Higher Polygenetic Predisposition for Asthma in Cow’s Milk Allergic Children
Abstract
1. Background
2. Methods
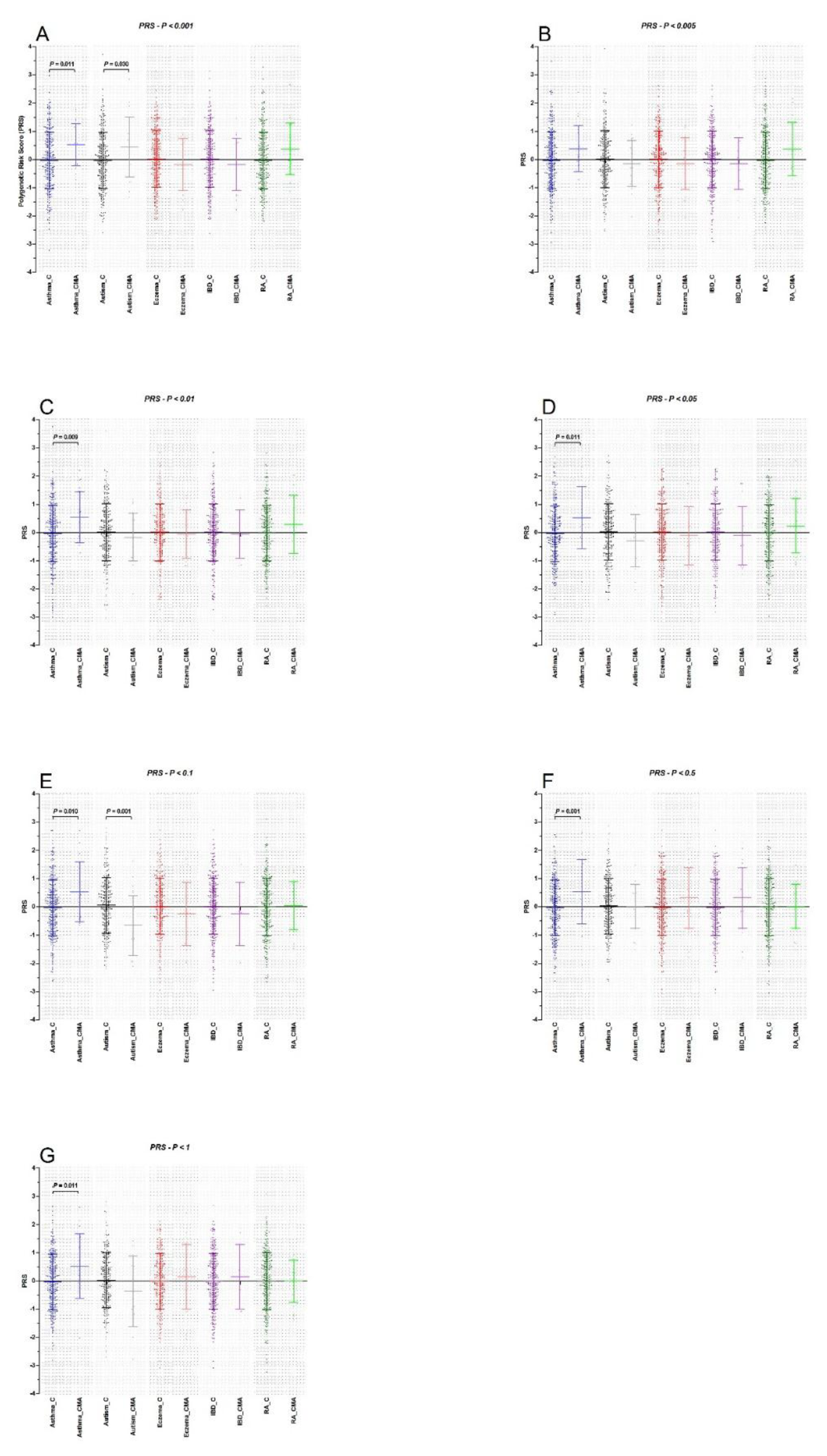
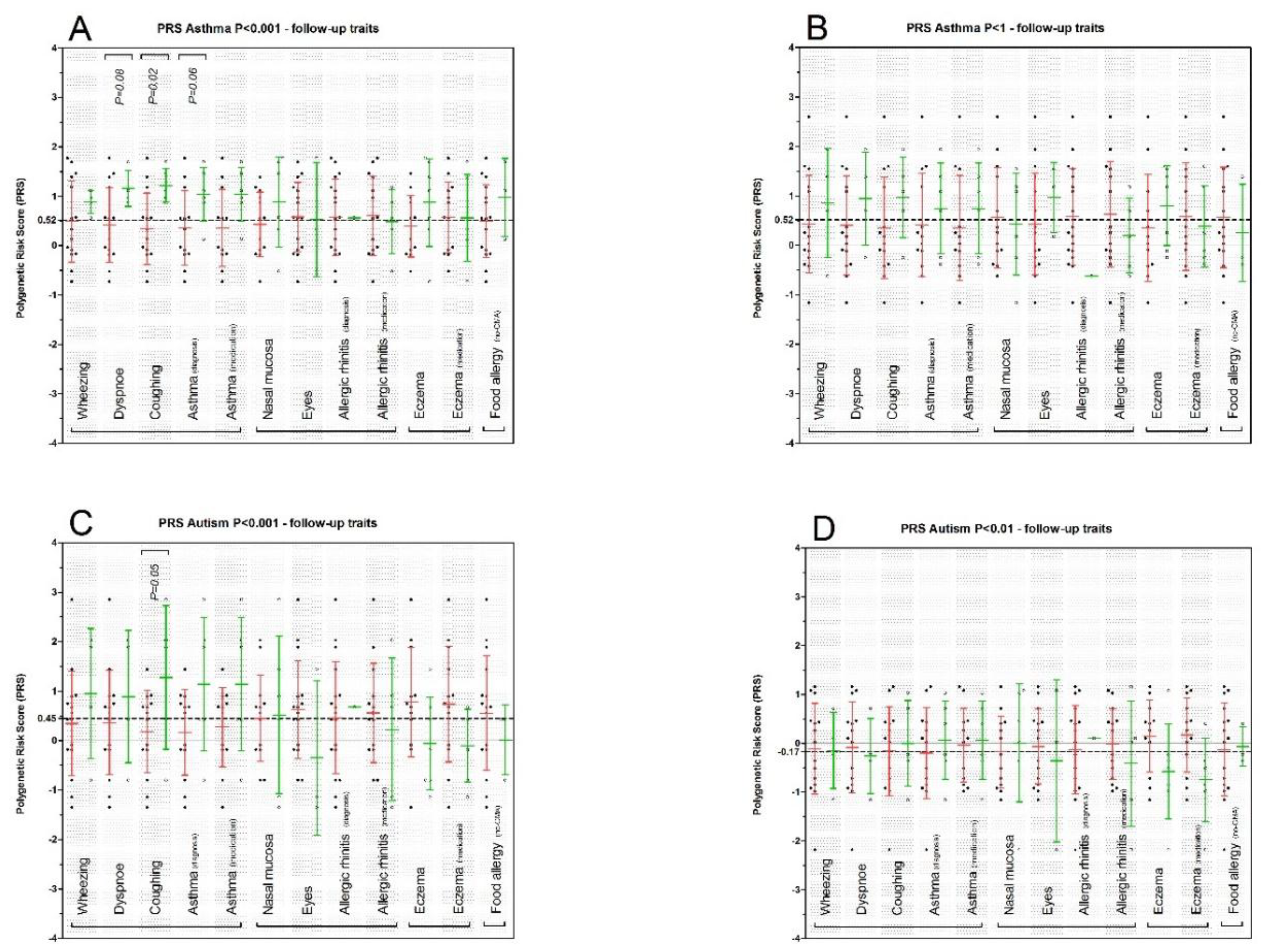
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gerrard, J.W.; MacKenzie, J.W.; Goluboff, N.; Garson, J.Z.; Maningas, C.S. Cow’s milk allergy: Prevalence and manifestations in an unselected series of newborns. Acta Paediatr. Scand. Suppl. 1973, 234, 3–21. [Google Scholar] [CrossRef] [PubMed]
- Petrus, N.C.; Schoemaker, A.F.; van Hoek, M.W.; Jansen, L.; Jansen-van der Weide, M.C.; van Aalderen, W.M.; Sprikkelman, A.B. Remaining symptoms in half the children treated for milk allergy. Eur. J. Pediatr. 2015, 174, 759–765. [Google Scholar] [CrossRef] [PubMed]
- Chafen, J.J.; Newberry, S.J.; Riedl, M.A.; Bravata, D.M.; Maglione, M.; Suttorp, M.J.; Sundaram, V.; Paige, N.M.; Towfigh, A.; Hulley, B.J.; et al. Diagnosing and managing common food allergies: A systematic review. JAMA 2010, 303, 1848–1856. [Google Scholar] [CrossRef] [PubMed]
- Schoemaker, A.A.; Sprikkelman, A.B.; Grimshaw, K.E.; Roberts, G.; Grabenhenrich, L.; Rosenfeld, L.; Siegert, S.; Dubakiene, R.; Rudzeviciene, O.; Reche, M.; et al. Incidence and natural history of challenge-proven cow’s milk allergy in european children—Europrevall birth cohort. Allergy 2015, 70, 963–972. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.J.; Kumar, R.; Pongracic, J.; Liu, X.; Story, R.; Yu, Y.; Caruso, D.; Costello, J.; Schroeder, A.; Fang, Y.; et al. Familial aggregation of food allergy and sensitization to food allergens: A family-based study. Clin. Exp. Allergy 2009, 39, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Benhamou, A.H.; Schappi Tempia, M.G.; Belli, D.C.; Eigenmann, P.A. An overview of cow’s milk allergy in children. Swiss Med. Wkly. 2009, 139, 300–307. [Google Scholar] [PubMed]
- Host, A.; Halken, S. A prospective study of cow milk allergy in danish infants during the first 3 years of life. Clinical course in relation to clinical and immunological type of hypersensitivity reaction. Allergy 1990, 45, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Host, A.; Halken, S.; Jacobsen, H.P.; Christensen, A.E.; Herskind, A.M.; Plesner, K. Clinical course of cow’s milk protein allergy/intolerance and atopic diseases in childhood. Pediatr. Allergy Immunol. 2002, 13 (Suppl. 15), 23–28. [Google Scholar] [CrossRef] [PubMed]
- Morita, H.; Nomura, I.; Matsuda, A.; Saito, H.; Matsumoto, K. Gastrointestinal food allergy in infants. Allergol. Int. 2013, 62, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Saarinen, K.M.; Pelkonen, A.S.; Makela, M.J.; Savilahti, E. Clinical course and prognosis of cow’s milk allergy are dependent on milk-specific ige status. J. Allergy Clin. Immunol. 2005, 116, 869–875. [Google Scholar] [CrossRef] [PubMed]
- Bonnelykke, K.; Sparks, R.; Waage, J.; Milner, J.D. Genetics of allergy and allergic sensitization: Common variants, rare mutations. Curr. Opin. Immunol. 2015, 36, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Marenholz, I.; Esparza-Gordillo, J.; Ruschendorf, F.; Bauerfeind, A.; Strachan, D.P.; Spycher, B.D.; Baurecht, H.; Margaritte-Jeannin, P.; Saaf, A.; Kerkhof, M.; et al. Meta-analysis identifies seven susceptibility loci involved in the atopic march. Nat. Commun. 2015, 6, 8804. [Google Scholar] [CrossRef] [PubMed]
- Paternoster, L.; Standl, M.; Waage, J.; Baurecht, H.; Hotze, M.; Strachan, D.P.; Curtin, J.A.; Bonnelykke, K.; Tian, C.; Takahashi, A.; et al. Multi-ancestry genome-wide association study of 21,000 cases and 95,000 controls identifies new risk loci for atopic dermatitis. Nat. Genet. 2015, 47, 1449–1456. [Google Scholar] [CrossRef] [PubMed]
- Bunyavanich, S.; Schadt, E.E.; Himes, B.E.; Lasky-Su, J.; Qiu, W.; Lazarus, R.; Ziniti, J.P.; Cohain, A.; Linderman, M.; Torgerson, D.G.; et al. Integrated genome-wide association, coexpression network, and expression single nucleotide polymorphism analysis identifies novel pathway in allergic rhinitis. BMC Med. Genom. 2014, 7, 48. [Google Scholar] [CrossRef] [PubMed]
- Henneman, P.; Petrus, N.C.; Venema, A.; van Sinderen, F.; van der Lip, K.; Hennekam, R.C.; Mannens, M.; Sprikkelman, A.B. Genetic susceptibility for cow’s milk allergy in dutch children: The start of the allergic march? Clin. Transl. Allergy 2015, 6, 7. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, A.; Curjuric, I.; Coin, L.J.; Kumar, A.; McArdle, W.L.; Imboden, M.; Leynaert, B.; Kogevinas, M.; Schmid-Grendelmeier, P.; Pekkanen, J.; et al. A genome-wide meta-analysis of genetic variants associated with allergic rhinitis and grass sensitization and their interaction with birth order. J. Allergy Clin. Immunol. 2011, 128, 996–1005. [Google Scholar] [CrossRef] [PubMed]
- Ierodiakonou, D.; Garcia-Larsen, V.; Logan, A.; Groome, A.; Cunha, S.; Chivinge, J.; Robinson, Z.; Geoghegan, N.; Jarrold, K.; Reeves, T.; et al. Timing of allergenic food introduction to the infant diet and risk of allergic or autoimmune disease: A systematic review and meta-analysis. JAMA 2016, 316, 1181–1192. [Google Scholar] [CrossRef] [PubMed]
- Alduraywish, S.A.; Standl, M.; Lodge, C.J.; Abramson, M.J.; Allen, K.J.; Erbas, B.; von Berg, A.; Heinrich, J.; Lowe, A.J.; Dharmage, S.C. Is there a march from early food sensitization to later childhood allergic airway disease? Results from two prospective birth cohort studies. Pediatr. Allergy Immunol. 2017, 28, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.C.; Mehl-Madrona, L. Autoimmune and gastrointestinal dysfunctions: Does a subset of children with autism reveal a broader connection? Expert Rev. Gastroenterol. Hepatol. 2011, 5, 465–477. [Google Scholar] [CrossRef] [PubMed]
- Lyall, K.; Van de Water, J.; Ashwood, P.; Hertz-Picciotto, I. Asthma and allergies in children with autism spectrum disorders: Results from the charge study. Autism Res. 2015, 8, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Theoharides, T.C. Is a subtype of autism an allergy of the brain? Clin. Ther. 2013, 35, 584–591. [Google Scholar] [CrossRef] [PubMed]
- Visscher, P.M.; Brown, M.A.; McCarthy, M.I.; Yang, J. Five years of gwas discovery. Am. J. Hum. Genet. 2012, 90, 7–24. [Google Scholar] [CrossRef] [PubMed]
- Eichler, E.E.; Flint, J.; Gibson, G.; Kong, A.; Leal, S.M.; Moore, J.H.; Nadeau, J.H. Missing heritability and strategies for finding the underlying causes of complex disease. Nat. Rev. Genet. 2010, 11, 446–450. [Google Scholar] [CrossRef] [PubMed]
- Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature 2014, 511, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Wray, N.R.; Lee, S.H.; Mehta, D.; Vinkhuyzen, A.A.; Dudbridge, F.; Middeldorp, C.M. Research review: Polygenic methods and their application to psychiatric traits. J. Child Psychol. Psychiatry 2014, 55, 1068–1087. [Google Scholar] [CrossRef] [PubMed]
- Dudbridge, F. Power and predictive accuracy of polygenic risk scores. PLoS Genet. 2013, 9, e1003348. [Google Scholar] [CrossRef]
- Keil, T.; McBride, D.; Grimshaw, K.; Niggemann, B.; Xepapadaki, P.; Zannikos, K.; Sigurdardottir, S.T.; Clausen, M.; Reche, M.; Pascual, C.; et al. The multinational birth cohort of europrevall: Background, aims and methods. Allergy 2010, 65, 482–490. [Google Scholar] [CrossRef] [PubMed]
- McBride, D.; Keil, T.; Grabenhenrich, L.; Dubakiene, R.; Drasutiene, G.; Fiocchi, A.; Dahdah, L.; Sprikkelman, A.B.; Schoemaker, A.A.; Roberts, G.; et al. The europrevall birth cohort study on food allergy: Baseline characteristics of 12,000 newborns and their families from nine european countries. Pediatr. Allergy Immunol. 2012, 23, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Lopuhaa, C.E.; Roseboom, T.J.; Osmond, C.; Barker, D.J.; Ravelli, A.C.; Bleker, O.P.; van der Zee, J.S.; van der Meulen, J.H. Atopy, lung function, and obstructive airways disease after prenatal exposure to famine. Thorax 2000, 55, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Roseboom, T.J.; van der Meulen, J.H.; Ravelli, A.C.; van Montfrans, G.A.; Osmond, C.; Barker, D.J.; Bleker, O.P. Blood pressure in adults after prenatal exposure to famine. J. Hypertens. 1999, 17, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Howie, B.; Marchini, J.; Stephens, M. Genotype imputation with thousands of genomes. G3 (Bethesda) 2011, 1, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Moffatt, M.F.; Gut, I.G.; Demenais, F.; Strachan, D.P.; Bouzigon, E.; Heath, S.; von Mutius, E.; Farrall, M.; Lathrop, M.; Cookson, W.O.; et al. A large-scale, consortium-based genomewide association study of asthma. N. Engl. J. Med. 2010, 363, 1211–1221. [Google Scholar] [CrossRef] [PubMed]
- Cross-Disorder Group of the Psychiatric Genomics, Consortium. Identification of risk loci with shared effects on five major psychiatric disorders: A genome-wide analysis. Lancet 2013, 381, 1371–1379. [Google Scholar]
- Liu, J.Z.; van Sommeren, S.; Huang, H.; Ng, S.C.; Alberts, R.; Takahashi, A.; Ripke, S.; Lee, J.C.; Jostins, L.; Shah, T.; et al. Association analyses identify 38 susceptibility loci for inflammatory bowel disease and highlight shared genetic risk across populations. Nat. Genet. 2015, 47, 979–986. [Google Scholar] [CrossRef] [PubMed]
- Stahl, E.A.; Raychaudhuri, S.; Remmers, E.F.; Xie, G.; Eyre, S.; Thomson, B.P.; Li, Y.; Kurreeman, F.A.; Zhernakova, A.; Hinks, A.; et al. Genome-wide association study meta-analysis identifies seven new rheumatoid arthritis risk loci. Nat. Genet. 2010, 42, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Saito, H. Epicutaneous immunity and onset of allergic diseases—per-”eczema”tous sensitization drives the allergy march. Allergol. Int. 2013, 62, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Tsakok, T.; Du Toit, G.; Lack, G. Prevention of food allergy. Chem. Immunol. Allergy 2015, 101, 253–262. [Google Scholar] [PubMed]
- Petrus, N.C.; Henneman, P.; Venema, A.; Mul, A.; van Sinderen, F.; Haagmans, M.; Mook, O.; Hennekam, R.C.; Sprikkelman, A.B.; Mannens, M. Cow’s milk allergy in dutch children: An epigenetic pilot survey. Clin. Transl. Allergy 2016, 6, 16. [Google Scholar] [CrossRef] [PubMed]
- Borad, S.G.; Kumar, A.; Singh, A.K. Effect of processing on nutritive values of milk protein. Crit. Rev. Food Sci. Nutr. 2017, 57, 3690–3702. [Google Scholar] [CrossRef] [PubMed]
- Sicherer, S.H.; Sampson, H.A. Food allergy: A review and update on epidemiology, pathogenesis, diagnosis, prevention, and management. J. Allergy Clin. Immunol. 2018, 141, 41–58. [Google Scholar] [CrossRef] [PubMed]
- Loh, W.; Tang, M.L.K. The epidemiology of food allergy in the global context. Int. J. Environ. Res. Public Health 2018, 15, 2043. [Google Scholar] [CrossRef] [PubMed]
- Alduraywish, S.A.; Lodge, C.J.; Campbell, B.; Allen, K.J.; Erbas, B.; Lowe, A.J.; Dharmage, S.C. The march from early life food sensitization to allergic disease: A systematic review and meta-analyses of birth cohort studies. Allergy 2016, 71, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Jeong, D.Y.; Peyrin-Biroulet, L.; Eisenhut, M.; Shin, J.I. Insight into the role of tslp in inflammatory bowel diseases. Autoimmun. Rev. 2017, 16, 55–63. [Google Scholar] [CrossRef] [PubMed]
| Genotype Data | N | Female | Male | Age | CR (mean) | CR (min) | CR (max) |
|---|---|---|---|---|---|---|---|
| CMA | 22 | 6 | 16 | 11.8 ± 4.9 * | 97.1% | 93.0% | 98.0% |
| Reference set | 307 | 130 | 177 | 53.3 ± 0.58 ** | 97.7% | 94.0% | 99.0% |
| PRS Analysis | Asthma | ASD | AD | IBD | RA |
|---|---|---|---|---|---|
| PT < 0.001 | 1.79 (1.14–2.84), 0.012 | 1.59 (1.04–2.44), 0.032 | 0.82 (0.53–1.28), 0.383 | 0.82 (0.53–1.28), 0.383 | 0.82 (0.53–1.28), 0.383 |
| PT < 0.005 | 1.50 (0.98–2.32), 0.065 | 0.85 (0.55–1.32), 0.481 | 0.86 (0.56–1.32), 0.483 | 0.86 (0.56–1.32), 0.483 | 0.86 (0.56–1.32), 0.483 |
| PT < 0.01 | 1.85 (1.16–2.94), 0.009 | 0.83 (0.54–1.28), 0.410 | 0.94 (0.61–1.45), 0.780 | 0.94 (0.61–145), 0.780 | 0.94 (0.61–1.45), 0.780 |
| PT < 0.05 | 1.73 (1.13–2.27), 0.012 | 0.73 (0.47–1.13), 0.161 | 0.89 (0.58–1.37), 0.596 | 0.89 (0.58–1.37), 0.596 | 0.89 (0.58–1.37), 0.596 |
| PT < 0.1 | 1.74 (1.13–2.66), 0.012 | 0.45 (0.28–0.74), 0.002 | 0.77 (0.50–1.18), 0.231 | 0.77 (0.50–1.18), 0.231 | 0.77 (0.50–1.18), 0.231 |
| PT < 0.5 | 1.74 (1.13–2.66), 0.011 | 0.67 (0.43–1.05), 0.078 | 1.42 (0.91–2.23), 0.124 | 1.42 (0.91–2.23 ), 0.124 | 1.42 (0.91–2.23), 0.124 |
| PT < 1 | 1.73 (1.13–2.65), 0.012 | 0.67 (0.43–1.04), 0.074 | 1.17 (0.76–1.81), 0.479 | 1.17 (0.76–1.81), 0.479 | 1.17 (0.76–1.81), 0.479 |
| General | |
|---|---|
| Total (N) | 19 |
| Female (N) | 6 |
| Male (N) | 13 |
| Age (mean ± SD) | 7.0 ± 1.0 * |
| 2 year IgE positive ** | 2 |
| Asthma related | N (%) |
| Wheezing | 4 (21.1%) |
| Dyspnoea | 4 (21.1%) |
| Coughing at night | 5 (26.3%) |
| Asthma diagnosed | 6 (33.3%) |
| Asthma medication | 6 (33.3%) |
| Allergic rhinitis related | N (%) |
| Irritated nasal mucosa) | 6 (33.3%) |
| Eyes | 3 (15.8%) |
| Allergic rhinitis diagnosed | 1 (5.3%) |
| Allergic rhinitis medication | 5 (26.3%) |
| Atopic dermatitis related | N (%) |
| Eczema | 7 (36.8%) |
| Topical steroids | 6 (31.6%) |
| Allergy related | N (%) |
| Food allergy | 3 (15.8%) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jansen, P.R.; Petrus, N.C.M.; Venema, A.; Posthuma, D.; Mannens, M.M.A.M.; Sprikkelman, A.B.; Henneman, P. Higher Polygenetic Predisposition for Asthma in Cow’s Milk Allergic Children. Nutrients 2018, 10, 1582. https://doi.org/10.3390/nu10111582
Jansen PR, Petrus NCM, Venema A, Posthuma D, Mannens MMAM, Sprikkelman AB, Henneman P. Higher Polygenetic Predisposition for Asthma in Cow’s Milk Allergic Children. Nutrients. 2018; 10(11):1582. https://doi.org/10.3390/nu10111582
Chicago/Turabian StyleJansen, Philip R., Nicole C. M. Petrus, Andrea Venema, Danielle Posthuma, Marcel M. A. M. Mannens, Aline B. Sprikkelman, and Peter Henneman. 2018. "Higher Polygenetic Predisposition for Asthma in Cow’s Milk Allergic Children" Nutrients 10, no. 11: 1582. https://doi.org/10.3390/nu10111582
APA StyleJansen, P. R., Petrus, N. C. M., Venema, A., Posthuma, D., Mannens, M. M. A. M., Sprikkelman, A. B., & Henneman, P. (2018). Higher Polygenetic Predisposition for Asthma in Cow’s Milk Allergic Children. Nutrients, 10(11), 1582. https://doi.org/10.3390/nu10111582






