Abstract
In recent years, the beneficial effect of n-3 polyunsaturated fatty acids (n-3 PUFAs) intake on human health has been widely accepted in the field of immunonutrition. Today, we find a diversity of supplements based on n-3 PUFAs and/or minerals, vitamins and other substances. The main objective of this review is to discuss the importance of n-3 PUFAs and their derivatives on immunity and inflammatory status related to liver disease and other non-communicable illnesses. Based on the burden of liver diseases in 2019, more than two million people die from liver pathologies per year worldwide, because it is the organ most exposed to agents such as viruses, toxins and medications. Consequently, research conducted on n-3 PUFAs for liver disease has been gaining prominence with encouraging results, given that these fatty acids have anti-inflammatory and cytoprotective effects. In addition, it has been described that n-3 PUFAs are converted into a novel species of lipid intermediaries, specialized pro-resolving mediators (SPMs). At specific levels, SPMs improve the termination of inflammation as well as the repairing and regeneration of tissues, but they are deregulated in liver disease. Since evidence is still insufficient to carry out pharmacological trials to benefit the resolution of acute inflammation in non-communicable diseases, there remains a call for continuing preclinical and clinical research to better understand SPM actions and outcomes.
1. Introduction
Inflammation is a response of the immune system that allows survival during an infection or injury, eliminating harmful stimuli and initiating the recovery process; therefore, inflammation is a vital defense mechanism for health [1]. During acute inflammatory responses, cellular and molecular events and interactions minimize impending injury or infection, processes that contribute to tissue homeostasis and resolution of acute inflammation [2]. However, uncontrolled acute inflammation may become chronic and lead to a variety of chronic inflammatory diseases including cardiovascular diseases, autoimmune diseases, fibrosis and cancer [1]. Currently, the resolution of inflammation is recognized as a dynamic, programmed and self-limited process, where its regulation is obtained through mechanisms of action based on endogenous anti-inflammatory and/or pro-resolutive agonists, called specific pro-resolving mediators (SPMs) [2].
Immunonutrition is the subject that deals with the study of the interactions between nutrition and immunity in its entirety, that is, the immune system, infections, inflammation and injury or tissue damage [3]. An adequate nutritional state is synonymous with a good defense function of an organism. Currently, scientific evidence has confirmed the crucial importance of dietary intake and its role in regulating the individual’s defenses, as well as in the risk of developing acute and chronic diseases [4], such as chronic liver disease [4,5]. In the context of liver disease, whose etiology can be generated by different agents such as viruses, toxins and drugs, is becoming one of the most serious public health problems [5]. Liver inflammation is a common trigger for liver disease and is considered the main driver of damage to liver tissue, causing the progression of non-alcoholic fatty liver disease (NAFLD) to severe fibrogenesis and, ultimately, hepatocellular carcinoma (HCC) [6].
Immunonutrition usually involves arginine, nucleotides and n-3 polyunsaturated fatty acids (n-3 PUFAs) [7]. Previously, the beneficial effects of n-3 PUFAs on the stress response and immune function it has been demonstrated in preclinical and clinical studies [8]. Additionally, n-3 PUFA supplementation has been used as a nutritional complementary approach in liver transplantation [9] and hepatectomy studies [10], with promising results related to a reduction in postoperative complications and an improvement in postoperative nutritional state. The latter aspect is of particular importance due to the protein deficiency and general malnutrition in patients with advanced chronic liver disease (CLD), which in the case of cirrhosis reaches rates of 50% to 90% of patients [11]. In the present work we focus on describing the role of n-3 PUFAs and their derivatives as immunonutrition in liver diseases; we specifically delve into the potential of SPMs as immunomodulatory and nutritional supplements related to complications in liver surgery, resections and CLD.
2. Methods
The review includes studies that considered the molecular pathways and beneficial effects of n-3 PUFAs, particularly EPA and DHA, and lipid mediators from EPA and DHA using in vivo and in vitro models of nutritional intervention in liver disease. Study searches were performed using the PubMed database from the National Library of Medicine—National Institutes of Health, the World Health Organization (WHO) and clinical trials from www.clinicaltrials.gov-U.S (accessed from March to September 2021), among others.
3. Nutritional States in Liver Diseases
According to the 2019 burden of the liver disease in the world, more than two million persons die of liver disease per year worldwide, half of them related to cirrhosis complications and the other half due to viral hepatitis and HCC [12]. Spontaneous survival related to ALF rates depends on the underlying disease, and there are reported ranges from 17% to 68%; however, when liver transplantation became available, survival rates have been reported at ranges from 60% to 79% [13,14,15]. Among the liver disease types, alcohol-associated liver disease (AALD) stands out as the major global cause of liver disease, since over 50% of mortality related to cirrhosis is attributable to alcohol [12]. Progression to cirrhosis is higher in patients with AALD compared to NAFLD. NAFLD is also related to two differential conditions: non-alcoholic fatty liver (NAFL) and non-alcoholic steatohepatitis (NASH), a condition that includes degrees of fibrosis, cirrhosis and HCC [12,13]. It should be considered that obesity could potentiate the increase in both AALD and NAFLD rates in the following years. Among the other prevalent liver diseases, it is important to mention (i) viral hepatitis, which is associated with 1.34 million deaths and affects principally low- and middle-income countries; (ii) primary sclerosing cholangitis and the related cholangiocarcinoma, gallbladder and colorectal cancer; (iii) primary biliary cholangitis; and (iv) autoimmune hepatitis, among other less prevalent pathologies [12,13,15].
One of the clinical challenges in liver disease is to normalize, revert or at least ameliorate the malnutrition status related to the chronicity and liver failure. Where malnutrition (undernutrition) is frequently a burden in patients with liver cirrhosis, occurring in 20–50% of them, it is associated with the progression of liver failure. Malnutrition has been reported in 20% of patients with compensated cirrhosis and in more than 50% of patients with decompensated liver disease [16]. In addition to undernutrition, overweight and/or obesity are observed in cirrhotic NASH patients. Muscle mass depletion (sarcopenia) may also occur in these patients, but it coexists with obesity and it might be overlooked. Obesity and sarcopenic obesity may worsen the prognosis of patients with liver cirrhosis [16,17].
In terms of AALD, nearly all patients with cirrhosis are malnourished and the degree of malnutrition correlates with disease severity and their complications. The malnutrition is multifactorial and is related in part to poor dietary intake (anorexia and dysgeusia), magnesium deficiency, disproportionate caloric intake from alcohol and the subsequent micronutrient and vitamin deficit in addition to impairment of gastrointestinal mucosal absorption caused by alcohol, among other factors [18]. Most of the micronutrients described as part of malnutrition are folate, vitamin B6, vitamin A, thiamine and minerals such as selenium, zinc, copper and magnesium, which in some instances are thought to be involved in its pathogenesis [19]. According to the current guidelines of the American Association for the Study of Liver Diseases (AASLD), all patients with alcoholic hepatitis or advanced ALD should be assessed for nutritional deficiencies and treated aggressively with enteral nutritional therapy [19,20].
4. Immunonutrition
Nutrients are initially recommended to provide energy, protein and micronutrients that are essential to prevent muscle fatigue and to avoid hunger-induced immune exhaustion. Currently, the field of nutritional support therapy has evolved since its conception in the eighties to immunonutrition, defined as the use of specific nutrients to increase the immune response and support trauma in the period of critical illness and stress, administered enterally or parenterally [21]. Immunonutrition has been incorporated largely into studies to improve the clinical course of critically ill and surgical patients, where they frequently require an exogenous supply of nutrients [22].
Historically, immunonutrition systems of administration are: (i) Enteral nutrition (EN), a nutritional support technique, in which nutritional substances are supplied to the gastrointestinal tract, distally to the oral cavity, nasally via implanted tubes or an enterostomy [23]. There is evidence that the enteral route has obtained more benefits and improvements in patients over the administration of parenteral nutrition and it ought to be preferred whenever there is a functioning gastrointestinal tract [24], EN being a safer and less expensive technique [25]. (ii) Parenteral nutrition (PN) provides lifesaving nutritional support in situations where the parenteral supply of calories does not meet the body’s demands or when EN is not possible to use for some reasons. PN preserves lean body mass, supports immune functions and reduces metabolic complications in patients who would otherwise be unable to feed. However, PN associated with intestinal failure exhibits adverse effects, such as deterioration of hepatic function [26], which normalized after the interruption of the procedure; therefore, it can represent a serious risk factor in people who receive this treatment in the long term [27]. The basis of a correct immunonutrition is to obtain the maximum benefits of this technique that decrease the days spent in hospital, diminish the incidence of infections and reduce hospital costs. The effects are more consistent in patients with severe trauma, including burned subjects, some critical pathologies, those undergoing a major surgical procedure, polytraumatized patients or those that in their processes generate a high level of organic stress, increasing nutritional requirements, especially malnourished individuals prior to a surgical event [28]. Nutrients that have been identified to generate an immune benefit include: (i) Glutamine, the most abundant non-essential amino acid, which is essential for fast-growing enterocytes and cells of the immune system [29], restores the immune depletion of critically ill patients [30] and regenerates the liver after weight gain and after hepatectomy [31]. (ii) Arginine, a non-essential amino acid that becomes essential during states of stress and critical illness [32]. This amino acid has been shown to be of vital importance in vasodilation, calcium and hormone release, neurotransmission, cell proliferation, secretory activities and immunity [33]. In the context of the liver, arginine has a regenerative effect after a hepatectomy [34], hepatoprotective properties against lipid peroxidation [35] and improves injury by preservation of liver transplantation [9]. (iii) Nucleotides, the endogenous, biologically active substances that play an important role in almost all biochemical processes as they are the precursors of DNA, RNA, ATP and coenzymes, among others. Dietary nucleotides may be necessary for the optimal growth and function of active metabolic cells, such as lymphocytes, macrophages and intestinal cells [36]. In this context, they contribute to correct the alterations in the levels of fatty acids in plasma and liver microsomes from rats with hepatic cirrhosis [37,38], besides acting as regenerative compounds in injured cirrhotic livers [39]. (iv) Finally, n-3 PUFAs are important for the structure and function of cell membranes and have additional important functions, such as being precursors of SPMs [40]. They are involved in physiological processes that modulate the state of health and the onset of chronic diseases, such as the regulation of plasma lipid levels, cardiovascular [41] and immune functions [42], neuronal development [43], visual activity, diabetes [44], rheumatoid arthritis [45,46] and cancer [47], in addition to the positive effects on the immune status of critically ill patients [48].
5. N-3 PUFAs and Inflammation
For some metabolic and structural functions, n-3 PUFAs and n-6 PUFAs undergo processes of elongation and desaturation, mainly in the liver [49]. In this way, linoleic acid (C18:2n-6; LA) from the n-6 PUFA series gives rise to arachidonic acid (C20:4n-6; AA), whereas α-linolenic acid (C18:3n-3; ALA) from the n-3 PUFA series generates eicosapentaenoic acid (C20:5n-3; EPA) and docosahexaenoic acid (C22:6n-3; DHA). AA, DHA and EPA are structural components of cell membranes, where they are released by the activation of the enzyme phospholipase A2 (PLA2) during the early stages of an inflammatory process. AA is metabolized by the enzymes lipoxygenase (LOX) and cyclooxygenase (COX), generating bioactive eicosanoids, such as prostaglandins (PG), leukotrienes (LT) and thromboxanes (TX) [50]. These include PGE2, TXA2 and LTB4, which are potent mediators of inflammation, fever, pain, increased vascular permeability [51], the release of inflammatory cytokines and enhanced activity of immune cells [52]. Instead, EPA and DHA give rise to anti-inflammatory and SPM derivatives such as resolvins, protectins and maresins [53] (Figure 1). A human diet rich in EPA and DHA allows for an increase in these fatty acids in cell membranes and reduces the AA content due to competition for the desaturation enzymes, decreasing the generation of pro-inflammatory products derived from n-6 fatty acids [51]. The molecular mechanisms involved in the anti-inflammatory effect of n-3 PUFAs in the liver are related to the downregulation of pro-inflammatory transcription factor nuclear factor-κB (NF-κB). These include (i) the formation of SPMs; (ii) binding to peroxisome-proliferator-activated receptor-α (PPAR-α) to form an inactive complex with NF-κB-p60; (iii) binding to G-protein coupled receptor 120 (GPR120) to abrogate NF-κB activation and derived inflammasome NLRP3 expression; and (iv) the formation of J3-isoprostanes, oxidation products that activate nuclear factor erythroid-2-related factor 2 (Nrf2), which induces an antioxidant response blocking the redox activation of NF-κB [54]. SPMs regulate the production of inflammatory mediators (chemokines, cytokines and inflammatory eicosanoids), preventing leukocyte infiltration and triggering macrophage phenotype changes. Thus, SPMs prevent inflammation from getting out of control and enable its termination. Furthermore, they have been shown to promote tissue repair and regeneration [55]. Furthermore, SPMs play a protective role in chronic inflammatory diseases such as atherosclerosis [56], cardiovascular disease [57], rheumatoid arthritis [58], cystic fibrosis [59], asthma [60], cancer [61,62] and periodontitis [63], and protect the liver cells against genotoxic damage and oxidative stress [64], among others. The discovery of SPMs provides a new perspective in the treatment of inflammatory disorders. These molecules are a group of endogenous chemical mediators biosynthesized in a transcellular way, and they were identified in inflammatory exudates. Within this group we find three distinctive families, namely resolvins, protectins and maresins [55].
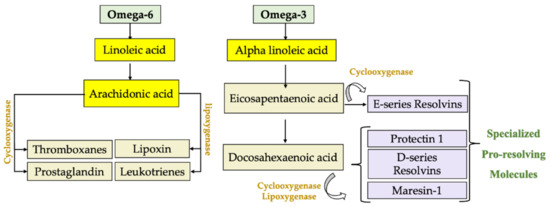
Figure 1.
Endogenous production of lipid mediators using omega-6 fatty acids, where linoleic acid is transformed and arachidonic acid is generated, which is metabolized by lipoxygenase and cyclooxygenase to generate prostaglandins (PG), leukotrienes (LT), thromboxanes (TX) and lipoxins (LX). On the other hand, omega-3 alpha-linolenic acid is metabolized into eicosapentaenoic acid to generate resolvin E1 and docosahexaenoic acid to form protectin 1, resolvin-D and maresin-1, products with powerful cytoprotective and especially anti-inflammatory properties.
5.1. Resolvins
These SPMs were the first described resolving molecules, and were identified in exudates obtained during spontaneous resolution, in a murine model of inflammation based on the formation of air pockets in the dorsal area of skin, which spontaneously resolve [65]. Their name derives from their ability to regulate resolution through multiple mechanisms, including the prevention of neutrophil infiltration, promotion of apoptotic neutrophil phagocytosis to clarify the lesion and enhancement of the resolution of inflammation within the lesion and thus promoting tissue regeneration [66]. In this way, the resolution interval is shortened, preventing the progression of an acute to chronic inflammatory response [67]. These lipid mediators have been shown to have anti-inflammatory, anti-fibrotic, anti-angiogenic and infection resolution properties [68].
5.2. Protectins
These molecules were initially described in human respiratory tracts and called D series protectins, because they derived from DHA and were later converted by leukocytes into protectin D1 or neuroprotectin, which protects neuronal tissues [69,70]. Protectin D1 is also produced by lymphocytes in the peripheral blood, and its action consists in reducing tumor necrosis factor-α (TNF-α) and interferon gamma (INFγ) secretion as well as blocking the migration of T cells, promoting apoptosis [71].
5.3. Maresins
The most recently discovered bioactive compound family are maresins, synthesized from DHA by macrophages that relate to the resolution of inflammation [41,72]. Their function is to limit tissue infiltration of polymorphonuclear (PMN) leukocytes, reduce collateral tissue damage by phagocytes, shorten the resolution interval, improve phagocytosis and counter-regulate pro-inflammatory chemical mediators. In addition, mitigation of hepatic steatosis is observed in obese mice [73] and they exert protection against ischemia–reperfusion (IR) liver injury [74].
6. Beneficial Effects of N-3 PUFAs
Currently, n-3 PUFAs are considered essential for normal growth and development, and these fatty acids also play an important role in the prevention and treatment of various diseases, as exemplified in the followed paragraphs.
6.1. Cardiovascular Health
Cardiovascular diseases are among the two leading causes of death in the world [75]. The n-3 PUFAs were shown to have numerous heart health benefits, preventing atherosclerosis by reducing total cholesterol concentrations, triglycerides [76] and blood pressure in patients with hypertension [77]. Besides, the administration of n-3 PUFAs increases high-density lipoprotein (HDL) cholesterol levels [78], prevents platelet clumping [79] and they have the potential to beneficially impact arterial wall remodeling and cardiovascular outcomes by targeting arterial wall hardening and endothelial dysfunction [80].
6.2. Nervous System
For several years, in vivo and in vitro studies with n-3 PUFA supplementation in humans revealed a neuroprotective effect, especially in lesions induced by ischemia and by excitotoxicity produced by neurotransmitters [25]. The administration of DHA prevented the anatomical functional deterioration in neurons of animals, characteristic of diabetic neuropathy, in addition to diminishing oxidative damage and learning problems in rats with traumatic brain injury [51]. It has also been determined that they can correct visual and brain problems in patients with demonstrated deficiency. Besides, n-3 PUFAs are part of the treatment of multiple neuropathologies, such as attention deficit hyperactivity disorder, multiple sclerosis, depression, schizophrenia and Alzheimer’s disease [81].
6.3. Cancer
Cancer is one of the leading causes of death in the Western world. The study of the use of n-3 PUFAs in cancer and cancer-related cachexia is of great interest. There are studies in mice and cell cultures showing that the administration of EPA and DHA can control the initiation and progression of prostate cancer [82]; specifically, DHA suppresses the NF-κB prosurvival pathway by arresting the growth of prostate cancer [83]. In rats with colon cancer, n-3 PUFAs directly affect the expression of genes related to tumorigenesis and apoptosis, reducing cellular and DNA damage [84]. Along with this, the study of cachexia, which generates loss of weight and tissue (mainly muscle) in cancer patients and is an indicator of a poor prognosis, revealed that the use of n-3 PUFAs supplements may increase appetite, decrease weight loss and augment lean mass, thus providing a better quality of life [82,85]. This is due to the fact that n-3 PUFAs decrease the levels of pro-inflammatory cytokines in plasma, benefiting the symptoms of patients with cancer-related cachexia that show elevated levels of inflammatory mediators [52]. In addition, n-3 PUFAs prevent breast cancer [86], skin cancer [87] and improve postoperative recovery in cirrhotic patients with liver cancer [88]. In hepatocellular carcinoma, Lim et al. (2009) demonstrated that n-3 PUFAs induce the degradation of β-catenin, a molecule related to cellular proliferation, which is associated with Wnt tumorigenesis pathways, where Wnt/β-catenin activation occurs in approximately 30% of HCC [89]. In patients with liver metastases due to bowel cancer having recurrence of the disease and death, the administration of n-3 PUFAs before liver surgery favors survival and lessens recurrence of the disease, outcomes also observed in liver metastasis from colorectal cancer [90].
6.4. Inflammatory Bowel Disease, Rheumatoid Arthritis and Other Autoimmune Diseases
N-3 PUFAs may contribute positively to intestinal pathologies [91]. For example, interleukins (IL)-1, IL-6 and TNF-α play an important role in the development of inflammatory bowel pathology, where the effects of n-3 PUFAs reduce pro-inflammatory cytokine levels in rat ulcerative colitis, improving several markers of cellular damage [91,92].
Rheumatoid arthritis, which afflicts 1% of the population worldwide, is a systemic autoimmune disease characterized by chronic inflammation of the synovial tissue and joint destruction. Treatment consists of anti-inflammatory and immunosuppressive drugs, which have unfavorable long-term side effects [93]. For this reason, studies have been conducted with the administration of n-3 PUFAs, reducing serum levels of IL-1, IL-2, IL-6, IL-8 and TNF-α [94]. They improve the symptoms of this disease and reduce joint tension and stiffness [45]. This supports the use of n-3 PUFAs as part of the treatment of autoimmune diseases, especially in the early stages. In addition, there have been studies in which n-3 PUFAs have benefits for other immune-related diseases, such as type II diabetes, metabolic syndrome and obesity, among others [95]. In patients with inflammatory bowel diseases, n-3 PUFAs induced a reduction in relapse rates, an improvement in symptoms and a diminution in oxidative stress in the intestine [51], and in psoriasis reduced the area of psoriasis, the severity index and the dermatological quality of life index [96].
6.5. Ischemia–Reperfusion-Induced Damage (IR)
There are numerous situations in which ischemia of organ/tissues can occur, with a lack or decrease in blood supply, oxygen and nutrients [97]. The IR damage is produced in situations such as myocardial infarction, acute kidney damage, systemic shock and major trauma related to surgeries, among others [97,98]. In the liver, IR damage is associated with transplantation, liver resection and trauma, where n-3 PUFA administration has had anti-inflammatory and cytoprotective effects, since it reduces the inflammatory response and increases antioxidant activity. In addition, n-3 PUFAs inhibit the translocation of NF-κB from cytosol to the nucleus [99,100], reducing the expression of pro-inflammatory cytokines through the activation of PPAR-α, which forms inactive complexes with NF-κBp65 to generate anti-inflammatory and cytoprotective effects [101].
7. Beneficial Effects of N-3 PUFA in Liver Diseases
The liver is especially sensitive to changes in the dietary fatty acids, and is an organ affected by diets with high levels of saturated fatty acids, trans fatty acids and other dietary components that can saturate its detoxifying functions, leading to metabolic disorders and liver damage. Supplementation with n-3 PUFAs is a focus of interest given their cytoprotective effects. Research conducted for liver disease has been gaining momentum recently, with the encouraging preliminary studies discussed below (Figure 2).
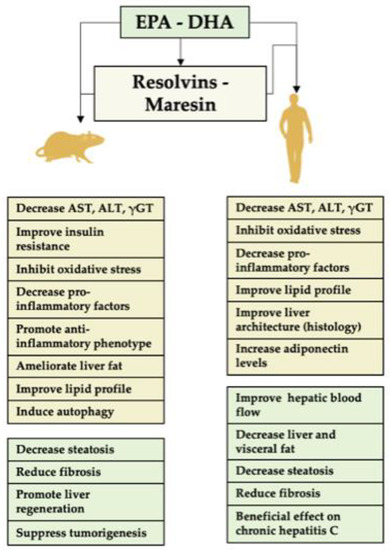
Figure 2.
Role of EPA and/or DHA and their derivatives in liver disease. A summary of the activity of these fatty acids in animals’ assays and clinical trials is shown. The specific pro-resolving mediators (SPMs), resolvins and maresins, derived from EPA and DHA represent major agents for the prevention or resolution of liver inflammation. The underlying mechanisms include (i) diminution in liver polymorphonuclear leukocyte (PMN) infiltration by blocking PMN transendothelial migration; (ii) downregulation of transcription factor nuclear factor-κB (NF-κB) activity to decrease the expression of the pro-inflammatory cytokines TNF-α and IL-1β; and (iii) stimulation of macrophage (Kupffer cell) phagocytosis of apoptotic PMNs. EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid; AST, aspartate aminotransferase; ALT, alanine aminotransferase; γGT, gamma glutamyl transferase; TNF-α, tumor necrosis factor-α; IL-1β, interleukin-1β.
7.1. Evidence for n-3 PUFA Effects in Animal Models of Liver Disease
In non-alcoholic fatty liver disease (NAFLD), the potentially beneficial effects of n-3 PUFAs are provided by animal studies, using several models that closely resemble the pathogenesis and metabolic changes of the disease, although the dosages of n-3 PUFAs supplied are greater than those used in the human studies. The molecular mechanisms of n-3 PUFAs on liver lipid metabolism leading to an anti-steatotic response include (i) the activation of the PPAR-α/FGF21/AMPK/PGC-1α signaling cascade to increase FA oxidation; (ii) diminution in the processing of lipogenic factor sterol responsive element-binding protein 1c (SREBP-1c); (iii) downregulation of the expression of the citrate carrier reducing the cytosolic availability of acetyl-CoA units for de novo lipogenesis; and (iv) abrogation of endoplasmic reticulum stress and the associated expression of lipogenic enzymes [54,102]. Sekiya et al. (2003) demonstrated that the administration of n-3 PUFAs, in the obesity model of leptin-deficient ob/ob mice, significantly decreased the triglyceride (TG) and alanine aminotransferase (ALT) serum levels [103]. Furthermore, the authors found improvements in hyperglycemia, hyperinsulinemia and the activation of PPAR-α, promoting fatty acid (FA) oxidation, with the concomitant suppression of lipogenic SREBP-1c, concluding that n-3 PUFAs improve symptoms associated with obesity, such as liver steatosis and insulin resistance [103]. In addition, similar results were observed in n-3-PUFA-fed rats in relation to the loss of hepatic TGs and weight [104]. Besides, the administration of n-3 PUFAs by parenteral nutrition of C57BL6 mice protected the liver against hepatic steatosis with improvement in liver architecture, decreased aspartate aminotransferase (AST) and ALT serum levels, an amelioration of liver fat content and a reversion of the essential fatty acid’s deficiency [105]. Interestingly, n-3 PUFAs had insulin sensitization actions in adipose tissue and liver, with an improved insulin tolerance in ob/ob mice, in addition to an enhancement in adiponectin levels and alleviation of liver steatosis, via the formation of resolvins and protectins [106]. In relation to lipid metabolism, Shang et al. (2017) studied the effects of dietary supplementation of different DHA/EPA ratios on a high-fat-diet-induced lipid metabolism disorder and the concurrent liver damage [107]. Under these conditions, decreased body weight and lipid profiles in serum and liver were observed, concomitantly with attenuation of liver steatosis, inflammatory reactions (lower serum TNF-α, IL-1β and IL-6 levels), lipid peroxidation and the protein expression of factors related to adipogenesis and inflammation [107,108]. In addition, a lower DHA/EPA ratio appears to be more beneficial in alleviating high-fat-diet-induced liver damage in mice and lowering inflammatory risk factors [108]. In other models of liver disease, n-3 PUFAs have been shown to decrease levels of γ-glutamyl transpeptidase (γ-GT), high-density lipoprotein (HDL), albumin and total protein in liver fibrosis induced by thioacetamide [109]. Compared to a lard diet, fish oil intake significantly decreased body weight gain, white blood cell count, levels of TG, total cholesterol, fat accumulation, HDL, oxidative stress and inflammatory cytokines [108]. Furthermore, it was demonstrated that n-3 PUFA supplementation by parenteral nutrition inhibits oxidative stress in an experimental model of liver regeneration [110], reduces liver fibrosis, promotes liver regeneration, even under cirrhotic conditions [111], and suppresses liver tumorigenesis [111,112,113].
7.2. Clinical Trials with n-3 PUFAs in NAFLD and Its Progressive Disease States
There is a bulk of information related to n-3 PUFA supplementation and PN on liver disease [88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116], with a significant number of clinical trials developed or in testing state (Table 1 and Supplementary Tables S1–S9), showing that n-3 PUFA supplementation is a noteworthy field in medicine.

Table 1.
Summary of official registers of clinical trials (in process or finished) related to the administration of omega-3 and liver conditions and/or disease *.
7.2.1. NAFLD
The efficacy of prolonged n-3 PUFA supplementation was evaluated in patients with NAFLD, the first study being conducted in humans by Capanni et al. (2006), where they found that after 12 months with treatment, the Doppler perfusion index (DPI) increased significantly, indicating an improvement in hepatic blood flow as a consequence of the reduction in intrahepatic fat accumulation [123]. Serum AST remained stable, while ALT and γ-GT decreased [123]. These results agree with a second trial by Lewis et al. (2006); Spadaro et al. (2008); Oscarsson et al. (2018); and Green et al. (2020), in which treatment with n-3 PUFAs showed better serum biochemistry with reduction in TG, γ-GT and TNF-α levels, and improved insulin sensitivity [120,121,122,124]. In the same line, n-3 PUFAs as seal oil were administrated to patients with NAFLD associated with hyperlipidemia, resulting in lowered ALT and TG levels, normal liver echo patterns and half of them had a regression of fatty liver [158]. A study with the same objective was carried out for 3 months and showed that EPA and DHA plasma concentrations increase, as well as those of serum levels of adiponectin, with a reduction in TNF-α, LTB4, fibroblast growth factor 21 (FGF21), cytokeratin fragment 18 and PGE2 [132]. The authors concluded that n-3 PUFAs may benefit from the metabolic abnormalities associated with NAFLD exhibiting hyperlipidemia [132]. Olive oil enriched with n-3 PUFA was also studied for one year of supplementation, and patients with NAFLD had decreased circulating values of liver enzymes and TGs, with a significant improvement in the levels of adiponectin [159]. Additionally, purified EPA and DHA administration was investigated until an endpoint defined by a decrease in liver fat and the improvement of histologically validated biomarkers of liver fibrosis was attained. The results showed a decrease in the percentage of liver fat that can be achieved with a high percentage of DHA enrichment in erythrocytes from NAFLD patients [118,119], results that were confirmed by two subsequent studies [160,161]. NAFLD in pediatric patients was similarly considered in the context of supplementation with EPA plus DHA or DHA alone [133,134,135]. In all the studies, the authors reported an improvement in liver laboratory tests and a difference in fat/lean body mass after intervention, particularly DHA supplementation, which decreases liver and visceral fat and improves metabolic abnormalities in children with NAFLD [133], however, no changes in the fibrosis score were observed [136]. More recently, Parker et al. (2019) analyzed the effect of fish oil supplementation on liver and visceral fat in overweight men subjected to 12 weeks of supplementation with 1000 mg/day n-3 PUFAs [136]. This protocol did not alter liver fat, aminotransferases or visceral adiposity in overweight men, concluding that the dose used may not be sufficient to recommend for the sole purpose of reducing liver and/or visceral fat [136].
7.2.2. NASH
From the studies that described NASH conditions in patients, in the first trial, highly purified EPA improves some NASH characteristics, including decreased serum ALT and AST levels as well as hepatic steatosis grade, with biopsy histology assessment showing improvement in several key features of NASH, such as hepatic steatosis, fibrosis, lobular inflammation and hepatocellular ballooning [162]. In particular, the authors found that the serum levels of pro-inflammatory cytokines such as TNFα tended to decrease after treatment, and the levels of the receptors TNF-R1 and sTNF-R2 also diminished [162]. Similar results were obtained by Nogueira et al. (2016), showing that increases in ALA and EPA are directly associated with better liver histology [137]. In the context of liver fibrosis, Li et al. (2015) examined whether n-3 PUFA administration for six months has an effect on NASH fibrosis parameters, where type IV collagen and pro-collagen type III were significantly diminished over control values [163]. In pediatric patients, DHA and vitamin E administration showed that the therapy is well-tolerated and can improve steatosis, ALT and fasting glucose levels [164]. It is important to mention, however, that Sanyal et al. (2014) [138] and Argo et al. (2015) [127] describe that EPA alone or combined EPA and DHA supplementation did not produce an improvement in the primary outcome of histological activity in patients with NASH.
7.2.3. Hepatitis C
A clinical trial where EPA was administered to patients with chronic hepatitis C (HCV) resulted in an enhancement in EPA content of the erythrocyte membrane, lymphocyte counts increased after 4 weeks of therapy and maintained baseline throughout therapy and the serum ALT levels improved significantly [165]. The serum levels of 8-hydroxy-2’-deoxyguanosine at 24 weeks in the EPA group were significantly lower than in the controls, suggesting a reduction in DNA oxidation, which implies a beneficial effect of EPA supplementation in the treatment of patients with chronic hepatitis C [165]. Reinforcing the above, Takaki et al. (2008) showed that the administration of EPA diminished the hemolytic anemia induced by ribavirin (RBV) in the hepatitis C treatment. The control of this adverse effect is important to maintain the RBV treatment and avoid the sustained virological response, which is responsible for the most common viral cirrhosis and HCC [166]. Interestingly, administration of n-3 PUFA was associated with an enhanced efficacy of interferon-based therapies and greater antifibrogenic effect in obese patients with HCV [167].
7.2.4. Liver Cirrhosis
Pazirandeh et al. (2007) conducted an assay where AA and DHA were supplemented in cirrhotic patients awaiting liver transplantation. The supplementation effectively raised the DHA levels in plasma lipid fractions and diminished the levels of n-6 PUFA derivatives, suggesting that DHA inhibited the elongation and desaturation of AA, which may have an anti-inflammatory outcome [168].
8. Supplementation with Specific Pro-Resolving Mediators (SPMs) in Liver Diseases
Exposure of the liver to different deleterious agents such as viruses, toxins and drugs determine alterations at the structural and functional level, generating chronic organ failure and liver cirrhosis in advanced stages. Nutritional therapy with essential fatty acids that are converted by the body into a novel genre of SPMs confer enhanced inflammation termination, tissue repair and regeneration [169]. Studies indicate that the production of these molecules is deregulated in several diseases, leading to a failure in resolution. It is noteworthy that numerous studies indicate that nutrition with n-3 PUFAs in the recommended daily doses generates an increase in several families of SPMs that correlate with improved responses of white blood cells in humans and reduce inflammation in mice [55].
8.1. SMP Effects in Experimental Animals
Among the SPM lipid autacoids, resolvins (Rv), are the best characterized, RvE1 being a drug candidate of the growing family of endogenous lipid mediators [169,170]. RvE1 reduces the levels of serum indicators of liver fibrosis, such as laminin, hyaluronic acid, pro-collagen type III and type IV collagen, slowing down the process of liver fibrosis, an effect that may be the result of adjustment of the immune and anti-inflammatory system [171]. Reinforcing the above contention, Kuang et al. (2016) demonstrated that RvD1 and RvE1 prevent concanavalin-A-induced liver damage and changes from hepatitis to liver cancer in mice by inhibiting transcription factors NF-κB and activating protein 1 (AP-1) as well as the respective inflammatory cytokine secretion [172]. In this context, it was recently shown that RvE1 and other eicosanoids were decreased in serum during NAFLD progression [173]. In relation to RvD1, an autacoid with anti-inflammatory and tissue restoration properties [174,175], when supplemented in a model of liver IR injury generates anti-inflammatory and resolutive effects in the liver. RvD1 attenuates IR-induced hepatocellular damage and the inflammatory response through promotion of anti-inflammatory M2 macrophages polarization, enhancement of AKT phosphorylation and/or regulation of mitochondrial redox homeostasis [176]. It is important to mention, however, that in a chronical model of cholestatic liver injury induced by bile duct ligation, the activity of hepatic stellate cells and the deposition of extracellular matrices were decreased by RvD1, without a notorious diminution in inflammation and the progression of fibrosis parameters [177]. Thus, RvD1 has limited therapeutic potential to treat cholestatic liver disease. Finally, the last SPM discovered, MaR1, also has important anti-inflammatory and resolution action on liver disease. In a murine model with carbon tetrachloride (CCl4)-induced liver damage, MaR1 attenuated liver damage, oxidative stress and lipid peroxidation [178]. MaR1 increased the activities of antioxidant mediators of the liver and decreased the production of inflammatory mediators such as TNFα, IL-6, IL-1β, monocyte chemotactic protein 1, myeloperoxidase, cyclooxygenase-2 and inducible nitric oxide synthase [178]. Furthermore, MaR1 inhibited the activation of NF-κB and MAPK in mouse liver, thus conferring antioxidant and anti-inflammatory properties in CCl4-induced liver injury [178]. In addition, MaR1 lowers lipogenic enzymes while inducing autophagy and fatty acid oxidation genes, which could be related to the activation of AMP-activated protein kinase (AMPK), an important regulator of cellular energy homeostasis [73]. More recently, Soto et al. (2020) showed in a murine model of liver IR injury that MaR1 generates activation of hepatocyte cell division via increased levels of IL-6 and nuclear localization of Nrf2, with decreased NF-κB activity [74]. These results open the possibility that MaR1 is a potential therapeutic tool in IR and other liver pathologies.
8.2. SPM Effects in the Clinical Setting
There is adequate evidence of the actions of the SPMs in animal models and several diseases or syndromes, including control of inflammatory pain [179], rheumatoid arthritis [180,181], diabetes [182,183,184] and atherogenesis [185], among others. However, studies related to the actions of SMPs on human liver disease have been scarcely explored. Previously, it was reported that supplementation with n-3 PUFAs to healthy voluntaries increased the concentration of plasma SPMs in relation to regulation of monocytes, neutrophiles and their inflammatory and adhesion molecules [186,187,188]. In relation to metabolic and liver disorders, Barden et al. (2015) described for the first time that subjects with metabolic syndrome had a diminution of resolvin precursors in plasma, and that the supplementation with n-3 PUFA increased total resolvin levels, except those of MaR-1 [189]. In this respect, the authors showed that when patients with metabolic syndrome lost weight (on average, body weight fell by 4.8 kg and reduction in waist circumference by 6.4 cm, described as a modest weight loss), neutrophil RvE1 clearly increased, but there were no significant changes in 18R-RvE3, RvD2 or Mar-1 [189]. Furthermore, Uno et al. (2016) showed that preoperative enteral immunonutrition reduced inflammatory responses and protected against the aggravation of postoperative complications in patients undergoing major hepatobiliary resection [154]. Furthermore, plasma levels of RvE1 were higher, whereas plasma levels of IL-6 decreased; therefore, RvE1 may play a key role in resolving acute inflammation when immunonutrition is supplemented by EPA [154]. More recently, Cata et al. (2017) demonstrated that the acute inflammatory response associated with liver resection in oncological patients (most of them where related to colorectal metastatic disease) is associated with an elevation in IL-6 and a depletion in RvD and lipoxin A4 at day one post-surgery, which tended to normalize at day five [190]. These studies allow understanding of the profiles of eicosanoids related to poor postoperative recovery and how to carry out pharmacological strategies to improve the resolution of acute inflammation related to liver disease, pointing to the need for more clinical trials to better understand the mechanisms of molecular action of these SPMs.
9. Conclusions
Research on nutrition with n-3 PUFAs has gained impetus with very encouraging results to improve the resolution of acute inflammation related to liver disease, due to their anti-inflammatory and cytoprotective properties. There is a large amount of information related to n-3 PUFA supplementation, with sufficient clinical trials to demonstrate their importance in liver disease. In addition, the supplementation with n-3 PUFAs as precursors of SPMs increase their tissue availability, therefore, studies have been carried out where it was possible to demonstrate a protective role in some liver diseases. However, evidence is still insufficient to be able to carry out a pharmacological strategy to improve the resolution of acute inflammation in liver diseases. This being the case, the situation calls for continuing clinical trials in order to better understand the mechanism of action of these lipid mediators.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/nu13103384/s1, Table S1: NAFLD clinical trial information, Table S2: NASH clinical trial information, Table S3: Liver parenteral nutrition clinical trial information, Table S4: HCC clinical trial information, Table S5: Healthy people clinical trial information, Table S6: HVC clinical trial information, Table S7: Mayor liver resection clinical trial information, Table S8: Hepatic metastases clinical trial information, Table S9: Liver Transplant clinical trial information.
Authors Contribution
Conceptualization: F.H.V., and J.Z.-H.; Data curation: F.H.V., and J.Z.-H.; Funding acquisition: J.Z.-H.; Methodology and resources: F.H.V., R.V.; Writing—review & editing: F.H.V., R.V., L.A.V.; All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by Proyecto Fondecyt Iniciación, Chilean National Fund for Scientific and Technological Development (Grant N° 11200258) to J.Z.-H.
Institutional Review Board Statement
Not Applicable.
Informed Consent Statement
Not Applicable.
Data Availability Statement
Not Applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tabas, I.; Glass, C.K. Anti-Inflammatory Therapy in Chronic Disease: Challenges and Opportunities. Science 2013, 339, 166–172. [Google Scholar] [CrossRef]
- Serhan, C.N. Systems Approach to Inflammation Resolution: Identification of Novel Anti-Inflammatory and pro-Resolving Mediators. J. Thromb. Haemost. 2009, 7, 44–48. [Google Scholar] [CrossRef]
- Zapatera, B.; Prados, A.; Gómez-Martínez, S.; Marcos, A. Immunonutrition: Methodology and Applications. Nutr. Hosp. 2015, 31, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Diaz, L.; Gómez-Martínez, S.; Nova, E.; Romeo, J.; Marcos, A. Inmunonutrición: Una Potente Herramienta Para Evaluar Situaciones Nutricionales y Beneficios de Nutrientes, Compuestos Bioactivos y Alimentos. In Manual Práctico de Nutrición y Salud Kellogg’s; Exlibris: Madrid, Spain; Digital.CSIC: Madrid, Spain, 2012; Volume 546, pp. 1–12. Available online: http://digital.csic.es/handle/10261/54350 (accessed on 12 January 2021).
- Canicoba, M.; Domínguez, N.; Gutiérrez, S. Nutrición En Las Enfermedades Hepáticas Crónicas. Nutr. Clín. Med. 2014, 8, 121–135. [Google Scholar] [CrossRef]
- Del Campo, J.A.; Gallego, P.; Grande, L. Role of Inflammatory Response in Liver Diseases: Therapeutic Strategies. World J. Hepatol. 2018, 10, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Mizock, B.A. Immunonutrition and Critical Illness: An Update. Nutrition 2010, 26, 701–707. [Google Scholar] [CrossRef] [PubMed]
- Aida, T.; Furukawa, K.; Suzuki, D.; Shimizu, H.; Yoshidome, H.; Ohtsuka, M.; Kato, A.; Yoshitomi, H.; Miyazaki, M. Preoperative Immunonutrition Decreases Postoperative Complications by Modulating Prostaglandin E2 Production and T-Cell Differentiation in Patients Undergoing Pancreatoduodenectomy. Surgery 2014, 155, 124–133. [Google Scholar] [CrossRef]
- Yagnik, G.; Takahashi, Y.; Tsoulfas, G.; Reid, K.; Murase, N.; Geller, D. Blockade of the L-Arginine/NO Synthase Pathway Worsens Hepatic Apoptosis and Liver Transplant Preservation Injury. Hepatology 2002, 36, 573–581. [Google Scholar] [CrossRef]
- Mikagi, K.; Kawahara, R.; Kinoshita, H.; Aoyagi, S. Effect of Preoperative Immunonutrition in Patients Undergoing Hepatectomy; a Randomized Controlled Trial. Kurume Med. J. 2011, 58, 1–8. [Google Scholar] [CrossRef]
- Eghtesad, S.; Poustchi, H.; Malekzadeh, R. Malnutrition in Liver Cirrhosis:The Influence of Protein and Sodium. Middle East J. Dig. Dis. 2013, 5, 65–75. [Google Scholar] [CrossRef]
- Asrani, S.K.; Devarbhavi, H.; Eaton, J.; Kamath, P.S. Burden of Liver Diseases in the World. J. Hepatol. 2019, 70, 151–171. [Google Scholar] [CrossRef] [PubMed]
- Hoyer, D.P.; Munteanu, M.; Canbay, A.; Hartmann, M.; Gallinat, A.; Paul, A.; Saner, F.H. Liver Transplantation for Acute Liver Failure: Are There Thresholds Not to Be Crossed? Transpl. Int. 2014, 27, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Germani, G.; Theocharidou, E.; Adam, R.; Karam, V.; Wendon, J.; O’Grady, J.; Burra, P.; Senzolo, M.; Mirza, D.; Castaing, D.; et al. Liver Transplantation for Acute Liver Failure in Europe: Outcomes over 20 Years from the ELTR Database. J. Hepatol. 2012, 57, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Bernal, W.; Cross, T.J.S.; Auzinger, G.; Sizer, E.; Heneghan, M.A.; Bowles, M.; Muiesan, P.; Rela, M.; Heaton, N.; Wendon, J.; et al. Outcome after Wait-Listing for Emergency Liver Transplantation in Acute Liver Failure: A Single Centre Experience. J. Hepatol. 2009, 50, 306–313. [Google Scholar] [CrossRef]
- Merli, M.; Berzigotti, A.; Zelber-Sagi, S.; Dasarathy, S.; Montagnese, S.; Genton, L.; Plauth, M.; Parés, A. Association for the Study of the Liver: EASL Clinical Practice Guidelines on Nutrition in Chronic Liver Disease. J. Hepatol. 2019, 70, 172–193. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, H.; Nishiguchi, S. Sarcopenia and Sarcopenic Obesity Are Prognostic Factors for Overall Survival in Patients with Cirrhosis. Int. Med. 2016, 55, 863–870. [Google Scholar] [CrossRef][Green Version]
- Rosato, V.; Abenavoli, L.; Federico, A.; Masarone, M.; Persico, M. Pharmacotherapy of Alcoholic Liver Disease in Clinical Practice. Int. J. Clin. Pract. 2016, 70, 119–131. [Google Scholar] [CrossRef]
- Osna, N.A.; Donohue, T.M.; Kharbanda, K.K. Alcoholic Liver Disease: Pathogenesis and Current Management. Alcohol Res. 2017, 38, 147–161. [Google Scholar]
- Frazier, T.H.; Mcclain, C.J.; Kershner, N.A.; Marsano, L.S. Treatment of Alcoholic Liver Disease. Ther. Adv. Gastroenterol. 2011, 4, 63–81. [Google Scholar] [CrossRef]
- González-Calatayud, M.; López Romero, S.; Athié Gutiérrez, C.; Valdovinos González, C.; Urbina León, D. Influence of Immunonutrition on the General and Nutritional State, and on the in-Hospital Stay, of Patients Operated Secondary to Abdominal Sepsis. Cir. Gen. 2011, 33, 236–242. [Google Scholar]
- Pollock, G.R.Y.; Van Way, C.W. Immune-Enhancing Nutrition in Surgical Critical Care. MO Med. 2012, 109, 388–392. [Google Scholar] [PubMed]
- Harkness, L. The history of enteral nutrition therapy: From Raw Eggs and Nasal Tubes to Purified Amino Acids and Early Postoperative Jejunal Delivery. J. Am. Diet. Assoc. 2002, 102, 399–404. [Google Scholar] [CrossRef]
- Uscátegui, H. Inmunonutrición: Enfoque en el paciente quirúrgico. Rev. Chil. Cir. 2010, 62, 87–92. [Google Scholar] [CrossRef][Green Version]
- Szefel, J.; Kruszewski, W.J.; Buczek, T. Enteral Feeding and Its Impact on the Gut Immune System and Intestinal Mucosal Barrier. Prz. Gastroenterol. 2015, 10, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Cahova, M.; Bratova, M.; Wohl, P. Parenteral Nutrition-Associated Liver Disease: The Role of the Gut Microbiota. Nutrients 2017, 9, 987. [Google Scholar] [CrossRef] [PubMed]
- Gabe, S.M.; Culkin, A. Abnormal Liver Function Tests in the Parenteral Nutrition Fed Patient. Frontline Gastroenterol. 2010, 1, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Duarte, M.J.; Díaz, M.S.; Vargas, G.R.B.; Rubio, G.J.; Fernández, P.L.; Lee Eng, C.V.E. Inmunonutrición: Logros y Promesas. Med. Crit. 2005, 19, 183–193. [Google Scholar]
- Planas, M.; Schwartz, S.; Arbos, M.A.; Farriol, M. Plasma Glutamine Levels in Septic Patients. J. Parenter. Enteral Nutr. 1993, 17, 299–300. [Google Scholar] [CrossRef]
- Ockenga, J.; Borchert, K.; Rifai, K.; Manns, M.P.; Bischoff, S.C. Effect of Glutamine-Enriched Total Parenteral Nutrition in Patients with Acute Pancreatitis. Clin. Nutr. 2002, 21, 409–416. [Google Scholar] [CrossRef]
- Ito, A.; Higashiguchi, T. Effects of Glutamine Administration on Liver Regeneration Following Hepatectomy. Nutrition 1999, 15, 23–28. [Google Scholar] [CrossRef]
- Morris, S.M. Arginine: Beyond Protein. Am. J. Clin. Nutr. 2006, 83, 508s–512s. [Google Scholar] [CrossRef]
- Peranzoni, E.; Marigo, I.; Dolcetti, L.; Ugel, S.; Sonda, N.; Taschin, E.; Mantelli, B.; Bronte, V.; Zanovello, P. Role of Arginine Metabolism in Immunity and Immunopathology. Immunobiology 2008, 212, 795–812. [Google Scholar] [CrossRef] [PubMed]
- Kurokawa, T.; An, J.; Tsunekawa, K.; Shimomura, Y.; Kazama, S.; Ishikawa, N.; Nonami, T.; Sugiyama, S. Effect of L-Arginine Supplement on Liver Regeneration after Partial Hepatectomy in Rats. World J. Surg. Oncol. 2012, 10, 99. [Google Scholar] [CrossRef] [PubMed]
- Al-Dalaen, S.; Alzyoud, J.; Al-Qtaitat, A. The Effects of L-Arginine in Modulating Liver Antioxidant Biomarkers Within Carbon Tetrachloride Induced Hepatotoxicity: Experimental Study in Rats. Biomedical and Pharmacology Journal. Biomed. Pharmacol. 2016, 9, 293–298. [Google Scholar] [CrossRef]
- Toro-Martin, J.; Arsenault, B.; Després, J.P.; Vohl, M.-C. Precision nutrition: A review of personalized nutritional approaches for the prevention and management of metabolic syndrome. Nutrients 2017, 22, 913. [Google Scholar] [CrossRef] [PubMed]
- Fontana, L.; Moreira, E.; Torres, M.I.; Fernández, I.; Ríos, A.; De Medina, F.S.; Gil, A. Dietary Nucleotides Correct Plasma and Liver Microsomal Fatty Acid Alterations in Rats with Liver Cirrhosis Induced by Oral Intake of Thioacetamide. J. Hepatol. 1998, 28, 662–669. [Google Scholar] [CrossRef]
- Pérez, M.; Sánchez-Medina, F.; Torres, M.; Gil, A.; Suárez, A. Dietary Nucleotides Enhance the Liver Redox State and Protein Synthesis in Cirrhotic Rats. J. Nutr. 2004, 134, 2504–2508. [Google Scholar] [CrossRef]
- Torres-López, M.I.; Fernandez, I.; Fontana, L.; Gil, A.; Rios, A. Influence of Dietary Nucleotides on Liver Structural Recovery and Hepatocyte Binuclearity in Cirrhosis Induced by Thioacetamide. Gut 1996, 38, 260–264. [Google Scholar] [CrossRef] [PubMed]
- Dennis, E.A.; Norris, P.C. Eicosanoid Storm in Infection and Inflammation. Nat. Rev. Immunol. 2015, 15, 511–523. [Google Scholar] [CrossRef]
- Kar, S.; Webel, R. Fish Oil Supplementation & Coronary Artery Disease: Does It Help? MO Med. 2012, 109, 142–145. [Google Scholar]
- Nova, E.; Montero, A.; Gómez, S.; Marcos, A. Capítulo I La Estrecha Relación Entre La Nutrición y El Sistema Inmunitario. In Soporte Nutricional en el Paciente Oncológico; Gomez, C., Sastre, A., Eds.; You & Us S.A. Publisher: Madrid, Spain, 2004; pp. 9–21. [Google Scholar]
- Gerbi, A.; Maixent, J.M.; Ansaldi, J.L.; Pierlovisi, M.; Coste, T.; Pelissier, J.F.; Vague, P.; Raccah, D. Fish Oil Supplementation Prevents Diabetes-Induced Nerve Conduction Velocity and Neuroanatomical Changes in Rats. J. Nutr. 1999, 129, 207–213. [Google Scholar] [CrossRef]
- Rudkowska, I. Fish Oils for Cardiovascular Disease: Impact on Diabetes. Maturitas 2010, 67, 25–28. [Google Scholar] [CrossRef] [PubMed]
- Hurst, S.; Zainal, Z.; Caterson, B.; Hughes, C.E.; Harwood, J.L. Dietary Fatty Acids and Arthritis. Prostaglandins Leukot. Essent. Fat. Acids 2010, 82, 315–318. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Immunomodulation by Omega-3 Fatty Acids. Prostaglandins Leukot. Essent. Fat. Acids 2007, 77, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Cockbain, A.J.; Toogood, G.J.; Hull, M.A. Omega-3 Polyunsaturated Fatty Acids for the Treatment and Prevention of Colorectal Cancer. Gut 2012, 61, 135–149. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, K.J.; Schallert, R.; Daniel, V. Immunonutrition—The Influence of Early Postoperative Glutamine Supplementation in Enteral/Parenteral Nutrition on Immune Response, Wound Healing and Length of Hospital Stay in Multiple Trauma Patients and Patients after Extensive Surgery. GMS Interdiscip. Plast. Reconstr. Surg. DGPW 2015, 4, Doc15. [Google Scholar] [CrossRef]
- Sambra, V.; Echeverria, F.; Valenzuela, A.; Chouinard-Watkins, R.; Valenzuela, R. Docosahexaenoic and Arachidonic Acids as Neuroprotective Nutrients throughout the Life Cycle. Nutrients 2021, 13, 986. [Google Scholar] [CrossRef]
- Kaur, N.; Chugh, V.; Gupta, A.K. Essential Fatty Acids as Functional Components of Foods—A Review. J. Food Sci. Technol. 2014, 51, 2289–2303. [Google Scholar] [CrossRef]
- Valenzuela, R.; Tapia, G.; González, M.; Valenzuela, A. Omega-3 Fatty Acids (EPA and DHA) and Its Application in Diverse Clinical Situations. Rev. Chil. Nutr. 2011, 38, 356–367. [Google Scholar] [CrossRef][Green Version]
- Xi, S.; Cohen, D.; Barve, S.; Chen, L.H. Fish Oil Suppressed Cytokines and Nuclear Factor-κB Induced by Murine AIDS Virus Infection. Nutr. Res. 2001, 21, 865–878. [Google Scholar] [CrossRef]
- Duvall, M.G.; Levy, B.D. DHA- and EPA-Derived Resolvins, Protectins, and Maresins in Airway Inflammation. Eur. J. Pharmacol. 2016, 785, 144–155. [Google Scholar] [CrossRef]
- Valenzuela, R.; Ortiz, M.; Hernández-Rodas, M.C.; Echeverría, F.; Videla, L.A. Targeting n-3 Polyunsaturated Fatty Acids in Non-Alcoholic Fatty Liver Disease. Curr. Med. Chem. 2020, 27, 5250–5272. [Google Scholar] [CrossRef]
- Norling, L.V.; Ly, L.; Dalli, J. Resolving Inflammation by Using Nutrition Therapy: Roles for Specialized Proresolving Mediators. Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 145–152. [Google Scholar] [CrossRef]
- Merched, A.J.; Ko, K.; Gotlinger, K.H.; Serhan, C.N.; Chan, L. Atherosclerosis: Evidence for Impairment of Resolution of Vascular Inflammation Governed by Specific Lipid Mediators. FASEB J. 2008, 22, 3595–3606. [Google Scholar] [CrossRef]
- Carracedo, M.; Artiach, G.; Arnardottir, H.; Bäck, M. The Resolution of Inflammation through Omega-3 Fatty Acids in Atherosclerosis, Intimal Hyperplasia, and Vascular Calcification. Semin. Immunopathol. 2019, 41, 757–766. [Google Scholar] [CrossRef]
- Perretti, M.; Norling, L.V. Actions of SPM in Regulating Host Responses in Arthritis. Mol. Asp. Med. 2017, 58, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Recchiuti, A.; Mattoscio, D.; Isopi, E. Roles, Actions, and Therapeutic Potential of Specialized Pro-Resolving Lipid Mediators for the Treatment of Inflammation in Cystic Fibrosis. Front. Pharmacol. 2019, 10, 252. [Google Scholar] [CrossRef] [PubMed]
- Miyata, J.; Arita, M. Role of Omega-3 Fatty Acids and Their Metabolites in Asthma and Allergic Diseases. Allergol. Int. 2015, 64, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Bassaganya-Riera, J.; Hontecillas, R. Dietary Conjugated Linoleic Acid and N-3 Polyunsaturated Fatty Acids in Inflammatory Bowel Disease. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 569–573. [Google Scholar] [CrossRef]
- Szymanski, K.M.; Wheeler, D.C.; Mucci, L.A. Fish Consumption and Prostate Cancer Risk: A Review and Meta-Analysis. Am. J. Clin. Nutr. 2010, 92, 1223–1233. [Google Scholar] [CrossRef] [PubMed]
- Azuma, M.M.; Gomes-Filho, J.E.; Cardoso, C.B.M.; Pipa, C.B.; Narciso, L.G.; Bomfim, S.R.M.; Jacinto, R.D.C.; Cintra, L.T.A. Omega 3 Fatty Acids Reduce the Triglyceride Levels in Rats with Apical Periodontitis. Braz. Dent. J. 2018, 29, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Rius, B.; López-Vicario, C.; González-Périz, A.; Morán-Salvador, E.; García-Alonso, V.; Clària, J.; Titos, E. Resolution of Inflammation in Obesity-Induced Liver Disease. Front. Immunol. 2012, 3, 257. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Arita, M.; Hong, S.; Gotlinger, K. Resolvins, Docosatrienes, and Neuroprotectins, Novel Omega-3-Derived Mediators, and Their Endogenous Aspirin-Triggered Epimers. Lipids 2004, 39, 1125–1132. [Google Scholar] [CrossRef]
- Schwab, J.M.; Chiang, N.; Arita, M.; Serhan, C.N. Resolvin E1 and Protectin D1 Activate Inflammation-Resolution Programmes. Nature 2007, 447, 869–874. [Google Scholar] [CrossRef] [PubMed]
- Hasturk, H.; Kantarci, A.; Goguet-Surmenian, E.; Blackwood, A.; Andry, C.; Serhan, C.N.; Van Dyke, T.E. Resolvin E1 Regulates Inflammation at the Cellular and Tissue Level and Restores Tissue Homeostasis In Vivo. J. Immunol. 2007, 179, 7021–7029. [Google Scholar] [CrossRef]
- Uddin, M.; Levy, B.D. Resolvins: Natural Agonists for Resolution of Pulmonary Inflammation. Prog. Lipid Res. 2011, 50, 75–88. [Google Scholar] [CrossRef]
- Levy, B.D. Prostaglandins, Leukotrienes and Essential Fatty Acids Resolvins and Protectins: Natural Pharmacophores for Resolution Biology. Prostaglandins Leukot. Essent. Fat. Acids 2010, 82, 327–332. [Google Scholar] [CrossRef]
- Levy, B.D.; Kohli, P.; Gotlinger, K.; Haworth, O.; Hong, S.; Kazani, S.; Israel, E.; Haley, K.J.; Serhan, C.N. Protectin D1 Is Generated in Asthma and Dampens Airway Inflammation and Hyperresponsiveness. J. Glob. Ethics 2010, 18, 147–162. [Google Scholar] [CrossRef]
- Freire, M.O.; Van Dyke, T.E. Natural Resolution of Inflammation. Periodontol. 2000 2013, 63, 149–164. [Google Scholar] [CrossRef]
- Buckley, C.D.; Gilroy, D.W.; Serhan, C.N. Proresolving Lipid Mediators and Mechanisms in the Resolution of Acute Inflammation. Immunity 2014, 40, 315–327. [Google Scholar] [CrossRef]
- Laiglesia, L.M.; Lorente-Cebrián, S.; Martínez-Fernández, L.; Sáinz, N.; Prieto-Hontoria, P.L.; Burrell, M.A.; Rodríguez-Ortigosa, C.M.; Martínez, J.A.; Moreno-Aliaga, M.J. Maresin 1 Mitigates Liver Steatosis in Ob/Ob and Diet-Induced Obese Mice. Int. J. Obes. 2018, 42, 572–579. [Google Scholar] [CrossRef]
- Soto, G.; Rodríguez, M.J.; Fuentealba, R.; Treuer, A.V.; Castillo, I.; González, D.R.; Zúñiga-Hernández, J. Maresin 1, a Proresolving Lipid Mediator, Ameliorates Liver Ischemia-Reperfusion Injury and Stimulates Hepatocyte Proliferation in Sprague-Dawley Rats. Int. J. Mol. Sci. 2020, 21, 540. [Google Scholar] [CrossRef]
- Mendoza-Torres, E.; Bravo-Sagua, R.; Villa, M.; Flores, N.; Olivares, M.J.; Calle, X.; Riquelme, J.A.; Bambs, C.; Castro, P.; Lavandero, S. Enfermedades Cardiovasculares y Cáncer: ¿Dos Entidades Mutuamente Relacionadas? Rev. Chil. Cardiol. 2019, 38, 54–63. [Google Scholar] [CrossRef]
- Cazzola, R.; Russo-Volpe, S.; Miles, E.A.; Rees, D.; Banerjee, T.; Roynette, C.E.; Wells, S.J.; Goua, M.; Wahle, K.W.J.; Calder, P.C.; et al. Age- and Dose-Dependent Effects of an Eicosapentaenoic Acid-Rich Oil on Cardiovascular Risk Factors in Healthy Male Subjects. Atherosclerosis 2007, 193, 159–167. [Google Scholar] [CrossRef]
- Simão, A.N.C.; Lozovoy, M.A.B.; Bahls, L.D.; Morimoto, H.K.; Simão, T.N.C.; Matsuo, T.; Dichi, I. Blood Pressure Decrease with Ingestion of a Soya Product (Kinako) or Fish Oil in Women with the Metabolic Syndrome: Role of Adiponectin and Nitric Oxide. Br. J. Nutr. 2012, 108, 1435–1442. [Google Scholar] [CrossRef]
- Eslick, G.D.; Howe, P.R.C.; Smith, C.; Priest, R.; Bensoussan, A. Benefits of Fish Oil Supplementation in Hyperlipidemia: A Systematic Review and Meta-Analysis. Int. J. Cardiol. 2009, 136, 4–16. [Google Scholar] [CrossRef]
- Manzur, F.; Suárez, A.; Farmacéutico, Q.; Moneriz, C. Efectos y controversias de los ácidos grasos omega-3. Colomb. Cardiol. 2006, 13, 180–184. [Google Scholar]
- Zanetti, M.; Grillo, A.; Losurdo, P.; Panizon, E.; Mearelli, F.; Cattin, L.; Barazzoni, R.; Carretta, R. Omega-3 Polyunsaturated Fatty Acids: Structural and Functional Effects on the Vascular Wall. BioMed Res. Int. 2015, 2015, 791978. [Google Scholar] [CrossRef]
- Dyall, S.C.; Michael-Titus, A.T. Neurological Benefits of Omega-3 Fatty Acids. NeuroMol. Med. 2008, 10, 219–235. [Google Scholar] [CrossRef] [PubMed]
- Cavazos, D.A.; Price, R.S.; Apte, S.S.; Degraffenried, L.A. Docosahexaenoic Acid Selectively Induces Human Prostate Cancer Cell Sensitivity to Oxidative Stress through Modulation of NF-ΚB. Prostate 2011, 71, 1420–1428. [Google Scholar] [CrossRef] [PubMed]
- Hebert, J.R.; Hurley, T.G.; Olendzki, B.C.; Teas, J.; Ma, Y.; Hampl, J.S. Nutritional and Socioeconomic Factors in Relation to Prostate Cancer Mortality: A Cross-National Study. J. Natl. Cancer Inst. 1998, 90, 1637–1647. [Google Scholar] [CrossRef]
- Davidson, L.A.; Nguyen, D.V.; Hokanson, R.M.; Callaway, E.S.; Isett, R.B.; Turner, N.D.; Dougherty, E.R.; Wang, N.; Lupton, J.R.; Carroll, R.J.; et al. Chemopreventive n-3 polyunsaturated fatty acids reprogram genetic signatures during colon cancer initiation and progretion in the rat. Cancer Res. 2015, 64, 6797–6804. [Google Scholar] [CrossRef] [PubMed]
- Deans, C.; Wigmore, S.J. Systemic Inflammation, Cachexia and Prognosis in Patients with Cancer. Curr. Opin. Clin. Nutr. Metab. Care 2005, 8, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Jourdan, M.L.; Mahéo, K.; Barascu, A.; Goupille, C.; De Latour, M.P.; Bougnoux, P.; Rio, P.G. Increased BRCA1 Protein in Mammary Tumours of Rats Fed Marine ω-3 Fatty Acids. Oncol. Rep. 2007, 17, 713–719. [Google Scholar] [CrossRef]
- Pilkington, S.M.; Massey, K.A.; Bennett, S.P.; Al-Aasswad, N.M.; Roshdy, K.; Gibbs, N.K.; Friedman, P.S.; Nicolau, A.; Rhodes, L.E. Randomized controlled trial of oral omega-3 PUFA in solar-stimulated radiation-induced supression of human cutaneous immune response. Am. J. Clin. Nutr. 2013, 97, 646–652. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Wei, G.; Li, R.; Wang, Y.; Yu, J.; Wang, R.; Xiao, H.; Wu, C.; Leng, C.; Zhang, B.; et al. N-3 Fatty Acid-Based Parenteral Nutrition Improves Postoperative Recovery for Cirrhotic Patients with Liver Cancer: A Randomized Controlled Clinical Trial. Clin. Nutr. 2017, 36, 1239–1244. [Google Scholar] [CrossRef]
- Lim, K.; Han, C.; Dai, Y.; Shen, M.; Wu, T. Omega-3 Polyunsaturated Fatty Acids Inhibit Hepatocellular Carcinoma Cell Growth through Blocking β-Catenin and Cyclooxygenase-2. Mol. Cancer Ther. 2009, 8, 3046–3055. [Google Scholar] [CrossRef]
- Wessex Health Line; Hull, M. Clinical Trial Study ID:34700. A Randomised Placebo-Controlled Phase III Trial of the Effect of the Omega-3 Fatty Acid Eicosapentaenoic Acid (EPA) on Colorectal Cancer Recurrence and Survival After Surgery for Resectable Liver Metastases. Available online: https://clinicaltrials.gov/ct2/history/NCT03428477?V_2=View (accessed on 23 July 2021).
- Hassan, A.; Ibrahim, A.; Mbodji, K.; Coëffier, M.; Ziegler, F.; Bounoure, F.; Chardigny, J.; Skiba, M.; Savoye, G.; Déchelotte, P.; et al. An α-Linolenic Acid-Rich Formula Reduces Oxidative Stress and Inflammation by Regulating NF-ΚB in Rats with TNBS-Induced Colitis. J. Nutr. 2010, 140, 1714–1721. [Google Scholar] [CrossRef]
- Camuesco, D.; Gálvez, J.; Nieto, A.; Comalada, M.; Rodríguez-Cabezas, M.E.; Concha, A.; Xaus, J.; Zarzuelo, A. Dietary Olive Oil Supplemented with Fish Oil, Rich in EPA and DHA (n-3) Polyunsaturated Fatty Acids, Attenuates Colonic Inflammation in Rats with DSS-Induced Colitis. J. Nutr. 2005, 135, 687–694. [Google Scholar] [CrossRef]
- McInnes, I.B.; O’Dell, J.R. State-of-the-Art: Rheumatoid Arthritis. Ann. Rheum. Dis. 2010, 69, 1898–1906. [Google Scholar] [CrossRef]
- Bahadori, B.; Uitz, E.; Thonhofer, R.; Trummer, M.; Pestemer-Lach, I.; McCarty, M.; Krejs, G.J. ω-3 Fatty Acids Infusions as Adjuvant Therapy in Rheumatoid Arthritis. J. Parenter. Enter. Nutr. 2010, 34, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Castellanos, T.L.; Rodriguez, D.M. The effect of omega 3 in human health and considerations to its intake. Rev. Chil. Nutr. 2015, 42, 90–95. [Google Scholar] [CrossRef]
- Márquez-Balbás, G.; Sánchez-Regaña, M.; Millet, U. Study on the Use of Omega-3 Fatty Acids as a Therapeutic Supplement in Treatment of Psoriasis. Clin. Cosmet. Investig. Dermatol. 2011, 4, 73. [Google Scholar] [CrossRef] [PubMed]
- Romanque, U.P.; Uribe, M.M.; Videla, L.A. Molecular Mechanisms in Liver Ischemic-Reperfusion Injury and Ischemic Preconditioning. Rev. Med. Chem. 2005, 133, 469–476. [Google Scholar] [CrossRef]
- Rampes, S.; Ma, D. Hepatic ischemia-reperfusion injury in liver transplant setting: Mechanisms and protective strategies. J. Biomed. Res. 2019, 33, 221–234. [Google Scholar] [CrossRef]
- Zúñiga, J.; Venegas, F.; Villarreal, M.; Núñez, D.; Chandía, M.; Valenzuela, R.; Tapia, G.; Varela, P.; Videla, L.A.; Fernández, V. Protection against in Vivo Liver Ischemia-Reperfusion Injury by n-3 Long-Chain Polyunsaturated Fatty Acids in the Rat. Free Radic. Res. 2010, 44, 854–863. [Google Scholar] [CrossRef]
- Meital, L.T.; Windsor, M.T.; Perissiou, M.; Schulze, K.; Magee, R.; Kuballa, A.; Golledge, J.; Bailey, T.G.; Askew, C.D.; Russell, F.D. Omega-3 Fatty Acids Decrease Oxidative Stress and Inflammation in Macrophages from Patients with Small Abdominal Aortic Aneurysm. Sci. Rep. 2019, 9, 12987. [Google Scholar] [CrossRef] [PubMed]
- Cipollina, C. Endogenous Generation and Signaling Actions of Omega-3 Fatty Acid Electrophilic Derivatives. Biomed. Res. Int. 2015, 2015, 501792. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, M.; Soto-Alarcón, S.A.; Orellana, P.; Espinosa, A.; Campos, C.; López-Arana, S.; Rincón, M.A.; Illesca, P.; Valenzuela, R.; Videla, L.A. Suppression of high-fat diet-induced obesity-associated liver mitochondrial dysfunction by docosahexaenoic acid and hydrpxytyrosol co-administration. Dig. Liver Dis. 2020, 52, 895–904. [Google Scholar] [CrossRef]
- Sekiya, M.; Yahagi, N.; Matsuzaka, T.; Najima, Y.; Nakakuki, M.; Nagai, R.; Ishibashi, S.; Osuga, J.I.; Yamada, N.; Shimano, H. Polyunsaturated Fatty Acids Ameliorate Hepatic Steatosis in Obese Mice by SREBP-1 Suppression. Hepatology 2003, 38, 1529–1539. [Google Scholar] [CrossRef]
- Levy, J.R.; Clore, J.N.; Stevens, W. Dietary N-3 Polyunsaturated Fatty Acids Decrease Hepatic Triglycerides in Fischer 344 Rats. Hepatology 2004, 39, 608–616. [Google Scholar] [CrossRef]
- Alwayn, I.P.J.; Gura, K.; Nosé, V.; Zausche, B.; Javid, P.; Garza, J.; Verbesey, J.; Voss, S.; Ollero, M.; Andersson, C.; et al. Omega-3 Fatty Acid Supplementation Prevents Hepatic Steatosis in a Murine Model of Nonalcoholic Fatty Liver Disease. Pediatr. Res. 2005, 57, 445–452. [Google Scholar] [CrossRef]
- González-Periz, A.; Horrillo, R.; Ferré, N.; Gronert, K.; Dong, B.; Morán-Salvador, E.; Titos, E.; Martínez-Clemente, M.; López-Parra, M.; Arroyo, V.; et al. Obesity-induced insulin resistance and hepatic steatosis are alleviated by omgea-3 fatty acids: A role for resolvins and protectins. FASEB J. 2009, 23, 1946–1957. [Google Scholar] [CrossRef] [PubMed]
- Shang, T.; Liu, L.; Zhou, J.; Zhang, M.; Hu, Q.; Fang, M.; Wu, Y.; Yao, P.; Gong, Z. Protective Effects of Various Ratios of DHA/EPA Supplementation on High-Fat Diet-Induced Liver Damage in Mice. Lipids Health Dis. 2017, 16, 65. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhao, F.; Wu, Q.; Li, M.; Zhu, Y.; Song, S.; Zhu, J.; Ma, Y.; Li, H.; Shi, X.; et al. Fish Oil Diet May Reduce Inflammatory Levels in the Liver of Middle-Aged Rats. Sci. Rep. 2017, 7, 6241. [Google Scholar] [CrossRef]
- Moghadamnia, D.; Mokhtari, M.; Khatamsaz, S. Protective Effects of Fish Oil Omega-3 Supplement on Liver-Related Biochemical Factors Changes Induced by Thioacetamide in Male Rats. Biosci. Biotechnol. Res. Asia 2016, 13, 1253–1258. [Google Scholar] [CrossRef]
- Firat, O.; Makay, O.; Yeniay, L.; Gokce, G.; Yenisey, C.; Coker, A. Omega-3 Fatty Acids Inhibit Oxidative Stress in a Rat Model of Liver Regeneration. Ann. Surg. Treat. Res. 2017, 93, 1–10. [Google Scholar] [CrossRef]
- Yang, Y.; Shao, C.; Zhang, W.; Wang, G.; Lu, D.-C.; Han, W.; Wu, Z.-S.; Chen, C. Omega-3 Polyunsaturated Fatty Acids Prevent Progression of Liver Fibrosis and Promote Liver Regeneration after Partial Hepatectomy in Cirrhotic Rats. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 10151–10160. [Google Scholar] [CrossRef]
- Weylandt, K.H.; Krause, L.F.; Gomolka, B.; Chiu, C.Y.; Bilal, S.; Nadolny, A.; Waechter, S.F.; Fischer, A.; Rothe, M.; Kang, J.X. Suppressed Liver Tumorigenesis in Fat-1 Mice with Elevated Omega-3 Fatty Acids Is Associated with Increased Omega-3 Derived Lipid Mediators and Reduced TNF-α. Carcinogenesis 2011, 32, 897–903. [Google Scholar] [CrossRef]
- Jump, D.B.; Depner, C.M.; Tripathy, S.; Lytle, K.A. Potential for Dietary ω-3 Fatty Acids to Prevent Nonalcoholic Fatty Liver Disease and Reduce the Risk of Primary Liver Cancer. Adv. Nutr. 2015, 6, 694–702. [Google Scholar] [CrossRef]
- Casson, C.; Nguyen, V.; Nayak, P.; Channabasappa, N.; Berris, K.; Panczuk, J.; Bhiladvala, C.; Dasgupta, T.; Piper, H.G. A Comparison of Smoflipid® and Intralipid® in the Early Management of Infants with Intestinal Failure. J. Pediatr. Surg. 2020, 55, 153–157. [Google Scholar] [CrossRef]
- Strijbosch, R.A.M.; van den Hoonaard, T.L.; Olieman, J.F.; Escher, J.C.; Alwayn, I.P.; Meijers-Ijsselstijn, H. Fish Oil in Prolonged Parenteral Nutrition in Children—Omega-3-Fatty Acids Have a Beneficial Effect on the Liver. Ned. Tijdschr. Geneeskd. 2010, 154, A2003. [Google Scholar]
- Wendel, D.; Mortensen, M.; Harmeson, A.; Shaffer, M.L.; Hsu, E.; Horslen, S. Resolving Malnutrition with Parenteral Nutrition Before Liver Transplant in Biliary Atresia. J. Pediatr. Gastroenterol. Nutr. 2018, 66, 212–217. [Google Scholar] [CrossRef]
- Kobyliak, N.; Abenavoli, L.; Falalyeyeva, T.; Mykhalchyshyn, G.; Boccuto, L.; Kononenko, L.; Kyriienko, D.; Komisarenko, I.; Dynnyk, O. Beneficial effects of probiotic combination with omega-3 fatty acids in NAFLD: A randomized clinical study. Minerva Med. 2018, 109, 418–428. [Google Scholar] [CrossRef]
- Scorletti, E.; Bhatia, L.; Mccormick, K.G.; Clough, G.F.; Nash, K.; Hodson, L.; Moyses, H.E.; Calder, P.C.; Byrne, C.D. Effects of Purified Eicosapentaenoic and Docosahexaenoic Acids in Nonalcoholic Fatty Liver Disease: Results from the WELCOME* Study. Hepatology 2014, 60, 1211–1221. [Google Scholar] [CrossRef]
- Scorletti, E.; Bhatia, L.; McCormick, K.G.; Clough, G.F.; Nash, K.; Calder, P.C.; Byrne, C.D. WELCOME Trial Investigators. Design and rationale of the WELCOME trial: A randomised, placebo controlled study to test the efficacy of purified long chainomega-3 fatty acid treatment in non-alcoholic fatty liver disease [corrected]. Contemp. Clin. Trials 2014, 37, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Green, C.J.; Pramfalk, C.; Charlton, C.A.; Gunn, P.J.; Cornfield, T.; Pavlides, M.; Karpe, F.; Hodson, L. Hepatic de novo lipogenesis is suppressed and fat oxidation is increased by omega-3 fatty acids at the expense of glucose metabolism. Open Diabetes Res. Care 2020, 8, e000871. [Google Scholar] [CrossRef] [PubMed]
- Oscarsson, J.; Önnerhag, K.; Risérus, U.; Sundén, M.; Johansson, L.; Jansson, P.A.; Moris, L.; Nilsson, P.M.; Eriksson, J.W.; Lind, L. Effects of free omega-3 carboxylic acids and fenofibrate on liver fat content in patients with hypertriglyceridemia and non-alcoholic fatty liver disease: A double-blind, randomized, placebo-controlled study. J. Clin. Lipidol. 2018, 12, 1390–1403.e4. [Google Scholar] [CrossRef] [PubMed]
- Lewis, M.C.; Phillips, M.L.; Slavotinek, J.P.; Kow, L.; Thompson, C.H.; Toouli, J. Change in liver size and fat content after treatment with Optifast very low calorie diet. Obes. Surg. 2006, 16, 697–701. [Google Scholar] [CrossRef] [PubMed]
- Capanni, M.; Calella, F.; Biagini, M.R.; Genise, S.; Raimondi, L.; Bedogni, G.; Svegliati-Baroni, G.; Sofi, F.; Milani, S.; Abbate, R.; et al. Prolonged N-3 Polyunsaturated Fatty Acid Supplementation Ameliorates Hepatic Steatosis in Patients with Non-Alcoholic Fatty Liver Disease: A Pilot Study. Aliment. Pharmacol. Ther. 2006, 23, 1143–1151. [Google Scholar] [CrossRef]
- Spadaro, L.; Magliocco, O.; Spampinato, D.; Piro, S.; Oliveri, C.; Alagona, C.; Papa, G.; Rabuazzo, A.M.; Purrello, F. Effects of N-3 Polyunsaturated Fatty Acids in Subjects with Nonalcoholic Fatty Liver Disease. Dig. Liver Dis. 2008, 40, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Iannelli, A.; Martini, F.; Schneck, A.S.; Ghavami, B.; Baudin, G.; Anty, R.; Gugenheim, J. Preoperative 4-week supplementation with omega-3 polyunsaturated fatty acids reduces liver volume and facilitates bariatric surgery in morbidly obese patients. Obes. Surg. 2013, 23, 1761–1765. [Google Scholar] [CrossRef] [PubMed]
- Van Name, M.A.; Savoye, M.; Chick, J.M.; Galuppo, B.T.; Feldstein, A.E.; Pierpont, B.; Johnson, C.; Shabanova, V.; Ekong, U.; Valentino, P.L.; et al. A Low ω-6 to ω-3 PUFA Ratio (n-6:n-3 PUFA) Diet to Treat Fatty Liver Disease in Obese Youth. J. Nutr. 2020, 150, 2314–2321. [Google Scholar] [CrossRef]
- Argo, C.K.; Patrie, J.T.; Lackner, C.; Henry, T.D.; De Lange, E.E.; Weltman, A.L.; Shah, N.L.; Al-Osaimi, A.M.; Pramoonjago, P.; Jayakumar, S.; et al. Effects of N-3 Fish Oil on Metabolic and Histological Parameters in NASH: A Double-Blind, Randomized, Placebo-Controlled Trial. J. Hepatol. 2015, 62, 190–197. [Google Scholar] [CrossRef]
- Della Pepa, G.; Vetrani, C.; Brancato, V.; Vitale, M.; Monti, S.; Annuzzi, G.; Lombardi, G.; Izzo, A.; Tommasone, M.; Cipriano, P. Effects of a multifactorial ecosustainable isocaloric diet on liver fat in patients with type 2 diabetes: Randomized clinical trial. Open Diabetes Res. Care 2020, 8, e001342. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Gramlich, T.; Matteoni, C.A.; Boparai, N.; McCullough, A.J. Nonalcoholic fatty liver disease in patients with type 2 diabetes. Clin. Gastroenterol. Hepatol. 2004, 2, 262–265, Erratum in Clin. Gastroenterol. Hepatol. 2004, 2, 522. [Google Scholar] [CrossRef]
- Dasarathy, S.; Dasarathy, J.; Khiyami, A.; Yerian, L.; Hawkins, C.; Sargent, R; McCullough, A.J. Double-blind randomized placebo-controlled clinical trial of omega 3 fatty acids for the treatment of diabetic patients with nonalcoholic steatohepatitis. J. Clin. Gastroenterol. 2015, 49, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, H.E.; Arendt, B.M.; Noureldin, S.A.; Therapondos, G.; Guindi, M.; Allard, J.P. A cross-sectional study assessing dietary intake and physical activity in Canadian patients with nonalcoholic fatty liver disease vs healthy controls. J. Acad. Nutr. Diet. 2014, 114, 1181–1194. [Google Scholar] [CrossRef]
- Qin, Y.; Zhou, Y.; Chen, S.H.; Zhao, X.L.; Ran, L.; Zeng, X.L.; Wu, Y.; Chen, J.L.; Kang, C.; Shu, F.R.; et al. Fish Oil Supplements Lower Serum Lipids and Glucose in Correlation with a Reduction in Plasma Fibroblast Growth Factor 21 and Prostaglandin E2 in Nonalcoholic Fatty Liver Disease Associated with Hyperlipidemia: A Randomized Clinical Trial. PLoS ONE 2015, 10, e0133496. [Google Scholar] [CrossRef]
- Janczyk, W.; Socha, P.; Lebensztejn, D.; Wierzbicka, A.; Mazur, A.; Neuhoff-Murawska, J.; Matusik, P. Omega-3 Fatty Acids for Treatment of Non-Alcoholic Fatty Liver Disease: Design and Rationale of Randomized Controlled Trial. BMC Pediatr. 2013, 13, 85. [Google Scholar] [CrossRef] [PubMed]
- Pacifico, L.; Bonci, E.; Di Martino, M.; Versacci, P.; Andreoli, G.; Silvestri, L.M.; Chiesa, C. A Double-Blind, Placebo-Controlled Randomized Trial to Evaluate the Efficacy of Docosahexaenoic Acid Supplementation on Hepatic Fat and Associated Cardiovascular Risk Factors in Overweight Children with Nonalcoholic Fatty Liver Disease. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 734–741. [Google Scholar] [CrossRef]
- Della Corte, C.; Carpino, G.; De Vito, R.; De Stefanis, C.; Alisi, A.; Cianfarani, S.; Overi, D.; Mosca, A.; Stronati, L.; Cucchiara, S.; et al. Docosahexanoic Acid plus Vitamin D Treatment Improves Features of NAFLD in Children with Serum Vitamin D Deficiency: Results from a Single Centre Trial. PLoS ONE 2016, 11, e0168216. [Google Scholar] [CrossRef]
- Parker, H.M.; Cohn, J.S.; O’connor, H.T.; Garg, M.L.; Caterson, I.D.; George, J.; Johnson, N.A. Effect of Fish Oil Supplementation on Hepatic and Visceral Fat in Overweight Men: A Randomized Controlled Trial. Nutrients 2019, 11, 475. [Google Scholar] [CrossRef]
- Nogueira, M.A.; Oliveira, C.P.; Ferreira Alves, V.A.; Stefano, J.T.; Rodrigues, L.S.; Torrinhas, R.S.; Cogliati, B.; Barbeiro, H.; Carrilho, F.J.; Waitzberg, D.L. Omega-3 Polyunsaturated Fatty Acids in Treating Non-Alcoholic Steatohepatitis: A Randomized, Double-Blind, Placebo-Controlled Trial. Clin. Nutr. 2016, 35, 578–586. [Google Scholar] [CrossRef] [PubMed]
- Sanyal, A.J.; Abdelmalek, M.F.; Suzuki, A.; Cummings, O.W.; Chojkier, M. No Significant Effects of Ethyl-Eicosapentanoic Acid on Histologic Features of Nonalcoholic Steatohepatitis in a Phase 2 TriaL. Gastroenterology 2014, 147, 377–384.e1. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, J.W.; Lundkvist, P.; Jansson, P.A.; Johansson, L.; Kvarnström, M.; Moris, L.; Miliotis, T.; Forsberg, G.B.; Risérus, U.; Lind, L.; et al. Effects of dapagliflozin and n-3 carboxylic acids on non-alcoholic fatty liver disease in people with type 2 diabetes: A double-blind randomised placebo-controlled study. Diabetologia 2018, 61, 1923–1934. [Google Scholar] [CrossRef] [PubMed]
- Diamond, I.R.; Grant, R.C.; Pencharz, P.B.; de Silva, N.; Feldman, B.M.; Fitzgerald, P.; Sigalet, D.; Dicken, B.; Turner, J.; Marchand, V.; et al. Preventing the Progression of Intestinal Failure-Associated Liver Disease in Infants Using a Composite Lipid Emulsion: A Pilot Randomized Controlled Trial of SMOFlipid. J. Parenter. Enteral Nutr. 2017, 41, 866–877. [Google Scholar] [CrossRef]
- Klek, S.; Szczepanek, K.; Scislo, L.; Walewska, E.; Pietka, M.; Pisarska, M.; Pedziwiatr, M. Intravenous lipid emulsions and liver function in adult chronic intestinal failure patients: Results from a randomized clinical trial. Nutrition 2018, 55–56, 45–50. [Google Scholar] [CrossRef]
- Gura, K.M.; Lee, S.; Valim, C.; Zhou, J.; Kim, S.; Modi, B.P.; Arsenault, D.A.; Strijbosch, R.A.; Lopes, S.; Duggan, C.; et al. Safety and efficacy of a fish-oil-based fat emulsion in the treatment of parenteral nutrition-associated liver disease. Pediatrics 2008, 121, e678–e686. [Google Scholar] [CrossRef]
- Puder, M.; Valim, C.; Meisel, J.A.; Le, H.D.; de Meijer, V.E.; Robinson, E.M.; Zhou, J.; Duggan, C.; Gura, K.M. Parenteral fish oil improves outcomes in patients with parenteral nutrition-associated liver injury. Ann. Surg. 2009, 250, 395–402. [Google Scholar] [CrossRef]
- Gura, K.M.; Premkumar, M.H.; Calkins, K.L.; Puder, M. Fish Oil Emulsion Reduces Liver Injury and Liver Transplantation in Children with Intestinal Failure-Associated Liver Disease: A Multicenter Integrated Study. J. Pediatr. 2021, 230, 46–54.e2. [Google Scholar] [CrossRef]
- Gura, K.; Premkumar, M.H.; Calkins, K.L.; Puder, M. Intravenous Fish Oil Monotherapy as a Source of Calories and Fatty Acids Promotes Age-Appropriate Growth in Pediatric Patients with Intestinal Failure-Associated Liver Disease. J. Pediatr. 2020, 219, 98–105.e4. [Google Scholar] [CrossRef]
- Premkumar, M.H.; Carter, B.A.; Hawthorne, K.M.; King, K.; Abrams, S.A. High rates of resolution of cholestasis in parenteral nutrition-associated liver disease with fish oil-based lipid emulsion monotherapy. J. Pediatr. 2013, 162, 793–798.e1. [Google Scholar] [CrossRef] [PubMed]
- Jurewitsch, B.; Gardiner, G.; Naccarato, M.; Jeejeebhoy, K.N. Omega-3-enriched lipid emulsion for liver salvage in parenteral nutrition-induced cholestasis in the adult patient. J. Parenter Enteral Nutr. 2011, 35, 386–390. [Google Scholar] [CrossRef]
- Fernandes, G.; Kaila, B.; Jeejeebhoy, K.N.; Gramlich, L.; Armstrong, D.; Allard, J.P. Canadian home parenteral nutrition (HPN) registry: Validation and patient outcomes. J. Parenter. Enteral. Nutr. 2012, 36, 407–414. [Google Scholar] [CrossRef]
- Lee, S.I.; Valim, C.; Johnston, P.; Le, H.D.; Meisel, J.; Arsenault, D.A.; Gura, K.M.; Puder, M. Impact of fish oil-based lipid emulsion on serum triglyceride, bilirubin, and albumin levels in children with parenteral nutrition-associated liver disease. Pediatr. Res. 2009, 66, 698–703. [Google Scholar] [CrossRef] [PubMed]
- De Meijer, V.E.; Le, H.D.; Meisel, J.A.; Gura, K.M.; Puder, M. Parenteral fish oil as monotherapy prevents essential fatty acid deficiency in parenteral nutrition-dependent patients. J. Pediatr. Gastroenterol. Nutr. 2010, 50, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Mercer, D.F.; Hobson, B.D.; Fischer, R.T.; Talmon, G.A.; Perry, D.A.; Gerhardt, B.K.; Grant, W.J.; Botha, J.F.; Langnas, A.N.; Quiros-Tejeira, R.E.; et al. Hepatic fibrosis persists and progresses despite biochemical improvement in children treated with intravenous fish oil emulsion. J. Pediatr. Gastroenterol. Nutr. 2013, 56, 364–369. [Google Scholar] [CrossRef]
- Holvik, K.; Madar, A.A.; Meyer, H.E.; Lofthus, C.M.; Stene, L.C. Changes in the vitamin D endocrine system and bone turnover after oral vitamin D3 supplementation in healthy adults: Results of a randomised trial. BMC Endocr. Disord. 2012, 12, 7. [Google Scholar] [CrossRef]
- Linecker, M.; Limani, P.; Botea, F.; Popescu, I.; Alikhanov, R.; Efanov, M.; Kim, P.; Khatkov, I.; Raptis, D.A.; Tschuor, C.; et al. A randomized, double-blind study of the effects of omega-3 fatty acids (Omegaven) on outcome after major liver resection. BMC Gastroenterol. 2015, 15, 102. [Google Scholar] [CrossRef] [PubMed]
- Linecker, M.; Botea, F.; Aristotele, D.; Nicolaescu, D.; Limani, P.; Alikhanov, R.; Kim, P.; Wirsching, A.; Kron, P.; Schneider, M.A.; et al. Perioperative omega-3 fatty acids fail to confer protection in liver surgery: Results of a multicentric, double-blind, randomized controlled trial. J. Hepatol. 2020, 72, 498–505. [Google Scholar] [CrossRef]
- Uno, H.; Furukawa, K.; Suzuki, D.; Shimizu, H.; Ohtsuka, M.; Kato, A.; Yoshitomi, H.; Miyazaki, M. Immunonutrition Suppresses Acute Inflammatory Responses through Modulation of Resolvin E1 in Patients Undergoing Major Hepatobiliary Resection. Surgery 2016, 160, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Cockbain, A.J.; Volpato, M.; Race, A.D.; Munarini, A.; Fazio, C.; Belluzzi, A.; Loadman, P.M.; Toogood, G.J.; Hull, M.A. Anticolorectal cancer activity of the omega-3 polyunsaturated fatty acid eicosapentaenoic acid. Gut 2014, 63, 1760–1768. [Google Scholar] [CrossRef] [PubMed]
- Al-Taan, O.; Stephenson, J.A.; Spencer, L.; Pollard, C.; West, A.L.; Calder, P.C.; Metcalfe, M.; Dennison, A.R. Changes in plasma and erythrocyte omega-6 and omega-3 fatty acids in response to intravenous supply of omega-3 fatty acids in patients with hepatic colorectal metastases. Lipids Health Dis. 2013, 12, 64. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.S.; Liu, S.; Chen, X.M.; Huang, Z.G.; Zhang, D.W. Effects of N-3 Polyunsaturated Fatty Acids from Seal Oils on Nonalcoholic Fatty Liver Disease Associated with Hyperlipidemia. World J. Gastroenterol. 2008, 14, 6395. [Google Scholar] [CrossRef]
- Sofi, F.; Giangrandi, I.; Cesari, F.; Corsani, I.; Abbate, R.; Gensini, G.F.; Casini, A. Effects of a 1-Year Dietary Intervention with n-3 Polyunsaturated Fatty Acid-Enriched Olive Oil on Non-Alcoholic Fatty Liver Disease Patients: A Preliminary Study. Int. J. Food Sci. Nutr. 2010, 61, 792–802. [Google Scholar] [CrossRef] [PubMed]
- Tobin, D.; Brevik-Andersen, M.; Qin, Y.; Innes, J.K.; Calder, P.C. Evaluation of a High Concentrate Omega-3 for Correcting the Omega-3 Fatty Acid Nutritional Deficiency in Non-Alcoholic Fatty Liver Disease (CONDIN). Nutrients 2018, 10, 1126. [Google Scholar] [CrossRef]
- Yan, J.H.; Guan, B.J.; Gao, H.Y.; Peng, X.E. Omega-3 polyunsaturated fatty acid supplementation and non-alcoholic fatty liver disease: A meta-analysis of randomized controlled trials. Medicine 2018, 97, e12271. [Google Scholar] [CrossRef]
- Tanaka, N.; Sano, K.; Horiuchi, A.; Tanaka, E.; Kiyosawa, K.; Aoyama, T. Highly-purified eicosapentaenoic acid treatment improves nonalcoholic steatohepatitis. J. Clin. Gastroenterol. 2008, 42, 413–418. [Google Scholar] [CrossRef]
- Li, Y.H.; Yang, L.H.; Sha, K.H.; Liu, T.G.; Zhang, L.G.; Liu, X.X. Efficacy of Poly-Unsaturated Fatty Acid Therapy on Patients with Nonalcoholic Steatohepatitis. World J. Gastroenterol. 2015, 21, 7008–7013. [Google Scholar] [CrossRef]
- Zöhrer, E.; Alisi, A.; Jahnel, J.; Mosca, A.; Della Corte, C.; Crudele, A.; Fauler, G.; Nobili, V. Efficacy of Docosahexaenoic Acid–Choline–Vitamin E in Paediatric NASH: A Randomized Controlled Clinical Trial. Appl. Physiol. Nutr. Metab. 2017, 42, 948–954. [Google Scholar] [CrossRef] [PubMed]
- Tomioka, K.; Kakibuchi, N.; Murakami, Y.; Kawakami, T.; Takaguchi, K.; Kita, K.; Okita, M. Effects of Eicosapentaenoic Acid Supplementation in the Treatment of Chronic Hepatitis C Patients. J. Nutr. Sci. Vitaminol. 2005, 51, 419–425. [Google Scholar] [CrossRef][Green Version]
- Takaki, S.; Kawakami, Y.; Imamura, M.; Aikata, H.; Takahashi, S.; Ishihara, H.; Tsuji, K.; Aimitsu, S.; Kawakami, H.; Nakanishi, T.; et al. Eicosapentaenoic Acid Could Permit Maintenance of the Original Ribavirin Dose in Chronic Hepatitis C Virus Patients during the First 12 Weeks of Combination Therapy with Pegylated Interferon-α and Ribavirin: A Prospective Randomized Controlled Trial. Intervirology 2008, 50, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Popa, S.G.; Mota, M. Impact of Obesity and Omega-3 Polyunsaturated Fatty Acids on Fibrogenesis and Responsiveness to Antiviral Therapy in Chronic Hepatitis C. Rom. J. Intern. Med. 2007, 45, 165–170. [Google Scholar] [PubMed]
- Pazirandeh, S.; Ling, P.R.; Ollero, M.; Gordon, F.; Burns, D.L.; Bistrian, B.R. Supplementation of Arachidonic Acid plus Docosahexaenoic Acid in Cirrhotic Patients Awaiting Liver Transplantation: A Preliminary Study. J. Parenter. Enter. Nutr. 2007, 31, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Mariqueo, T.A.; Zúñiga-Hernández, J. Omega-3 Derivatives, Specialized pro-Resolving Mediators: Promising Therapeutic Tools for the Treatment of Pain in Chronic Liver Disease. Prostaglandins Leukot. Essent. Fat. Acids 2020, 158, 102095. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N. Controlling the Resolution of Acute Inflammation: A New Genus of Dual Anti-Inflammatory and Proresolving Mediators. J. Periodontol. 2008, 79, 1520–1526. [Google Scholar] [CrossRef] [PubMed]
- Qiu, W.; Guo, K.; Yi, L.; Gong, Y.; Huang, L.; Zhong, W. Resolvin E1 Reduces Hepatic Fibrosis in Mice with Schistosoma Japonicum Infection. Exp. Ther. Med. 2014, 7, 1481–1485. [Google Scholar] [CrossRef]
- Kuang, H.; Hua, X.; Zhou, J.; Yang, R. Resolvin D1 and E1 Alleviate the Progress of Hepatitis toward Liver Cancer in Long-Term Concanavalin A-Induced Mice through Inhibition of NF-ΚB Activity. Oncol. Rep. 2016, 35, 307–317. [Google Scholar] [CrossRef]
- Maciejewska, D.; Drozd, A.; Skonieczna-Żydecka, K.; Skórka-Majewicz, M.; Dec, K.; Jakubczyk, K.; Pilutin, A.; Stachowska, E. Eicosanoids in Nonalcoholic Fatty Liver Disease (NAFLD) Progression. Do Serum Eicosanoids Profile Correspond with Liver Eicosanoids Content during NAFLD Development and Progression? Molecules 2020, 25, 2026. [Google Scholar] [CrossRef]
- Jung, T.W.; Hwang, H.J.; Hong, H.C.; Choi, H.Y.; Yoo, H.J.; Baik, S.H.; Choi, K.M. Resolvin D1 Reduces ER Stress-Induced Apoptosis and Triglyceride Accumulation through JNK Pathway in HepG2 Cells. Mol. Cell. Endocrinol. 2014, 391, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Wang, Y.; Wang, L.; Yao, B.; Chen, T.; Li, Q.; Liu, Z.; Liu, R.; Niu, Y.; Song, T.; et al. Resolvin D1 Prevents Epithelial-Mesenchymal Transition and Reduces the Stemness Features of Hepatocellular Carcinoma by Inhibiting Paracrine of Cancer-Associated Fibroblast-Derived COMP. J. Exp. Clin. Cancer Res. 2019, 38, 170. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.W.; Lee, S.M. Resolvin D1 Protects the Liver from Ischemia/Reperfusion Injury by Enhancing: M2 Macrophage Polarization and Efferocytosis. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2016, 1861, 1025–1035. [Google Scholar] [CrossRef]
- Abshagen, K.; Hartmann, A.; Grüner, L.; Liebig, M.; Vollmar, B. Limited Potential of Resolvin D1 in Treatment of Cholestatic Liver Fibrosis. Hepatobiliary Surg. Nutr. 2019, 9, 587–596. [Google Scholar] [CrossRef]
- Li, R.; Wang, Y.; Zhao, E.; Wu, K.; Li, W.; Shi, L.; Wang, D.; Xie, G.; Yin, Y.; Deng, M.; et al. Maresin 1, a Proresolving Lipid Mediator, Mitigates Carbon Tetrachloride-Induced Liver Injury in Mice. Oxid. Med. Cell. Longev. 2016, 2016, 9203716. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.M.; Chung, G.; Kim, Y.H.; Park, C.K. The Role of Maresins in Inflammatory Pain: Function of Macrophages in Wound Regeneration. Int. J. Mol. Sci. 2019, 21, 5849. [Google Scholar] [CrossRef]
- Abdolmaleki, F.; Kovanen, P.T.; Mardani, R.; Gheibi-hayat, S.M.; Bo, S.; Sahebkar, A. Resolvins: Emerging Players in Autoimmune and Inflammatory Diseases. Clin. Rev. Allergy Immunol. 2020, 58, 82–91. [Google Scholar] [CrossRef]
- Giera, M.; Ioan-Facsinay, A.; Toes, R.; Gao, F.; Dalli, J.; Deelder, A.M.; Serhan, C.N.; Mayboroda, O.A. Lipid and Lipid Mediator Profiling of Human Synovial Fluid in Rheumatoid Arthritis Patients by Means of LC-MS/MS. Biochim. Biophys. Acta 2012, 1821, 1415–1421. [Google Scholar] [CrossRef]
- Herrera, B.S.; Hasturk, H.; Kantarci, A.; Freire, M.O.; Nguyen, O.; Kansal, S.; van Dyke, T.E. Impact of Resolvin E1 on Murine Neutrophil Phagocytosis in Type 2 Diabetes. Infect. Immun. 2015, 83, 792–801. [Google Scholar] [CrossRef][Green Version]
- Sima, C.; Paster, B.; Van Dyke, T.E. Function of Pro-Resolving Lipid Mediator Resolvin E1 in Type 2 Diabetes. Crit. Rev. Immunol. 2018, 38, 343–365. [Google Scholar] [CrossRef]
- Freire, M.O.; Dalli, J.; Serhan, C.N.; Van Dyke, T.E. Neutrophil Resolvin E1 Receptor Expression and Function in Type 2 Diabetes. J. Immunol. 2017, 198, 718–728. [Google Scholar] [CrossRef]
- Hasturk, H.; Abdallah, R.; Kantarci, A.; Nguyen, D.; Giordano, N.; Hamilton, J.; Van Dyke, T.E. Resolvin E1 (RvE1) Attenuates Atherosclerotic Plaque Formation in Diet and Inflammation-Induced Atherogenesis. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 1123–1133. [Google Scholar] [CrossRef] [PubMed]
- Colas, R.A.; Shinohara, M.; Dalli, J.; Chiang, N.; Serhan, C.N. Identification and Signature Profiles for Pro-Resolving and Inflammatory Lipid Mediators in Human Tissue. Am. J. Physiol. Cell Physiol. 2014, 307, C39–C54. [Google Scholar] [CrossRef] [PubMed]
- Mas, E.; Croft, K.D.; Zahra, P.; Barden, A.; Mori, T.A. Resolvins D1, D2, and Other Mediators of Self-Limited Resolution of Inflammation in Human Blood Following n-3 Fatty Acid Supplementation. Clin. Chem. 2012, 58, 1476–1484. [Google Scholar] [CrossRef] [PubMed]
- Souza, P.R.; Marques, R.M.; Gomez, E.A.; Colas, R.A.; De Matteis, R.; Zak, A.; Patel, M.; Collier, D.J.; Dalli, J. Enriched Marine Oil Supplements Increase Peripheral Blood Specialized Pro-Resolving Mediators Concentrations and Reprogram Host Immune Responses: A Randomized Double-Blind Placebo-Controlled Study. Circ. Res. 2020, 126, 75–90. [Google Scholar] [CrossRef] [PubMed]
- Barden, A.; Shinde, S.; Tsai, I.J.; Croft, K.D.; Beilin, L.J.; Puddey, I.B.; Mori, T.A. Effect of Weight Loss on Neutrophil Resolvins in the Metabolic Syndrome. Prostaglandins Leukot. Essent. Fat. Acids 2019, 148, 25–29. [Google Scholar] [CrossRef]
- Cata, J.P.; Velasquez, J.F.; Ramirez, M.F.; Vauthey, J.N.; Gottumukkala, V.; Conrad, C.; Kim, B.J.; Aloia, T. Inflammation and Pro-Resolution Inflammation after Hepatobiliary Surgery. World J. Surg. Oncol. 2017, 15, 152. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).