Effects of Different Types of Carbohydrates on Arterial Stiffness: A Comparison of Isomaltulose and Sucrose
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Study Design
2.3. Body Composition
2.4. Arterial Stiffness
2.5. Upper Arm and Ankle Blood Pressure
2.6. Heart Rate
2.7. Blood Glucose
2.8. Isomaltulose Solution and Sucrose Solution Ingestion
2.9. Statistical Analysis
3. Results
3.1. Physical Characteristics
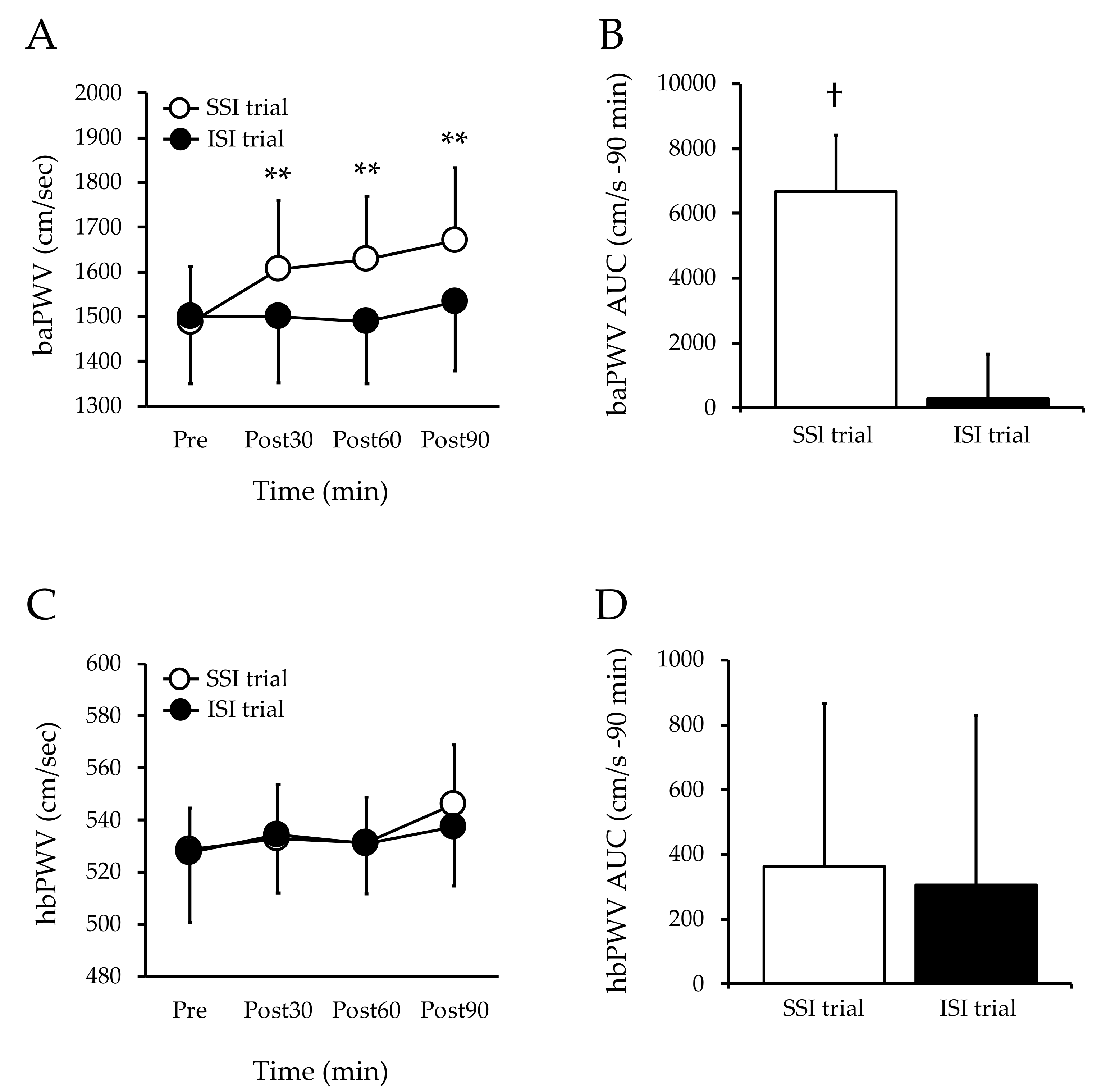
3.2. Arterial Stiffness
3.3. Heart Rate
3.4. Brachial Blood Pressure
3.5. Ankle Blood Pressure
3.6. Blood Glucose
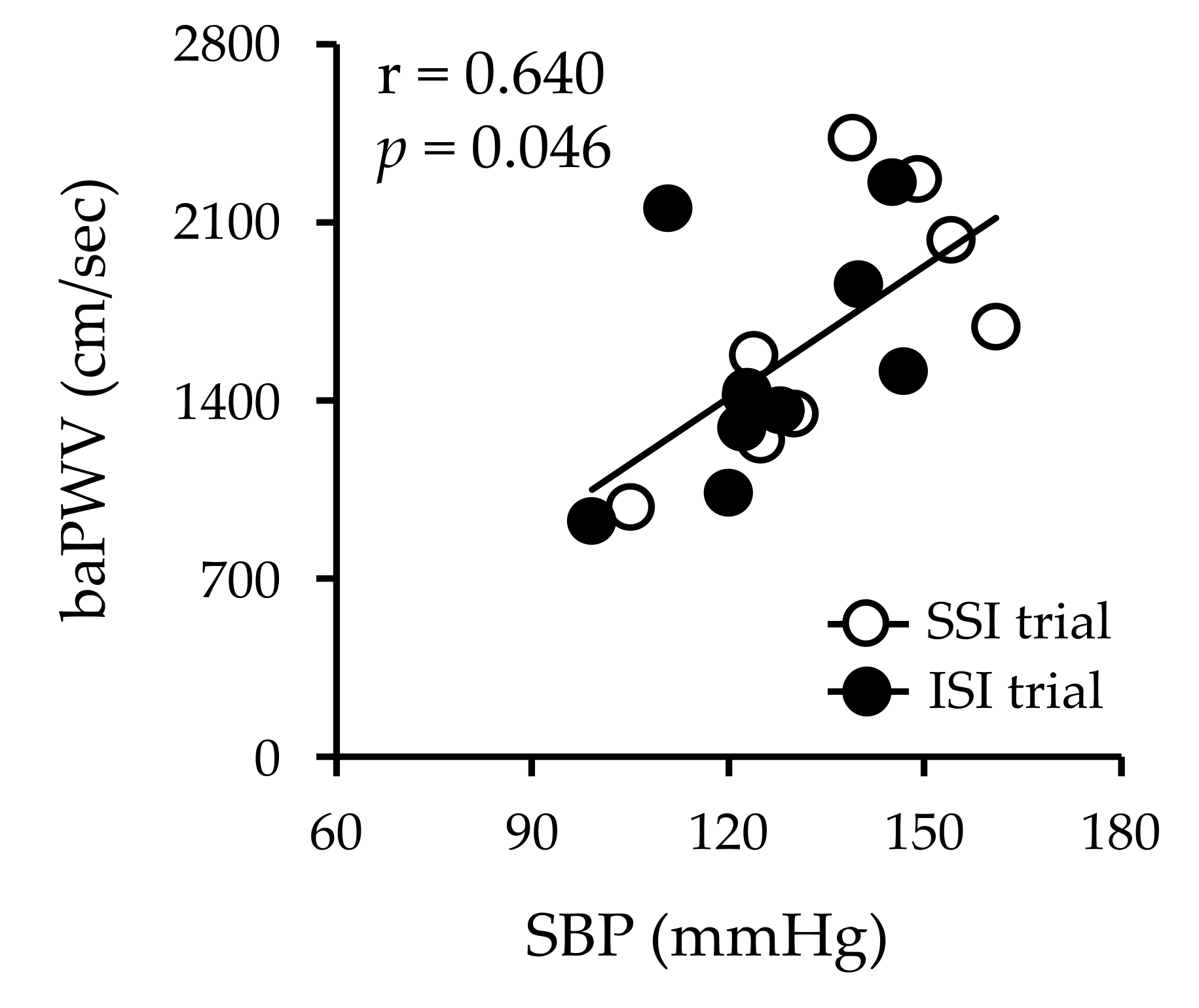
3.7. Arterial Stiffness and Brachial SBP at 90 Min after Sucrose and Isomaltulose Solution Ingestion
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tominaga, M.; Eguchi, H.; Manaka, H.; Igarashi, K.; Kato, T.; Sekikawa, A. Impaired Glucose Tolerance Is a Risk Factor for Cardiovascular Disease, but Not Impaired Fasting Glucose. The Funagata Diabetes Study. Diabetes Care 1999, 22, 920–924. [Google Scholar] [CrossRef] [PubMed]
- DECODE Study Group, the European Diabetes Epidemiology Group. Glucose Tolerance and Cardiovascular Mortality: Comparison of Fasting and 2-Hour Diagnostic Criteria. Arch. Intern. Med. 2001, 161, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Jacome-Sosa, M.; Parks, E.J.; Bruno, R.S.; Tasali, E.; Lewis, G.F.; Schneeman, B.O.; Rains, T.M. Postprandial Metabolism of Macronutrients and Cardiometabolic Risk: Recent Developments, Emerging Concepts, and Future Directions. Adv. Nutr. 2016, 7, 364–374. [Google Scholar] [CrossRef] [PubMed]
- Gordin, D.; Saraheimo, M.; Tuomikangas, J.; Soro-Paavonen, A.; Forsblom, C.; Paavonen, K.; Steckel-Hamann, B.; Vandenhende, F.; Nicolaou, L.; Pavo, I.; et al. Influence of Postprandial Hyperglycemic Conditions on Arterial Stiffness in Patients With Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2016, 101, 1134–1143. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, R.; Sato, K.; Sakazaki, M.; Nagai, Y.; Iwanuma, S.; Ohashi, N.; Hashiguchi, T. Acute Effects of Difference in Glucose Intake on Arterial Stiffness in Healthy Subjects. Cardiol. J. 2021, 28, 446–452. [Google Scholar] [CrossRef]
- Ko, G.T.C.; Wai, H.P.S.; Tang, J.S.F. Effects of Age on Plasma Glucose Levels in Non-Diabetic Hong Kong Chinese. Croat. Med. J. 2006, 47, 709–713. [Google Scholar]
- Tsuboi, A.; Ito, C.; Fujikawa, R.; Yamamoto, H.; Kihara, Y. Association between the Postprandial Glucose Levels and Arterial Stiffness Measured According to the Cardio-Ankle Vascular Index in Non-Diabetic Subjects. Intern. Med. 2015, 54, 1961–1969. [Google Scholar] [CrossRef] [PubMed]
- Arai, H.; Ouchi, Y.; Toba, K.; Endo, T.; Shimokado, K.; Tsubota, K.; Matsuo, S.; Mori, H.; Yumura, W.; Yokode, M.; et al. Japan as the Front-Runner of Super-Aged Societies: Perspectives from Medicine and Medical Care in Japan. Geriatr. Gerontol. Int. 2015, 15, 673–687. [Google Scholar] [CrossRef]
- Kelsch, E.; Diana, J.C.; Burnet, K.; Hanson, E.D.; Fryer, S.F.; Credeur, D.P.; Stone, K.J.; Stoner, L. Arterial Stiffness Responses to Prolonged Sitting Combined with a High-Glycemic-Index Meal: A Double-Blind, Randomized Crossover Trial. J. Appl. Physiol. (1985) 2021, 131, 229–237. [Google Scholar] [CrossRef]
- Gaesser, G.A.; Rodriguez, J.; Patrie, J.T.; Whisner, C.M.; Angadi, S.S. Effects of Glycemic Index and Cereal Fiber on Postprandial Endothelial Function, Glycemia, and Insulinemia in Healthy Adults. Nutrients 2019, 11, 2387. [Google Scholar] [CrossRef]
- Sanchez-Aguadero, N.; Patino-Alonso, M.C.; Mora-Simon, S.; Gomez-Marcos, M.A.; Alonso-Dominguez, R.; Sanchez-Salgado, B.; Recio-Rodriguez, J.I.; Garcia-Ortiz, L. Postprandial Effects of Breakfast Glycemic Index on Vascular Function among Young Healthy Adults: A Crossover Clinical Trial. Nutrients 2017, 9, 712. [Google Scholar] [CrossRef]
- Low, N.H.; Nelson, D.L.; Sporns, P. Carbohydrate Analysis of Western Canadian Honeys and Their Nectar Sources to Determine the Origin of Honey Oligosaccharides. J. Apic. Res. 1988, 27, 245–251. [Google Scholar] [CrossRef]
- Kawai, K.; Yoshikawa, H.; Murayama, Y.; Okuda, Y.; Yamashita, K. Usefulness of Palatinose as a Caloric Sweetener for Diabetic Patients. Horm. Metab. Res. 1989, 21, 338–340. [Google Scholar] [CrossRef]
- Kawai, K.; Okuda, Y.; Yamashita, K. Changes in Blood Glucose and Insulin after an Oral Palatinose Administration in Normal Subjects. Endocrinol. Jpn 1985, 32, 933–936. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, R.; Sato, K.; Takahashi, T.; Asaki, K.; Iwanuma, S.; Ohashi, N.; Hashiguchi, T. Arterial Stiffness during Hyperglycemia in Older Adults with High Physical Activity vs Low Physical Activity. J. Clin. Biochem. Nutr. 2019, 65, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, R.; Sato, K.; Takahashi, T.; Asaki, K.; Iwanuma, S.; Ohashi, N.; Hashiguchi, T. Effects of a Short-Term Increase in Physical Activity on Arterial Stiffness during Hyperglycemia. J. Clin. Biochem. Nutr. 2020, 66, 238–244. [Google Scholar] [CrossRef]
- Guideline: Sugars Intake for Adults and Children; WHO Guidelines Approved by the Guidelines Review Committee; World Health Organization: Geneva, Switzerland, 2015; ISBN 978-92-4-154902-8.
- Bonora, E. Postprandial Peaks as a Risk Factor for Cardiovascular Disease: Epidemiological Perspectives. Int. J. Clin. Pract. Suppl. 2002, 5–11. [Google Scholar]
- Wolever, T.M.; Jenkins, D.J.; Ocana, A.M.; Rao, V.A.; Collier, G.R. Second-Meal Effect: Low-Glycemic-Index Foods Eaten at Dinner Improve Subsequent Breakfast Glycemic Response. Am. J. Clin. Nutr. 1988, 48, 1041–1047. [Google Scholar] [CrossRef]
- Fang, F.-S.; Liu, M.-Y.; Cheng, X.-L.; Zhong, W.-W.; Miao, X.-Y.; Li, J.; Li, C.-L.; Tian, H. Insulin Resistance Correlates with the Arterial Stiffness before Glucose Intolerance. Intern. Med. 2014, 53, 189–194. [Google Scholar] [CrossRef][Green Version]
- Yokoyama, H.; Shoji, T.; Kimoto, E.; Shinohara, K.; Tanaka, S.; Koyama, H.; Emoto, M.; Nishizawa, Y. Pulse Wave Velocity in Lower-Limb Arteries among Diabetic Patients with Peripheral Arterial Disease. J. Atheroscler. Thromb. 2003, 10, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, R.; Yoshida, S.; Okamoto, T. Arterial Stiffness after Glucose Ingestion in Exercise-Trained versus Untrained Men. Appl. Physiol. Nutr. Metab. 2015, 40, 1151–1156. [Google Scholar] [CrossRef]
- Tucker, W.J.; Sawyer, B.J.; Jarrett, C.L.; Bhammar, D.M.; Ryder, J.R.; Angadi, S.S.; Gaesser, G.A. High-Intensity Interval Exercise Attenuates but Does Not Eliminate Endothelial Dysfunction after a Fast Food Meal. Am. J. Physiol. Heart. Circ. Physiol. 2018, 314, H188–H194. [Google Scholar] [CrossRef]
- Sugawara, J.; Tanaka, H. Brachial-Ankle Pulse Wave Velocity: Myths, Misconceptions, and Realities. Pulse 2015, 3, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, J.; Tomoto, T.; Tanaka, H. Heart-to-Brachium Pulse Wave Velocity as a Measure of Proximal Aortic Stiffness: MRI and Longitudinal Studies. Am. J. Hypertens 2019, 32, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Gao, H.; Li, X.; Liu, Y.; Wang, M. Correlation between Brachial-Ankle Pulse Wave Velocity and Arterial Compliance and Cardiovascular Risk Factors in Elderly Patients with Arteriosclerosis. Hypertens Res. 2006, 29, 309–314. [Google Scholar] [CrossRef]
- Nakao, M.; Nomura, K.; Karita, K.; Nishikitani, M.; Yano, E. Relationship between Brachial-Ankle Pulse Wave Velocity and Heart Rate Variability in Young Japanese Men. Hypertens Res. 2004, 27, 925–931. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.L.; Zou, X.; Orr, W.C.; Chen, J.D. Postprandial Changes of Sympathovagal Balance Measured by Heart Rate Variability. Dig. Dis. Sci. 1999, 44, 857–861. [Google Scholar] [CrossRef]
- Takei, Y.; Tomiyama, H.; Tanaka, N.; Yamashina, A. Close Relationship between Sympathetic Activation and Coronary Microvascular Dysfunction during Acute Hyperglycemia in Subjects with Atherosclerotic Risk Factors. Circ. J. 2007, 71, 202–206. [Google Scholar] [CrossRef]
- Mah, E.; Noh, S.K.; Ballard, K.D.; Matos, M.E.; Volek, J.S.; Bruno, R.S. Postprandial Hyperglycemia Impairs Vascular Endothelial Function in Healthy Men by Inducing Lipid Peroxidation and Increasing Asymmetric Dimethylarginine:Arginine. J. Nutr. 2011, 141, 1961–1968. [Google Scholar] [CrossRef]


| Value | |
|---|---|
| Age, years | 62.8 ± 4.4 |
| Height, cm | 162.5 ± 2.9 |
| Weight, kg | 60.9 ± 3.0 |
| BMI, kg/m2 | 23.1 ± 1.1 |
| Body fat, % | 27.7 ± 2.7 |
| Brachial SBP, mmHg | 123.5 ± 6.2 |
| Ankle SBP, mmHg | 153.8 ± 8.6 |
| Heart rate, bpm | 62.0 ± 3.7 |
| Fasting blood glucose, mg/dL | 98.8 ± 4.2 |
| Variable | Trial | Baseline | Post 30 min | Post 60 min | Post 90 min | p-Value (Group) |
|---|---|---|---|---|---|---|
| Brachial SBP, mmHg | SSI trial | 123.5 ± 6.2 | 128.6 ± 6.3 | 131.0 ± 6.1 | 134.4 ± 5.9 * | 0.93 |
| ISI trial | 124.2 ± 4.6 | 127.1 ± 4.1 | 129.4 ± 6.1 | 126.1 ± 5.3 | ||
| Brachial MBP, mmHg | SSI trial | 88.2 ± 2.9 | 90.5 ± 3.2 | 91.5 ± 2.9 | 93.4 ± 3.1 | 0.85 |
| ISI trial | 88.6 ± 2.9 | 89.8 ± 2.7 | 91.8 ± 3.8 | 90.4 ± 3.4 | ||
| Brachial DBP, mmHg | SSI trial | 70.6 ± 2.5 | 71.5 ± 2.3 | 71.7 ± 2.2 | 72.8 ± 3.0 | 0.80 |
| ISI trial | 70.8 ± 2.6 | 71.1 ± 2.6 | 73.0 ± 3.3 | 72.6 ± 3.0 | ||
| Brachial PP, mmHg | SSI trial | 52.9 ± 6.0 | 57.0 ± 5.3 | 59.3 ± 5.7 | 61.6 ± 6.2 * | 0.80 |
| ISI trial | 53.4 ± 3.8 | 56.0 ± 3.7 | 56.4 ± 4.9 | 53.6 ± 4.2 | ||
| HR, beats/min | SSI trial | 62.0 ± 3.7 | 60.8 ± 2.7 | 58.1 ± 3.1 | 58.7 ± 2.5 | 0.50 |
| ISI trial | 58.1 ± 4.1 | 54.7 ± 3.2 | 55.4 ± 2.7 | 57.4 ± 3.0 |
| Variable | Trial | Baseline | Post 30 min | Post 60 min | Post 90 min | p-Value (Group) |
|---|---|---|---|---|---|---|
| Ankle SBP, mmHg | SSI trial | 153.8 ± 8.6 | 160.6 ± 8.8 | 165.3 ± 10.2 | 167.4 ± 8.0 * | 0.93 |
| ISI trial | 147.9 ± 9.9 | 155.1 ± 8.2 | 154.0 ± 8.1 | 155.7 ± 9.6 | ||
| Ankle MBP, mmHg | SSI trial | 99.0 ± 3.1 | 102.4 ± 3.5 | 104.1 ± 3.4 | 106.3 ± 3.2 * | 0.85 |
| ISI trial | 95.4 ± 4.7 | 99.9 ± 3.3 | 99.8 ± 3.8 | 100.0 ± 4.4 | ||
| Ankle DBP, mmHg | SSI trial | 71.6 ± 1.7 | 73.2 ± 1.7 | 73.6 ± 2.0 | 75.8 ± 2.8 | 0.80 |
| ISI trial | 69.2 ± 3.2 | 72.2 ± 2.6 | 72.7 ± 2.9 | 72.2 ± 3.1 | ||
| Ankle PP, mmHg | SSI trial | 82.2 ± 8.8 | 87.4 ± 8.4 | 91.7 ± 10.7 | 91.6 ± 8.5 * | 0.80 |
| ISI trial | 78.7 ± 8.9 | 82.9 ± 8.5 | 81.3 ± 7.6 | 83.4 ± 9.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kobayashi, R.; Sakazaki, M.; Nagai, Y.; Asaki, K.; Hashiguchi, T.; Negoro, H. Effects of Different Types of Carbohydrates on Arterial Stiffness: A Comparison of Isomaltulose and Sucrose. Nutrients 2021, 13, 4493. https://doi.org/10.3390/nu13124493
Kobayashi R, Sakazaki M, Nagai Y, Asaki K, Hashiguchi T, Negoro H. Effects of Different Types of Carbohydrates on Arterial Stiffness: A Comparison of Isomaltulose and Sucrose. Nutrients. 2021; 13(12):4493. https://doi.org/10.3390/nu13124493
Chicago/Turabian StyleKobayashi, Ryota, Miki Sakazaki, Yukie Nagai, Kenji Asaki, Takeo Hashiguchi, and Hideyuki Negoro. 2021. "Effects of Different Types of Carbohydrates on Arterial Stiffness: A Comparison of Isomaltulose and Sucrose" Nutrients 13, no. 12: 4493. https://doi.org/10.3390/nu13124493
APA StyleKobayashi, R., Sakazaki, M., Nagai, Y., Asaki, K., Hashiguchi, T., & Negoro, H. (2021). Effects of Different Types of Carbohydrates on Arterial Stiffness: A Comparison of Isomaltulose and Sucrose. Nutrients, 13(12), 4493. https://doi.org/10.3390/nu13124493






