Abstract
Behavioral disinhibition is observed to be an important characteristic of many neurodevelopmental and psychiatric disorders. Recent studies have linked dietary quality to levels of behavioral inhibition. However, it is currently unclear whether brain factors might mediate this. The current study investigates whether cortical and subcortical brain volumes mediate part of the association between dietary composition and behavioral disinhibition. A total of 15,258 subjects from the UK Biobank project were included in the current study. Dietary composition and behavioral disinhibition were based on Principle Component Analyses of self-reported dietary composition). As a further data reduction step, cortical and subcortical volume segmentations were input into an Independent Component Analysis. The resulting four components were used as mediator variables in the main mediation analyses, where behavioral disinhibition served as the outcome variable and dietary components as predictors. Our results show: (1) significant associations between all dietary components and brain volume components; (2) brain volumes are associated with behavioral disinhibition; (3) the mediation models show that part of the variance in behavioral disinhibition explained by dietary components (for healthy diet, restricted diet, and high-fat dairy diet) is mediated through the frontal-temporal/parietal brain volume component. These results are in part confirming our hypotheses and offer a first insight into the underlying mechanisms linking dietary composition, frontal-parietal brain volume, and behavioral disinhibition in the general adult population.
1. Introduction
Behavioral disinhibition refers to the problematic and uncontrolled expression of impulsive behavior [1] and is observed to be an important characteristic of several neurodevelopmental and psychiatric disorders. Disorders like Attention-Deficit Hyperactivity Disorder (ADHD), Obsessive-Compulsive Disorder (OCD), Tourette’s Disorder, Mania and Substance Use Disorder (SUD) are characterized by a poor ability to control behavior [2,3,4]. Severe behavioral disinhibition is also more generally linked to increased negative health outcomes and increased mortality over the lifespan [5,6], demonstrating the need to study factors underlying behavioral disinhibition at the population level.
A wealth of studies have indicated that lifestyle factors, including dietary composition, may be associated with cognitive performance and mental health factors. Specifically, previous work has largely focused on the effects of a healthy diet and physical fitness on improved cognitive performance and reduced symptoms of major depressive disorder [7,8,9] and anxiety disorders [10,11]. More recently, research has shown neurodevelopmental disorders like ADHD [12,13] or Tourette Disorder [14,15], and impulse control disorders like substance use disorder [16,17] to be also influenced by dietary composition, indicating an effect of diet on a broad range of mental health disorders. We postulate here that behavioral disinhibition may play a key role as an overarching factor linking the association between diet and mental health. Indeed, studies have consistently linked overall dietary quality to levels of behavioral inhibition [18,19,20,21]. In particular, a recent large-scale population-based study using the UKbiobank cohort was able to show associations between behavioral disinhibition and four main dietary composition components, namely overall diet quality, presence of specific dietary restrictions, meat/fish intake, and high-fat dairy intake [22].
A second factor to take into consideration when investigating the link between mental health and diet is the brain. Several studies have established associations between a poor diet and lower brain volumes [23,24,25]. Studies using a Mediterranean diet intervention report larger prefrontal brain regions, crucially implicated in behavioral disinhibition [26], in cross-sectional cohorts [27,28]. Therefore in the current study, we aim to investigate the neurobiological mechanisms underlying the association between diet and behavioral disinhibition by testing the links between diet, brain morphology, and behavioral disinhibition. An extensive body of literature shows that behavioral disinhibition is associated with both structural and functional changes in the brain [29,30,31,32,33,34,35,36]. Specifically, differences in the cortical-striatal pathways associated with cognitive control have been associated with high levels of disinhibited behavior. Both subcortical volumes of the striatum and frontal cortical thickness have been found to be decreased in association with disinhibition [37,38]. Functionally, frontal-parietal and striatal hypoactivation has been found in impulsive subjects during inhibition tasks [31,39,40,41,42,43]. These studies suggest that both diet and disinhibition are associated with the frontal–striatal pathways in the brain, making this a likely target to mediate the association between diet and disinhibition.
The current study, therefore, aims to test whether the association between dietary composition and behavioral disinhibition is mediated by cortical and subcortical brain volumes in a large scale adult population sample (UKbiobank [44]). We hypothesize that (1) dietary composition is associated with brain volumes. (2) Brain volumes and (3) dietary composition are each associated with behavioral disinhibition, and (4) part of the association between dietary composition and behavioral disinhibition is mediated by cortical and subcortical brain volumes, particularly by frontal-parietal and subcortical areas.
2. Methods
2.1. Sample
UK (United Kingdom) Biobank is an open-access, population-based cohort study consisting of in-depth biomedical and health information, as described in [44] or https://www.ukbiobank.ac.uk/ (29 July 2019). Of the full UKBiobank sample, all subjects were included who had segmented T1 MRI data available, as well as the Mental Health Questionnaire (MHQ), the Food Frequency Questionnaire (FFQ), and self-reported fitness measures. Both MRI measurement and MHQ assessment were obtained in a subsample of the full UKBiobank cohort. Importantly, the subsample of UKbiobank with MRI measurement available was designed to be a random selection of the full cohort and did not differ on major demographic variables. A detailed flowchart and description of the subsample inclusion and exclusion criteria can be found [45]. The sample used in the current analyses included a total of 15,258 subjects for which both MRI and MHQ assessments were available (mean age = 55, 7037 males). All analyses were based on a copy of the UKB data downloaded on 29 July 2019.
2.2. Behavioral Disinhibition
Behavioral disinhibition is not a standard measure available in the UKBiobank. Hence, we used a single aggregated measure for disinhibition that has been calculated on a much larger sample of the UKBiobank by [22]. In that study, a principal component analysis (PCA) was performed on all available disinhibition-related items from different questionnaires (e.g., items covering addictions including smoking, risk behaviors such as heavy drinking, self-reported and hospital diagnoses of mental health disorders, self-harm behaviors, and personality questionnaire items, among others. A full list of items is available in Supplementary Tables S1 and S2). To obtain a balanced representation of different manifestations of disinhibition, these items were grouped into nine types of disinhibited behaviors. Schweren et al. [22] performed the PCA based on tetrachoric correlations between these behavioral groups using the psych package in R ( Supplementary Table S3). The single-component model, preferred a priori, presented with no interpretational shortcomings: all behaviors loaded positively on the principal component with factor loadings ranging from 0.335 to 0.708 (Supplementary Table S3). For each subject, a factor score was extracted, with higher scores indicating a higher tendency for disinhibition. We did not re-run this analysis on our subsample but used the factor scores from the original PCA.
2.3. Dietary Components
Dietary composition parameters per subject were obtained from the [22] paper, where dietary composition was calculated based on the 29 items of the Food Frequency Questionnaire (FFQ), a self-report questionnaire assessing the participants’ past-year average food consumption. Individual items of the FFQ are highly correlated, reflecting underlying dietary patterns. To derive these patterns, Schweren et al. [22] performed a PCA with Promax rotation implemented in the psych package in R. The PCA started with one principal component, and new components were added one-by-one. Components were retained when they contributed unique information, were interpretable and plausible, and remained stable upon including additional components to the model. The optimal model contained four dietary components. These were used as input for a PCA, rendering four dietary components: Diet PC1 was associated with healthy foods including vegetables, fruits, whole grain bread, and oily fish, which we will therefore label as the ‘healthy diet’. Diet PC2 reflected specific restrictions in bread, milk, and wheat intake, which will therefore be labeled ‘restricted diet’. Diet PC3 was associated with meat and fish consumption, labeled the ‘meat/fish diet’. Diet PC4 was associated with a specific intake of high-fat dairy products, labeled the ‘high-fat dairy diet’. We did not re-calculate these data in our smaller subsample but used the factor loadings from [22] as input in our analyses (see Supplementary Tables S4 and S5 for additional details on FFQ items and PCA factor loadings).
2.4. Structural Brain Measures
For all subjects with available MRI scans in UKBiobank, T1 images have been automatically segmented by UK Biobank based on the Harvard–Oxford atlas using FLS-FIRST. Cortical volume measures of the resulting 47 cortical regions of interest (ROIs) were included, as well as those of the 6 subcortical segmentations (amygdala, caudate, hippocampus, pallidum, putamen, and thalamus). For all ROIs, left and right hemisphere volumes were averaged to obtain one input measure per region. Outliers were removed at ±3×SD.
2.5. Independent Component Analysis of Structural Brain Measures
To reduce the number of brain measures while maintaining meaningful mediators, we carried out data-reduction in the form of an Independent Component Analysis (ICA), using the z-transformed of the 53 standard subcortical and cortical volume measures as input.
The number of components for this ICA was started at 2, and components were added based on their interpretability, uniqueness, and stability, with the underlying assumption that all resulting ICs should be meaningful as input in the upcoming mediation analysis. This led to the final selection of an ICA with 4 components since this selection included maximally dissociable individual PCs without including any ICs that could not be functionally interpreted (see Table 1 for the resulting factor loadings for the 4 factor ICA solution. See Figure 1A–D for the visualized factor loadings per IC.).

Table 1.
Factor loadings of all cortical and subcortical structural brain volume segmentations resulting from Independent Component Analysis. Loadings higher than 0.3 are indicated in bold and are plotted in Figure 1A–D.
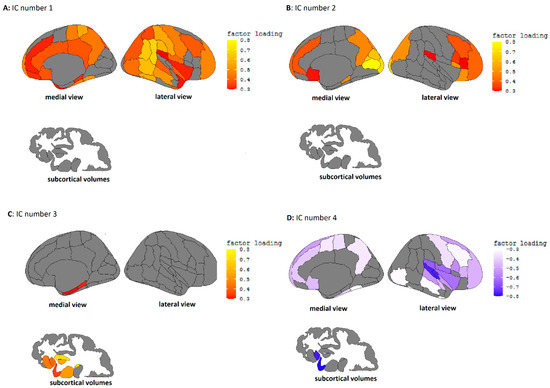
Figure 1.
(A). Independent component 1, characterized by high factor loadings in the temporal/parietal and frontal cortex. (B). Independent component 2, characterized by high loadings in the occipital and frontal cortex. (C). Independent component 3, characterized by high loading in the temporal cortex and subcortical volumes. (D). Independent component 4, characterized by high loadings in the temporal cortex.
Independent component 1 (IC1) could be characterized by high loadings in frontal and cingulate areas, as well as around the temporal/parietal cortex. IC2 showed high loadings in frontal and occipital cortices. IC3 was characterized by positive loadings mainly in the subcortical areas, and IC4 showed high negative loadings on frontal/temporal areas, particularly around the superior temporal cortex.
2.6. Covariates
Age, gender, social-economic status (SES) (educational attainment (years of education), total household income, Indices of Multiple Deprivation (IMD), employment (employed/unemployed)) and ethnicity (white/non-white) were used as covariates. To account for a generally healthier lifestyle outside of dietary composition, we also included BMI and moderate-to-vigorous physical activity (MVPA) as covariates, in line with the previous publication by [22]. MVPA was based on the Recent Physical Activity Questionnaire (RPAQ) [46] which included a self-reported number of days per week that subjects performed physical activity, as well as the number of minutes that they performed this activity on these days. Taken together, this gives us the total minutes of MVPA for the last week for each subject. To account for outliers, the highest 2.5% of MVPA values were removed from the analysis.
2.7. Mediation Analyses
Using the LAVAAN package in R, four separate multiple mediation models were constructed (see Figure 2). Each model used one dietary component as the predictor variable, the behavioral disinhibition factor as the dependent variable, and all four brain volume ICs as mediator variables, thereby testing the full mediation of all the brain measures for each dietary component within the same model. We report 4 pathways within each model, namely: pathway A from dietary composition to the brain, path B from the brain to behavioral disinhibition, C between dietary composition and behavioral disinhibition, and D as the indirect pathway, which is part of the total effect on behavioral disinhibition mediated through the brain. For each pathway, we report the standard Beta coefficient to indicate the direction and size of the effect, as well as FDR (false-discovery rate) corrected p-values. Within the mediation models, all the above-mentioned covariates were included to correct for these variables on all pathways.
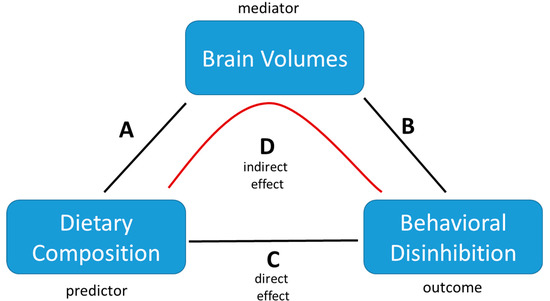
Figure 2.
Schematic illustration of the multiple mediation model employed to analyze the associations between dietary composition, brain volumes, and behavioral disinhibition. The model tests for the significance of any of the direct effects (A–C) as well as whether the indirect effect of (D) explains (part of) the total effect. Covariates are not depicted but were included in all analyses (A–D) as described in the text.
3. Results
3.1. Demographic and Lifestyle Factors
The association between demographic factors and dininhibition are displayed in Table 2. Disinhibition was higher in men compared to women (B = 0.145, p < 0.001). Disinhibition was significantly associated with younger age (B = −0.204, p < 0.001), unemployment (B = 0.23, p = 0.014), white ethnicity (B = 0.12, p = 0.035), and neighborhood deprivation (B = 0.09, p < 0.01). MVPA was associated with higher disinhibition (B=0.029, p = 0.003), as was BMI (B = 0.031, p = 0.002). Disinhibition was not associated with adjusted income and years of education.

Table 2.
Sample characteristics and associations with Behavioral Disinhibition. Unemployment refers to current employment status (0 = no employment, 1 = currently employed). Ethnicity was coded as 0 = white, 1 = non-white. IMD = Indices of Multiple Deprivation. MVPA = Moderate/Vigorous Physical Activity.
3.2. Multiple Mediation Models
The first multiple mediation model used healthy diet as the predictor and IC1–4 as the mediator variables (see Table 3). This model showed a significant association between healthy diet and all four brain ICs (range B estimates A1–A4 = −1.05–0.064; p-values < 0.001), as well as a significant association between brain IC1 and disinhibition (B estimate B1 = 0.038; p < 0.001). Healthy diet was significantly negatively associated with disinhibition (B estimate C = −0.035; p < 0.001). Part of the total effect of this model was mediated through the brain IC1 (B estimate D1 = −0.004, p < 0.001, 10%). None of the other brain ICs mediated the association between healthy diet and disinhibition (Table 3).

Table 3.
Multiple mediation model for the healthy diet component. Bold values indicate significant associations.
The second mediation model used restricted diet as the predictor and IC1-4 as the mediator variables (see Table 4). Here we observed a significant association between restricted diet and brain IC3 and IC4 (B estimates A3 = 0.029; p < 0.001. A4 = 0.029, p < 0.01). The association between restricted diet and disinhibition was positive and significant (B estimate C = 0.018; p < 0.01). No part of the total effects was mediated through the brain ICs.

Table 4.
Multiple mediation model for the restricted diet component. Bold values indicate significant associations.
The third mediation model used meat/fish diet as the predictor and IC1–4 as the mediator variables (see Table 5). In this model we observe significant associations between meat/fish diet and brain ICs 1, 2, and 4 (B estimate A1 = 0.052; p < 0.001; A2 = −0.058; p < 0.001; A4 = −0.148; p < 0.001). Meat/fish diet is significantly positively associated with disinhibition (B estimate C = 0.018, p < 0.021). Part of the effect in this model is mediated through brain IC1 (B estimate D1 = 0.002; p < 0.001).

Table 5.
Multiple mediation model for the meat/fish diet component. Bold values indicate significant associations.
The fourth mediation model used high-fat dairy diet as the predictor and IC1–4 as the mediator variables (see Table 6). High-fat dairy diet was associated with IC1 (B estimate A1 = 0.048; p < 0.001), IC3 (B estimate A3 = −0.042; p < 0.001), and IC4 (B estimate A4 = −0.035; p < 0.001). High-fat dairy diet did not significantly associate with disinhibition in this model, though there was a significant indirect effect through IC1 (B estimate D1 = 0.002, p < 0.002).

Table 6.
Multiple mediation model for the high-fat dairy diet component. Bold values indicate significant associations.
4. Discussion
In this study, we investigated the associations between dietary composition, cortical and subcortical brain volume, and behavioral disinhibition. Our models showed that: (1) there are significant associations between all dietary components and brain volume components; (2) brain volumes (for the first three components) are associated with behavioral disinhibition; (3) the mediation models show that part of the variance in behavioral disinhibition explained by dietary components (for healthy diet, restricted diet, and high-fat dairy diet) is mediated through the frontal-temporal/parietal brain volume component. These results partially confirm our hypotheses on the relation between dietary composition, frontal-parietal brain volume, and behavioral disinhibition.
The first step in our analyses was an ICA of the cortical and subcortical brain volumes in order to reduce the input data for our mediation models. This analysis resulted in four uniquely interpretable and stable brain components, namely a frontal-parietal-temporal component (IC1), a frontal-occipital component (IC2), a subcortical component (IC3), and a superior temporal component (IC4). The frontal-parietal-temporal and subcortical ICs were the primary components that we hypothesized to associate with both diet and disinhibition. In fact, IC1 was the only brain component associated with disinhibited behavior, the positive association indicating that higher volumes in the frontal-temporal/parietal areas were associated with higher disinhibition scores, which was surprising as we had expected overall lower brain volumes to relate to disinhibition based on previous literature [37,38]. IC1 (frontal-parietal-temporal volume) was also the brain component most strongly associated with all dietary components except the restricted diet. Interestingly, larger brain volumes of IC1 were negatively associated with a healthy diet but positively with meat/fish and high-fat dairy intake. IC2 (frontal-occipital volumes) was negatively associated with the meat/fish diet, and IC3 (subcortical volumes) was positively associated with the healthy diet and restricted diet but negatively with the high-fat dairy diet. IC4 (superior temporal volume) was associated with all diets, showing a positive association with a healthy and restricted diet but a negative association with meat/fish and high-fat dairy intake.
Our mediation models show that for healthy diet, restricted diet, and high-fat dairy diet, part of the association between diet and disinhibition is mediated through the frontal-temporal/parietal brain volume component. The healthy diet shows a negative association with disinhibition, suggesting an overall protective effect of a healthy diet, of which part may be due to the influence of a healthy diet on frontal-temporal/parietal brain volumes. For the restricted diet, no part of the positive association between dietary restrictions and disinhibition was mediated through the brain. For the meat/fish diet, there was also a positive association between diet and disinhibition, indicating more meat/fish consumption was associated with more disinhibitory behavior, part of which was mediated through the association between more meat/fish consumption and higher frontal-temporal/parietal volumes. For the high-fat dairy diet, we could not replicate the direct effect between diet and disinhibition observed by [22], meaning that although an indirect effect was statistically significant, this was not meaningfully interpretable.
Overall, the results above indicate a primary role for the frontal-parietal/temporal network in linking diet and disinhibition, which is in line with both inhibition literature and our hypotheses that the frontal cognitive control areas would be involved. This is the first study to show such mediation within a large general population sample. No subcortical effects were discovered, suggesting that higher cortical areas might be more sensitive than subcortical ones to dietary effects. Our mediation models further show that only a small portion of the association between diet and disinhibition is mediated through brain volumes. We expect that more detailed volumetric measures (i.e., voxel-based analyses) or functional brain imaging (fMRI or resting-state fMRI) may explain further parts of this variance. However, we must also remain open to other mediating factors beyond brain metrics that may explain parts of the links between diet and disinhibition. Biological factors like inflammation [47] or (epi)genetics [48,49] have also been linked to both diet and inhibition and may explain further parts of the association.
The disinhibition score that we used in this study was based on a mixed set of interview questions, online questionnaires, and general health records. The resulting disinhibition scale represents impulsive, compulsive, and emotionally unstable features [22]. It can be interpreted as an overarching impulsivity construct linked to impulsive behavior within different psychiatric disorders. This interpretation fits well within the categorical interpretation of mental health constructs within the RDoC approach [50]. Previously, we showed that the disinhibition score was associated in the expected directions with gender, age, socioeconomic status, and MPVA [22]; see also above for the current subsample), and both in the previous work and the current study, disinhibition was found to be correlated with dietary patterns. It must be noted that within this cross-sectional study, we can only observe associations between covariates, diet, the brain, and disinhibition and are unable to make claims about causality. Our assumption, though, reflected in the way we have set up our mediation models, is that diet influences the brain, which in turn contributes to disinhibitory behavior. Specifically, several known causal pathways exist through which diet can influence brain functioning. For instance, intake of healthy foods is known to decrease inflammation markers and thereby decrease neuroinflammation [51,52]. Similarly, HPA-axis activation is shown to be downregulated through decreased pro-inflammatory cytokines and glucocorticoid levels in response to dietary alterations. Upregulated histone acetylation and BDNF expressions can also lead to epigenetic changes in response to dietary interventions. In these ways, we postulate that unhealthy dietary composition over the lifespan could lead to altered brain structure and functioning, which may subsequently lead to issues like increased disinhibitory behavior. Further studies in the UKBiobank and other samples should take these pathways into account to further complete the causal relations between diet, disinhibition, and the brain.
However, as shown in the Supplementary Materials, these mediation models can equally validly be turned around. Indeed, it is plausible and has been shown in scientific studies that impulsivity may influence food choices [53,54,55]. More insight into the causal directions underlying the association between diet, brain, and disinhibition, therefore, requires a large-scale longitudinal study, tracking these variables over development. Intervention studies where diet is experimentally manipulated would also be able to shed light on these causal pathways.
Limitations
Apart from the cross-sectional nature of the UKBiobank cohort described above, there are several limitations inherent in the current study. First, the UKBiobank project was not aimed at indexing disinhibition, meaning our inhibition measure is not easily comparable to other studies using impulsive populations, like subjects with ADHD. The flip-side of the argument is that we used a transdiagnostic construct, it is unlikely that individual differences in diet and brain volume are associated with specific psychiatric conditions. Another limitation lies in the unknown presence of confounding variables. We carefully selected the variables we assumed a priori to potentially confound associations between diet, disinhibition, and brain volumes, but without experimental intervention, we cannot rule out unmeasured confounding that may influence the observed associations. Particularly, factors like medication use and the presence of specific medical disorders may influence the relation between diet, disinhibition, and the brain. We suggest that further analyses of the UKBiobank dataset should be aimed at discerning additional influential factors in this framework. The nature of the UKBiobank cohort reflects only an older part (40+ years) of the general population of adults. In this cohort, we find overall small effects of the general dietary pattern on brain and behavior, but it might be the case that specific subgroups or specific age ranges are more sensitive to dietary effects. The publication by [22] shows differential associations between dietary composition and disinhibition for men and women. Given that gender influences both brain structure and disinhibitory behavior, one of the main directions of future research should be regarding the influence of gender on the link between diet, brain, and disinhibition.
5. Conclusions
To conclude, this study shows that frontal-parietal brain volumes partly mediate the association between dietary composition and behavioral disinhibition. Given the strong association between behavioral disinhibition and functional brain alterations, we recommend that further research investigate a wider range of structural and functional brain measures. We further postulate that large-scale dietary intervention, as well as longitudinal observational studies, may be necessary to definitively identify the causal relations between diet, brain, and behavioral disinhibition. Additionally, part of the association between diet and disinhibition that is not mediated directly by neural factors may be explained by other biological factors like inflammation markers or (epi)genetics, which would also be prospective targets for future research.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/nu13103542/s1, Table S1: UK Biobank items related to disinhibition, impulsivity, compulsivity and/or emotional instability; Table S2: ICD-10 diagnoses related to impulsivity, compulsivity and/or emotional instability per diagnostic group; Table S3: Cases per behavioural group, its sensitivity and factor loading; Table S4: UK Biobank items related to diet; Table S5: Factor loading for each of the dietary components on the dietary components (DC1-4).
Author Contributions
Conceptualization, L.S., D.v.R., H.S., J.K.B. and C.A.H.; Data curation, L.S. and D.v.R.; Formal analysis, D.v.R. Funding acquisition, J.K.B.; Methodology, D.v.R., L.S.; Supervision, J.K.B., C.A.H.; Writing—original draft, D.v.R.; Writing—review & editing, L.S., D.v.R., H.S., J.K.B. and C.A.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the European Union’s Horizon 2020 Research and Innovation Program, grant number 728018. The APC was funded by the same grant.
Institutional Review Board Statement
The UK Biobank study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board of the North West Multi-centre Research Ethics Committee. We have complied with all relevant ethical regulations for work with UK Biobank data and all participants provided informed consent.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Bona fide researchers can apply to access the UK Biobank research resource to conduct health-related research that is in the public interest. Access to the data was granted by UK Biobank following registration with their system and approval of our research project (project number 23668). All derived variables (behavioral disinhibition, four dietary components, and one dietary grouping variable) will be returned and shared through UK Biobank. For details, see https://www.ukbiobank.ac.uk/ (accessed on 29 July 2019).
Conflicts of Interest
J.K.B. declares that he has been in the past 3 years a consultant to/member of advisory board of/and/or speaker for Janssen Cilag BV, Eli Lilly, Medice, Shire, Roche, and Servier. He is not an employee of any of these companies, and not a stock shareholder of any of these companies. He has no other financial or material support, including expert testimony, patents, royalties. L.S., D.v.R., H.S. and C.A.H. declare that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Abbreviations
| ADHD | Attention-Deficit/Hyperactivity Disorder |
| DSM-IV | Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition |
| FFQ | Food frequency questionnaire |
| MHQ | Mental Health Questionnaire |
| MRI | Magnetic Resonance Imaging |
| PCA | Principle Component Analysis |
References
- Sharma, L.; Markon, K.E.; Clark, L.A. Toward a theory of distinct types of “impulsive” behaviors: A meta-analysis of self-report and behavioral measures. Psychol. Bull. 2014, 140, 374–408. [Google Scholar] [CrossRef]
- Chamberlain, S.R.; Blackwell, A.D.; Fineberg, N.A.; Robbins, T.W.; Sahakian, B.J. The neuropsychology of obsessive compulsive disorder: The importance of failures in cognitive and behavioral inhibition as candidate endophenotypic markers. Neurosci. Biobehav. Rev. 2005, 29, 399–419. [Google Scholar] [CrossRef] [PubMed]
- Alderson, R.M.; Rapport, M.D.; Kofler, M. Attention-Deficit/Hyperactivity Disorder and Behavioral Inhibition: A Meta-Analytic Review of the Stop-signal Paradigm. J. Abnorm. Child Psychol. 2007, 35, 745–758. [Google Scholar] [CrossRef] [PubMed]
- Kurvits, L.; Martino, D.; Ganos, C. Clinical Features That Evoke the Concept of Disinhibition in Tourette Syndrome. Front. Psychiatry 2020, 11, 21. [Google Scholar] [CrossRef] [PubMed]
- Amirian, E.; Baxter, J.; Grigsby, J.; Curran-Everett, D.; Hokanson, J.E.; Bryant, L.L. Executive function (capacity for behavioral self-regulation) and decline predicted mortality in a longitudinal study in Southern Colorado. J. Clin. Epidemiol. 2010, 63, 307–314. [Google Scholar] [CrossRef] [Green Version]
- Kubzansky, L.D.; Park, N.; Peterson, C.; Vokonas, P.; Sparrow, D. Healthy psychological functioning and incident coronary heart disease: The importance of self-regulation. Arch. Gen. Psychiatry 2011, 68, 400–408. [Google Scholar] [CrossRef] [Green Version]
- Lopresti, A.L.; Hood, S.D.; Drummond, P.D. A review of lifestyle factors that contribute to important pathways associated with major depression: Diet, sleep and exercise. J. Affect. Disord. 2013, 148, 12–27. [Google Scholar] [CrossRef] [Green Version]
- Jacka, F.N.; O’Neil, A.; Opie, R.; Itsiopoulos, C.; Cotton, S.; Mohebbi, M.; Castle, D.; Dash, S.; Mihalopoulos, C.; Chatterton, M.L.; et al. A randomised controlled trial of dietary improvement for adults with major depression (the ‘SMILES’ trial). BMC Med. 2017, 15, 23. [Google Scholar] [CrossRef] [Green Version]
- Parletta, N.; Zarnowiecki, D.; Cho, J.; Wilson, A.; Bogomolova, S.; Villani, A.; Itsiopoulos, C.; Niyonsenga, T.; Blunden, S.; Meyer, B.; et al. A Mediterranean-style dietary intervention supplemented with fish oil improves diet quality and mental health in people with depression: A ran-domised controlled trial (HELFIMED). J. Australas. Coll. Nutr. Environ. Med. 2018, 37, 6–18. [Google Scholar] [CrossRef] [Green Version]
- Opie, R.; O’Neil, A.; Itsiopoulos, C.; Jacka, F.N. The impact of whole-of-diet interventions on depression and anxiety: A systematic review of randomised controlled trials. Public Health Nutr. 2015, 18, 2074–2093. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.M.; Holscher, H.D. A review of dietary and microbial connections to depression, anxiety, and stress. Nutr. Neurosci. 2020, 23, 237–250. [Google Scholar] [CrossRef]
- Loewen, O.K.; Maximova, K.; Ekwaru, J.P.; Asbridge, M.; Ohinmaa, A.; Veugelers, P.J. Adherence to Life-Style Recommendations and Attention-Deficit/Hyperactivity Disorder: A Population-Based Study of Children Aged 10 to 11 Years. Psychosom. Med. 2020, 82, 305–315. [Google Scholar] [CrossRef]
- Ríos-Hernández, A.; Alda, J.A.; Farran-Codina, A.; Ferreira-García, E.; Izquierdo-Pulido, M. The Mediterranean Diet and ADHD in Children and Adolescents. Pediatrics 2017, 139, e20162027. [Google Scholar] [CrossRef] [Green Version]
- Briguglio, M.; Dell’Osso, B.; Galentino, R.; Dina, C.Z.; Banfi, G.; Porta, M. Tics and obsessive-compulsive disorder in relation to diet: Two case reports. L’Encephale 2018, 44, 479–481. [Google Scholar] [CrossRef]
- Ludlow, A.K.; Rogers, S. Understanding the impact of diet and nutrition on symptoms of Tourette syndrome: A scoping review. J. Child Health Care 2018, 22, 68–83. [Google Scholar] [CrossRef]
- Alkerwi, A.; Baydarlioglu, B.; Sauvageot, N.; Stranges, S.; Lemmens, P.; Shivappa, N.; Hébert, J.R. Smoking status is inversely associated with overall diet quality: Findings from the ORISCAV-LUX study. Clin. Nutr. 2017, 36, 1275–1282. [Google Scholar] [CrossRef] [PubMed]
- Ross, L.J.; Wilson, M.; Banks, M.; Rezannah, F.; Daglish, M. Prevalence of malnutrition and nutritional risk factors in patients undergoing alcohol and drug treatment. Nutrition 2012, 28, 738–743. [Google Scholar] [CrossRef]
- Guerrieri, R.; Nederkoorn, C.; Stankiewicz, K.; Alberts, H.; Geschwind, N.; Martijn, C.; Jansen, A. The influence of trait and induced state impulsivity on food intake in normal-weight healthy women. Appetite 2007, 49, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Terracciano, A.; Sutin, A.R.; McCrae, R.R.; Deiana, B.; Ferrucci, L.; Schlessinger, D.; Uda, M.; Costa, P.T. Facets of per-sonality linked to underweight and overweight. Psychosom. Med. 2009, 71, 682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nederkoorn, C.; Houben, K.; Hofmann, W.; Roefs, A.; Jansen, A. Control yourself or just eat what you like? Weight gain over a year is predicted by an interactive effect of response inhibition and implicit preference for snack foods. Health Psychol. 2010, 29, 389–393. [Google Scholar] [CrossRef] [Green Version]
- Lumley, J.; Stevenson, R.J.; Oaten, M.J.; Mahmut, M.; Yeomans, M.R. Individual differences in impulsivity and their relationship to a Western-style diet. Pers. Individ. Differ. 2016, 97, 178–185. [Google Scholar] [CrossRef] [Green Version]
- Schweren, L.J.S.; van Rooij, D.; Shi, H.; Larsson, H.; Arias-Vasquez, A.; Li, L.; Grimstvedt Kvalvik, L.; Haavik, J.; Buitelaar, J.; Hartman, C. Diet, physical activity and disinhibition in middle-aged and older adults: A UK Biobank study. Nutrients 2021, 13, 1607. [Google Scholar] [CrossRef]
- Ho, A.J.; Raji, C.A.; Becker, J.; Lopez, O.L.; Kuller, L.H.; Hua, X.; Lee, S.; Hibar, D.; Dinov, I.D.; Stein, J.; et al. Obesity is linked with lower brain volume in 700 AD and MCI patients. Neurobiol. Aging 2010, 31, 1326–1339. [Google Scholar] [CrossRef] [Green Version]
- Croll, P.H.; Voortman, T.; Ikram, M.A.; Franco, O.H.; Schoufour, J.D.; Bos, D.; Vernooij, M.W. Better diet quality relates to larger brain tissue volumes: The Rotterdam Study. Neurology 2018, 90, e2166–e2173. [Google Scholar] [CrossRef]
- Akbaraly, T.; Sexton, C.; Zsoldos, E.; Mahmood, A.; Filippini, N.; Kerleau, C.; Verdier, J.-M.; Virtanen, M.; Gabelle, A.; Ebmeier, K.P.; et al. Association of Long-Term Diet Quality with Hippocampal Volume: Longitudinal Cohort Study. Am. J. Med. 2018, 131, 1372-1381.e4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aron, A.R.; Robbins, T.; Poldrack, R.A. Inhibition and the right inferior frontal cortex: One decade on. Trends Cogn. Sci. 2014, 18, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Titova, O.E.; Ax, E.; Brooks, S.J.; Sjögren, P.; Cederholm, T.; Kilander, L.; Kullberg, J.; Larsson, E.-M.; Johansson, L.; Åhlström, H.; et al. Mediterranean diet habits in older individuals: Associations with cognitive functioning and brain volumes. Exp. Gerontol. 2013, 48, 1443–1448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Staubo, S.C.; Aakre, J.A.; Vemuri, P.; Syrjanen, J.A.; Mielke, M.M.; Geda, Y.E.; Kremers, W.K.; Machulda, M.M.; Knopman, D.S.; Petersen, R.C.; et al. Mediterranean diet, micronutrients and macronutrients, and MRI measures of cortical thickness. Alzheimer’s Dement. 2017, 13, 168–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luna, B.; Sweeney, J.A. The emergence of collaborative brain function: FMRI studies of the development of response inhibition. In Adolescent Brain Development: Vulnerabilities and Opportunities; New York Academy of Sciences: New York, NY, USA, 2004. [Google Scholar]
- Fineberg, A.N.; Potenza, M.N.; Chamberlain, S.; Berlin, A.H.; Menzies, L.; Bechara, A.; Sahakian, B.; Robbins, T.; Bullmore, E.; Hollander, E. Probing Compulsive and Impulsive Behaviors, from Animal Models to Endophenotypes: A Narrative Review. Neuropsychopharmacology 2009, 35, 591–604. [Google Scholar] [CrossRef] [Green Version]
- Bari, A.; Robbins, T.W. Inhibition and impulsivity: Behavioral and neural basis of response control. Prog. Neurobiol. 2013, 108, 44–79. [Google Scholar] [CrossRef]
- Cao, M.; Shu, N.; Cao, Q.; Wang, Y.; He, Y. Imaging functional and structural brain connectomics in attention-deficit/hyperactivity disorder. Mol. Neurobiol. 2014, 50, 1111–1123. [Google Scholar] [CrossRef] [PubMed]
- Zandbelt, B.B.; Vink, M. On the Role of the Striatum in Response Inhibition. PLoS ONE 2010, 5, e13848. [Google Scholar] [CrossRef] [PubMed]
- Zeeuw, P.D.; Durston, S. Cognitive control in attention deficit hyperactivity disorder. In The Wiley Handbook of Cognitive Control; Egner, T., Ed.; Wiley Blackwell: Hoboken, NJ, USA, 2017; pp. 602–618. [Google Scholar]
- Zhang, R.; Geng, X.; Lee, T.M.C. Large-scale functional neural network correlates of response inhibition: An fMRI meta-analysis. Brain Struct. Funct. 2017, 222, 3973–3990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Rooij, D.; Hoekstra, P.J.; Mennes, M.; von Rhein, D.; Thissen, A.J.; Heslenfeld, D.; Zwiers, M.P.; Faraone, S.V.; Oosterlaan, J.; Franke, B.; et al. Distinguishing adolescents with ADHD from their unaffected siblings and healthy comparison subjects by neural activation pat-terns during response inhibition. Am. J. Psychiatry 2015, 172, 674–683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ide, J.S.; Li, H.T.; Chen, Y.; Le, T.M.; Li, C.S.; Zhornitsky, S.; Li, C.S.R. Gray matter volumetric correlates of behavioral activation and inhibition system traits in children: An exploratory voxel-based morphometry study of the ABCD project data. Neuroimage 2020, 220, 117085. [Google Scholar] [CrossRef] [PubMed]
- Pan, N.; Wang, S.; Zhao, Y.; Lai, H.; Qin, K.; Li, J.; Biswal, B.B.; Sweeney, J.A.; Gong, Q. Brain gray matter structures associated with trait impulsivity: A systematic review and voxel-based meta-analysis. Hum. Brain Mapp. 2021, 42, 2214–2235. [Google Scholar] [CrossRef]
- Smith, A.B.; Taylor, E.; Brammer, M.; Toone, B.; Rubia, K. Task-specific hypoactivation in prefrontal and temporoparietal brain regions during motor inhibition and task switching in medication-naive children and adolescents with attention deficit hyperactivity disorder. Am. J. Psychiatry 2006, 163, 1044–1051. [Google Scholar] [CrossRef]
- Lansbergen, M.M.; Böcker, K.B.; Bekker, E.M.; Kenemans, J.L. Neural correlates of stopping and self-reported impulsivity. Clin. Neurophysiol. 2007, 118, 2089–2103. [Google Scholar] [CrossRef] [PubMed]
- Cubillo, A.; Halari, R.; Ecker, C.; Giampietro, V.; Taylor, E.; Rubia, K. Reduced activation and inter-regional functional connectivity of fronto-striatal networks in adults with childhood Attention-Deficit Hyperactivity Disorder (ADHD) and persisting symptoms during tasks of motor inhibition and cognitive switching. J. Psychiatr. Res. 2010, 44, 629–639. [Google Scholar] [CrossRef]
- Sebastian, A.; Gerdes, B.; Feige, B.; Klöppel, S.; Lange, T.; Philipsen, A.; van Elst, L.T.; Lieb, K.; Tüscher, O. Neural correlates of interference inhibition, action withholding and action cancelation in adult ADHD. Psychiatry Res. Neuroimaging 2012, 202, 132–141. [Google Scholar] [CrossRef]
- Verbruggen, F.; Aron, A.R.; Band, G.P.; Beste, C.; Bissett, P.G.; Brockett, A.T.; Brown, J.W.; Chamberlain, S.R.; Chambers, C.D.; Colonius, H.; et al. A consensus guide to capturing the ability to inhibit actions and impulsive behaviors in the stop-signal task. eLife 2019, 8, 46323. [Google Scholar] [CrossRef] [PubMed]
- Bycroft, C.; Freeman, C.; Petkova, D.; Band, G.; Elliott, L.T.; Sharp, K.; Motyer, A.; Vukcevic, D.; Delaneau, O.; O’Connell, J.; et al. The UK Biobank resource with deep phenotyping and genomic data. Nature 2018, 562, 203–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Littlejohns, T.J.; Holliday, J.; Gibson, L.M.; Garratt, S.; Oesingmann, N.; Alfaro-Almagro, F.; Bell, J.D.; Boultwood, C.; Collins, R.; Conroy, M.C.; et al. The UK Biobank imaging enhancement of 100,000 participants: Rationale, data collection, management and future directions. Nat. Commun. 2020, 11, 2624. [Google Scholar] [CrossRef] [PubMed]
- Golubic, R.; May, A.M.; Benjaminsen Borch, K.; Overvad, K.; Charles, M.A.; Diaz, M.J.; Amiano, P.; Palli, D.; Valanou, E.; Vigl, M.; et al. Validity of electroni-cally administered Recent Physical Activity Questionnaire (RPAQ) in ten European countries. PLoS ONE 2014, 9, e92829. [Google Scholar]
- Steele, C.C.; Steele, T.J.; Gwinner, M.; Rosenkranz, S.K.; Kirkpatrick, K. The relationship between dietary fat intake, impulsive choice, and metabolic health. Appetite 2021, 165, 105292. [Google Scholar] [CrossRef] [PubMed]
- Archer, T.; Oscar-Berman, M.; Blum, K.; Gold, M. Neurogenetics and Epigenetics in Impulsive Behaviour: Impact on Reward Circuitry. J. Genet. Syndr. Gene Ther. 2012, 3, 1000115. [Google Scholar] [CrossRef] [Green Version]
- Grissom, N.M.; Herdt, C.T.; Desilets, J.; Lidsky-Everson, J.; Reyes, T.M. Dissociable Deficits of Executive Function Caused by Gestational Adversity are Linked to Specific Transcriptional Changes in the Prefrontal Cortex. Neuropsychopharmacology 2014, 40, 1353–1363. [Google Scholar] [CrossRef] [Green Version]
- Cuthbert, B.N.; Insel, T.R. Toward the future of psychiatric diagnosis: The seven pillars of RDoC. BMC Med. 2013, 11, 126. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Lee, I.-S.; Braun, C.; Enck, P. Effect of Probiotics on Central Nervous System Functions in Animals and Humans: A Systematic Review. J. Neurogastroenterol. Motil. 2016, 22, 589–605. [Google Scholar] [CrossRef]
- Johnson, D.; Thurairajasingam, S.; Letchumanan, V.; Chan, K.-G.; Lee, L.-H. Exploring the Role and Potential of Probiotics in the Field of Mental Health: Major Depressive Disorder. Nutrients 2021, 13, 1728. [Google Scholar] [CrossRef]
- Guerrieri, R.; Nederkoorn, C.; Jansen, A. How impulsiveness and variety influence food intake in a sample of healthy women. Appetite 2007, 48, 119–122. [Google Scholar] [CrossRef]
- Sarmugam, R.; Worsley, A. Dietary Behaviours, Impulsivity and Food Involvement: Identification of Three Consumer Segments. Nutrients 2015, 7, 8036–8057. [Google Scholar] [CrossRef] [PubMed]
- Hardcastle, S.J.; Thøgersen-Ntoumani, C.; Chatzisarantis, N.L. Food Choice and Nutrition: A Social Psychological Perspective. Nutrients 2015, 7, 8712–8715. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).