Abstract
Avocado is a rich source of numerous nutrients, such as micro- and macroelements, essential unsaturated fatty acids, and vitamins essential for the correct functioning of the body. Consequently, its consumption has significantly increased in recent years. The primary edible part of the fruit is the flesh, while the seed is still considered biowaste. Currently, various methods for utilization of this biowaste are being explored, prompting the authors of this work to investigate the catalytic properties of ground avocado seeds. Dried, ground avocado seeds were used as the catalyst in the environmentally friendly oxidation of limonene with oxygen. The process was carried out in mild conditions, without the use of any solvent and at atmospheric pressure. The studies examined the influence of temperature (70–110 °C), the amount of the catalyst (0.5–5.0 wt%), and the reaction time (15–360 min). The analyses of the post-reaction mixtures were performed using the gas chromatography method (GC). The maximum value of the conversion of limonene obtained during the tests was 36 mol%. The main products of this process were as follows: 1,2-epoxylimonene, carveol, and perillyl alcohol. Also, the following compounds were determined in the post-reaction mixtures: carvone and 1,2-epoxylimonene diol. The studied process is interesting, taking into account both the management of waste in the form of avocado seeds and possible wide applications of limonene transformation products in medicine, cosmetics and the food industry. Given that limonene is now increasingly being extracted from waste orange peels, this is also a good way to manage the future naturally derived limonene and reduce the amount of waste orange peels. The presented studies fit perfectly with the goals of sustainable development and circular economy and may be the basis for the future development of “green technology” for obtaining value-added oxygenated derivatives of limonene. These studies show the use of waste biomass in the form of avocado seeds to obtain a green catalyst. In this context, our research presents an effective way of waste valorization.
1. Introduction
Currently, observed climate changes, as well as high levels of air, water and soil pollution, may threaten human life and cause an increased incidence of respiratory, cardiovascular and neurodegenerative diseases, as well as contribute to the development of cancer. Therefore, it is important to use natural resources rationally while paying attention to the appropriate management of waste. One of the directions of the rational use of natural resources is currently the conversion of waste biomass into highly active catalytic materials used in organic syntheses as active catalysts, e.g., activated carbons [1]. Another direction may be the application of raw materials of natural origin as reagents in organic syntheses (for example, such reagents can be terpenes). A rich source of terpenes are essential oils obtained from plants, including essential oils obtained from waste plant parts.
In recent decades, there has been a visible, gradual increase in the annual production of avocados. More than half a century ago, this fruit was not popular, and its world production was only 716,353 tons (in 1961), while in 2022, this production increased to 8.98 × 106 tons [2]. This is partly due to the fact that the flesh of this fruit is recommended for inclusion in the diet for its health-promoting properties [3]. Avocados are also a rich source of beneficial bioactive compounds and can be found in cosmetic formulations, such as avocado oil. Research to date has shown that not only the flesh of the fruit has beneficial properties for the human body, but also the seed, which is the main biowaste from this fruit. Avocado seed extract has been shown to neutralize free radicals and reduce the growth of microorganisms, which may have applications in dermatology [4]. According to the studies performed by Kaio V. L. da Silva Bastos et al. [5], it has even more powerful antioxidant activity than quercetin or ascorbic acid, which could make it the object of interest in both the cosmetic and pharmaceutical industries. Historical data suggest that ancient Aztec and Mayan communities used avocado seed decoctions to treat fungal infections. Research has shown that the seeds contain biologically active compounds that have anti-inflammatory and insulin-lowering properties, stimulate the regeneration of skin cells, and also have antibacterial and antifungal properties [6]. The seeds may also be the source of orange dye, which can be an alternative to synthetic dyes. Compounds such as furfural, phenolic, and catechin compounds may be responsible for producing the color [7]. The analysis of acetone and ethanolic extracts of avocado seeds revealed the presence in these extracts such compounds as terpenes and fatty acid derivatives, for example, estragole, linoleic acid, methyl ester, linolenic acid and tridecanoic acid, 11-dodecen-2-one, and 9,12-octadecadien-1-ol [8]. It is also possible to obtain avocado seed oil, which is rich in polyphenols and has health and skin care properties [4]. Avocado seed oil contains palmitic, oleic and linoleic acids as the main compounds and small traces of lauric, myristic, stearic, linolenic or arachidonic acids [9]. In addition, the composition of avocado seeds was examined, and the presence of perseitol, quinic acid, citric acid and penstemide was established. Moreover, hydroxytyrosol-1-glucoside, hydroxytyrosol, four hydroxycinnamic acid derivatives, vanillic acid, cavoylquinic acid isomers, quinic acid, vanillic acid glucoside, 4-caffeoylquinic acid, 3-O-p-coumaroylquinic acid, tyrosol glucoside, 1-caffeoylquinic acid, hydroxytyrosol glucoside, and citric acid were detected [10]. Also, chlorogenic acid, catechins, caffeic acid and ferulic acid were identified in dried avocado seeds [11].
However, it is not only the biologically active compounds contained in waste avocado seeds that attract the attention of the cosmetics and pharmaceutical industries. Currently, other ways of using avocado seeds are also being sought. An interesting and new way of using avocado seeds could also be their transformation into active catalysts for the transformation of organic compounds. Activated carbons can be obtained from avocado seeds, for example, which can later be impregnated with metals to increase their activity, but a more interesting way seems to be to simply use powder from dried avocado seeds as catalysts. This way, it does not require large financial and energy outlays to obtain the final product. That is why we decided to conduct research on the catalytic activity of ordinary ground avocado seeds.
In the case of essential oils, it is important that they can be obtained from various parts of plants, such as roots, stems, leaves, fruits and flowers. This allows the entire plant to be used, not just its part, from which, for example, biologically active substances for medicinal purposes, fragrance compounds for the perfume industry, or flavor compounds for the food industry are obtained [12]. Essential oils obtained from waste plant parts are also rich in terpene compounds, which can also be successfully used as ingredients for drugs preventing the occurrence of many diseases and in the treatment of, for example, cancers [13]. Terpene compounds obtained from plant materials (including waste plant materials) can also be subjected to various chemical reactions (e.g., isomerization or oxidation), as a result of which other compounds can be obtained (value-added derivatives) that are very valuable for medicine, cosmetics, or perfumery, among others: 1,2-epoxylimonene, carvone, carveol, perillyl alcohol and 1,2-epoxylimonene diol [14]. An example of the terpene compound that can now be successfully obtained from waste biomass (waste orange peels) is limonene. Limonene is currently enjoying great interest from researchers in terms of its use in medicine, in the cosmetics industry, in organic synthesis, and in obtaining polymers. Limonene (1-methyl-4-(prop-1-en-2-yl)cyclohex-1-ene), (C10H16), is the monocyclic terpene with two optical isomers: D(+)-limonene and L(-)-limonene, with D(+)-limonene being the dominant isomer of limonene in nature. D(+)-limonene naturally occurs in the essential oils isolated from the peels of citrus fruits, such as Citrus × sinensis, Citrus × aurantium, and Citrus reticulata [15]. D(+)-limonene (commonly called limonene) is an edible raw material with a sweet taste and a pleasant citrus fragrance. It is used in the food industry for flavoring food and in the cosmetic industry to provide a pleasant scent to cosmetic products, personal care items, and household chemicals. Limonene is also applied in the fragrance industry as a component of scent compositions. Additionally, limonene is employed as the industrial solvent [16]. Limonene is also used in medicine due to its analgesic, antifungal, antiviral, anti-inflammatory, antimicrobial, neuroprotective, and antioxidant properties. Limonene helps prevent internal organ damage caused by diabetes. It also aids in lowering blood glucose levels and may contribute to reducing insulin resistance. It exhibits anticancer properties, facilitates the repair of damaged DNA, and inhibits cell proliferation. Limonene may alleviate symptoms of depression, anxiety, and mental disorders. It supports the regeneration of tissues and internal organs after injuries. It also has insecticidal effects and helps combat plant pests in agriculture [15]. Limonene is a safe, non-toxic, non-mutagenic raw material that is environmentally friendly [17]. It is worth emphasizing that limonene is currently obtained in large quantities from waste orange peels, which also highlights the usefulness of our research, as the presented method may also use natural limonene in the oxidation process in the future.
The aim of our research was to present a new way of utilizing the waste avocado seeds to obtain the active catalyst for the process of limonene oxidation. This catalyst was obtained in a simple way: by grinding the dried avocado seeds. No additional operations were carried out to increase its activity. The aim of catalytic tests, conducted with the use of powder from ground avocado seeds as the catalyst, was to investigate the possibility of effective transformation of limonene into valuable oxygenated derivatives (value-added derivatives of limonene), which are 1,2-epoxylimonene, 1,2-epoxylimonene diol, carveol, carvone and perillyl alcohol. During these studies, the most favourable conditions for conducting the process of limonene oxidation in the presence of powder from ground avocado seeds as the catalyst were determined. The method proposed for limonene oxidation is ecological not only because it uses the catalytic material obtained from the plant waste in the form of avocado seeds but also because the oxidation is carried out with oxygen, and no organic solvent is used in the reaction mixture during oxidation—only limonene and catalyst are added to the reactor. In these conditions, limonene is both the organic raw material and the solvent for the reaction products being formed.
Our research addresses key aspects of sustainable development: Waste Valorization and Circular Economy, Green Chemistry and Environmentally Friendly Technologies, Sustainable Material Development, and Potential Socio-economic Impact. The proposed application of dried and ground avocado seeds, a common biowaste, as a green catalyst exemplifies sustainable waste valorization. This approach supports the principles of the circular economy by converting agricultural waste into a value-added product, thereby minimizing environmental burden and contributing to resource efficiency. The catalytic oxidation of limonene is performed under mild conditions, without the use of organic solvents, and utilizing molecular oxygen as the oxidant. These features are consistent with green chemistry principles and help to reduce the environmental footprint of chemical transformations. Our catalyst is derived directly from plant biomass without the need for energy-intensive activation or chemical modifications. This contributes to the development of low-cost, sustainable catalytic materials derived from renewable resources. The valorization of agro-waste, such as avocado seeds, may have socio-economic benefits in regions where avocados are produced in large quantities. The creation of new applications for waste materials can stimulate local economies and support sustainable agricultural practices.
2. Materials and Methods
2.1. Preparation of the Catalyst from the Dried Avocado Seeds
Before the catalytic tests, the studied catalyst was appropriately prepared. The avocados were purchased in Polish supermarkets. First, the flesh of the avocado fruit was separated from the seed. The seeds were stripped of their husk and ground in a laboratory grinder to obtain smaller particles. The material was then placed in a laboratory dryer at 50 °C for 20 h. After the drying process, the material was ground again to achieve a powder-like consistency and subsequently dried the second time under the same conditions to remove any remaining water content (Figure 1).
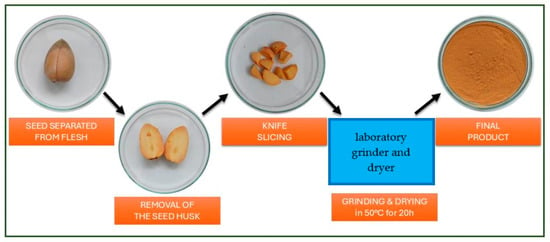
Figure 1.
A scheme of steps involved in crushing the avocado seed. The final step is obtaining the catalytic material in the form of powder.
2.2. Instrumental Methods Used in the Analysis of the Catalyst Obtained from the Dried Avocado Seeds
The morphological analysis of the obtained catalyst sample was carried out using an SU8020 Ultra-High Resolution Field Emission Scanning Electron Microscope equipped with EDX (Hitachi Ltd., Ibaraki, Japan). X-ray Diffraction (XRD) pattern was recorded using an Empyrean Panalytical diffractometer employing a Cu lamp as the radiation source over a 2θ range of 10–40° with a step size of 0.026° (Malvern, UK). Fourier-transform infrared spectroscopy (FT-IR) measurements were performed in the 400–4000 cm−1 wavenumber range using a Thermo Electron Nicolet 380 spectrometer (Malente, Germany). UV-VIS spectrum was recorded in the range of wavelength 190–900 nm with a Jasco 650 spectrometer (Tokyo, Japan).
In addition to the above-mentioned instrumental studies, we also carried out a total acidity test for our catalyst. The acid site concentration was measured using the method described by Vilcocq et al. [18]. A total of 20 mg of materials was added to 10 cm3 of NaOH 0.01 M. The solution was shaken at room temperature for 2 h. The material was then filtered off, and the pH of the filtrate was determined by titration with 0.01 m HCL in the presence of phenolphthalein as an indicator. The acid site concentration was calculated from the following formula:
where:
[OH−]: the hydroxide molar concentration determined by the titration (mol/dm3), V: the volume of NaOH solution added to tested material, m: the mass of tested material.
2.3. Oxidation of Limonene on the Catalyst Obtained from the Dried Avocado Seeds
The laboratory apparatus that was used for the oxidation of limonene on the catalyst in the form of powder obtained from the dried avocado seeds consisted of a magnetic stirrer (stirring rate of 500 rpm) equipped with a heating function, a cylinder that contained oxygen with the purity of 99.99% (Messer, Szczecin, Poland), a 40 mL/min. An oxygen flow controller, a reflux condenser, a glass reactor with a capacity of 25 cm3, and a glass babbler to introduce oxygen into the reactor.
During the studies on the catalytic activity of the catalyst in the form of powder obtained from the dried avocado seeds, the influence of temperature, the amount of the catalyst and the reaction time were tested. Depending on the studied parameters, 10 g of limonene (studies on the influence of temperature and the amount of the catalysts) or 20 g of limonene (studies on the influence of reaction time) with a purity of 93% (Sigma Aldrich, Poznań, Poland) were introduced into the reactor. The influence of temperature was tested in the range of 70–110 °C. The amount of the catalyst used in the studies changed in the range of 0.05 wt%–5.00 wt%. The reaction time for the oxidation of limonene on the catalyst in the form of powder obtained from dried avocado seeds was 15–360 min. After the oxidation was completed, each post-reaction mixture was taken into Eppendorf-type tubes in the amount of 1 cm3. Then, the reaction solution was centrifuged in a laboratory centrifuge at 5000 rpm for 5 min. The sample was next diluted with acetone (99.5% purity) (Sigma Aldrich, Poznań, Poland) at the ratio of 1:4.
2.4. Identification of Limonene Oxidation Products Using the Gas Chromatography Method
In the gas chromatographic analyses, a FOCUS GC chromatograph (Thermo Scientific, Waltham, MA, USA), equipped with an FID detector that enabled quantitative analyses of the post-reaction mixtures, was used. Analyses were performed with the ZB-1701 chromatographic column, with the dimensions of 30 m × 0.53 mm × 1 µm and filled with 14% cyanopropylphenyl and 86% dimethylpolysiloxane. The detector temperature was 250 °C, and the inlet temperature was 230 °C. The flow rate of hydrogen and helium was 0.8 mL/min. Before GC analyses, the post-reaction mixture samples were diluted with acetone in a ratio of 8:2. The internal normalization method was used to identify and determine the content of individual products in the post-reaction mixtures.
Mass balances were prepared for all post-reaction mixtures obtained in the studies on the effect of temperature, the amount of the catalyst, and the reaction time. Based on the obtained mass balances, the main functions describing the process of oxidation were calculated. These functions included the conversion of limonene and the selectivities of the appropriate products, such as 1,2-epoxylimonene, 1,2-epoxylimonene diol, carveol, carvone, and perillyl alcohol. Figure 2 shows the main products of limonene oxidation, which were detected in the post-reaction mixtures.
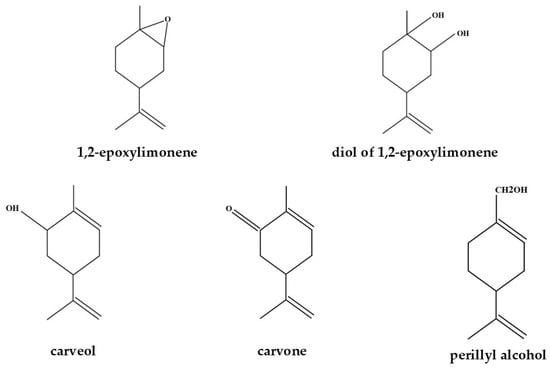
Figure 2.
The main products identified in the post-reaction mixtures using the gas chromatography method.
The conversion of limonene and the selectivities of the appropriate products were calculated according to the following formulas:
Conversion of limonene = (number of moles of limonene reacted/number of moles of limonene introduced into the reactor) × 100 [mol%]
Selectivity of the appropriate product = (number of moles of the appropriate product/number of moles of reacted limonene) × 100 [mol%].
3. Results and Discussion
3.1. Characterization of the Catalyst Obtained from the Dried Avocado Seeds
The EDX spectrum (Figure 3) represents the elemental composition of the powder obtained from the dried avocado seeds, showing peaks corresponding to specific elements and their respective weight percentages. A significant peak at around 0.3 keV indicates the presence of carbon with a weight percentage of 31.79%. This indicates the possible organic nature of the sample. A pronounced peak at around 0.5 keV, with a weight percentage of 63.02%, reflecting the presence of oxygen-rich organic compounds such as cellulose, hemicellulose, and possibly starch. A distinct peak near 3.3 keV, with a weight percentage of 5.19%, suggests the presence of potassium salts or other potassium-based compounds, which are common in plant materials and play a role in biological processes. The dominance of oxygen and carbon aligns with the composition of organic plant material, where carbohydrates and structural polysaccharides are primary constituents.

Figure 3.
EDX patterns for dried avocado seeds powder.
Figure 4 presents the XRD spectrum of the powder obtained from the dried avocado seeds. The XRD pattern presented in Figure 4 exhibits a broad peak centered at the 2θ angle of 20–25°, which suggests the presence of an amorphous phase or poorly crystalline material. The in-depth analysis using the X’Pert HighScore software ver. 1.0 indicated the presence of starch in the sample (JCPDS No. 39-1912). The starch’s reference peaks are marked on the figure as vertical bars.
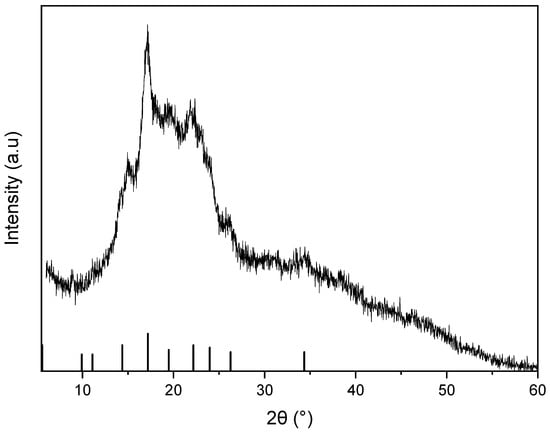
Figure 4.
XRD spectrum of dried avocado seeds powder. Vertical bars indicate reference peaks for starch (JCPDS No. 39-1912).
Figure 5 shows the FT-IR spectrum of the powder obtained from the dried avocado seeds. The Fourier Transform Infrared spectroscopy method was utilized to analyze and identify the functional groups that existed in the tested material. Dried and ground avocado seeds (in the form of powder) were tested in the wavelength range of 4000 to 500 cm−1. In the obtained spectrum, the broad band between 3590 and 2990 cm−1 is visible, corresponding to the presence of hydroxyl groups intermolecularly connected by hydrogen bonds [19]. The narrow, low-intensity band at 2922 cm−1 corresponds to the presence of the C-H bond. Another narrow, low-intensity peak, which appeared near 1614 cm−1, is considered to be associated with an aromatic ring (C=C) [20]. The peak with the highest intensity appeared at 1007 cm−1, indicating the presence of C-OH or C-O-C groups [21]. The narrow, intense band at 537 cm−1 is identified as basic vibrational movements related to the structure of the pyranose ring found in starch [22]. In the work of J.Br. Tarigan et al. [23], which presents the application of the waste passion fruit peel as the heterogeneous catalyst for biodiesel production, the characteristic stretching and bending vibration of C−O groups from potassium carbonate in the FT-IR spectrum of this material were described at peaks of 1654, 1386, and 1110 cm−1. These bands are also present in the FT-IR spectrum of dried avocado seeds powder studied in this work (these are low-intensity bands which are marked with green arrows), which confirms the composition results obtained by the EDX method for this material that showed the presence of potassium in the dried avocado seeds powder sample [23].
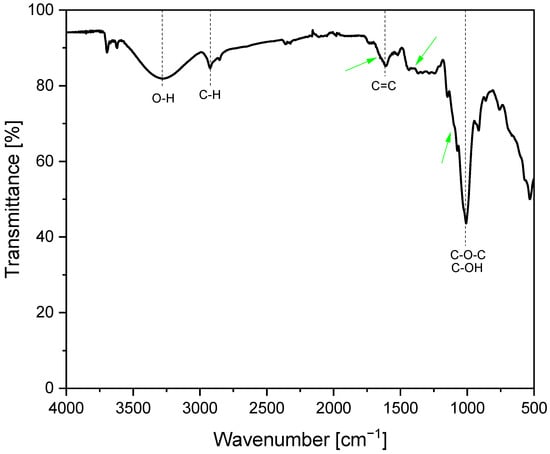
Figure 5.
FT-IR spectrum of dried avocado seeds powder.
Figure 6 shows the UV-VIS spectrum of the powder obtained from the dried avocado seeds. The analysis was carried out to observe characteristic bands for the tested material, which is an avocado seed-based catalyst. Only specific functional groups in organic compounds known as chromophores, which have valence electrons with low excitation energies, are capable of absorbing ultraviolet and visible light [24].
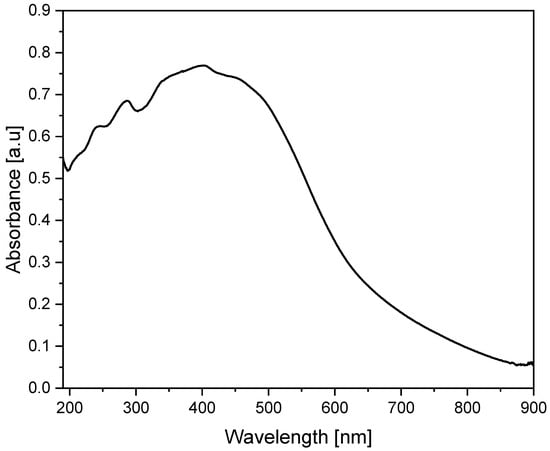
Figure 6.
UV-VIS spectrum of dried avocado seeds powder.
The UV-VIS studies were conducted in the range of 190 to 900 nm. Avocado seed powder showed strong absorbance from 190 to 600 nm. The ultraviolet range shows several narrow band peaks reaching a maximum at wavelengths of approximately 190 nm, 247 nm, 290 nm and 340 nm. This may indicate the content of flavonoids and their derivatives [25]. In the visible light region, there is a noticeable broad band from 400 to 500 nm with high intensity with an absorption maximum (0.769) at 403 nm. The significant absorption of wavelengths in the range of 400–500 nm (corresponding to blue and green light) is a characteristic feature of red-orange objects, as these wavelengths correspond to their complementary colors. Absorbance decreased significantly above 580 nm, consistent with the obtained reddish-orange color of our catalyst powder.
Figure 7 shows the SEM images of the powder obtained from the dried avocado seeds.
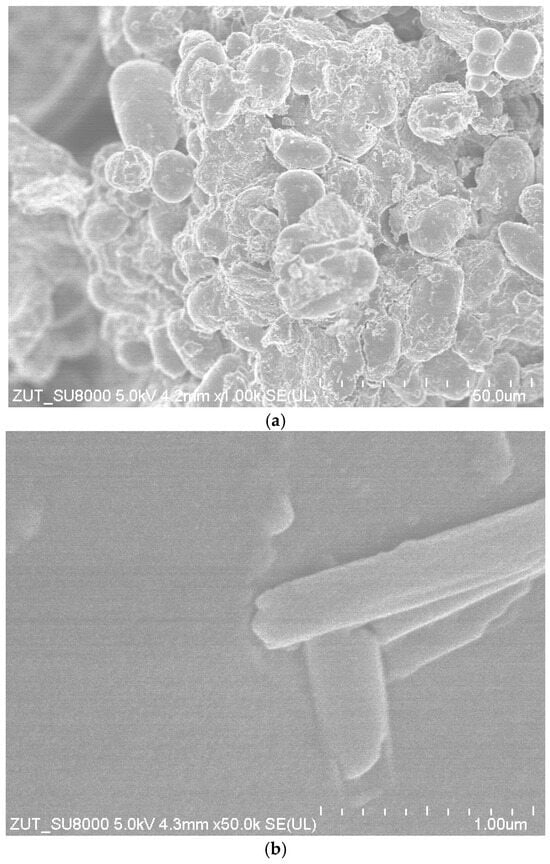
Figure 7.
The SEM images of dried avocado seeds powder. Magnification 1000× (a) and 50,000× (b).
At the magnification of 1000×, the surface morphology of the dried avocado seeds powder reveals the agglomeration of irregularly oval-shaped particles. Most of these particles exhibit a rough surface. Only a few have a smooth surface. The size distribution appears to be heterogeneous, with clusters forming dense agglomerates, suggesting a compact microstructure. The SEM image prepared at the magnification of 50,000× provides a detailed view of the microstructure, highlighting individual micro- and nano-sized elements. Rod-like structures are observed on the surface of particles. The smoothness of these structures contrasts with the coarser texture seen in the lower magnification, offering insights into the potential internal organization of the material.
3.2. Catalytic Investigation of the Catalyst Obtained from the Dried Avocado Seeds
The first parameter studied during the catalytic tests was temperature. The influence of temperature was tested in the range of 70–100 °C. The amount of the catalyst used in the oxidation process was 1 wt%. The reaction time amounted to 5 h. Figure 8 shows the results of studies on the influence of temperature on the course of limonene oxidation in the presence of the powder obtained from the dried avocado seeds as the catalyst.
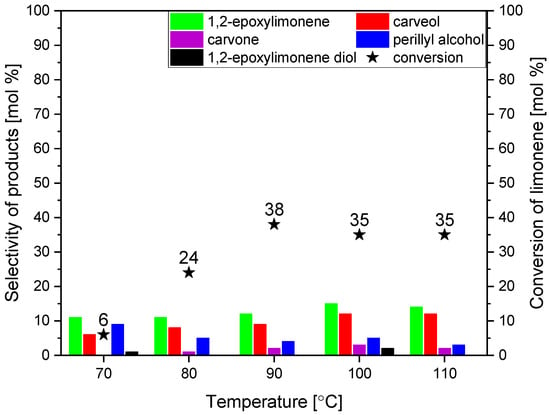
Figure 8.
The influence of temperature on the selectivities of the transformation to the main limonene oxidation products and on the conversion of limonene in the presence of powder obtained from the dried avocado seeds as the catalyst (amount of the catalyst was 1 wt%, and the reaction time was 5 h).
Figure 8 shows that the conversion of limonene increases from 6 to 38 mol% in the temperature range of 70 to 90 °C, and later, despite the temperature increase, it does not undergo any significant changes. The conversion of limonene is one of the main functions of the process, based on which the most favorable values of the tested process parameters will be selected. The second is the selectivity of the transformation to 1,2-epoxylimonene—the main product of the studied process. At the same time, it can be said that the highest selectivities of most of the obtained products are obtained at the temperature of 100 °C (except for perillyl alcohol, which is formed with the highest selectivity at the lowest tested temperature, i.e., at 70 °C). It should be noted that the differences in the selectivity values of individual compounds above 80 °C compared to the values obtained at 80 °C, visible in Figure 8, are insignificant and amount to about 2–3% mol, which means that they are within the error limits of the GC method. It can, therefore, be assumed that there are no significant changes in the selectivity of the transformation to the main products above 80 °C. However, considering that the next two stages of the study will examine the effect of the amount of catalyst and the reaction time, it was decided to adopt a temperature higher than 80 °C, i.e., 90 °C, as the most advantageous. Perhaps by selecting these two remaining parameters, it will be possible to increase the conversion of limonene and the selectivity of the transformation to the main products.
The two compounds that are formed with the highest selectivities at this stage of research are 1,2-epoxylimonene and carveol. Moreover, the comparison of the obtained results shows that the epoxy compound formed during oxidation is relatively stable in the studied conditions and is converted to diol only in a small amount, regardless of the tested temperature of the oxidation process. The same conclusion can also be drawn in relation to carveol, which is only slightly converted to carvone. The explanation of this phenomenon is presented later in the manuscript, where a probable mechanism of limonene oxidation using avocado seeds powder as a catalyst is proposed. Carvone is formed in the studied process only in small amounts, and at the temperature of 70 °C, the formation of this compound was not observed at all.
As mentioned above, considering the results obtained at this stage of the research, the temperature of 90 °C was considered the most favorable (mainly taking into account the values of the conversion of limonene) and at this temperature, the studies on the effect of the amount of catalyst and the reaction time were carried out.
The studies on the effect of the amount of the catalyst were performed in the range of catalyst content of 0.5–5.0 wt%, with a reaction time of 5 h. The results of these studies are presented in Figure 9. Figure 9 shows that with the increase in the catalyst content in the reaction mixture, very slight changes in the values of limonene conversion are observed, which changes from 39 mol% to 32–34 mol%. The decrease in limonene conversion with an increase in the catalyst content in the reaction mixture is probably related to the formation of oligomeric products from limonene and its transformation products, which are not determined by the GC method in our work. Similarly to the previous stage of the studies, the main products of the process are: 1,2-epoxylimonene and carveol, which are formed with very similar selectivities (11–13 mol% and 9–12 mol%), with slightly more 1,2-epoxylimonene always being formed in relation to carveol (this difference is about 2–3 mol%). Both products are stable in the reaction mixtures because there is no visible increase in the selectivity of the transformation to carvone (the product of carveol oxidative dehydrogenation or direct oxidation of limonene) and 1,2-epoxylimonene diol (the product of epoxy ring hydrolysis) with increasing in the amount of catalyst in the reaction mixture. As we mentioned above, the explanation of this phenomenon is presented later in the manuscript, where the probable mechanism of limonene oxidation using avocado seeds powder as a catalyst is proposed. After analyzing the obtained results, the most favorable catalyst content was found to be 2 wt%, mainly taking into account the selectivity of the transformation to 1,2-epoxylimonene (for this catalyst content, the highest value of this function was obtained—13 mol%) and the conversion of limonene (35 mol% was one of the highest values of this function during studies on the influence of catalyst content in the range of 0.5 to 5.0 wt%).
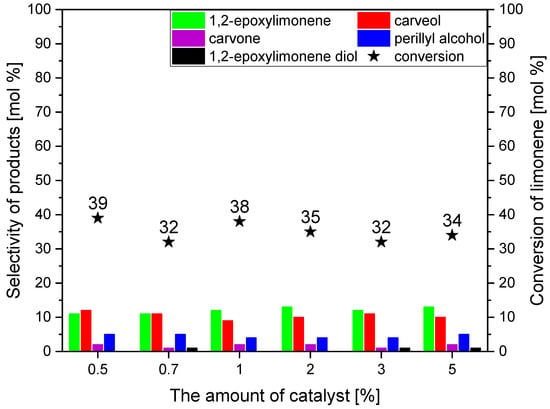
Figure 9.
The influence of the amount of the catalyst on the selectivities of the transformation to the main limonene oxidation products and on the conversion of limonene in the presence of powder obtained from the dried avocado seeds as the catalyst (temperature 90 °C and reaction time 5 h).
The last parameter studied was reaction time. The studies at this stage were performed at a temperature of 90 °C and for the amount of catalyst of 2 wt%. The obtained results are presented in Figure 10.
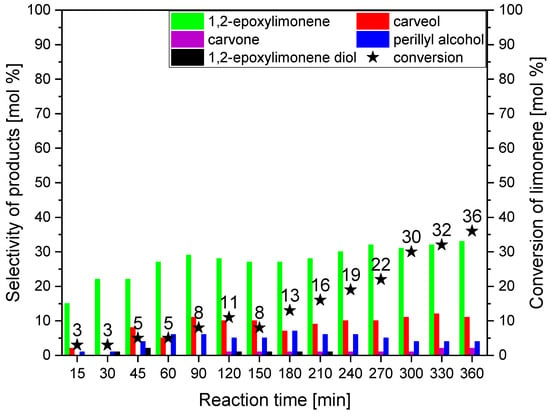
Figure 10.
The influence of reaction time on the selectivities of the transformation to the main limonene oxidation products and on the conversion of limonene in the presence of powder obtained from the dried avocado seeds as the catalyst (temperature 90 °C, amount of catalyst 2 wt%).
Analysis of Figure 10 shows that with the extension of the reaction time, there is a significant increase in the selectivity of the transformation to 1,2-epoxylimonene from 15 to 33 mol%. At the same time, the selectivities of the transformation to carveol are not high (changes from 1 to 11 mol% are only observed), and the dominant product is still 1,2-epoxylimonene. The epoxy compound is stable under the reaction conditions, and no hydration of the epoxy ring and transformation of 1,2-epoxylimonene to 1,2-epoxylimonene diol is observed here. Carvone is not formed at all for reaction times from 15 min to 90 min, and for longer reaction times, its selectivities are low and amount below 2 mol%.
The summary of the conducted studies shows that the main direction of limonene oxidation with the participation of powder from dried and ground avocado seeds is the product of oxidation of the bond in the position of 1–2 in the limonene molecule, namely 1,2-epoxylimonene (Figure 11). The second main product in this process is carveol—the product of oxidation at the allylic position 6. During the studies of the effect of temperature and the amount of the catalyst, the differences in the selectivities of both compounds were not significant. Only, they were very noticeable during the studies of the effect of reaction time, where for the longest reaction times, almost three-fold higher values of the selectivity of the transformation to 1,2-epoxylimonene were obtained. It should also be noted that both products are very stable, which only to a small extent underwent transformations in subsequent reactions: 1,2-epoxylimonene in the hydrolysis of the epoxy ring and carveol in the oxidative dehydrogenation to carvone. Thanks to the high stability of these two compounds in the reaction mixture, it was possible to limit the amount of other products, which is beneficial from the point of view of separating products from the post-reaction mixtures. The third important product of the studied process was perillyl alcohol—the product of the oxidation at the allylic position 7. This product was formed during the time effect studies (for the longest reaction times) with a selectivity two times lower than carveol. This indicates that the preferred direction of oxidation of the limonene molecule was the oxidation of the double bond in position 1–2, followed by the oxidation at allylic position 6, and the least favorable was oxidation at allylic position 7. At the same time, conducting the reaction only in limonene, which played the role of both organic substrate and solvent, provided favorable conditions for the stability of 1,2-epoxylimonene and carveol molecules.
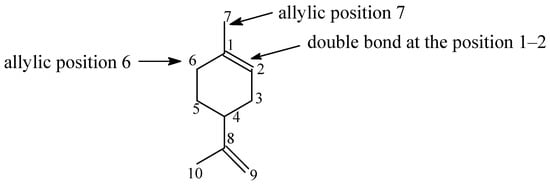
Figure 11.
Main directions of the oxidation in the limonene molecule.
After analyzing the obtained results and presenting the above main directions of the limonene oxidation process, a question arises related to explaining the mechanism of formation of limonene oxygenated products on the avocado seed powder catalyst. First of all, the role of starch and potassium, present in the composition of the tested catalyst, seems to be very important in this mechanism. Starch has a lot of hydroxyl groups, but its chemical character is neutral. Protons in these hydroxyl groups do not exhibit acidic properties because there are no electron-withdrawing groups that could stabilize the O2− anions formed after the splitting of protons. However, if the catalyst containing starch also contains potassium in the form of, e.g., KOH or K2CO3 (based on the FT-IR spectrum presented earlier, we can state that our material contains K2CO3), then a change in the chemical character of—–OH groups in starch and activation of the catalyst surface may occur. Probably, as a result of the interaction of K+ ions with –OH groups of starch, acidic centers of the catalyst with easily cleaved (mobile) protons are formed. In order to verify these conclusions, we measured the total acidity of the catalyst in the form of powder from dried avocado seeds. We obtained the following result: 0.36286 mmol/g. The obtained value indicates the acidic nature of the catalyst we tested. Below, in Figure 12, we present a possible way of formation of acid centers in the starch structure in the presence of potassium ions.
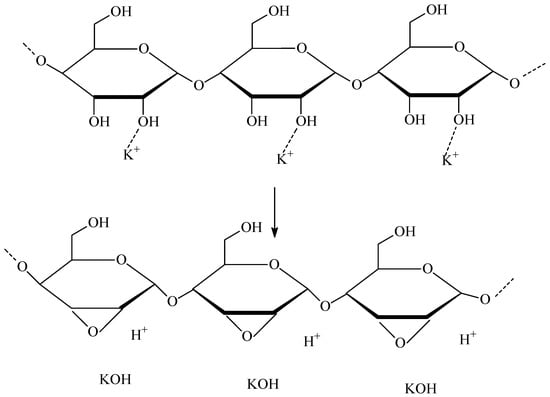
Figure 12.
The possible way of forming acid centers in the starch structure in the presence of potassium ions.
Considering the key role of potassium ions in the formation of acidic centers in the starch structure, we can say that potassium plays the role of a catalytic promoter, which increases the catalytic activity of dried avocado seed powder. Next, we decided to compare the acidity of the catalyst obtained by us from dried avocado seeds with the acidity of other catalysts that we had also previously used in the oxidation of limonene or other terpene compounds, i.e., catalysts: TS-1, Ti-MCM-41 and Ti-SBA-15. For these catalysts, the total acidity amounted to [in mmol/g] 0.27, 0.50, and 0.37, respectively. The catalyst from avocado seeds, therefore, has a comparable total acidity to these catalysts, and especially to the catalyst Ti-SBA-15 and TS-1, and this is a moderate acidity value.
Based on the earlier publication by A. Wróblewska [26], we would like to propose a probable mechanism for obtaining the main products of limonene oxidation (1,2-epoxylimonene, carveol and perillyl alcohol)—Figure 13 and Figure 14. However, this is only a preliminary proposal, which will require further research in the future and consideration of other routes of limonene oxidation.
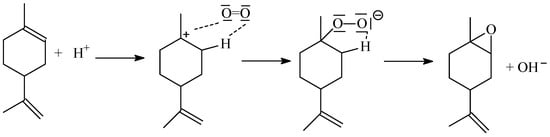
Figure 13.
The possible way of 1,2-epoxylimonene formation on acid centers formed in a starch structure in the presence of potassium ions.
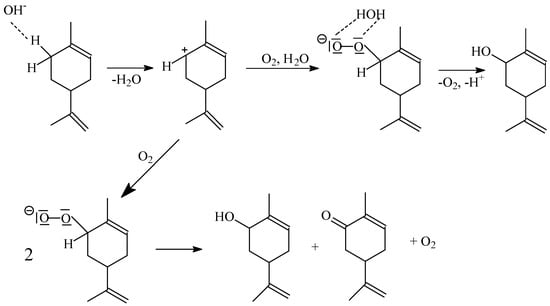
Figure 14.
The possible way of carveol formation on acid centers formed in a starch structure in the presence of potassium ions.
Figure 13 shows the attachment of the proton from the acid center in starch to the double bound in the position of 1–2 in the limonene molecule. The attachment of the proton in position 2 causes the formation of a carbocation in position 1. If there is an oxygen molecule in the vicinity of the carbocation, an interaction occurs between this carbocation and the electron pair of an oxygen atom. In the next stage, the oxygen molecule attaches to position 1 in the limonene molecule. A peroxy group is formed, with which the proton bound in position 2 interacts. In the next stage, the hydroxyl group is eliminated, and an epoxy group is formed—1,2-epoxylimonene is formed, which is the main product of limonene oxidation.
Figure 14 shows two ways of carveol formation (perillyl alcohol is formed in a similar way). Hydroxyl groups formed in the stage of 1,2-epoxylimonene formation interact with the hydrogen atom bound in position 6 (allylic position) in the limonene molecule. As a result of this interaction, a proton is separated, and the carbocation is formed, while water is also released. The resulting carbocation can undergo changes in two directions. The first direction is the attachment of the oxygen molecule and the formation of the peroxy group, with which the water molecule can interact (the process is carried out under atmospheric pressure in the air atmosphere, and water vapor is also present in the air). As a result of this interaction, carveol is formed, and oxygen molecules and protons are regenerated. In the second way, we obtain both carveol and carvone from two compounds with the peroxy group. Since we did not observe the formation of carvone in large amounts during our studies, it can be assumed that the first route of transformation, the peroxy group, is dominant. Otherwise, both products would be formed with similar selectivities. The formation of carvone, according to the first route, can be explained by the interaction of the proton released in the previous stage with the hydroxyl group in position 6, as a result of which a hydrogen molecule and a carbonyl group are formed. However, the selectivity of this compound is not high, which may indicate that, most likely, free hydroxyl groups (not involved in the formation of active centers) in the starch structure can interact with the hydroxyl group in carveol molecule via hydrogen bonds, which stabilizes this compound and the reaction with proton is not observed. A similar stabilizing effect may apply to the epoxy group in 1,2-epoxylimonene because, during studies on the oxidation of limonene, the formation of 1,2-epoxylimonene diol was observed with very low selectivity, even for very long reaction times. In the case of perillyl alcohol, its formation can also be explained in a similar way to the formation of carveol, but oxidative dehydrogenation was not observed here because perillyl aldehyde was not detected in the post-reaction mixtures. In perillyl alcohol, the hydroxyl group is located on the outside of the molecule and is distant from the six-membered ring. In connection with this fact, here, the much greater influence of the hydroxyl groups present in starch is most likely noticeable, which stabilizes the hydroxyl group in perillyl alcohol via hydrogen bonds and therefore it does not undergo oxidative dehydrogenation reaction.
Due to the fact that the acidity of the avocado seeds powder that we used in our studies was moderate, other routes of limonene oxidation by this catalytic material can also be considered. The possibility of oxidation of starch hydroxyl groups to carbonyl and carboxyl groups should probably also be taken into account here. Both hydroxyl groups, carbonyl groups and carboxyl groups can participate in the transfer of oxygen to the limonene molecule by forming peroxy and hydroperoxy groups. Taking this into account, below we would like to present another probable route of limonene oxidation occurring according to the free-radical mechanism, in which the key role is played by hydroxyl groups present in the starch structure (groups not involved in the formation of acid centers). In this transformation pathway, which we would like to present starting from the hydroxyl groups present in the starch structure, first, the hydroxyl groups react with oxygen, as a result of which diradical structure is formed in the starch structure, and hydroxyl radicals are generated—Figure 15.
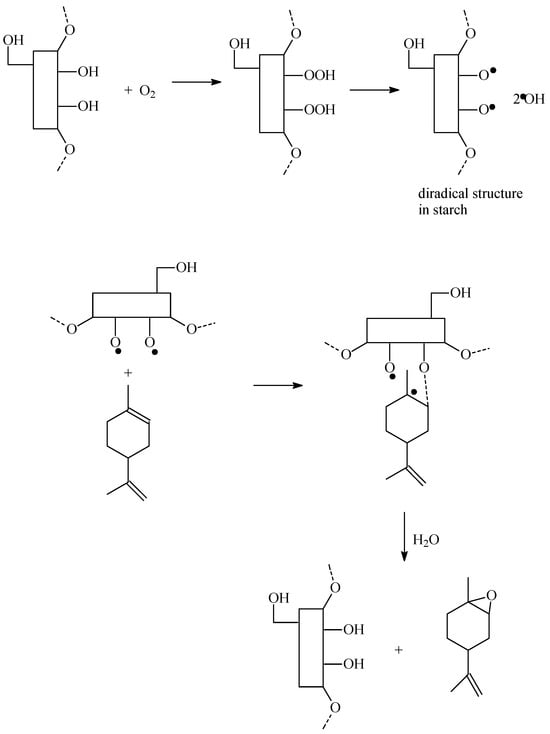
Figure 15.
The reaction of oxygen with hydroxyl groups present in the starch structure—in this way a diradical structure is formed, which reacts with the limonene molecule.
Figure 15 shows that the diradical structure formed in the starch structure then reacts with the limonene molecule, and a second diradical structure is formed, in which the limonene molecule is bound. As a result of the reaction of this structure with water, the starch structure with hydroxyl groups is reconstructed, and 1,2-epoxylimonene is formed. In the formation of carveol and perillyl alcohol, hydroxyl radicals produced from hydroperoxy groups are involved. The scheme of transformations involving these radicals is very similar to that shown above for the ionic mechanism in which the hydroxyl groups took part. Also, in the case of this mechanism, it is possible to stabilize the epoxy group in 1,2-epoxylimonene and the hydroxyl group in carveol and perillyl alcohol by the hydroxyl groups present in the starch structure. If the limonene oxidation reaction proceeds according to this mechanism, then also, in this case, there is an explanation for the low selectivity of the transformation to 1,2-epoxylimonene diol and carvone and the absence of perillyl aldehyde among the reaction products. We presented the proposals for the free radical mechanism of limonene oxidation in this article based on our earlier publication concerning the oxidation of α-pinene on the Ti-SBA-15 catalyst [27].
The considerations presented above on the oxidation of limonene using the catalyst in the form of power from dried avocado seeds are only preliminary theoretical considerations. These should be thoroughly investigated and confirmed in our future broader studies. Considering that the acidity of the tested catalyst is only moderate, it can be assumed that in addition to the reactions occurring according to the ionic mechanism, reactions occur simultaneously according to the free radical mechanism. However, this requires confirmation in the course of further studies. At this stage of our research, which was only preliminary, the main goal was to investigate whether the catalyst obtained from waste biomass would be active in the process of limonene oxidation.
The comparison of the results of limonene oxidation using the catalyst in the form of dried avocado seeds powder with the earlier results of studies on limonene oxidation using titanium-silicate catalysts TS-1 and Ti-SBA-15, presented by A. Wróblewska [26], shows that different directions of limonene molecule oxidation are preferred using the avocado seeds catalyst than using the TS-1 and Ti-SBA-15 catalysts. In the case of our catalyst, as described above, epoxidation of the double bond in position 1–2 is preferred, and this is the main direction of the reaction. Moreover, oxidation in the allylic position on carbon atom no. 6 (formation of carveol) and, to a lesser extent, oxidation in the allylic position on carbon atom no. 7 (formation of perillyl alcohol) also occurs—Figure 11. In the above-mentioned publication by A. Wróblewska [26], the studies were conducted using 60% hydrogen peroxide as the oxidant, with the content of titanium-silicate catalyst in the reaction mixture being 3 wt%, with the use of methanol as the solvent, the concentration of which in the reaction mixture was 80 wt% and with the molar ratio of limonene/H2O2 = 1:2. For the TS-1 catalyst (microporous catalyst) at 80 °C the main reaction product was perillyl alcohol, which was formed with the selectivity of 46–64 mol%. The second reaction direction was the oxidation of the double bond in position 1–2 in the limonene molecule, whereby the formed 1,2-epoxylimonene was largely hydrated to the diol (30–50% in relation to the amount of 1,2-epoxylimonene formed). The third reaction direction was the formation of carveol. The selectivities of the transformation to this compound were low, 1–11 mol%. However, the formation of carvone was observed with a selectivity of 15–18 mol%. Limonene conversion was lower during the tests with the TS-1 catalyst, especially for longer reaction times, starting from 180 min (for the reaction time of 180 min, the limonene conversion was 7 mol%). The results presented for the TS-1 catalyst differ significantly from the results obtained with the catalyst in the form of dried avocado seeds powder. The lack of porosity and active centers in the form of Ti4+ ions, but on the other hand, the formation of acid centers with the participation of potassium ions and the presence of hydroxyl groups, acting as oxygen carriers to the limonene molecule and stabilizers of groups such as epoxy and hydroxyl in the limonene molecule, are the key reasons for the activity of our catalyst. The catalyst proposed in this work, which was obtained from renewable biomass, is, therefore, the effective catalyst for the oxidation process, allowing for obtaining different selectivities of individual reaction products than the TS-1 catalyst. In the case of the Ti-SBA-15 catalyst, similar directions of transformations were observed as for the TS-1 catalyst, although perillyl alcohol (the main product of the process) was formed with even slightly higher selectivities than for the TS-1 catalyst (selectivities up to 68 mol%). Also, in the presence of the Ti-SBA-15 catalyst, more carvone than carveol was determined in the reaction mixture, whereas less was formed for longer reaction times of 1,2-epoxylimonene. In the case of Ti-SBA-15, significantly higher conversions of limonene were observed than for the TS-1 catalyst and the avocado powder catalyst. These conversions varied in the range of 17–46 mol%. The difference in limonene conversion may result in the case of the Ti-SBA-15 catalyst from the larger pores of this material and, thus, from greater accessibility to active sites, resulting from lower steric constraints.
The catalyst in the form of powder from dried avocado seeds is, therefore, the active catalyst for limonene oxidation. Its use provides benefits in the form of the increase in the amount of the epoxy compound formed and alcohols formed by the oxidation in allylic positions 6 and 7. At the same time, compared to the process carried out with the participation of titanium-silicate catalysts, the proposed method of limonene oxidation on the catalyst from waste avocado seeds, using oxygen and without the participation of a solvent, seems to be a more economical and environmentally friendly solution. In addition, this method of limonene oxidation does not generate a large amount of waste that is difficult to manage, and all the main products formed in this process (1,2-epoxylimonene, carveol and perillyl alcohol) find many applications, both in industry and in medicine and cosmetics. We described these applications in our previous article [28], and they show, among others, that perillyl alcohol, carveol, and 1,2-epoxylimonene have a lot of applications as components of fragrant compositions for perfumery and cosmetics, as food additives, and as components of polymers. The applications of perillyl alcohol and carveol in medicine deserve special attention. Perillyl alcohol and carveol have been reported as compounds with significant anticancer properties. Perillyl alcohol has been efficacious against the formation and progression of various cancers: colon, skin, head, lung and neck. Carveol was used in therapy for pancreatic and breast cancer. Carveol also shows anti-yeast and antifungal activity [28]. For this reason, research on limonene oxidation using natural catalysts obtained from waste biomass is worth further development.
4. Conclusions
The research presented in this work shows that raw materials of natural origin—including wastes, which often cause us problems when it comes to their management—can be successfully used as catalytic materials for reactions in organic chemistry (avocado seeds), as well as substrates for these reactions (limonene). The preparation of powdered, dry avocado seeds is not difficult and does not require high costs. As can be seen from the results we have shown, such a powder can be an effective catalyst for terpene oxidation processes. Further research on this catalytic material should go towards increasing its activity, e.g., by washing it with acid solutions or by introducing metals into the structure of this catalyst, which could constitute its active centers.
Author Contributions
Conceptualization, S.G., J.S., A.W. and B.M.; methodology, S.G., J.S., A.W. and B.M.; validation, S.G., J.S., A.W. and B.M.; formal analysis, A.W. and B.M.; investigation, S.G., J.S., A.W. and B.M.; resources, S.G., J.S., A.W. and B.M.; data curation, S.G., J.S., A.W. and B.M.; writing—original draft preparation, S.G., J.S., A.W. and B.M.; writing—review and editing, S.G., J.S., A.W. and B.M.; visualization, S.G. and J.S.; supervision, A.W. and B.M.; project administration, A.W.; writing—review and editing, A.W. and B.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author(s).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Manisalidis, I.; Stavropoulou, E.; Stavropoulos, A.; Bezirtzoglou, E. Environmental and Health Impacts of Air Pollution: A Review. Front. Public Health 2020, 8, 505570. [Google Scholar] [CrossRef]
- Ritchie, H.; Rosado, P.; Roser, M. Avocado Production. In Agricultural Production; Food and Agriculture Organization of the United Nations: Rome, Italy, 2023; Available online: https://ourworldindata.org/grapher/avocado-production (accessed on 14 March 2025).
- Ford, N.A.; Spagnuolo, P.; Kraft, J.; Bauer, E. Nutritional Composition of Hass Avocado Pulp. Foods 2023, 12, 2516. [Google Scholar] [CrossRef] [PubMed]
- Marra, A.; Manousakis, V.; Zervas, G.P.; Koutis, N.; Finos, M.A.; Adamantidi, T.; Panoutsopoulou, E.; Ofrydopoulou, A.; Tsoupras, A. Avocado and its By-products as Natural Sources of Valuable Anti-inflammatory and Antioxidant Bioactives for Functional Foods and Cosmetics with Health-Promoting Properties. Appl. Sci. 2024, 14, 5978. [Google Scholar] [CrossRef]
- Da Silva Bastos, K.V.L.; Souza, A.B.; Gomes, R.R.; de Souza, L.C.; Aquino, I.P.; de Moura Souza, F. Phytochemicals Present in Ethanol Extract of Avocado Seed and its Potential Antioxidant Effect. Curr. Organocatalysis 2024, 11, 71–77. [Google Scholar] [CrossRef]
- Dabas, D.; Shegog, R.M.; Ziegler, G.R.; Lambert, J.D. Avocado (Persea americana) Seed as a Source of Bioactive Phytochemicals. Curr. Pharm. Des. 2013, 19, 6133–6140. [Google Scholar] [CrossRef]
- Arlene, A.A.; Prima, K.A.; Utama, L.; Anggraini, S.A. The Preliminary Study of the Dye Extraction from the Avocado Seed using Ultrasonic Assisted Extraction. Procedia Chem. 2015, 16, 334–340. [Google Scholar] [CrossRef]
- Cid-Pérez, T.S.; Hernández-Carranza, P.; Ochoa-Velasco, C.E.; Ruiz-López, I.I.; Nevárez-Moorillón, G.V.; Ávila-Sosa, R. Avocado Seeds (Persea americana cv. Criollo sp.): Lipophilic Compounds Profile and Biological Activities. Saudi J. Biol. Sci. 2021, 28, 3384–3390. [Google Scholar]
- Rodrigues, C.E.C.; Meirelles, A.J.A. Extraction of Free Fatty Acids from Peanut Oil and Avocado Seed Oil: Liquid−Liquid Equilibrium Data at 298.2 K. J. Chem. Eng. Data 2008, 53, 1698–1704. [Google Scholar] [CrossRef]
- López-Cobo, A.; Gómez-Caravaca, A.M.; Pasini, F.; Caboni, M.F.; Segura-Carretero, A.; Fernández-Gutiérrez, A. HPLC-DAD-ESI-QTOF-MS and HPLC-FLD-MS as Valuable Tools for the Determination of Phenolic and other Polar Compounds in the Edible Part and By-products of Avocado. LWT-Food Sci. Technol. 2016, 73, 505–513. [Google Scholar] [CrossRef]
- Saavedra, J.; Córdova, A.; Navarro, R.; Díaz-Calderón, P.; Fuentealba, C.; Astudillo-Castro, C.; Toledo, L.; Enrione, J.; Galvez, L. Industrial Avocado Waste: Functional Compounds Preservation by Convective Drying process. J. Food Eng. 2017, 198, 81–90. [Google Scholar] [CrossRef]
- Lego, N.; Shadap, A.; Momin, K.C. Essential Oils and the Methods of Extraction. In Aromatic Plants. The Technology, Human Welfare and Beyond; Nova Science Publishers: New York, NY, USA, 2021; Chapter 7; pp. 145–168. [Google Scholar]
- Gould, M.N. Cancer Chemoprevention and Therapy by Monoterpenes. Environ. Health Perspect. 1997, 105, 977–979. [Google Scholar]
- Wróblewska, A.; Miądlicki, P.; Makuch, E.; Benedyczak, N. Epoxidation of Natural Limonene Extracted from Orange Peels with Hydrogen Peroxide over Ti-MCM-41 Catalyst. Pol. J. Chem. Technol. 2018, 20, 1–6. [Google Scholar] [CrossRef]
- Lin, H.; Li, Z.; Sun, Y.; Zhang, Y.; Wang, S.; Zhang, Q.; Cai, T.; Xiang, W.; Zeng, C.; Tang, J. D-Limonene: Promising and Sustainable Natural Bioactive Compound. Appl. Sci. 2024, 14, 4605. [Google Scholar] [CrossRef]
- Pagliaro, M.; Fabiano-Tixier, A.-S.; Ciriminna, R. Limonene as a Natural Product Extraction Solvent. Green Chem. 2023, 25, 6108–6119. [Google Scholar] [CrossRef]
- Chen, X.; Ding, Y.; Guan, H.; Zhou, C.; He, X.; Shao, Y.; Wang, Y.; Wang, N.; Li, B.; Lv, G.; et al. The Pharmacological Effects and Potential Applications of Limonene from Citrus Plants: A review. Nat. Prod. Commun. 2024, 19, 1–12. [Google Scholar] [CrossRef]
- Vilcocq, L.; Spinola, V.; Moniz, P.; Duarte, L.C.C.; Carvalheiro, F.; Fernandes, C.; Castilho, P. Acid-Modified Clays as Green Catalysts for the Hydrolysis of Hemicellulosic Oligosaccharides. Catal. Sci. Technol. 2015, 5, 4072–4080. [Google Scholar] [CrossRef]
- Bakti, A.I.; Gareso, P.L. Characterization of Active Carbon Prepared from Coconuts Shells using FTIR, XRD and SEM Techniques. J. Ilm Pendidik. Fis. Al-Biruni. 2018, 7, 33–39. [Google Scholar] [CrossRef]
- Kamińska, A.; Sreńscek-Nazzal, J.; Serafin, J.; Miądlicki, P.; Kiełbasa, K.; Wróblewska, A. Biomass-Based Activated Carbons Produced by Chemical Activation with H3PO4 as Catalysts for the Transformation of α-Pinene to High-Added Chemicals. Environ. Sci. Pollut. Res. Int. 2024, 31, 40063–40082. [Google Scholar] [CrossRef]
- Ojrzynska, M.; Wroblewska, A.; Judek, J.; Malolepszy, A.; Duzynska, A.; Zdrojek, M. Study of Optical Properties of Graphene Flakes and its Derivatives in Aqueous Solutions. Opt. Express 2020, 28, 7274–7281. [Google Scholar] [CrossRef]
- Kizil, R.; Irudayaraj, J.; Seetharaman, K. Characterization of Irradiated Starches by using FT-Raman and FTIR Spectroscopy. J. Agric. Food Chem. 2002, 50, 3912–3918. [Google Scholar] [CrossRef]
- Tarigan, J.B.; Singh, K.; Sinuraya, J.S.; Supeno, M.; Sembiring, H.; Tarigan, K.; Rambe, S.M.; Karo-karo, J.A.; Sitepu, E.K. Waste Passion Fruit Peel as a Heterogeneous Catalyst for Room-Temperature Biodiesel Production. ACS Omega 2022, 7, 7885–7892. [Google Scholar] [CrossRef] [PubMed]
- Pentassuglia, S.; Agostino, V.; Tommasi, T. EAB—Electroactive Biofilm: A Biotechnological Resource. In Encyclopedia of Interfacial Chemistry, Surface Science and Electrochemistry; Elsevier: Amsterdam, The Netherlands, 2018; pp. 110–123. [Google Scholar]
- Singh, R.; Mendhulkar, V.D. FTIR Studies and Spectrophotometric Analysis of Natural Antioxidants, Polyphenols and Flavonoids in Abutilon Indicum (Linn) Sweet Leaf Extract. J. Chem. Pharm. Res. 2015, 7, 205–211. [Google Scholar]
- Wróblewska, A. The Epoxidation of Limonene over the TS-1 and Ti-SBA-15 Catalysts. Molecules 2014, 19, 19907–19922. [Google Scholar] [CrossRef] [PubMed]
- Grzeszczak, J.; Wróblewska, A.; Michalkiewicz, B.; Dzięcioł, M.; Katarzyna Janda-Milczarek, K. Oxidation of α-Pinene on the Ti-SBA-15 Catalyst Obtained Using Orange Peel Waste as Components of the Synthesis Gel. Molecules 2025, 30, 1627. [Google Scholar] [CrossRef]
- Glonek, K.; Wróblewska, A.; Makuch, E.; Ulejczyk, B.; Krawczyk, K.; Wróbel, R.J.; Koren, Z.C.; Michalkiewicz, B. Oxidation of Limonene using Activated Carbon Modified in Dielectric Barrier Discharge Plasma. Appl. Surf. Sci. 2017, 420, 873–881. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).