In Vitro Antimicrobial Activity and Probiotic Potential of Bifidobacterium and Lactobacillus against Species of Clostridium
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Growth Conditions
2.2. Antimicrobial Activity Screening
2.2.1. Agar Spot Test
2.2.2. Inhibition of Gas Production
2.3. Antimicrobial Activity after pH Adjustment
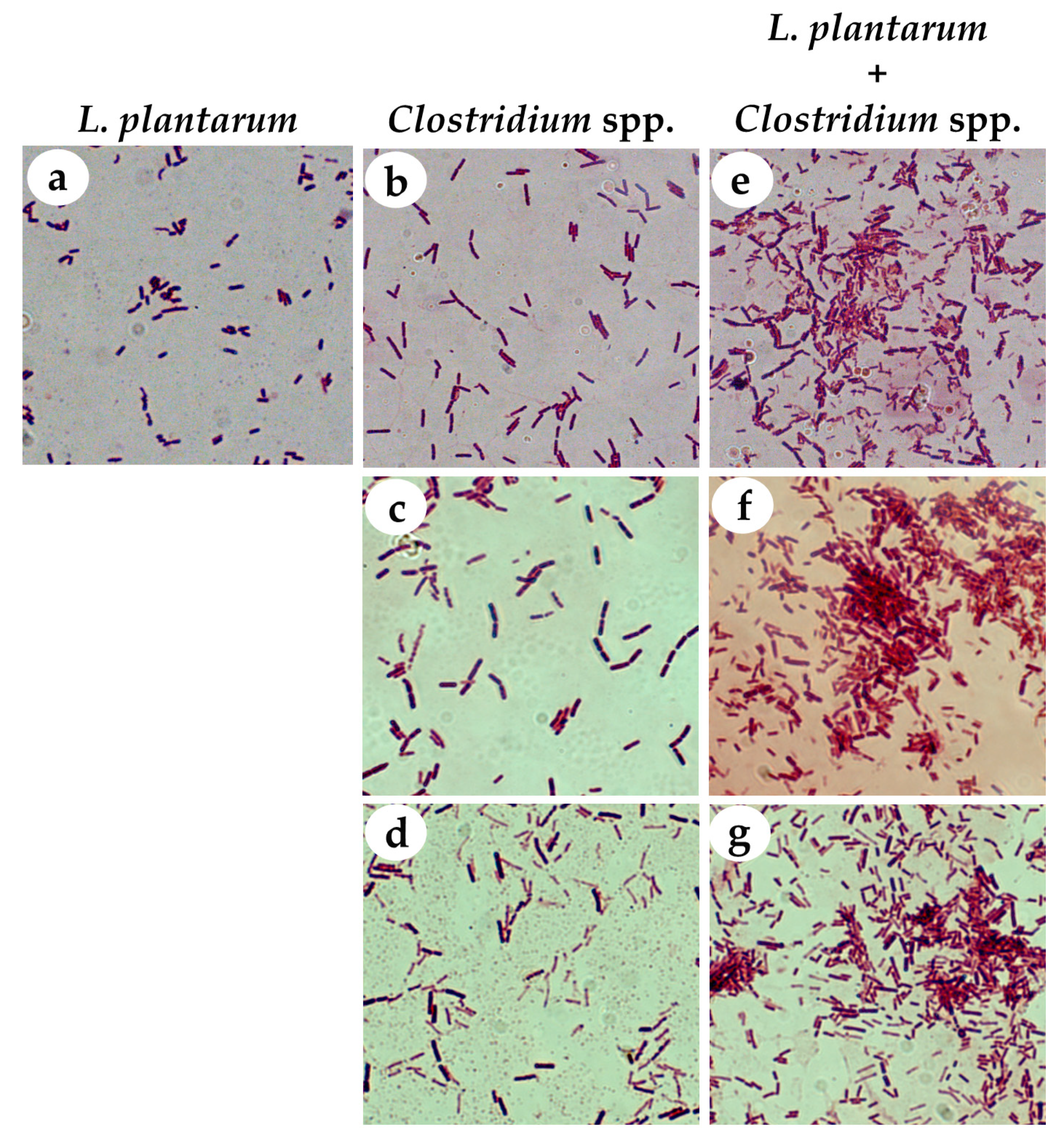
2.4. Coaggregation Test
2.5. Mucin Binding Assay
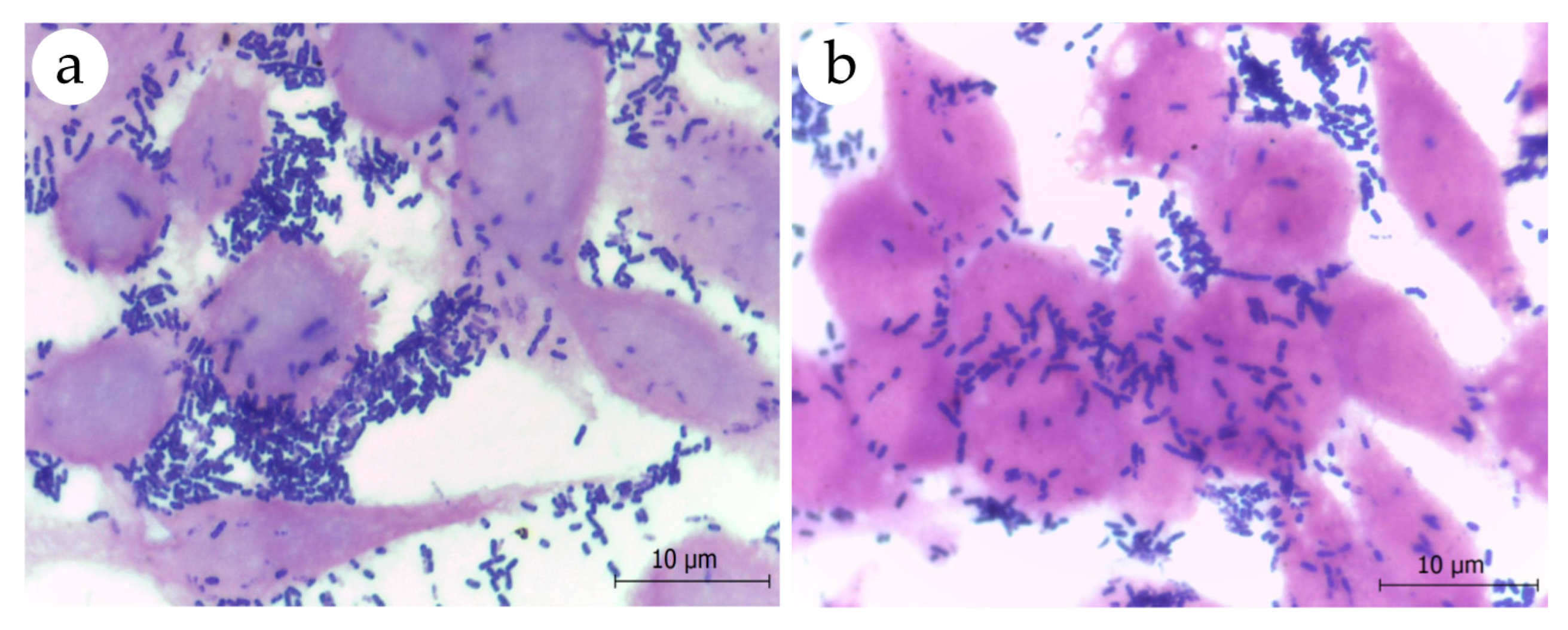
2.6. Adhesion to Eukaryotic Cells
2.7. Tolerance to Acidic pH and Bile Salts
2.8. Antibiotic Susceptibility Testing
2.9. Statistical Analyses
3. Results
3.1. Selection of Strains with Antimicrobial Activity against Clostridium spp.
3.2. L. plantarum ATCC 8014 Presents Probiotic Potential
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Perez-Lopez, A.; Behnsen, J.; Nuccio, S.P.; Raffatellu, M. Mucosal immunity to pathogenic intestinal bacteria. Nat. Rev. Immunol. 2016, 16, 135–148. [Google Scholar] [CrossRef]
- Reinoso Webb, C.; Koboziev, I.; Furr, K.L.; Grisham, M.B. Protective and pro-inflammatory roles of intestinal bacteria. Pathophysiology 2016, 23, 67–80. [Google Scholar] [CrossRef]
- Sonnenburg, J.L.; Bäckhed, F. Diet–microbiota interactions as moderators of human metabolism. Nature 2016, 535, 56. [Google Scholar] [CrossRef]
- Kau, A.L.; Ahern, P.P.; Griffin, N.W.; Goodman, A.L.; Gordon, J.I. Human nutrition, the gut microbiome and the immune system. Nature 2011, 474, 327–336. [Google Scholar] [CrossRef]
- Littman, D.R.; Pamer, E.G. Role of the commensal microbiota in normal and pathogenic host immune responses. Cell Host Microbe 2011, 10, 311–323. [Google Scholar] [CrossRef]
- Conlon, M.A.; Bird, A.R. The impact of diet and lifestyle on gut microbiota and human health. Nutrients 2014, 7, 17–44. [Google Scholar] [CrossRef]
- Donaldson, G.P.; Lee, S.M.; Mazmanian, S.K. Gut biogeography of the bacterial microbiota. Nat. Rev. Microbiol. 2016, 14, 20–32. [Google Scholar] [CrossRef]
- Prosberg, M.; Bendtsen, F.; Vind, I.; Petersen, A.M.; Gluud, L.L. The association between the gut microbiota and the inflammatory bowel disease activity: A systematic review and meta-analysis. Scand. J. Gastroenterol. 2016, 51, 1407–1415. [Google Scholar] [CrossRef]
- McFarland, L.V.; Ozen, M.; Dinleyici, E.C.; Goh, S. Comparison of pediatric and adult antibiotic-associated diarrhea and Clostridium difficile infections. World J. Gastroenterol. 2016, 22, 3078–3104. [Google Scholar] [CrossRef]
- Bennet, S.M.; Ohman, L.; Simren, M. Gut microbiota as potential orchestrators of irritable bowel syndrome. Gut Liver 2015, 9, 318–331. [Google Scholar] [CrossRef]
- Pammi, M.; Cope, J.; Tarr, P.I.; Warner, B.B.; Morrow, A.L.; Mai, V.; Gregory, K.E.; Kroll, J.S.; McMurtry, V.; Ferris, M.J.; et al. Intestinal dysbiosis in preterm infants preceding necrotizing enterocolitis: A systematic review and meta-analysis. Microbiome 2017, 5, 31. [Google Scholar] [CrossRef]
- Sircana, A.; Framarin, L.; Leone, N.; Berrutti, M.; Castellino, F.; Parente, R.; De Michieli, F.; Paschetta, E.; Musso, G. Altered gut microbiota in type 2 diabetes: Just a coincidence? Curr. Diab. Rep. 2018, 18, 98. [Google Scholar] [CrossRef]
- Durack, J.; Boushey, H.A.; Lynch, S.V. Airway microbiota and the implications of dysbiosis in asthma. Curr. Allergy Asthma Rep. 2016, 16, 52. [Google Scholar] [CrossRef]
- Boursier, J.; Mueller, O.; Barret, M.; Machado, M.; Fizanne, L.; Araujo-Perez, F.; Guy, C.D.; Seed, P.C.; Rawls, J.F.; David, L.A. The severity of nonalcoholic fatty liver disease is associated with gut dysbiosis and shift in the metabolic function of the gut microbiota. Hepatology 2016, 63, 764–775. [Google Scholar] [CrossRef]
- Tilg, H.; Adolph, T.E.; Gerner, R.R.; Moschen, A.R. The intestinal microbiota in colorectal cancer. Cancer Cell 2018, 33, 954–964. [Google Scholar] [CrossRef]
- Tremlett, H.; Bauer, K.C.; Appel-Cresswell, S.; Finlay, B.B.; Waubant, E. The gut microbiome in human neurological disease: A review. Ann. Neurol. 2017, 81, 369–382. [Google Scholar] [CrossRef]
- Lezutekong, J.N.; Nikhanj, A.; Oudit, G.Y. Imbalance of gut microbiome and intestinal epithelial barrier dysfunction in cardiovascular disease. Clin. Sci. 2018, 132, 901–904. [Google Scholar] [CrossRef]
- Rupnik, M.; Wilcox, M.H.; Gerding, D.N. Clostridium difficile infection: New developments in epidemiology and pathogenesis. Nat. Rev. Microbiol. 2009, 7, 526. [Google Scholar] [CrossRef]
- Olsen, M.A.; Yan, Y.; Reske, K.A.; Zilberberg, M.D.; Dubberke, E.R. Recurrent Clostridium difficile infection is associated with increased mortality. Clin. Microbiol. Infect. 2015, 21, 164–170. [Google Scholar] [CrossRef]
- Coursey, C.A.; Hollingsworth, C.L.; Wriston, C.; Beam, C.; Rice, H.; Bisset, G., 3rd. Radiographic predictors of disease severity in neonates and infants with necrotizing enterocolitis. AJR Am. J. Roentgenol. 2009, 193, 1408–1413. [Google Scholar] [CrossRef]
- Shah, T.A.; Meinzen-Derr, J.; Gratton, T.; Steichen, J.; Donovan, E.F.; Yolton, K.; Alexander, B.; Narendran, V.; Schibler, K.R. Hospital and neurodevelopmental outcomes of extremely low-birth-weight infants with necrotizing enterocolitis and spontaneous intestinal perforation. J. Perinatol. 2012, 32, 552–558. [Google Scholar] [CrossRef]
- Fitzgibbons, S.C.; Ching, Y.; Yu, D.; Carpenter, J.; Kenny, M.; Weldon, C.; Lillehei, C.; Valim, C.; Horbar, J.D.; Jaksic, T. Mortality of necrotizing enterocolitis expressed by birth weight categories. J. Pediatr. Surg. 2009, 44, 1072–1075, discussion 1075–1076. [Google Scholar] [CrossRef]
- Neu, J.; Walker, W.A. Necrotizing enterocolitis. N. Engl. J. Med. 2011, 364, 255–264. [Google Scholar] [CrossRef]
- Coggins, S.A.; Wynn, J.L.; Weitkamp, J.-H. Infectious causes of necrotizing enterocolitis. Clin. Perinatol. 2015, 42, 133–154. [Google Scholar] [CrossRef]
- Cassir, N.; Benamar, S.; Khalil, J.B.; Croce, O.; Saint-Faust, M.; Jacquot, A.; Million, M.; Azza, S.; Armstrong, N.; Henry, M. Clostridium butyricum strains and dysbiosis linked to necrotizing enterocolitis in preterm neonates. Clin. Infect. Dis. 2015, 61, 1107–1115. [Google Scholar] [CrossRef]
- Sato, Y.; Kujirai, D.; Emoto, K.; Yagami, T.; Yamada, T.; Izumi, M.; Ano, M.; Kase, K.; Kobayashi, K. Necrotizing enterocolitis associated with Clostridium butyricum in a Japanese man. Acute Med. Surg. 2018, 5, 194–198. [Google Scholar] [CrossRef]
- Schonherr-Hellec, S.; Klein, G.L.; Delannoy, J.; Ferraris, L.; Roze, J.C.; Butel, M.J.; Aires, J. Clostridial strain-specific characteristics associated with necrotizing enterocolitis. Appl. Environ. Microbiol. 2018, 84, e02428-17. [Google Scholar] [CrossRef]
- Cassir, N.; Benamar, S.; La Scola, B. Clostridium butyricum: From beneficial to a new emerging pathogen. Clin. Microbiol. Infect. 2016, 22, 37–45. [Google Scholar] [CrossRef]
- Uzal, F.A.; Freedman, J.C.; Shrestha, A.; Theoret, J.R.; Garcia, J.; Awad, M.M.; Adams, V.; Moore, R.J.; Rood, J.I.; McClane, B.A. Towards an understanding of the role of Clostridium perfringens toxins in human and animal disease. Future Microbiol. 2014, 9, 361–377. [Google Scholar] [CrossRef]
- Asha, N.; Tompkins, D.; Wilcox, M. Comparative analysis of prevalence, risk factors, and molecular epidemiology of antibiotic-associated diarrhea due to Clostridium difficile, Clostridium perfringens, and Staphylococcus aureus. J. Clin. Microbiol. 2006, 44, 2785–2791. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Kelly, C.R.; de Leon, L.; Jasutkar, N. Fecal microbiota transplantation for relapsing Clostridium difficile infection in 26 patients: Methodology and results. J. Clin. Gastroenterol. 2012, 46, 145–149. [Google Scholar] [CrossRef]
- Walker, A.W.; Lawley, T.D. Therapeutic modulation of intestinal dysbiosis. Pharmacol. Res. 2013, 69, 75–86. [Google Scholar] [CrossRef]
- Hempel, S.; Newberry, S.J.; Maher, A.R.; Wang, Z.; Miles, J.N.; Shanman, R.; Johnsen, B.; Shekelle, P.G. Probiotics for the prevention and treatment of antibiotic-associated diarrhea: A systematic review and meta-analysis. JAMA 2012, 307, 1959–1969. [Google Scholar] [CrossRef]
- Lau, C.S.; Chamberlain, R.S. Probiotics are effective at preventing Clostridium difficile-associated diarrhea: A systematic review and meta-analysis. Int. J. Gen. Med. 2016, 9, 27. [Google Scholar]
- AlFaleh, K.; Anabrees, J. Probiotics for prevention of necrotizing enterocolitis in preterm infants. Evid. Based Child Health 2014, 9, 584–671. [Google Scholar] [CrossRef]
- Underwood, M.A. Impact of probiotics on necrotizing enterocolitis. Semin. Perinatol. 2017, 41, 41–51. [Google Scholar] [CrossRef]
- Sanders, M.E.; Benson, A.; Lebeer, S.; Merenstein, D.J.; Klaenhammer, T.R. Shared mechanisms among probiotic taxa: Implications for general probiotic claims. Curr. Opin. Biotechnol. 2018, 49, 207–216. [Google Scholar] [CrossRef]
- Lebeer, S.; Bron, P.A.; Marco, M.L.; Van Pijkeren, J.P.; O’Connell Motherway, M.; Hill, C.; Pot, B.; Roos, S.; Klaenhammer, T. Identification of probiotic effector molecules: Present state and future perspectives. Curr. Opin. Biotechnol. 2018, 49, 217–223. [Google Scholar] [CrossRef]
- Singhi, S.C.; Kumar, S. Probiotics in critically ill children. F1000Res 2016, 5. [Google Scholar] [CrossRef]
- Anas, M.; Eddine, H.J.; Mebrouk, K. Antimicrobial activity of Lactobacillus species isolated from Algerian raw goat’s milk against Staphylococcus aureus. World J. Dairy Food Sci. 2008, 3, 39–49. [Google Scholar]
- Golic, N.; Veljovic, K.; Popovic, N.; Djokic, J.; Strahinic, I.; Mrvaljevic, I.; Terzic-Vidojevic, A. In vitro and in vivo antagonistic activity of new probiotic culture against Clostridium difficile and Clostridium perfringens. BMC Microbiol. 2017, 17, 108. [Google Scholar] [CrossRef]
- Gaspar, C.; Donders, G.G.; Palmeira-de-Oliveira, R.; Queiroz, J.A.; Tomaz, C.; Martinez-de-Oliveira, J.; Palmeira-de-Oliveira, A. Bacteriocin production of the probiotic Lactobacillus acidophilus KS400. AMB Express 2018, 8, 153. [Google Scholar] [CrossRef]
- Reid, G.; McGroarty, J.A.; Domingue, P.G.; Chow, A.W.; Bruce, A.W.; Eisen, A.; Costerton, J.W. Coaggregation of urogenital bacteria in vitro and in vivo. Curr. Microbiol. 1990, 20, 47–52. [Google Scholar] [CrossRef]
- Do Carmo, M.S.; Noronha, F.M.; Arruda, M.O.; Costa, E.P.; Bomfim, M.R.; Monteiro, A.S.; Ferro, T.A.; Fernandes, E.S.; Giron, J.A.; Monteiro-Neto, V. Lactobacillus fermentum ATCC 23271 displays in vitro inhibitory activities against Candida spp. Front. Microbiol. 2016, 7, 1722. [Google Scholar] [CrossRef]
- Tallon, R.; Arias, S.; Bressollier, P.; Urdaci, M. Strain-and matrix-dependent adhesion of Lactobacillus plantarum is mediated by proteinaceous bacterial compounds. J. Appl. Microbiol. 2007, 102, 442–451. [Google Scholar] [CrossRef]
- Munoz-Quezada, S.; Chenoll, E.; Vieites, J.M.; Genoves, S.; Maldonado, J.; Bermudez-Brito, M.; Gomez-Llorente, C.; Matencio, E.; Bernal, M.J.; Romero, F.; et al. Isolation, identification and characterisation of three novel probiotic strains (Lactobacillus paracasei CNCM I-4034, Bifidobacterium breve CNCM I-4035 and Lactobacillus rhamnosus CNCM I-4036) from the faeces of exclusively breast-fed infants. Br. J. Nutr. 2013, 109 (Suppl. 2), S51–S62. [Google Scholar] [CrossRef]
- Charteris, W.P.; Kelly, P.M.; Morelli, L.; Collins, J.K. Antibiotic susceptibility of potentially probiotic Lactobacillus species. J. Food Prot. 1998, 61, 1636–1643. [Google Scholar] [CrossRef]
- CLSI. Perfomance Standards for Antimicrobial Susceptibility Testing (Supplement M100), 27th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2017; p. 250. [Google Scholar]
- Iannitti, T.; Palmieri, B. Therapeutical use of probiotic formulations in clinical practice. Clin. Nutr. 2010, 29, 701–725. [Google Scholar] [CrossRef]
- Aceti, A.; Gori, D.; Barone, G.; Callegari, M.L.; Di Mauro, A.; Fantini, M.P.; Indrio, F.; Maggio, L.; Meneghin, F.; Morelli, L.; et al. Probiotics for prevention of necrotizing enterocolitis in preterm infants: Systematic review and meta-analysis. Ital. J. Pediatr. 2015, 41, 89. [Google Scholar] [CrossRef]
- Coman, M.; Verdenelli, M.; Cecchini, C.; Silvi, S.; Orpianesi, C.; Boyko, N.; Cresci, A. In vitro evaluation of antimicrobial activity of Lactobacillus rhamnosus IMC 501®, Lactobacillus paracasei IMC 502® and SYNBIO® against pathogens. J. Appl. Microbiol. 2014, 117, 518–527. [Google Scholar] [CrossRef] [PubMed]
- Do Carmo, M.S.; Santos, C.I.D.; Araujo, M.C.; Giron, J.A.; Fernandes, E.S.; Monteiro-Neto, V. Probiotics, mechanisms of action, and clinical perspectives for diarrhea management in children. Food Funct. 2018, 9, 5074–5095. [Google Scholar] [CrossRef]
- Nes, I.F.; Kjos, M.; Diep, D.B. Antimicrobial Components of Lactic Acid Bacteria. In Lactic Acid Bacteria: Microbiological and Functional Aspects, 4th ed.; Lahtinen, A., Ouwehand, A.C., Salminen, S., Wright, A., Eds.; CRC Press: Boca Raton, FL, USA, 2012; pp. 285–330. [Google Scholar]
- Lee, Y.K.; Salminen, S. Handbook of Probiotics and Prebiotics; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Amin, M.; Moradi Choghakabodi, P.; Alhassan Hamidi, M.; Najafian, M.; Farajzadeh Sheikh, A. In vitro antimicrobial activities of metabolites from vaginal Lactobacillus strains against Clostridium perfringens isolated from a woman’s vagina. J. Chin. Med. Assoc. 2017, 80, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Wiedemann, I.; Bottiger, T.; Bonelli, R.R.; Schneider, T.; Sahl, H.G.; Martinez, B. Lipid II-based antimicrobial activity of the lantibiotic plantaricin C. Appl. Environ. Microbiol. 2006, 72, 2809–2814. [Google Scholar] [CrossRef] [PubMed]
- Rea, M.C.; Clayton, E.; O’Connor, P.M.; Shanahan, F.; Kiely, B.; Ross, R.P.; Hill, C. Antimicrobial activity of lacticin 3,147 against clinical Clostridium difficile strains. J. Med. Microbiol. 2007, 56, 940–946. [Google Scholar] [CrossRef]
- Kos, B.; Šušković, J.; Vuković, S.; Šimpraga, M.; Frece, J.; Matošić, S. Adhesion and aggregation ability of probiotic strain Lactobacillus acidophilus M92. J. Appl. Microbiol. 2003, 94, 981–987. [Google Scholar] [CrossRef]
- Van Tassell, M.L.; Miller, M.J. Lactobacillus adhesion to mucus. Nutrients 2011, 3, 613–636. [Google Scholar] [CrossRef]
- Devirgiliis, C.; Zinno, P.; Perozzi, G. Update on antibiotic resistance in foodborne Lactobacillus and Lactococcus species. Front. Microbiol. 2013, 4, 301. [Google Scholar] [CrossRef]
- FAO/WHO. Joint FAO/WHO Expert Consultation on Evaluation of Health and Nutritional Properties of Probiotics in Food Including Powder Milk with Live Lactic Acid Bacteria; FAO/WHO: Rome, Italy, 2001; pp. 1–4. [Google Scholar]
- Posno, M.; Leer, R.J.; van Luijk, N.; van Giezen, M.J.; Heuvelmans, P.T.; Lokman, B.C.; Pouwels, P.H. Incompatibility of Lactobacillus vectors with replicons derived from small cryptic Lactobacillus plasmids and segregational instability of the introduced vectors. Appl. Environ. Microbiol. 1991, 57, 1822–1828. [Google Scholar]
- Fraqueza, M.J. Antibiotic resistance of lactic acid bacteria isolated from dry-fermented sausages. Int. J. Food Microbiol. 2015, 212, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Zhang, W.; Guo, H.; Pan, L.; Zhang, H.; Sun, T. Comparative studies on antibiotic resistance in Lactobacillus casei and Lactobacillus plantarum. Food Control 2015, 50, 250–258. [Google Scholar] [CrossRef]
- Brook, I. Clostridial Infections in Children: Spectrum and Management. Curr. Infect. Dis. Rep. 2015, 17, 47. [Google Scholar] [CrossRef] [PubMed]
- Debast, S.; Bauer, M.; Kuijper, E.; Committee. European Society of Clinical Microbiology and Infectious Diseases: Update of the treatment guidance document for Clostridium difficile infection. Clin. Microbiol. Infect. 2014, 20, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Agustina, R.; Kok, F.J.; van de Rest, O.; Fahmida, U.; Firmansyah, A.; Lukito, W.; Feskens, E.J.; van den Heuvel, E.G.; Albers, R.; Bovee-Oudenhoven, I.M. Randomized trial of probiotics and calcium on diarrhea and respiratory tract infections in Indonesian children. Pediatrics 2012, 129, e1155–e1164. [Google Scholar] [CrossRef] [PubMed]
- Bron, P.A.; Tomita, S.; Mercenier, A.; Kleerebezem, M. Cell surface-associated compounds of probiotic lactobacilli sustain the strain-specificity dogma. Curr. Opin. Microbiol. 2013, 16, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Snel, J.; Vissers, Y.M.; Smit, B.A.; Jongen, J.M.; van der Meulen, E.T.; Zwijsen, R.; Ruinemans-Koerts, J.; Jansen, A.P.; Kleerebezem, M.; Savelkoul, H.F. Strain-specific immunomodulatory effects of Lactobacillus plantarum strains on birch-pollen-allergic subjects out of season. Clin. Exp. Allergy 2011, 41, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Hughes, D.; Andersson, D.I. Evolutionary Trajectories to Antibiotic Resistance. Annu. Rev. Microbiol. 2017, 71, 579–596. [Google Scholar] [CrossRef] [PubMed]
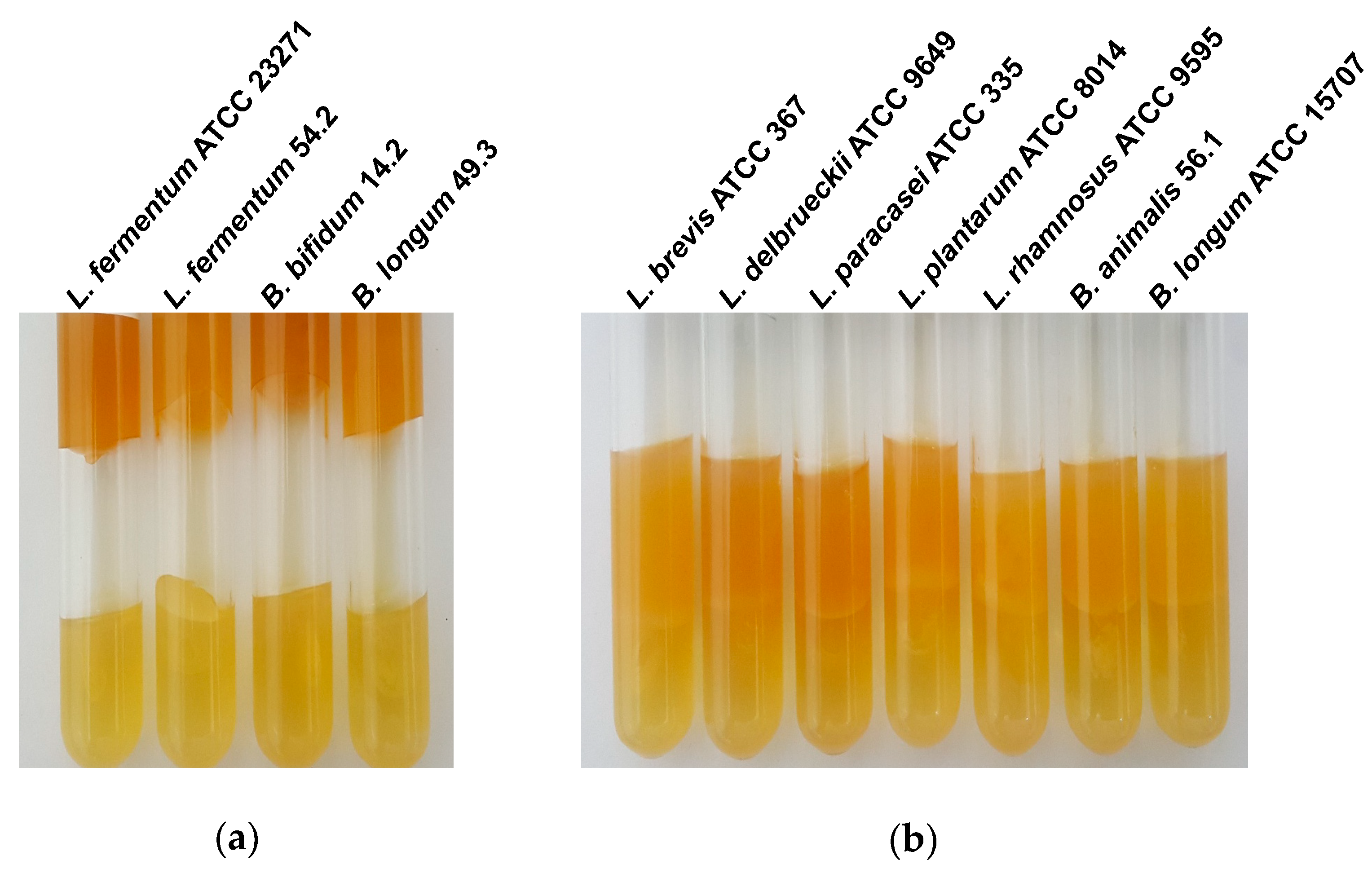
| Potential Probiotics | Diameter of Inhibition Zones (mm ±SD) of: | ||
|---|---|---|---|
| Clostridium butyricum | Clostridium difficile | Clostridium perfringens | |
| Bifidobacterium animalis 56.1 | 0 | 0 | 0 |
| Bifidobacterium bifidum 14.2 | 12 (1.6) | 12 (0.4) | 11 (1.7) |
| Bifidobacterium longum ATCC 15707 | 11 (0.4) | 12 (0.2) | 11 (1.4) |
| Bifidobacterium longum 49.3 | 12 (0.4) | 11 (0.0) | 10 (0.7) |
| Lactobacillus brevis ATCC 367 | 9 (0.4) | 0 | 0 |
| Lactobacillus delbrueckii ATCC 9649 | 10 (1.5) | 11 (0.7) | 12 (2.4) |
| Lactobacillus fermentum ATCC 23271 | 10 (1.1) | 10 (0.5) | 10 (0.6) |
| Lactobacillus fermentum 54.2 | 13 (0.7) | 9 (0,4) | 10 (0.0) |
| Lactobacillus paracasei ATCC 335 | 11 (0.5) | 12 (0.3) | 11 (0.4) |
| Lactobacillus plantarum ATCC 8014 | 17 (0.8) | 13 (1.1) | 13 (0.6) |
| Lactobacillus rhamnosus ATCC 9595 | 11 (0.0) | 0 | 0 |
| Lactobacillus rhamnosus GG ATCC 53103 | 10 (0.9) | 0 | 0 |
| Strains | Without Buffer | With Buffer |
|---|---|---|
| B. animalis 56.1 | + | + |
| B. bifidum 14.2 | + | − |
| B. longum ATCC 15707 | + | + |
| B. longum 49.3 | − | − |
| L. brevis ATCC 367 | + | + |
| L. delbrueckii ATCC 9649 | + | + |
| L. fermentum ATCC 23271 | − | − |
| L. fermentum 54.2 | − | − |
| L. paracasei ATCC 335 | + | + |
| L. plantarum ATCC 8014 | + | + |
| L. rhamnosus ATCC 9595 | + | + |
| Clostridium spp. | Inhibition Zone Diameters of CFSN in mm (±SD) at: | t1 | p Value 1 | |
|---|---|---|---|---|
| pH 4.3 | pH 6.5 | |||
| C. butyricum ATCC 860 | 16.5 (0.5) | 14.7 (0.5) | 5.97 | 0.002 |
| C. difficile ATCC 9689 | 14.2 (0.8) | 13.2 (0.4) | 2.24 | 0.076 |
| C. perfringens ATCC 12924 | 14.3 (0.8) | 13.7 (0.5) | 1.35 | 0.235 |
| Assays 1 | L. plantarum ATCC 8014 | L. fermentum ATCC 23271 | p Value 2 |
|---|---|---|---|
| Cell adhesion | 7.602 (±0.135) | 7.349 (±0.053) | 0.0037 |
| Mucin binding | 5.057 (±0.062) | 5.370 (±0.031) | <0.0001 |
| Conditions | % Survival (±SD) 1 | p Value 2 | |
|---|---|---|---|
| L. plantarum | L. fermentum | ||
| pH 2.0 | 70.3 (±4.46) | 64.7 (±6.49) | 0.1155 |
| pH 4.0 | 97.8 (±5.67) | 106.2 (±9.36) | 0.7606 |
| Bile salts 0.5% | 110.8 (±12.04) | 112.7 (±8.79) | 0.0912 |
| Bile salts 1.0% | 90.1 (±3.77) | 92.6 (±3.07) | 0.2419 |
| Antibiotics | Inhibition Zone Diameters mm (±SD) | Interpretation 1 |
|---|---|---|
| Clindamycin | 25.7 (1.1) | Susceptible |
| Chloramphenicol | 20.3 (1.5) | |
| Erythromycin | 30.0 (1.0) | |
| Gentamicin | 14.5 (0.5) | |
| Penicillin | 33.7 (1.2) | |
| Rifampicin | 23.3 (0.6) | |
| Tetracycline | 31.0 (1.0) | |
| Co-trimoxazole | 14.3 (0.6) | Moderately susceptible |
| Ciprofloxacin | 9.3 (0.6) | Resistant |
| Vancomycin | 0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monteiro, C.R.A.V.; do Carmo, M.S.; Melo, B.O.; Alves, M.S.; dos Santos, C.I.; Monteiro, S.G.; Bomfim, M.R.Q.; Fernandes, E.S.; Monteiro-Neto, V. In Vitro Antimicrobial Activity and Probiotic Potential of Bifidobacterium and Lactobacillus against Species of Clostridium. Nutrients 2019, 11, 448. https://doi.org/10.3390/nu11020448
Monteiro CRAV, do Carmo MS, Melo BO, Alves MS, dos Santos CI, Monteiro SG, Bomfim MRQ, Fernandes ES, Monteiro-Neto V. In Vitro Antimicrobial Activity and Probiotic Potential of Bifidobacterium and Lactobacillus against Species of Clostridium. Nutrients. 2019; 11(2):448. https://doi.org/10.3390/nu11020448
Chicago/Turabian StyleMonteiro, Cinara R. A. V., Monique S. do Carmo, Bruna O. Melo, Matheus S. Alves, Camilla I. dos Santos, Sílvio G. Monteiro, Maria Rosa Q. Bomfim, Elizabeth S. Fernandes, and Valério Monteiro-Neto. 2019. "In Vitro Antimicrobial Activity and Probiotic Potential of Bifidobacterium and Lactobacillus against Species of Clostridium" Nutrients 11, no. 2: 448. https://doi.org/10.3390/nu11020448
APA StyleMonteiro, C. R. A. V., do Carmo, M. S., Melo, B. O., Alves, M. S., dos Santos, C. I., Monteiro, S. G., Bomfim, M. R. Q., Fernandes, E. S., & Monteiro-Neto, V. (2019). In Vitro Antimicrobial Activity and Probiotic Potential of Bifidobacterium and Lactobacillus against Species of Clostridium. Nutrients, 11(2), 448. https://doi.org/10.3390/nu11020448






