Tryptophan-Tyrosine Dipeptide, the Core Sequence of β-Lactolin, Improves Memory by Modulating the Dopamine System
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animals
2.3. Spontaneous Alteration Test
2.4. Monoamine Analysis
2.5. Monoamine Oxidase (MAO) Activity Assay
2.6. Injection of Adeno-Associated Viruses (AAV) to the Hippocampus
2.7. Statistical Analysis
3. Results
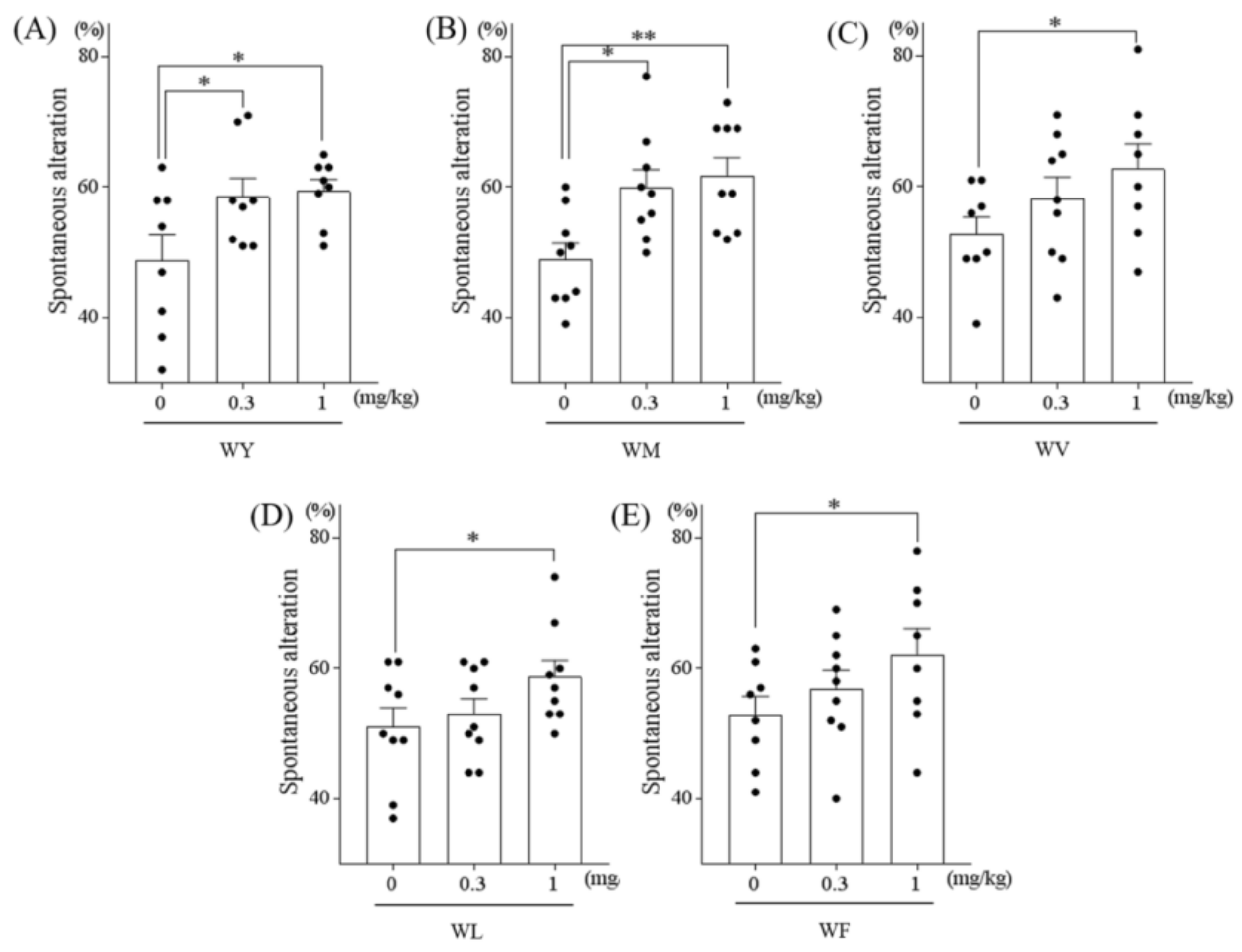
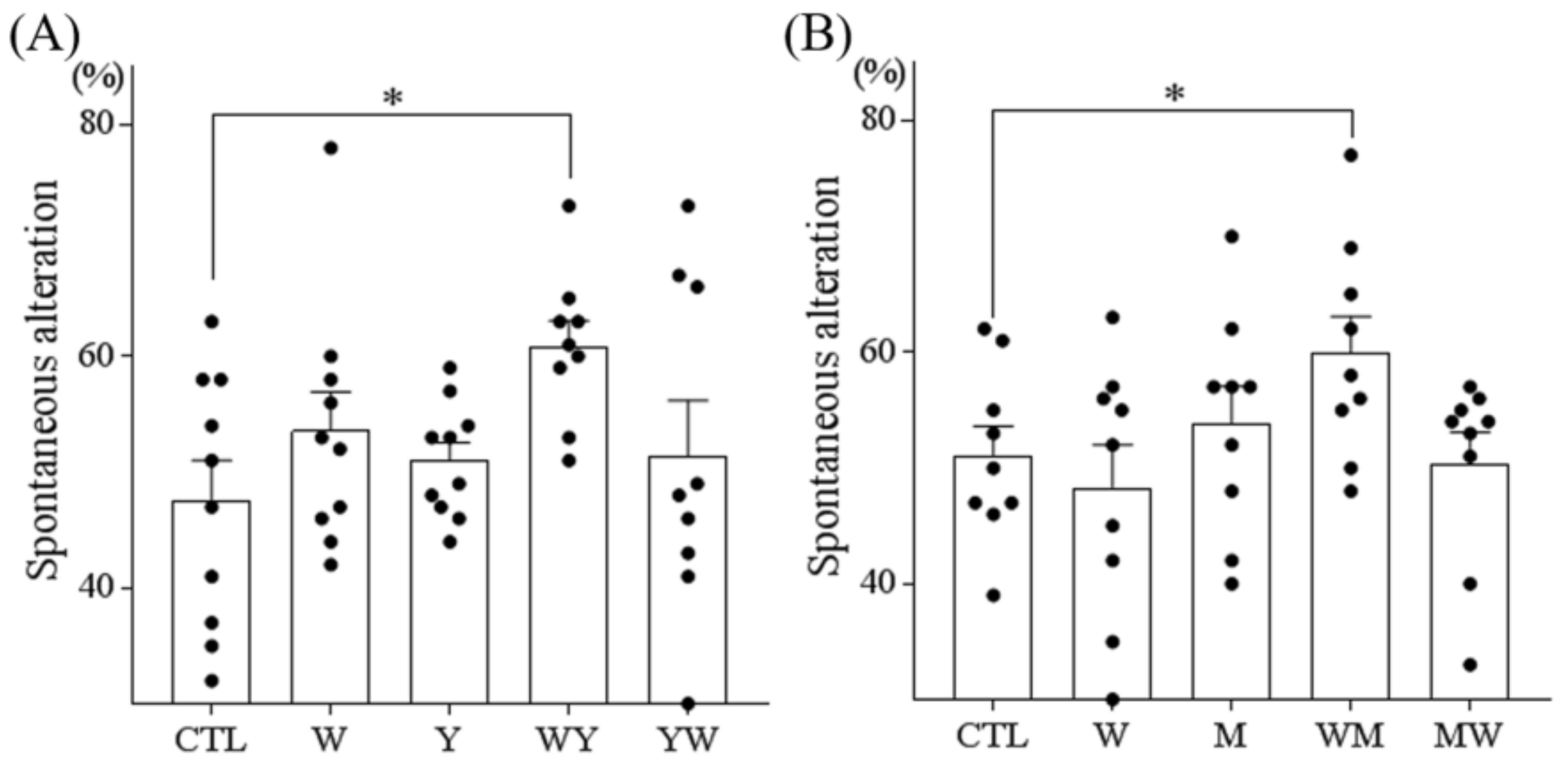
3.1. Tryptophan-Containing Dipeptides Improved Memory Impairment in Amnesic Mice
3.2. Dipeptides Containing Tryptophan at the N-Terminus But Not at the C-Terminus Improved Memory Impairment
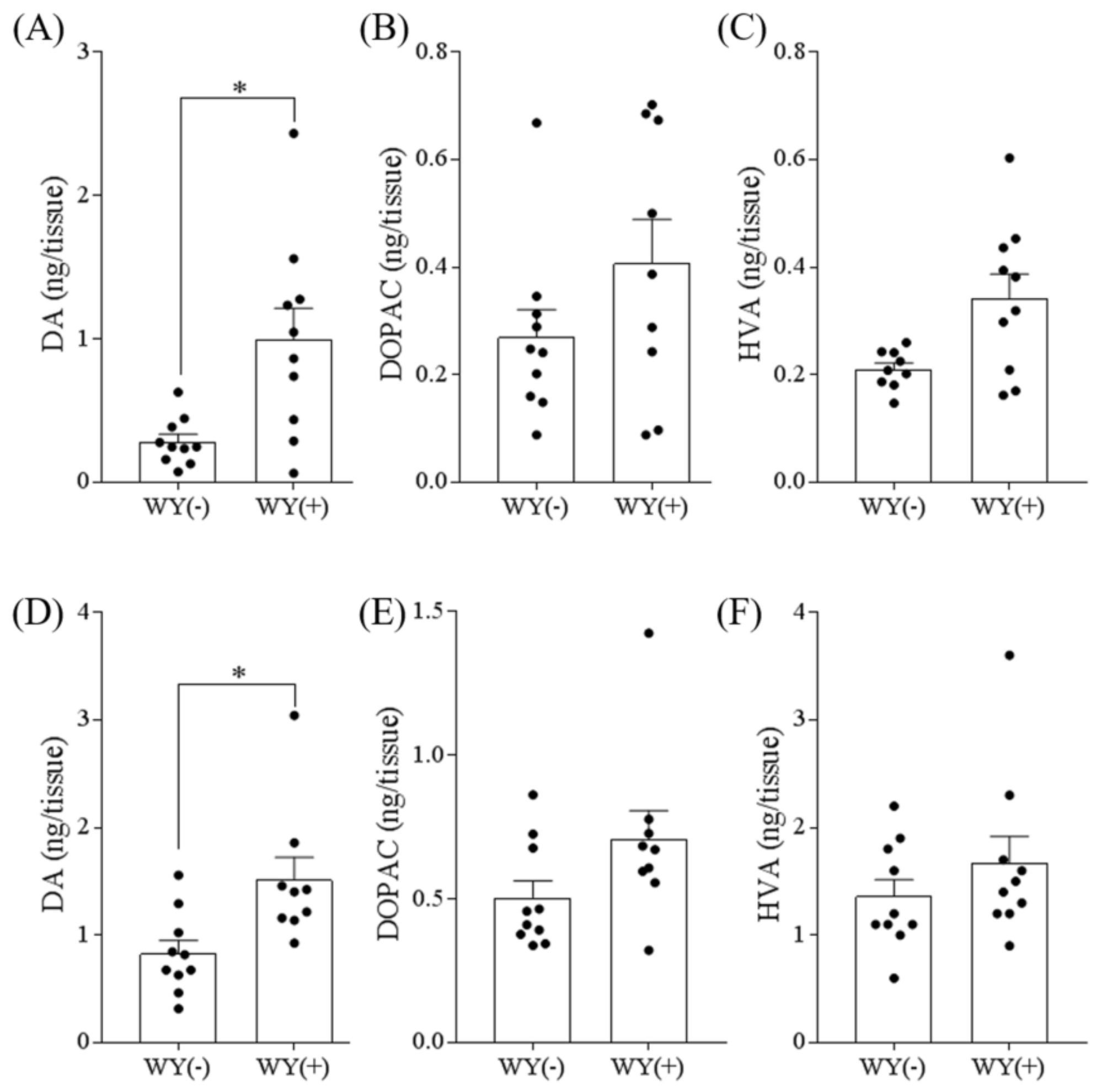
3.3. WY Peptide Increased Dopamine Levels in the Hippocampus and Frontal Cortex
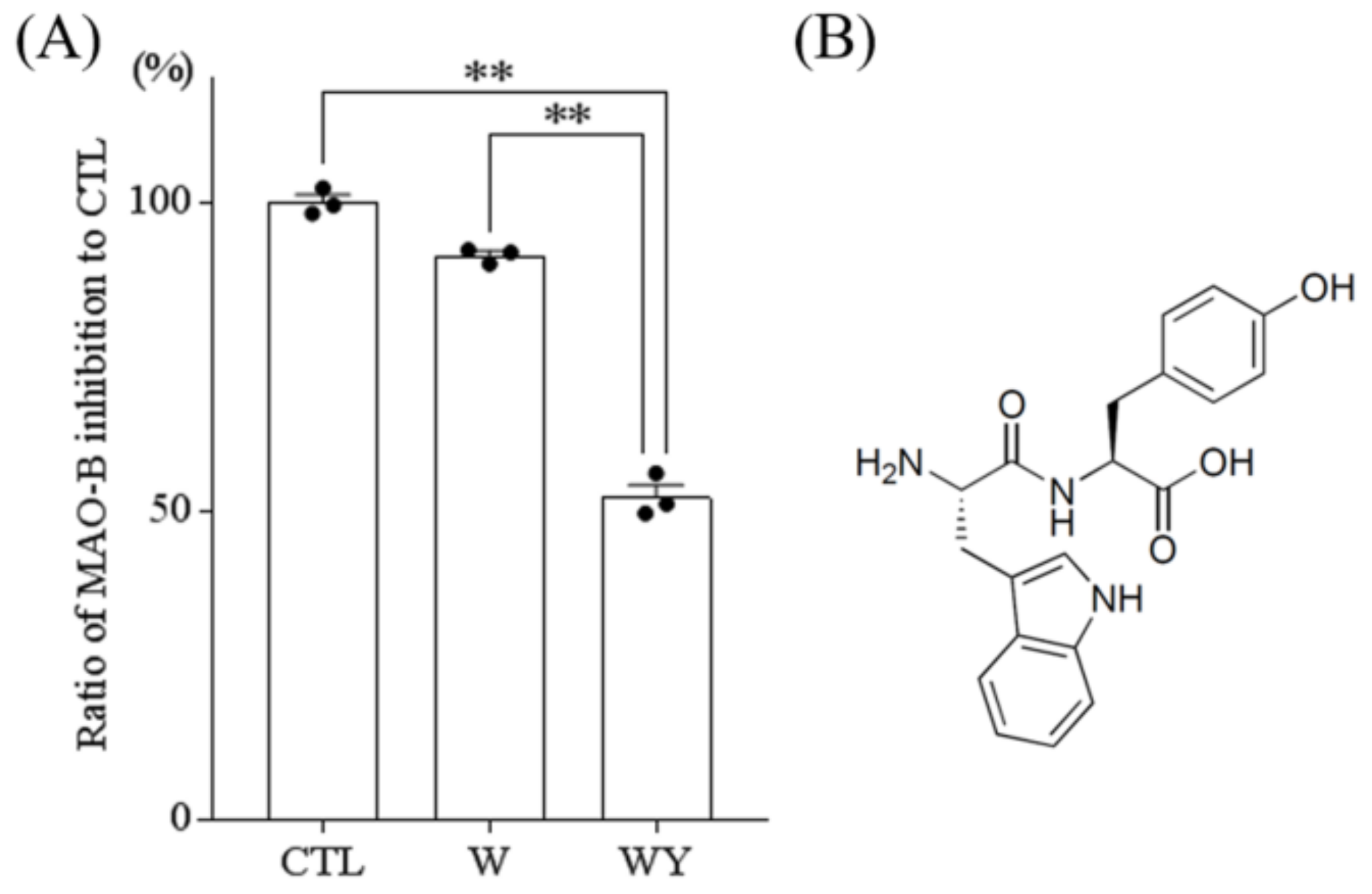
3.4. WY Peptide Inhibited the Activity of MAO
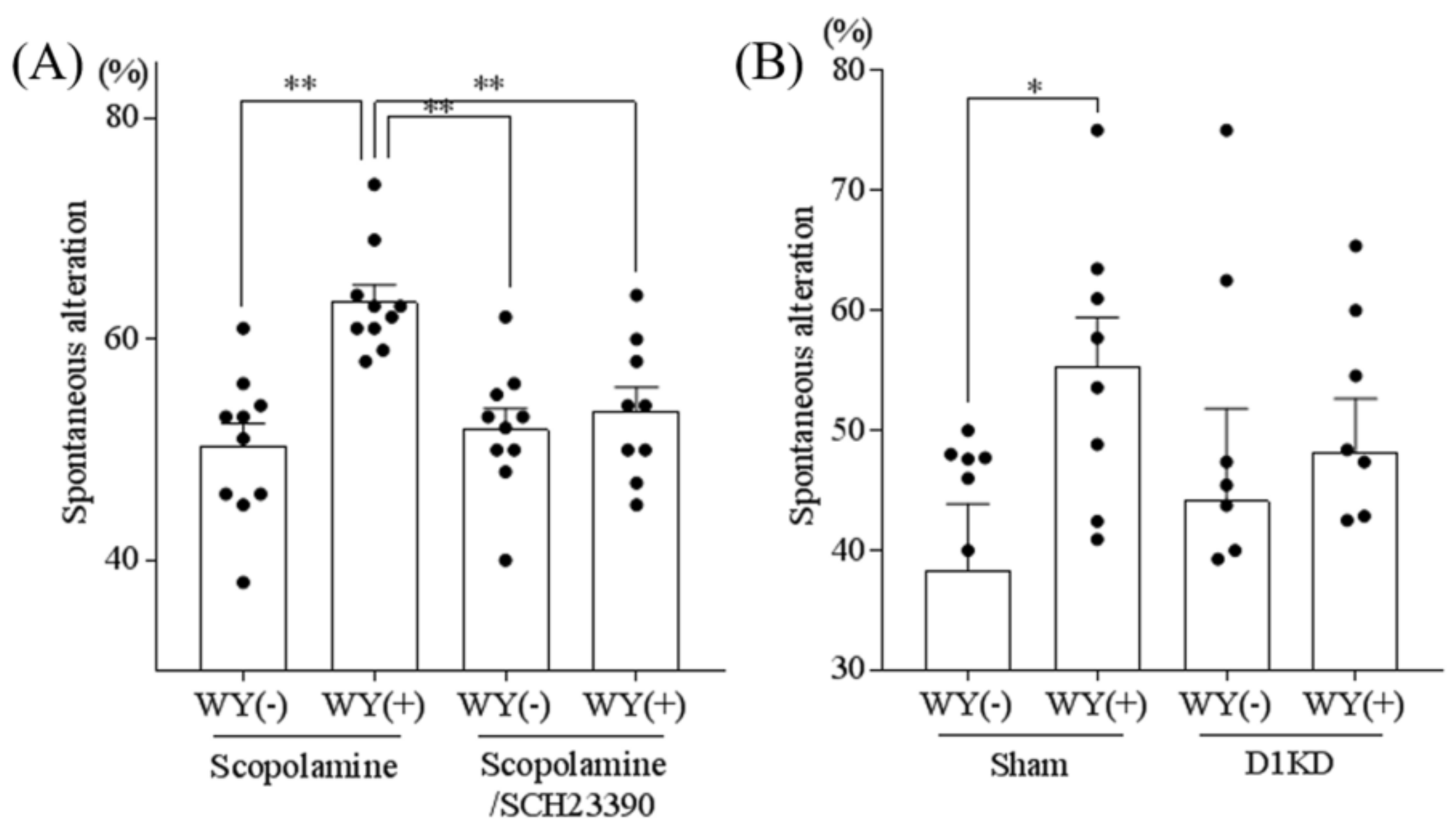
3.5. Inhibition of the Dopamine D1 Receptor Attenuated the WY-Induced Memory Improvement
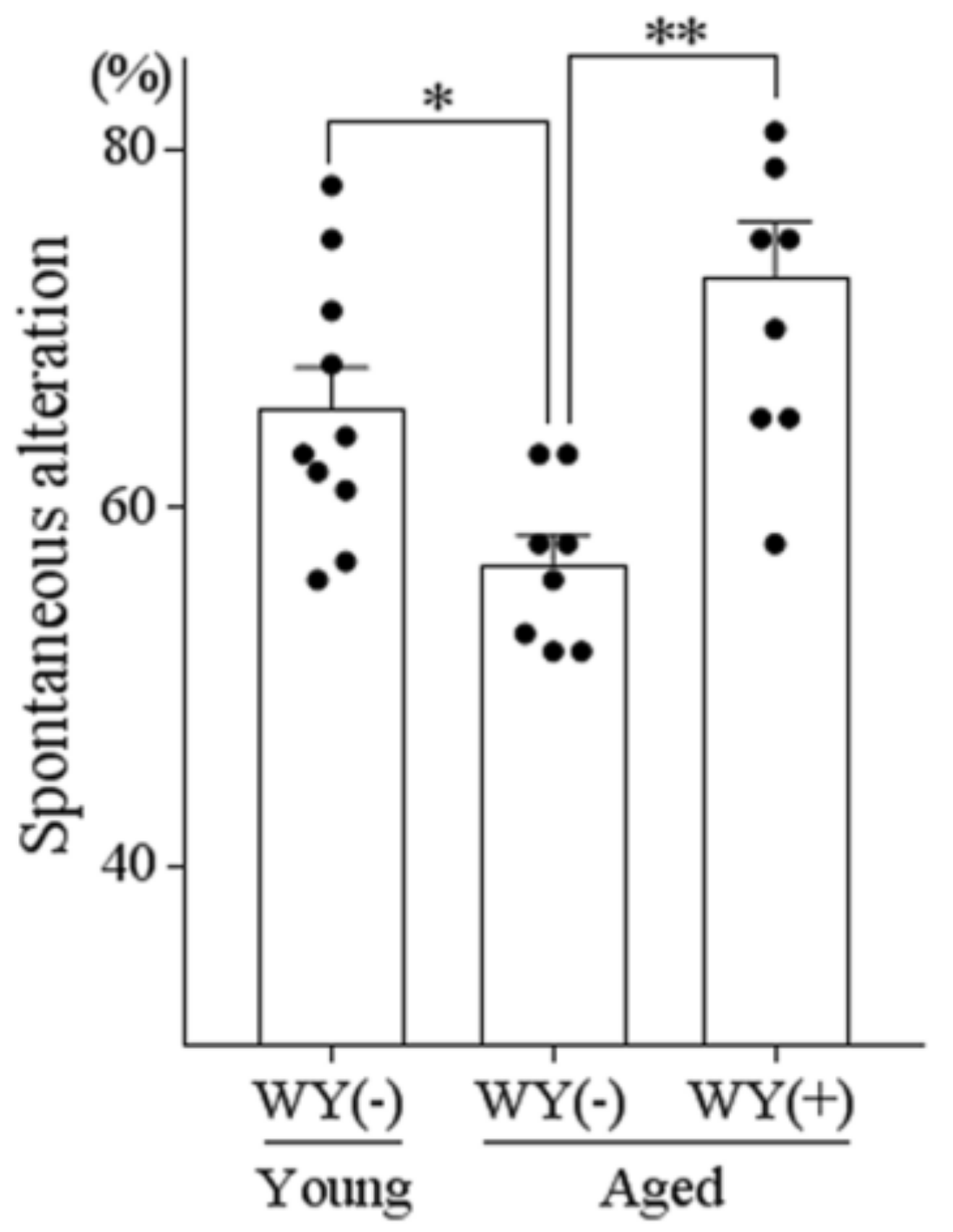
3.6. WY Peptide Improved Age-Related Memory Impairment
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ano, Y.; Nakayama, H. Preventive effects of dairy products on dementia and the underlying mechanisms. Int. J. Mol. Sci. 2018, 19, 1927. [Google Scholar] [CrossRef] [PubMed]
- Crichton, G.E.; Bryan, J.; Murphy, K.J.; Buckley, J. Review of dairy consumption and cognitive performance in adults: Findings and methodological issues. Dement. Geriatr. Cogn. Disord. 2010, 30, 352–361. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, M.; Ninomiya, T.; Ohara, T.; Doi, Y.; Uchida, K.; Shirota, T.; Yonemoto, K.; Kitazono, T.; Kiyohara, Y. Dietary patterns and risk of dementia in an elderly Japanese population: The Hisayama Study. Am. J. Clin. Nutr. 2013, 97, 1076–1082. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, M.; Ohara, T.; Ninomiya, T.; Hata, J.; Yoshida, D.; Mukai, N.; Nagata, M.; Uchida, K.; Shirota, T.; Kitazono, T.; et al. Milk and dairy consumption and risk of dementia in an elderly Japanese population: The Hisayama Study. J. Am. Geriatr. Soc. 2014, 62, 1224–1230. [Google Scholar] [CrossRef] [PubMed]
- Ogata, S.; Tanaka, H.; Omura, K.; Honda, C.; Hayakawa, K. Association between intake of dairy products and short-term memory with and without adjustment for genetic and family environmental factors: A twin study. Clin. Nutr. (Edinburgh, Scotland) 2016, 35, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Ano, Y.; Ozawa, M.; Kutsukake, T.; Sugiyama, S.; Uchida, K.; Yoshida, A.; Nakayama, H. Preventive effects of a fermented dairy product against Alzheimer’s disease and identification of a novel oleamide with enhanced microglial phagocytosis and anti-inflammatory activity. PloS ONE 2015, 10, e0118512. [Google Scholar] [CrossRef] [PubMed]
- Ano, Y.; Ayabe, T.; Kutsukake, T.; Ohya, R.; Takaichi, Y.; Uchida, S.; Yamada, K.; Uchida, K.; Takashima, A.; Nakayama, H. Novel lactopeptides in fermented dairy products improve memory function and cognitive decline. Neurobiol. Aging 2018, 72, 23–31. [Google Scholar] [CrossRef]
- Kita, M.; Obara, K.; Kondo, S.; Umeda, S.; Ano, Y. Effect of supplementation of a whey peptide rich in tryptophan-tyrosine-related peptides on cognitive performance in healthy adults: A randomized, double-blind, placebo-controlled study. Nutrients 2018, 10, 899. [Google Scholar] [CrossRef]
- Shinohara, R.; Taniguchi, M.; Ehrlich, A.T.; Yokogawa, K.; Deguchi, Y.; Cherasse, Y.; Lazarus, M.; Urade, Y.; Ogawa, A.; Kitaoka, S.; et al. Dopamine D1 receptor subtype mediates acute stress-induced dendritic growth in excitatory neurons of the medial prefrontal cortex and contributes to suppression of stress susceptibility in mice. Mol. Psychiatr. 2017, 23, 1717. [Google Scholar] [CrossRef]
- Ano, Y.; Hoshi, A.; Ayabe, T.; Ohya, R.; Uchida, S.; Yamada, K.; Kondo, K.; Kitaoka, S.; Furuyashiki, T. Iso-alpha-acids, the bitter components of beer, improve hippocampus-dependent memory through vagus nerve activation. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2019. [Google Scholar] [CrossRef]
- Franklin, K.; Paxinos, G. The Mouse Brain in Stereotaxic Coordinates, Compact; Academic Press: San Diego, CA, USA, 2008. [Google Scholar]
- McNamara, C.G.; Dupret, D. Two sources of dopamine for the hippocampus. Trends Neurosci. 2017, 40, 383–384. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, T.; Duszkiewicz, A.J.; Sonneborn, A.; Spooner, P.A.; Yamasaki, M.; Watanabe, M.; Smith, C.C.; Fernandez, G.; Deisseroth, K.; Greene, R.W.; et al. Locus coeruleus and dopaminergic consolidation of everyday memory. Nature 2016, 537, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, R.; Guitart-Masip, M.; Lambert, C.; Dayan, P.; Huys, Q.; Duzel, E.; Dolan, R.J. Dopamine restores reward prediction errors in old age. Nat. Neurosci. 2013, 16, 648–653. [Google Scholar] [CrossRef] [PubMed]
- Bar-Am, O.; Amit, T.; Kupershmidt, L.; Aluf, Y.; Mechlovich, D.; Kabha, H.; Danovitch, L.; Zurawski, V.R.; Youdim, M.B.; Weinreb, O. Neuroprotective and neurorestorative activities of a novel iron chelator-brain selective monoamine oxidase-A/monoamine oxidase-B inhibitor in animal models of Parkinson’s disease and aging. Neurobiol. Aging 2015, 36, 1529–1542. [Google Scholar] [CrossRef] [PubMed]
- Justo, L.A.; Duran, R.; Alfonso, M.; Fajardo, D.; Faro, L.R.F. Effects and mechanism of action of isatin, a MAO inhibitor, on in vivo striatal dopamine release. Neurochem. Int. 2016, 99, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Schulz, D.; Mirrione, M.M.; Henn, F.A. Cognitive aspects of congenital learned helplessness and its reversal by the monoamine oxidase (MAO)-B inhibitor deprenyl. Neurobiol. Learn. Mem. 2010, 93, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Gaweska, H.; Fitzpatrick, P.F. Structures and mechanism of the monoamine oxidase family. Biomol. Concepts 2011, 2, 365–377. [Google Scholar] [CrossRef]
- Cai, Z. Monoamine oxidase inhibitors: Promising therapeutic agents for Alzheimer’s disease (Review). Mol. Med. Rep. 2014, 9, 1533–1541. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, Y.; Guo, Y.; Wang, Z.; Huang, L.; Li, X. Dual functional cholinesterase and MAO inhibitors for the treatment of Alzheimer’s disease: Synthesis, pharmacological analysis and molecular modeling of homoisoflavonoid derivatives. J. Enzyme Inhib. Med. Chem. 2016, 31, 389–397. [Google Scholar] [CrossRef]
- Borroni, E.; Bohrmann, B.; Grueninger, F.; Prinssen, E.; Nave, S.; Loetscher, H.; Chinta, S.J.; Rajagopalan, S.; Rane, A.; Siddiqui, A.; et al. Sembragiline: A novel, selective monoamine oxidase type B inhibitor for the treatment of Alzheimer’s disease. J. Pharmacol. Exp. Ther. 2017, 362, 413–423. [Google Scholar] [CrossRef]
- Da Silva, W.C.; Kohler, C.C.; Radiske, A.; Cammarota, M. D1/D5 dopamine receptors modulate spatial memory formation. Neurobiol. Learn. Mem. 2012, 97, 271–275. [Google Scholar] [CrossRef] [PubMed]
- De Bundel, D.; Femenia, T.; DuPont, C.M.; Konradsson-Geuken, A.; Feltmann, K.; Schilstrom, B.; Lindskog, M. Hippocampal and prefrontal dopamine D1/5 receptor involvement in the memory-enhancing effect of reboxetine. Int. J. Neuropsychopharmacol. 2013, 16, 2041–2051. [Google Scholar] [CrossRef] [PubMed]
- Yuan Xiang, P.; Janc, O.; Grochowska, K.M.; Kreutz, M.R.; Reymann, K.G. Dopamine agonists rescue Abeta-induced LTP impairment by Src-family tyrosine kinases. Neurobiol. Aging 2016, 40, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.R.; Sun, N.; Lei, L.; Li, X.Y.; Yao, B.; Sun, K.; Hu, R.; Zhang, X.; Shi, X.D.; Gao, C. L-Stepholidine rescues memory deficit and synaptic plasticity in models of Alzheimer’s disease via activating dopamine D1 receptor/PKA signaling pathway. Cell Death Dis. 2015, 6, e1965. [Google Scholar] [CrossRef] [PubMed]
- Pioli, E.Y.; Gaskill, B.N.; Gilmour, G.; Tricklebank, M.D.; Dix, S.L.; Bannerman, D.; Garner, J.P. An automated maze task for assessing hippocampus-sensitive memory in mice. Behav. Brain Res. 2014, 261, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Hikida, A.; Kawai, S.; Lan, V.T.; Motoyama, T.; Kitagawa, S.; Yoshikawa, Y.; Kato, R.; Kawarasaki, Y. Analysing the substrate multispecificity of a proton-coupled oligopeptide transporter using a dipeptide library. Nat. Commun. 2013, 4, 2502. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ano, Y.; Ayabe, T.; Ohya, R.; Kondo, K.; Kitaoka, S.; Furuyashiki, T. Tryptophan-Tyrosine Dipeptide, the Core Sequence of β-Lactolin, Improves Memory by Modulating the Dopamine System. Nutrients 2019, 11, 348. https://doi.org/10.3390/nu11020348
Ano Y, Ayabe T, Ohya R, Kondo K, Kitaoka S, Furuyashiki T. Tryptophan-Tyrosine Dipeptide, the Core Sequence of β-Lactolin, Improves Memory by Modulating the Dopamine System. Nutrients. 2019; 11(2):348. https://doi.org/10.3390/nu11020348
Chicago/Turabian StyleAno, Yasuhisa, Tatsuhiro Ayabe, Rena Ohya, Keiji Kondo, Shiho Kitaoka, and Tomoyuki Furuyashiki. 2019. "Tryptophan-Tyrosine Dipeptide, the Core Sequence of β-Lactolin, Improves Memory by Modulating the Dopamine System" Nutrients 11, no. 2: 348. https://doi.org/10.3390/nu11020348
APA StyleAno, Y., Ayabe, T., Ohya, R., Kondo, K., Kitaoka, S., & Furuyashiki, T. (2019). Tryptophan-Tyrosine Dipeptide, the Core Sequence of β-Lactolin, Improves Memory by Modulating the Dopamine System. Nutrients, 11(2), 348. https://doi.org/10.3390/nu11020348





