRef] [PubMed]
- Ravn-Haren, G.; Dragsted, L.O.; Buch-Andersen, T.; Jensen, E.N.; Jensen, R.I.; Németh-Balogh, M.; Paulovicsová, B.; Bergström, A.; Wilcks, A.; Licht, T.R.; et al. Intake of whole apples or clear apple juice has contrasting effects on plasma lipids in healthy volunteers. Eur. J. Nutr. 2013, 52, 1875–1889. [Google Scholar] [CrossRef] [PubMed]
- De Morais, E.C.; Stefanuto, A.; Klein, G.A.; Boaventura, B.C.; de Andrade, F.; Wazlawik, E.; Di Pietro, P.F.; Maraschin, M.; da Silva, E.L. Consumption of yerba mate ( Ilex paraguariensis ) improves serum lipid parameters in healthy dyslipidemic subjects and provides an additional LDL-cholesterol reduction in individuals on statin therapy. J. Agric. Food. Chem. 2009, 57, 8316–8324. [Google Scholar] [CrossRef]
- Lockyer, S.; Rowland, I.; Spencer, J.P.E.; Yaqoob, P.; Stonehouse, W. Impact of phenolic-rich olive leaf extract on blood pressure, plasma lipids and inflammatory markers: A randomised controlled trial. Eur. J. Nutr. 2017, 56, 1421–1432. [Google Scholar] [CrossRef]
- Martinez-Lopez, S.; Sarriá, B.; Sierra-Cinos, J.L.; Goya, L.; Mateos, R.; Bravo, L. Realistic intake of a flavanol-rich soluble cocoa product increases HDL-cholesterol without inducing anthropometric changes in healthy and moderately hypercholesterolemic subjects. Food Funct. 2014, 5, 364–374. [Google Scholar] [CrossRef] [PubMed]
- Arzola-Paniagua, M.A.; García-Salgado López, E.R.; Calvo-Vargas, C.G.; Guevara-Cruz, M. Efficacy of an orlistat-resveratrol combination for weight loss in subjects with obesity: A randomized controlled trial. Obesity 2016, 24, 1454–1463. [Google Scholar] [CrossRef]
- Bo, S.; Ciccone, G.; Castiglione, A.; Gambino, R.; De Michieli, F.; Villois, P.; Durazzo, M.; Cavallo-Perin, P.; Cassader, M. Anti-inflammatory and antioxidant effects of resveratrol in healthy smokers a randomized, double-blind, placebo-controlled, cross-over trial. Curr. Med. Chem. 2013, 20, 1323–1331. [Google Scholar] [CrossRef] [PubMed]
- Sarria, B.; Martínez-López, S.; Sierra-Cinos, J.L.; García-Diz, L.; Mateos, R.; Bravo-Clemente, L. Regularly consuming a green/roasted coffee blend reduces the risk of metabolic syndrome. Eur. J. Nutr. 2018, 57, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Morel, S.; Leahy, J.; Fournier, M.; Lamarche, B.; Garofalo, C.; Grimard, G.; Poulain, F.; Delvin, E.; Laverdière, C.; Krajinovic, M.; et al. Lipid and lipoprotein abnormalities in acute lymphoblastic leukemia survivors. J. Lipid Res. 2017, 58, 982–993. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yuan, W.; Fang, J.; Wang, W.; He, P.; Lei, J.; Wang, C. Efficacy of Resveratrol Supplementation against Non-Alcoholic Fatty Liver Disease: A Meta-Analysis of Placebo-Controlled Clinical Trials. PLoS ONE 2016, 11, e0161792. [Google Scholar] [CrossRef]
- Iannelli, P.; Zarrilli, V.; Varricchio, E.; Tramontano, D.; Mancini, F.P. The dietary antioxidant resveratrol affects redox changes of PPARalpha activity. Nutr. Metab. Cardiovasc. Dis. 2007, 17, 247–256. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, J.; Ungvari, Z.; Zhang, C. Resveratrol improves endothelial function: Role of TNF{alpha} and vascular oxidative stress. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 1164–1171. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.Y.; Hsiao, G.; Liu, C.L.; Fong, T.H.; Lin, K.H.; Chou, D.S.; Sheu, J.R. Inhibitory mechanisms of resveratrol in platelet activation: Pivotal roles of p38 MAPK and NO/cyclic GMP. Br. J. Haematol. 2007, 139, 475–485. [Google Scholar] [CrossRef] [PubMed]
- Rivera, L.; Morón, R.; Zarzuelo, A.; Galisteo, M. Long-term resveratrol administration reduces metabolic disturbances and lowers blood pressure in obese Zucker rats. Biochem. Pharmacol. 2009, 77, 1053–1063. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Castillejo, S.; Valls, R.M.; Castañer, O.; Rubió, L.; Catalán, Ú.; Pedret, A.; Macià, A.; Sampson, M.L.; Covas, M.I.; Fitó, M.; et al. Polyphenol rich olive oils improve lipoprotein particle atherogenic ratios and subclasses profile: A randomized, crossover, controlled trial. Mol. Nutr. Food. Res. 2016, 60, 1544–1554. [Google Scholar] [CrossRef]
- Covas, M.I.; Nyyssönen, K.; Poulsen, H.E.; Kaikkonen, J.; Zunft, H.J.; Kiesewetter, H.; Gaddi, A.; de la Torre, R.; Mursu, J.; Bäumler, H.; et al. The effect of polyphenols in olive oil on heart disease risk factors: A randomized trial. Ann. Intern. Med. 2006, 145, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Devaraj, S.; Vega-López, S.; Kaul, N.; Schönlau, F.; Rohdewald, P.; Jialal, I. Supplementation with a pine bark extract rich in polyphenols increases plasma antioxidant capacity and alters the plasma lipoprotein profile. Lipids 2002, 37, 931–934. [Google Scholar] [CrossRef] [PubMed]
- Baba, S.; Natsume, M.; Yasuda, A.; Nakamura, Y.; Tamura, T.; Osakabe, N.; Kanegae, M.; Kondo, K. Plasma LDL and HDL cholesterol and oxidized LDL concentrations are altered in normo- and hypercholesterolemic humans after intake of different levels of cocoa powder. J. Nutr. 2007, 137, 1436–1441. [Google Scholar] [CrossRef]
- Souza, S.J.; Petrilli, A.A.; Teixeira, A.M.; Pontilho, P.M.; Carioca, A.A.; Luzia, L.A.; Souza, J.M.; Damasceno, N.R.; Segurado, A.A.; Rondó, P.H. Effect of chocolate and mate tea on the lipid profile of individuals with HIV/AIDS on antiretroviral therapy: A clinical trial. Nutrition 2017, 43–44, 61–68. [Google Scholar] [CrossRef]
- Ruiz-Roso, B.; Quintela, J.C.; de la Fuente, E.; Haya, J.; Pérez-Olleros, L. Insoluble carob fiber rich in polyphenols lowers total and LDL cholesterol in hypercholesterolemic sujects. Plant Foods Hum. Nutr. 2010, 65, 50–56. [Google Scholar] [CrossRef]
- Trautwein, E.A.; Du, Y.; Meynen, E.; Yan, X.; Wen, Y.; Wang, H.; Molhuizen, H.O. Purified black tea theaflavins and theaflavins/catechin supplements did not affect serum lipids in healthy individuals with mildly to moderately elevated cholesterol concentrations. Eur. J. Nutr. 2010, 49, 27–35. [Google Scholar] [CrossRef]
- Dash, S.; Xiao, C.; Morgantini, C.; Szeto, L.; Lewis, G.F. High-dose resveratrol treatment for 2 weeks inhibits intestinal and hepatic lipoprotein production in overweight/obese men. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 2895–2901. [Google Scholar] [CrossRef]
- Novotny, J.A.; Baer, D.J.; Khoo, C.; Gebauer, S.K.; Charron, C.S. Cranberry juice consumption lowers markers of cardiometabolic risk, including blood pressure and circulating C-reactive protein, triglyceride, and glucose concentrations in adults. J. Nutr. 2015, 145, 1185–1193. [Google Scholar] [CrossRef] [PubMed]
- Gliozzi, M.; Walker, R.; Muscoli, S.; Vitale, C.; Gratteri, S.; Carresi, C.; Musolino, V.; Russo, V.; Janda, E.; Ragusa, S. Bergamot polyphenolic fraction enhances rosuvastatin-induced effect on LDL-cholesterol, LOX-1 expression and protein kinase B phosphorylation in patients with hyperlipidemia. Int. J. Cardiol. 2013, 170, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Upadya, H.; Prabhu, S.; Prasad, A.; Subramanian, D.; Gupta, S.; Goel, A. A randomized, double blind, placebo controlled, multicenter clinical trial to assess the efficacy and safety of Emblica officinalis extract in patients with dyslipidemia. BMC Complement. Altern. Med. 2019, 19, 27. [Google Scholar] [CrossRef]
- Kardum, N.; Milovanović, B.; Šavikin, K.; Zdunić, G.; Mutavdžin, S.; Gligorijević, T.; Spasić, S. Beneficial Effects of Polyphenol-Rich Chokeberry Juice Consumption on Blood Pressure Level and Lipid Status in Hypertensive Subjects. J. Med. Food 2015, 18, 1231–1238. [Google Scholar] [CrossRef]
- Rahbar, A.R.; Mahmoudabadi, M.M.; Islam, M.S. Comparative effects of red and white grapes on oxidative markers and lipidemic parameters in adult hypercholesterolemic humans. Food Funct. 2015, 6, 1992–1998. [Google Scholar] [CrossRef]
- Mansur, A.P.; Roggerio, A.; Goes, M.F.S.; Avakian, S.D.; Leal, D.P.; Maranhão, R.C.; Strunz, C.M.C. Serum concentrations and gene expression of sirtuin 1 in healthy and slightly overweight subjects after caloric restriction or resveratrol supplementation: A randomized trial. Int. J. Cardiol. 2017, 227, 788–794. [Google Scholar] [CrossRef]
- Chachay, V.S.; Macdonald, G.A.; Martin, J.H.; Whitehead, J.P.; O’Moore-Sullivan, T.M.; Lee, P.; Franklin, M.; Klein, K.; Taylor, P.J.; Ferguson, M.; et al. Resveratrol does not benefit patients with nonalcoholic fatty liver disease. Clin. Gastroenterol. Hepatol. 2014, 12, e1–e6. [Google Scholar] [CrossRef]
- Morissette, A.; Kropp, C.; Songpadith, J.P.; Junges Moreira, R.; Costa, J.; Mariné-Casadó, R.; Pilon, G.; Varin, T.V.; Dudonné, S.; Boutekrabt, L.; et al. Blueberry proanthocyanidins and anthocyanins improve metabolic health through a gut microbiota-dependent mechanism in diet-induced obese mice. Am. J. Physiol. Endocrinol. Metab. 2020, 318, E965–E980. [Google Scholar] [CrossRef] [PubMed]
- Anhe, F.F.; Varin, T.V.; Le Barz, M.; Desjardins, Y.; Levy, E.; Roy, D.; Marette, A. Gut Microbiota Dysbiosis in Obesity-Linked Metabolic Diseases and Prebiotic Potential of Polyphenol-Rich Extracts. Curr. Obes. Rep. 2015, 4, 389–400. [Google Scholar] [CrossRef]
- Anhe, F.F.; Roy, D.; Pilon, G.; Dudonné, S.; Matamoros, S.; Varin, T.V.; Garofalo, C.; Moine, Q.; Desjardins, Y.; Levy, E.; et al. A polyphenol-rich cranberry extract protects from diet-induced obesity, insulin resistance and intestinal inflammation in association with increased Akkermansia spp. population in the gut microbiota of mice. Gut 2015, 64, 872–883. [Google Scholar] [CrossRef]
- Anhe, F.F.; Nachbar, R.T.; Varin, T.V.; Vilela, V.; Dudonné, S.; Pilon, G.; Fournier, M.; Lecours, M.A.; Desjardins, Y.; Roy, D.; et al. A polyphenol-rich cranberry extract reverses insulin resistance and hepatic steatosis independently of body weight loss. Mol. Metab. 2017, 6, 1563–1573. [Google Scholar] [CrossRef]
- Vidal, R.; Hernandez-Vallejo, S.; Pauquai, T.; Texier, O.; Rousset, M.; Chambaz, J.; Demignot, S.; Lacorte, J.M. Apple procyanidins decrease cholesterol esterification and lipoprotein secretion in Caco-2/TC7 enterocytes. J. Lipid Res. 2005, 46, 258–268. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, A.; Natsume, M.; Osakabe, N.; Kawahata, K.; Koga, J. Cacao polyphenols influence the regulation of apolipoprotein in HepG2 and Caco2 cells. J. Agric. Food. Chem. 2011, 59, 1470–1476. [Google Scholar] [CrossRef]
- Bo, S.; Ponzo, V.; Ciccone, G.; Evangelista, A.; Saba, F.; Goitre, I.; Procopio, M.; Pagano, G.F.; Cassader, M.; Gambino, R. Six months of resveratrol supplementation has no measurable effect in type 2 diabetic patients. A randomized, double blind, placebo-controlled trial. Pharmacol. Res. 2016, 111, 896–905. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.C.; Kim, S.H.; Park, Y.; Lee, B.H.; Hwang, H.J. Effects of 12-week oral supplementation of Ecklonia cava polyphenols on anthropometric and blood lipid parameters in overweight Korean individuals: A double-blind randomized clinical trial. Phytother. Res. 2012, 26, 363–368. [Google Scholar] [CrossRef]
- Millar, C.L.; Duclos, Q.; Garcia, C.; Norris, G.H.; Lemos, B.S.; DiMarco, D.M.; Fernandez, M.L.; Blesso, C.N. Effects of Freeze-Dried Grape Powder on High-Density Lipoprotein Function in Adults with Metabolic Syndrome: A Randomized Controlled Pilot Study. Metab. Syndr. Relat. Disord. 2018, 16, 464–469. [Google Scholar] [CrossRef]
- Annuzzi, G.; Bozzetto, L.; Costabile, G.; Giacco, R.; Mangione, A.; Anniballi, G.; Vitale, M.; Vetrani, C.; Cipriano, P.; Della Corte, G.; et al. Diets naturally rich in polyphenols improve fasting and postprandial dyslipidemia and reduce oxidative stress: A randomized controlled trial. Am. J. Clin. Nutr. 2014, 99, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Betts, N.M.; Ortiz, J.; Simmons, B.; Wu, M.; Lyons, T.J. Low-energy cranberry juice decreases lipid oxidation and increases plasma antioxidant capacity in women with metabolic syndrome. Nutr. Res. 2011, 31, 190–196. [Google Scholar] [CrossRef]
- Paquette, M.; Medina Larqué, A.S.; Weisnagel, S.J.; Desjardins, Y.; Marois, J.; Pilon, G.; Dudonné, S.; Marette, A.; Jacques, H. Strawberry and cranberry polyphenols improve insulin sensitivity in insulin-resistant, non-diabetic adults: A parallel, double-blind, controlled and randomised clinical trial. Br. J. Nutr. 2017, 117, 519–531. [Google Scholar] [CrossRef]
- Chiva-Blanch, G.; Urpi-Sarda, M.; Ros, E.; Valderas-Martinez, P.; Casas, R.; Arranz, S.; Guillén, M.; Lamuela-Raventós, R.M.; Llorach, R.; Andres-Lacueva, C.; et al. Effects of red wine polyphenols and alcohol on glucose metabolism and the lipid profile: A randomized clinical trial. Clin. Nutr. 2013, 32, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Shema-Didi, L.; Kristal, B.; Sela, S.; Geron, R.; Ore, L. Does Pomegranate intake attenuate cardiovascular risk factors in hemodialysis patients? Nutr. J. 2014, 13, 18. [Google Scholar] [CrossRef] [PubMed]
- Brull, V.; Burak, C.; Stoffel-Wagner, B.; Wolffram, S.; Nickenig, G.; Müller, C.; Langguth, P.; Alteheld, B.; Fimmers, R.; Naaf, S. Effects of a quercetin-rich onion skin extract on 24 h ambulatory blood pressure and endothelial function in overweight-to-obese patients with (pre-)hypertension: A randomised double-blinded placebo-controlled cross-over trial. Br. J. Nutr. 2015, 114, 1263–1277. [Google Scholar] [CrossRef]
- Pfeuffer, M.; Auinger, A.; Bley, U.; Kraus-Stojanowic, I.; Laue, C.; Winkler, P.; Rüfer, C.E.; Frank, J.; Bösch-Saadatmandi, C.; Rimbach, G.; et al. Effect of quercetin on traits of the metabolic syndrome, endothelial function and inflammation in men with different APOE isoforms. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Timmers, S.; Konings, E.; Bilet, L.; Houtkooper, R.H.; van de Weijer, T.; Goossens, G.H.; Hoeks, J.; van der Krieken, S.; Ryu, D.; Kersten, S.; et al. Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab. 2011, 14, 612–622. [Google Scholar] [CrossRef] [PubMed]
- Kusunoki, M.; Sato, D.; Tsutsumi, K.; Tsutsui, H.; Nakamura, T.; Oshida, Y. Black soybean extract improves lipid profiles in fenofibrate-treated type 2 diabetics with postprandial hyperlipidemia. J. Med. Food 2015, 18, 615–618. [Google Scholar] [CrossRef] [PubMed]
- Zuniga, L.Y.; Aceves-de la Mora, M.C.A.; González-Ortiz, M.; Ramos-Núñez, J.L.; Martínez-Abundis, E. Effect of Chlorogenic Acid Administration on Glycemic Control, Insulin Secretion, and Insulin Sensitivity in Patients with Impaired Glucose Tolerance. J. Med. Food 2018, 21, 469–473. [Google Scholar] [CrossRef]
- Rahmani, S.; Asgary, S.; Askari, G.; Keshvari, M.; Hatamipour, M.; Feizi, A.; Sahebkar, A. Treatment of Non-alcoholic Fatty Liver Disease with Curcumin: A Randomized Placebo-controlled Trial. Phytother. Res. 2016, 30, 1540–1548. [Google Scholar] [CrossRef] [PubMed]
- Cases, J.; Romain, C.; Dallas, C.; Gerbi, A.; Cloarec, M. Regular consumption of Fiit-ns, a polyphenol extract from fruit and vegetables frequently consumed within the Mediterranean diet, improves metabolic ageing of obese volunteers: A randomized, double-blind, parallel trial. Int. J. Food Sci. Nutr. 2015, 66, 120–125. [Google Scholar] [CrossRef]
- Farras, M.; Valls, R.M.; Fernández-Castillejo, S.; Giralt, M.; Solà, R.; Subirana, I.; Motilva, M.J.; Konstantinidou, V.; Covas, M.I.; Fitó, M. Olive oil polyphenols enhance the expression of cholesterol efflux related genes in vivo in humans. A randomized controlled trial. J. Nutr. Biochem. 2013, 24, 1334–1339. [Google Scholar] [CrossRef]
- Richter, C.K.; Skulas-Ray, A.C.; Gaugler, T.L.; Lambert, J.D.; Proctor, D.N.; Kris-Etherton, P.M. Incorporating freeze-dried strawberry powder into a high-fat meal does not alter postprandial vascular function or blood markers of cardiovascular disease risk: A randomized controlled trial. Am. J. Clin. Nutr. 2017, 105, 313–322. [Google Scholar] [CrossRef]
- Ochiai, R.; Sugiura, Y.; Otsuka, K.; Katsuragi, Y.; Hashiguchi, T. Coffee bean polyphenols ameliorate postprandial endothelial dysfunction in healthy male adults. Int. J. Food Sci. Nutr. 2015, 66, 350–354. [Google Scholar] [CrossRef]
- Mathew, A.S.; Capel-Williams, G.M.; Berry, S.E.; Hall, W.L. Acute effects of pomegranate extract on postprandial lipaemia, vascular function and blood pressure. Plant Foods Hum. Nutr. 2012, 67, 351–357. [Google Scholar] [CrossRef]
- Naissides, M.; Mamo, J.C.; James, A.P.; Pal, S. The effect of acute red wine polyphenol consumption on postprandial lipaemia in postmenopausal women. Atherosclerosis 2004, 177, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Guerci, B.; Paul, J.L.; Hadjadj, S.; Durlach, V.; Vergès, B.; Attia, N.; Girard-Globa, A.; Drouin, P. Analysis of the postprandial lipid metabolism: Use of a 3-point test. Diabetes Metab. 2001, 27, 449–457. [Google Scholar] [PubMed]
- O’Doherty, A.F.; Sathyapalan, T.; Rigby, A.S.; Ingle, L.; Carroll, S. The repeatability of the abbreviated (4-h) Oral Fat Tolerance Test and influence of prior acute aerobic exercise. Eur. J. Nutr. 2018, 57, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Harbis, A.; Perdreau, S.; Vincent-Baudry, S.; Charbonnier, M.; Bernard, M.C.; Raccah, D.; Senft, M.; Lorec, A.M.; Defoort, C.; Portugal, H.; et al. Glycemic and insulinemic meal responses modulate postprandial hepatic and intestinal lipoprotein accumulation in obese, insulin-resistant subjects. Am. J. Clin. Nutr. 2004, 80, 896–902. [Google Scholar] [CrossRef] [PubMed]
- Kolovou, G.D.; Mikhailidis, D.P.; Kovar, J.; Lairon, D.; Nordestgaard, B.G.; Ooi, T.C.; Perez-Martinez, P.; Bilianou, H.; Anagnostopoulou, K.; Panotopoulos, G. Assessment and clinical relevance of non-fasting and postprandial triglycerides: An expert panel statement. Curr. Vasc. Pharmacol. 2011, 9, 258–270. [Google Scholar] [CrossRef]
- Burton-Freeman, B.; Linares, A.; Hyson, D.; Kappagoda, T. Strawberry Modulates LDL Oxidation and Postprandial Lipemia in Response to High-Fat Meal in Overweight Hyperlipidemic Men and Women. J. Am. Coll. Nutr. 2010, 29, 46–54. [Google Scholar] [CrossRef]
- Basu, A.; Betts, N.M.; Leyva, M.J.; Fu, D.; Aston, C.E.; Lyons, T.J. Acute Cocoa Supplementation Increases Postprandial HDL Cholesterol and Insulin in Obese Adults with Type 2 Diabetes after Consumption of a High-Fat Breakfast. J. Nutr. 2015, 145, 2325–2332. [Google Scholar] [CrossRef] [PubMed]
- Natella, F.; Macone, A.; Ramberti, A.; Forte, M.; Mattivi, F.; Matarese, R.M.; Scaccini, C. Red wine prevents the postprandial increase in plasma cholesterol oxidation products: A pilot study. Br. J. Nutr. 2011, 105, 1718–1723. [Google Scholar] [CrossRef][Green Version]
- Hutchison, A.J. Oral phosphate binders. Kidney Int. 2009, 75, 906–914. [Google Scholar] [CrossRef] [PubMed]
- Del Bo, C.; Bernardi, S.; Marino, M.; Porrini, M.; Tucci, M.; Guglielmetti, S.; Cherubini, A.; Carrieri, B.; Kirkup, B.; Kroon, P. Systematic Review on Polyphenol Intake and Health Outcomes: Is there Sufficient Evidence to Define a Health-Promoting Polyphenol-Rich Dietary Pattern? Nutrients 2019, 11, 1355. [Google Scholar]
- Grosso, G.; Micek, A.; Godos, J.; Pajak, A.; Sciacca, S.; Galvano, F.; Giovannucci, E.L. Dietary Flavonoid and Lignan Intake and Mortality in Prospective Cohort Studies: Systematic Review and Dose-Response Meta-Analysis. Am. J. Epidemiol. 2017, 185, 1304–1316. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the substantiation of a health claim related to polyphenols in olive and maintenance of normal blood HDL cholesterol concentrations (ID 1639, further assessment) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2012, 10, e2848. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the substantiation of a health claim related to cocoa flavanols and maintenance of normal endothelium-dependent vasodilation pursuant to Article 13(5) of Regulation (EC) No 1924/2006. EFSA J. 2012, 10, 2809. [Google Scholar] [CrossRef]
- Bhagwat, S.; Haytowitz, D.B.; Holden, J.M. USDA Database for the Flavonoid Content of Selected Foods. 2013. Available online: https://www.ars.usda.gov/arsuserfiles/80400525/data/flav/flav_r03-1.pdf (accessed on 22 November 2020).




| Type | Name | Molecular Defect | Lipoprotein Elevated | Clinical Features | Incidence |
|---|---|---|---|---|---|
| 1 | Familial Hyperchylomicronemia | LPL, Apo C-II | CM | Juvenile or early adulthood onset; Eruptive xanthomas; Lipemia retinalis; Pancreatitis; Hepatosplenomegaly; Dyspnea; Lymphadenopathy; Neurologic dysfunction | 1:1,000,000 |
| 2a | Familial Hypercholesterolemia | a. LDLR b. Apo B-100 c. PCSK9 | LDL | Onset at all ages; Tendon xanthomas, Arthralgia; Xanthelasmas; Corneal arcus | a. 1:500 b. <1:1000 c. 1:1,000,000 |
| 2b | Combined HLP | Polygenetic | LDL VLDL | CVD | 1:50–1:200 |
| 3 | Dysbetalipoproteinemia | Apo E | IDL CM-remnants | Palmar xanthomas; CVD | 1:1000–1:5000 |
| 4 | Primary or simple hypertriglyceridemia | Unknown | VLDL | Adult onset; Eruptive xanthomas; Hepatosplenomegaly; Hyperglycemia; Hyperuricemia | 1:50–1:100 |
| 5 | Mixed hypertriglyceridemia | Unknown | CM VLDL | Eruptive xanthomas; Pancreatitis; CVD | Rare |
| Polyphenols | Protocol | Participants | Variation of Lipid Profile 1 | Reference | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dietary Source (Main PLP) 2 | Dosage (mg/Day) | Matrix | Intake Repartition | Length S.D. (Days) | n (Female) | Age 3 (Years) | D.-O. (%) | TG | TC | LDL-C | HDL-C | |
| Red grape (anthocyanidins, quercetin, myricetin) | 640 | Diet (drink) | Bid | 14 P | 15 (7) | 34.4 ± 3.3 | 10 | ↑19% | ↓6% * | ↓13% * | ↑16% * | [89] |
| Potato (anthocyanidins) | 288 | Diet (whole food) | Die | 14 CO | 14 (8) | 33.5 ± 2.9 | 0 | ↓11% * | ↑9% | ↓11% * | NV | [137] |
| Shampion apple (quercetin, epicatechin) + pectin | 75 | Diet (apple pomace) | Die | 28 CO | 23 (14) | 36.2 ± 3.7 | 32 | ↓11% | ↓5% * | ↓10% * | NV | [143] |
| Shampion apple (Procyanidin, Epicatechin) + pectin | 239 | Diet (whole apple) | Die | 28 CO | 23 (14) | 36.2 ± 3.7 | 32 | ↓7% * | ↓7% * | ↓8% * | ↓6% | [143] |
| Shampion apple (Procyanidin, chlorogenic acid) | 145 | Diet (cloudy apple juice) | Die | 28 CO | 23 (14) | 36.2 ± 3.7 | 32 | ↑1% | ↓3% * | ↓4% * | NV | [143] |
| Shampion apple (Procyanidin, chlorogenic acid) | 108 | Diet (clear apple juice) | Die | 28 CO | 23 (14) | 36.2 ± 3.7 | 32 | ↑4% | ↑2% * | ↑6% * | ↓1% | [143] |
| Yerba mate tea (green or roasted) (cholorogenic acid, 4,5-dicaffeolquinic acid, gallocatechin) | 3589 | Diet (drink) | Tid | 40 P | 15 (14) | 42.0 ± 3.2 | 11 | NV | ↓3% * | ↓7% * | ↑2% | [144] |
| Yerba mate tea (green or roasted) (cholorogenic acid, 4,5-dicaffeolquinic acid, gallocatechin) | 3589 | Diet (drink) | Tid | 20 P | 15 (14) | 42.0 ± 3.2 | 11 | ↑13% | ↓2% | ↓9% * | ↑4% | [144] |
| Olive leaf extract (oleuropein) | 167 | Liquid supplement | Bid | 42 CO | 60 (0) | 45.3 ± 1.6 | 2 | ↓12% * | ↓6% * | ↓6% * | ↓4% | [145] |
| Chocolate (flavanol, epicatechin) + fibers | 45.3 | Diet (drink) | Bid | 28 CO | 24 (13) | 27.0 ± 4.8 | 12 | ↓2% | ↑4% | ↑1% | ↑16% * | [146] |
| Resveratrol | 150 | Capsule | Die | 30 CO | 15 (12) | 38.2 ± 2.1 | 18 | ↓1% | ↑2% | ↑2% | ↑1% | [85] |
| Resveratrol (+300mg Orlistat die) | 300 | Capsule | Tid | 168 P | 24 (21) | 40.9 ± 1.6 | 48 | ↓7% | N/A | N/A | N/A | [147] |
| Resveratrol | 300 | Capsule | Tid | 168 P | 15 (12) | 33.7 ± 2.0 | 48 | ↑10% | N/A | N/A | N/A | [147] |
| Resveratrol | 500 | Capsule | Die | 30 CO | 49 (42) | 35.9 ± 1.6 | 0 | ↓0.4% * | NV | N/A | ↓1% | [148] |
| Coffee (hydroxycinnamic acids, methylxanthines) | 510.6 | Diet (drink) | Tid | 56 CO | 25 (15) | 26.2 ± 1.4 | 4 | NV | N/A | N/A | ↑4% | [149] |
| Polyphenol | Length of Chronic Intake (Days; if Available) 1 | Composition of High-Fat Meal | Length of Challenge (Hours) | Participants N (Female); Baseline Characteristics | Reported Effects | Reference | |||
|---|---|---|---|---|---|---|---|---|---|
| Dietary Source | Dosage (mg) | Matrix | Energy (kcal) | Fat (g) | |||||
| Quercetin dihydrates | 150 | Capsule | 56 | N/A | 60 | 8 | 19 (0) MetS, ApoE3 homozygotes | ↓11% * of AUC 0–4 h—TG vs placebo; No effect overall on other lipid parameters, glucose and insulin levels. | [186] |
| Quercetin dihydrates | 150 | Capsule | 56 | N/A | 60 | 8 | 30 (0) MetS, ApoE3/E4 heterozygotes | ↓11% * AUC 0–4 h—TG vs placebo; No effect overall on other lipid parameters, glucose and insulin levels. | [186] |
| Tea, coffee, chocolate, fruits, olive oil | 2903 | Diet | 21 | 1000 | N/A | 6 | 20 (11) MetS | ↓39% * AUC 0–6 h—TG vs baseline; ↓39% * AUC 0–6 h—VLDL-TG ↓90% * AUC 0–6 h—VLDL-TC ↓81% * AUC 0–6 h—Apo B-48; No effect on CM composition. | [180] |
| Resveratrol | 1500 | Capsule | 14 | N/A | 49% | 10 | 8 (0) DLP | ↓22% * Apo B-48 and ↓27% * ApoB100 production rates; No effect on plasma TG, TRL-TG, glucose and insulin levels. | [163] |
| Strawberry | 338 | Liquid supplement | 42 | 960 | 31 | 6 | 24 (14) DLP | ↓5% * TG, ↓4% * TC, ↓4% * LDL-C ↓3% * HDL-C ↓48% oxLDL vs placebo. | [201] |
| Strawberry | 338 | Liquid supplement | 0 | 960 | 31 | 6 | 24 (14) DLP | ↓3% * TG; ↑1% * LDL-C; ↓1% * HDL-C; ↓115% * oxLDL vs placebo; No effect on TC. | [201] |
| Cocoa | 960 | Liquid supplement | 0 | 766 | 50 | 6 | 18 (14) T2D | ↑2% * HDL-C; ↑overall insulin levels * vs placebo; No effect overall on other lipid parameters and glucose levels. | [202] |
| Red wine (no alcohol) | 880 | Liquid supplement | 0 | N/A | 25 | 7 | 17 (17) DLP | No effect on TG, Apo B-48 and insulin levels vs placebo. | [196] |
| Olive oil | 8 | Diet | 0 | N/A | 27 | 5 | 13 (6) Healthy | ↑15% * TG; ↓9% * oxLDL; ↓7% * glucose vs baseline; no other effect on lipid, OxS or inflammation parameters. | [192] |
| Olive oil | 26 | Diet | 0 | N/A | 27 | 5 | 13 (6) Healthy | ↑24% * TG; ↓7% * oxLDL; ↓6% * glucose vs baseline; no other effect on lipid, OxS or inflammation parameters. | [192] |
| Pomegranate | 652–948 | Liquid supplement | 0 | N/A | 50 | 2 | 19 (0) Healthy | No effect overall on lipid parameters. | [195] |
| Red wine | 561 | Diet | 0 | N/A | 26 | 3 | 12 (6) Healthy | ↑15% * TG ↓lipid hydroperoxides *, oxyCHOLs *, 7-ketoCHOL * and 7-β-hydroxyCHOL * vs placebo. | [203] |
| Strawberry | 196 | Powder | 0 | N/A | 50 | 4 | 30 (13) DLP | No effect overall on TG, glucose, insulin and OxS levels. | [193] |
| Coffee | 600 | Liquid supplement | 0 | N/A | 30 | 6 | 13 (0) Healthy | No effect overall on TG, TC, glucose, insulin, OxS and inflammation levels. | [194] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feldman, F.; Koudoufio, M.; Desjardins, Y.; Spahis, S.; Delvin, E.; Levy, E. Efficacy of Polyphenols in the Management of Dyslipidemia: A Focus on Clinical Studies. Nutrients 2021, 13, 672. https://doi.org/10.3390/nu13020672
Feldman F, Koudoufio M, Desjardins Y, Spahis S, Delvin E, Levy E. Efficacy of Polyphenols in the Management of Dyslipidemia: A Focus on Clinical Studies. Nutrients. 2021; 13(2):672. https://doi.org/10.3390/nu13020672
Chicago/Turabian StyleFeldman, Francis, Mireille Koudoufio, Yves Desjardins, Schohraya Spahis, Edgard Delvin, and Emile Levy. 2021. "Efficacy of Polyphenols in the Management of Dyslipidemia: A Focus on Clinical Studies" Nutrients 13, no. 2: 672. https://doi.org/10.3390/nu13020672
APA StyleFeldman, F., Koudoufio, M., Desjardins, Y., Spahis, S., Delvin, E., & Levy, E. (2021). Efficacy of Polyphenols in the Management of Dyslipidemia: A Focus on Clinical Studies. Nutrients, 13(2), 672. https://doi.org/10.3390/nu13020672








