Abstract
Polyethylene microplastics (PE MPs) pose a severe threat to aquatic ecosystems and human health, demanding urgent, sustainable remediation strategies. While the electro-Fenton process is widely used for treating refractory pollutants in wastewater, its standalone application remains inadequate for PE MPs due to their stable chemical structure and complex molecular chains. This study introduces a green and sustainable magnetite-activated persulfate electro-Fenton (Mt-PS-EF) system designed to address these limitations while aligning with circular-economy principles. By synergizing Fe₃O₄ catalysis, persulfate activation, and electrochemical processes, the Mt-PS-EF system achieves efficient PE MP degradation through hydroxyl (·OH) and sulfate (SO₄·⁻) radical-driven oxidation. Under optimized conditions (60 mg/L PE, 40 mM persulfate, 150 mg Fe3O₄, 20 h treatment), a 90.6% degradation rate was attained, with PE MPs undergoing chain scission, surface erosion, and release of low-molecular-weight organics. Crucially, the magnetic property of magnetite facilitated the recovery and reuse of the catalyst, significantly reducing material costs and minimizing waste generation. By integrating catalytic efficiency with resource recovery, this work advances scalable, eco-friendly solutions for microplastic pollution mitigation, directly contributing to UN Sustainable Development Goals (SDGs) 6 (Clean Water) and 14 (Life Below Water). The findings highlight the potential of hybrid electro-Fenton technologies in achieving sustainable wastewater treatment and plastic waste management.
1. Introduction
Since the inception of the pioneering work [1,2], the electro-Fenton (EF) process has gained increasing popularity through the in-depth development by pioneering teams such as Oturan [3,4] and Brillas [5], emerging as an environmentally significant technology for treating refractory wastewaters contaminated with toxic pollutants [6,7]. However, EF technology has demonstrated certain inadequacies in practical applications [8]. Firstly, the reactions involved in the EF process are three-phase interface reactions occurring at the solid–liquid–gas interface boundary [9], where the solid catalyst is in contact with both the liquid and gas phases [10]. Most EF experiments are conducted in beaker-scale setups with limited treatment capacity [11], rendering them unable to process large volumes of wastewater samples, which, in turn, restricts the representativeness and practicality of the experimental results [12]. Furthermore, the hydroxyl radicals (·OH) generated electrochemically have a very short lifetime (3.15 × 10−6 s) and cannot be stored, necessitating their in situ generation in practical applications [13]. It is noteworthy that in the classic Fenton reaction, the effective range of the ·OH oxidation process is only 50 nm, whereas in electrochemical oxidation systems, the effective range of the ·OH oxidation process is approximately 1 μm [13]. To address these issues and enhance the efficiency of pollutant degradation, considerable efforts have been made. For instance, hydrophilic electrode materials have been adopted [14]; larger-scale experimental setups have been selected [15]; and materials or electrodes with high adsorption capacities can enhance the adsorption of pollutants due to their shorter mass transfer distances, thereby improving the utilization efficiency of hydroxyl radicals (·OH) [16].
In recent years, advanced oxidation processes (AOPs) based on sulfate radicals (SO4·−) have gradually emerged as a research hotspot [17]. Compared to hydroxyl radicals (·OH), SO4·− persists longer in aqueous solutions, exhibits stronger oxidizing properties, and demonstrates more pronounced degradation effects on water-soluble organic pollutants, with more thorough degradation products and broader applicability [18]. SO4·− is typically generated through the activation of persulfates, including peroxymonosulfate (PMS) and peroxidisulfate (PS) [19,20]. Doumbi et al. compared the treatment of wastewater from a tannery in Cameroon using two processes: EF and electro-persulfate (EP), demonstrating that the organic removal rate was 67.25% using the EF process, whereas the removal rate was as high as 95.62% using the EP process [21]. Furthermore, calculations showed that EP had a higher removal efficiency at the same cost [21]. O. Ganiyu et al. achieved complete degradation of the organic pollutant 5-phenylpentanoic acid through an electrocatalytic peroxymonosulfate (EC-PMS) activation process. The results indicated that sulfate radicals (SO4·−) generated by EC-PMS were the primary radical oxidants for the degradation of 5-phenylpentanoic acid, contributing over 93%, while ·OH produced through the electro-Fenton process was responsible for the mineralization of the organic solution in the later stages of the coupled process [22]. Lei et al. designed a novel electro-oxidation-electro-Fenton-peroxidisulfate (EO-PS-EF) system for the degradation of the antibiotic cefotaxime. The results showed that the EO-EF-PS treatment system shortened the electrolysis time required for complete removal of cefotaxime, and radical quenching experiments indicated that the mechanism of the EO-EF-PS oxidation process involved the combined action of two types of radicals (·OH and SO4·−) [7]. In summary, both ·OH and SO4·− radicals can efficiently degrade organic pollutants in the electro-Fenton system.
There exist significant differences in chemical composition and sources between traditional organic pollutants and microplastics [23]. Polyethylene microplastics (PE MPs) are amongst the most widely produced synthetic polymers globally, accounting for over 50% of total plastic production [1]. PE microplastics, derived primarily from the fragmentation of packaging materials, agricultural films, and consumer products, dominate microplastic pollution in aquatic ecosystems due to their low density and persistence 113. Recent studies estimate that freshwater systems globally exhibit PE microplastic concentrations ranging from 3.5 × 103 units L−1 in densely populated regions (e.g., Lake Huron, USA) to as low as 1.2 × 10−4 units L−1 in remote areas (e.g., Mongolian lakes) 110. Their small size (<500 µm) and high surface area facilitate the adsorption of toxic additives, such as phthalates (e.g., dibutyl phthalate, detected at 389.38 ng mL−1 in PE/PP mixtures), which exacerbate ecological risks. PE MPs, due to their stable chemical structures and complex molecular chains, are resistant to degradation by conventional methods [24]. Therefore, how to effectively activate persulfate to generate sufficient radicals for disrupting the molecular structure of PE MPs has become a research challenge [25]. Magnetite (Fe3O4), as a green and efficient heterogeneous catalyst, exhibits excellent performance in electro-Fenton catalytic AOPs and sulfate radical-based AOPs [26]. Magnetite-based materials, generally sourced from mined ores, are inexpensive and readily available, featuring large specific surface areas, good adsorption properties, and ease of separation and recovery [27]. Furthermore, magnetite can overcome the issue of iron sludge production in traditional electro-Fenton technology, thereby enhancing reaction efficiency [28]. Wu et al. demonstrated through research that the application of magnetite in a peroxodisulfate electro-Fenton system can accelerate the activation process of peroxodisulfate, generating a large amount of SO4·− and ·OH radicals [29]. However, research on the application of magnetite in a peroxodisulfate electro-Fenton system for the degradation of PE MPs is still rare.
This study advances sustainable water treatment technologies by developing a magnetite-activated EO-PS-EF system for PE MPs degradation. We specifically address two sustainability gaps:
1. Scalability: Implementing 3-L reactor trials (vs. conventional <1 L systems [11]) to bridge lab–practice gaps in sustainable technology deployment.
2. Resource efficiency: Optimizing PS/Fe3O4 ratios to minimize chemical consumption while maximizing radical yield.
By establishing quantitative relationships between operational parameters and degradation efficiency, this work provides actionable insights for implementing sustainable microplastic remediation technologies at ecosystem-relevant scales.
2. Experimental Method
2.1. Materials and Reagents
Polyethylene microplastics (300 mesh), acetylene black (C), polyvinylidene fluoride (PVDF), titanium anode sheets, nickel foam mesh, sodium peroxodisulfate, hydrochloric acid, ethanol, N-methylpyrrolidone (NMP), and sulfuric acid (H2SO4, GR, 95.0–98.0%) were purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China), while nano-sized iron(III) oxide (Fe3O4) was obtained from Macklin Biochemical Co., Ltd. (Shanghai, China). The nickel foam mesh was pretreated by ultrasonic cleaning in ethanol to remove surface oxides, followed by drying at 60 °C for subsequent use. Unless otherwise specified, all water used in the experiments was purified water. All degradation experiments of PE MPs were conducted in a 3-L experimental setup.
2.2. Working Condition Setting
Figure 1 presents the orthographic views of the electro-Fenton setup for the degradation of PE MPs. The experimental reactor had dimensions of 350 × 100 × 100 mm and was constructed from acrylic plexiglass. Fe(II) serves as a catalyst to activate H2O2 (electro-Fenton) and persulfate (PS), generating reactive oxygen species (·OH and SO4·−) while undergoing oxidation to Fe(III). Subsequent electrochemical reduction regenerates Fe(II), enabling sustained catalytic activity.
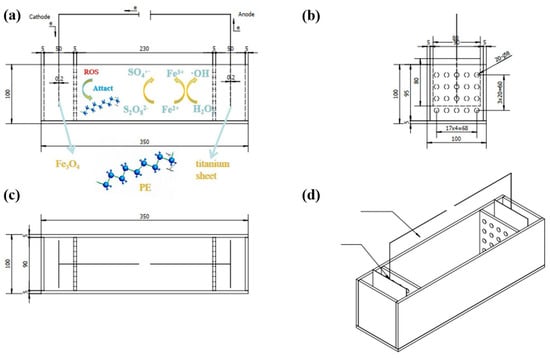
Figure 1.
Three views of the electro-Fenton setup for the degradation of PE MPs, (a) front views, (b) side views, (c) top views, and (d) full views.
The cathode was prepared as follows: Fe3O4, C, and PVDF were mixed in a ratio of 7:2:1, and ethanol was added and ground to ensure homogeneous mixing. This process was repeated three times. Finally, an appropriate amount of NMP was added to form a paste, which was then uniformly coated onto a nickel mesh using a brush. After drying at 60 °C, it could be used as the cathode. The anode was a commercial titanium sheet, and the electrolyte was Na2S2O8. To analyze and study the effect of electro-Fenton treatment on the degradation of PE MPs under different operating conditions, we designed 20 sets of conditions, including varying concentrations of PE MPs, Na2S2O8, Fe3O4 dosage, and electro-Fenton treatment duration. To expose the wires connecting the electrodes above the liquid level, the electrode sheets were cut to a size of 80 × 80 × 2 mm. Additionally, through preliminary electro-Fenton experiments, we found that a voltage of 30 V could effectively reduce the electro-Fenton treatment time without causing electrode fusing; thus, the voltage for subsequent experiments was uniformly set at 30 V.
After the electro-Fenton process, PE MPs were filtered using a 0.45 μm pre-weighed membrane, washed several times with ethanol and deionized water, and then dried in a vacuum oven at 80 °C for 6 h. The dried samples were labeled as PE-E, and the filtrate was labeled as PE-E filtrate. In addition, a blank experiment without electro-Fenton treatment was conducted, and the filtrate was labeled as PE-W filtrate. All experiments were repeated three times, with new Fe3O4 cathodes and titanium anodes replaced before each experiment.
Based on the density of microplastics, the mass change of PE MPs before and after electro-Fenton treatment was determined. The degradation rate of PE MPs was calculated as (mi − mf)/mi × 100%, where mi represents the initial mass before electro-Fenton treatment and mf represents the final mass after treatment (g).
2.3. Characterization of PE MPs, Fe3O4, and Filtrate Before and After Electro-Fenton Treatment
The morphology and particle size of PE MPs and Fe3O4 before and after electro-Fenton treatment were characterized using a GeminiSEM 500 ultra-high-resolution scanning electron microscope (Zeiss, Oberkochen, Germany). To reveal changes in the chemical properties of PE MPs and Fe3O4 before and after electro-Fenton treatment, a Vertex 80 FTIR (Bruker, Ettlingen, MA, USA) and Renishaw inVia Raman (Gloucestershire, UK) were used to analyze the molecular structures and chemical bonds. Dissolved organic carbon (DOC) in the filtrate before and after electro-Fenton treatment was obtained using a TOC analyzer (TOC-l, Shimadzu, Kyoto, Japan). Finally, qualitative and quantitative analyses of the elemental composition, chemical states, and molecular structures of PE MPs and Fe3O4 before and after electro-Fenton treatment were conducted using an ESCALAB 250Xi XPS (Thermo Fisher Scientific, MA, USA).
3. Results
3.1. Effect of Operation Conditions on the Degradation of PE MPs
To evaluate the performance of magnetite-activated PS in the electro-Fenton degradation of PE MPs, an electro-Fenton treatment was conducted on PE MPs at room temperature. The study investigated the degradation of PE MPs under various conditions, including different concentrations of PE, PS, magnetite dosage, and electro-Fenton treatment duration, with an applied voltage of 30 V, an initial pH of 2.6, and an electrolysis time of 20 h.
3.1.1. Effect of Initial PE MPs Concentration
The influence of different initial concentrations of PE MPs on the electro-Fenton treatment was investigated, as shown in Figure 2a. When the initial concentrations of PE MPs were 40, 50, 60, 70, and 100 mg/L, the weight loss percentages of PE MPs were 39.5%, 48%, 90.6%, 71.1%, and 30%, respectively. According to previous reports, the relationship between the initial concentration of microplastics and the degradation rate was not absolutely positive [30]. When the concentration of PE MPs was not higher than 60 mg/L, the number of radicals generated in the system could be effectively allocated to each microplastic particle, leading to an increase in the degradation rate [31]. Additionally, PE MPs could serve as a medium for electron transfer, accelerating the Fe(III)/Fe(II) cycle and thus increasing the production rate of radicals [32]. When the concentration of PE MPs exceeded 60 mg/L, the increase in the number of radicals generated in the system was limited, and the number of radicals that each microplastic particle may contact could decrease, resulting in a decrease in the degradation rate [33]. Furthermore, interactions such as aggregation and adsorption might occur between PE MPs particles [34], which could also affect the diffusion and reaction rates of radicals, leading to reduced degradation efficiency [35].
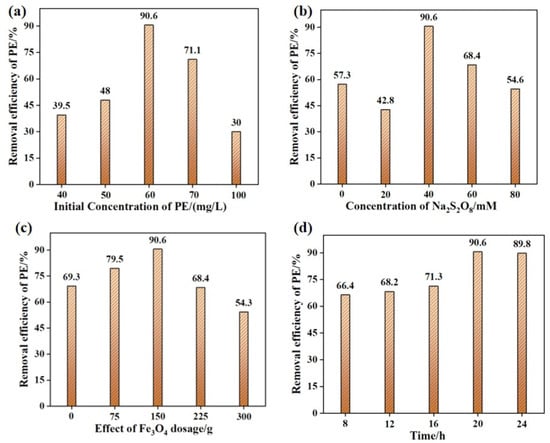
Figure 2.
The effect of initial PE MPs concentration (a) (mFe3O4 = 150 mg, CNa2S2O8 = 40 mM, V = 30 V, Time = 20 h), electrolyte concentration (b) (mFe3O4 = 150 mg, CPE = 60 mg/L, V = 30 V, Time = 20 h), Fe3O4 dosage (c) (CPE = 60 mg/L, CNa2S2O8 = 40 mM, V = 30 V, Time = 20 h), and time (d) (mFe3O4 = 150 mg, CPE = 60 mg/L, CNa2S2O8 = 40 mM, V = 30 V) on the weight loss rates of PE MPs. In the sample names, the absence of a suffix following PE or Fe3O4 indicates the sample before electro-Fenton treatment, while the suffix “-E” denotes the sample after electro-Fenton treatment.
3.1.2. Effect of Electrolyte Concentration
To enhance the conductivity of the solution and reduce energy consumption, while simultaneously improving the degradation efficiency of PE MPs via electro-Fenton treatment, this study investigated the effect of different Na2S2O8 concentrations, ranging from 0 to 80 mM, on the degradation efficiency of PE MPs using Na2S2O8 as the supporting electrolyte (Figure 2b). The results indicated that when the Na2S2O8 concentrations were 0, 20, 40, 60, and 80 mM, the weight loss percentages of PE MPs were 57.3%, 42.8%, 90.6%, 68.4%, and 54.6%, respectively. This might be attributed to the following reasons: (1) Higher Na2S2O8 concentrations resulted in better solution conductivity and higher charge transfer rates [36]; (2) During the electro-Fenton process, Fe2+ activated Na2S2O8 to convert it into SO4·− radicals, and within a certain range, the concentration of SO4·− radicals was proportional to the Na2S2O8 concentration [37]; (3) When the Na2S2O8 concentration was excessively high, reaching 60 mM, free radical self-quenching, side-reaction, diffusion restriction and other mechanisms dominate, resulting in a decrease in efficiency. To be specific, the substantial increase in the concentration of sulfate radicals (SO4·−) generated through activation processes leads to enhanced collision probabilities among these radical species, thereby inducing a self-quenching reaction. This critical side-reaction converts active radicals into inert persulfate ions (SO82−), consequently diminishing the effective radical concentration. Excessive sodium persulfate dosage further exacerbates radical depletion through direct reactions with SO4·−, generating secondary oxidation products with diminished oxidative capacity. Such parasitic reactions substantially reduce the total available radical population for polyethylene microplastics (PE MPs) degradation [20]. Moreover, elevated sodium persulfate concentrations induce increased solution viscosity, which significantly reduces the diffusion coefficients of both SO4·− radicals and PE MPs. This transport limitation impedes effective radical–particle surface interactions, ultimately deteriorating the reaction kinetics. Particularly, the restricted mobility of radicals substantially compromises their accessibility to reactive sites on PE surfaces, resulting in inefficient utilization of oxidative species [38]. It was noteworthy that excessively high electrolyte concentrations might result in increased energy consumption [39], requiring more energy to maintain the electro-Fenton process. Therefore, selecting an appropriate electrolyte concentration was crucial.
3.1.3. Effect of Fe3O4 Dosage
The influence of Fe3O4 dosage on the degradation rate was investigated, as shown in Figure 2c. It was observed that the degradation efficiency of PE MPs reached a maximum of 90.6% when the Fe3O4 dosage was 150 mg. This was attributed to the fact that during the electro-Fenton process, the incorporation of Fe3O4 provided an increased number of reaction sites, thereby facilitating the accelerated generation of two types of radicals (·OH and SO4·−) and the degradation of PE MPs [40]. However, although the number of active sites on the electrode surface increases when the Fe3O4 dosage is increased to a certain extent (225 mg), an excessive number of active sites may obstruct electron transfer on the electrode surface, reducing the reaction rate [41]. Additionally, an excessive amount of electrode material might lead to excessive current density on the electrode surface, causing overheating, corrosion, or detachment of the electrode, ultimately affecting the degradation rate of PE MPs [42]. Hence, the selection of an optimal dosage of Fe3O4 served as a pivotal factor in enhancing the degradation efficiency of PE MPs.
3.1.4. Effect of Time
The treatment duration of the electro-Fenton process had a significant impact on the degradation of PE MPs [43,44]. In practical applications, it was imperative to appropriately regulate the duration of the electro-Fenton treatment to ensure compliance with requirements pertaining to degradation efficiency and cost. Generally, the radicals generated during the electro-Fenton process require a certain amount of time to fully contact and react with PE MPs as the treatment time increases [45], leading to a gradual increase in the degradation efficiency of PE MPs. Furthermore, shorter treatment times might result in partial degradation of the PE MPs surface, while longer treatment times could lead to more thorough degradation of PE MPs, potentially even completely decomposing them into small molecular substances [46,47,48]. As observed in Figure 2d, when the electro-Fenton treatment time reached 24 h, the degradation rate decreased. This might be due to the prolonged treatment time causing the Fe3O4 coated on the nickel mesh to fall off and become difficult to separate from the PE-E MPs, ultimately affecting the degradation effect of PE MPs.
3.2. Comparison of PE MPs Before and After Electro-Fenton Treatment
To investigate the mechanism of PE MPs degradation in the triple-coupled system of EO, PS, and EF, physicochemical characterizations were conducted on PE MPs, Fe3O4, and the filtrate before and after the electro-Fenton treatment for one experimental group. The specific parameters for this experimental condition were as follows: voltage of 30 V, electrolyte volume of 2 L, pH of 2.6, PE concentration of 60 mg/L, PS concentration of 40 mM, magnetite dosage of 150 mg, and electro-Fenton treatment time of 20 h.
The morphology and size distribution of PE MPs and Fe3O4 changed after 20 h of electro-Fenton treatment, as shown in Figure 3. SEM images revealed that the electro-Fenton process caused physical damage to PE MPs (Figure 3a,b). Specifically, the initial PE MPs had a size of 300 mesh (approximately 48 μm), and after electro-Fenton treatment, the particle size of PE-E MPs decreased from 34.74 μm to 29.13 μm (Figures S3 and S4), representing a reduction of 16.1%, which was consistent with previous research findings [49]. Concurrently, we identified abundant surface defects on PE-E MPs, which facilitated the permeation of hydroxyl radicals (·OH) and sulfate radicals (SO4⁻·) while accelerating internal oxidation processes, thereby promoting particle fragmentation. Additionally, irregular edges observed in PE-E MPs exhibited correlation with XPS analytical data, revealing a 2.2% elevation in oxygen content (O/C ratio increased from 0.03 to 0.05), indicative of oxidative chain scission phenomena. Meanwhile, as observed in Figure 3c,d, after electro-Fenton treatment, Fe3O4 with an initial average particle size of 28.42 μm exhibited significant size enlargement and aggregation. This aggregation was also evident on the nickel mesh, as observed in the SEM images (Figures S1 and S2). XPS analysis further confirmed the homogeneous distribution of iron and oxygen species (Fe/O atomic ratio: ~3:4), with no detectable iron leaching observed throughout the experimental protocol (Table S1). Clearly, both PE-E MPs and Fe3O4-E underwent certain changes in morphology and size after the electro-Fenton process, indicating that both exhibited reactive activity during the process.
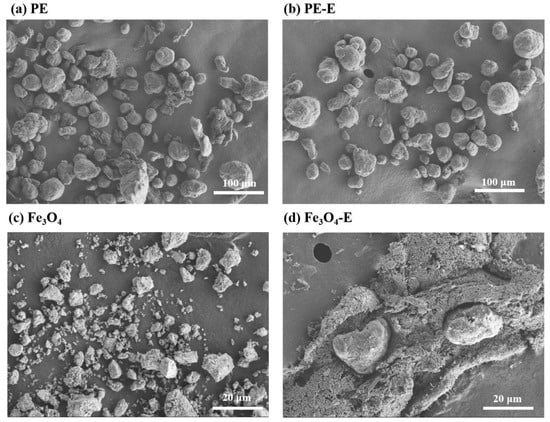
Figure 3.
Changes in the physical properties of PE MPs and Fe3O4 before and after electro-Fenton treatment, (a,b) SEM images of original and electro-Fenton-treated PE MPs, and (c,d) SEM images of original and electro-Fenton-treated Fe3O4. In the sample names, the absence of a suffix following PE or Fe3O4 indicates the original sample, while the suffix “-E” denotes the sample after electro-Fenton treatment.
3.3. Functional Groups Analyses
The electro-Fenton process also resulted in changes in the chemical properties of PE MPs and Fe3O4, including surface functional groups and elemental composition (Figure 4a–c). The FTIR spectrum of the original PE MPs (Figure 4a) confirmed the presence of characteristic peaks corresponding to the stretching vibrations of the main chain C-H2 at 2919 and 2851 cm−1, the bending vibration of C-H2 at 1475 cm−1, and the rocking vibration of C-H2 at 721 cm−1 [49,50,51]. The increased intensity of the C-O absorption peak at 1184 and 1100 cm−1 and the C=O absorption peak at 1750 cm−1 in PE-S MPs suggested the formation of oxygen-containing functional groups during the electro-Fenton process. To further investigate the changes in the functional groups of PE MPs during the electro-Fenton process, Raman analysis was conducted using a laser with a wavelength of 532 nm. In the Raman spectrum of PE MPs (Figure 4b), the intensity of the absorption peak of the O-H bond at 2881 cm−1 in PE-E MPs increased significantly, indicating an increase in the oxygen content. The bond at 2847 cm−1 was related to the Fermi resonance doublet of CH2 [52]. Additionally, the bands at 1295 cm−1, 1130 cm−1, and 1062 cm−1 corresponded to methyl (CH) twisting and carbon-carbon stretching, respectively [53,54]. Table 1 listed the displacements corresponding to CH2 and CH3 bands in the Raman spectrum of PE MPs. These results indicated the presence of C-O and C=O functional groups in PE-E MPs, further confirming the formation of carboxyl groups during the electro-Fenton process of PE MPs [49].
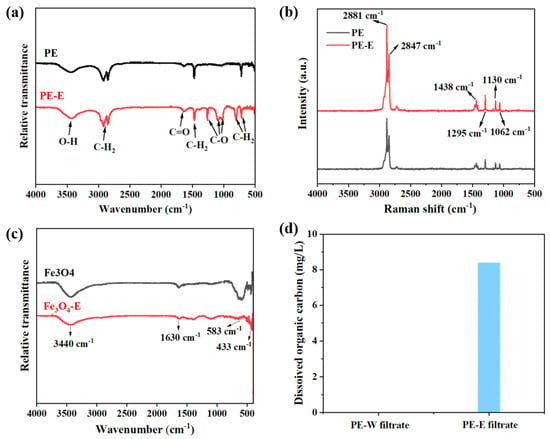
Figure 4.
Changes in the chemical properties of PE MPs, Fe3O4, and filtrate after electro-Fenton treatment, (a,b) FTIR spectrum (a) and Raman spectrum (b) of original and electro-Fenton-treated PE MPs, (c) infrared spectrum of original and electro-Fenton-treated Fe3O4, and (d) TOC plots of PE MPs filtrate with and without electro-Fenton treatment. In the sample names, the absence of a suffix following PE or Fe3O4 indicates the original sample, the suffix “-E” denotes the sample after electro-Fenton treatment, and the suffix “-W” denotes the sample without electro-Fenton treatment.

Table 1.
The CH2 and CH3 PE bands of Raman spectra [54].
Figure 4c presents the infrared spectra of Fe3O4 and Fe3O4-E. A sharp band at 583 cm−1 and a weaker band at 669 cm−1 were observed, with the positions and shapes of these bands attributed to magnetite [50]. The strong band within the range of 570–585 cm−1 is characteristic of Fe-O absorption, resulting from the symmetric stretching vibration of Fe-O [55]. It was noticeable that the Fe-O peak in Fe3O4-E nearly disappeared, potentially due to substitution by other chemical bonds. Among them, the band at 1630 cm−1 corresponded to the stretching of H-O-H, attributed to the bending of H-O-H from water vapor adsorbed on the surface of magnetite [56]. The band at 3440 cm−1 represented the bending vibration peak of H-O-H [55]. Meanwhile, the aging process was often accompanied by the release of organic compounds, leading to new pollution [57]. To explore the release of organic compounds during the transformation process, PE MPs filtrate with and without electro-Fenton treatment was characterized using TOC. The TOC results (Figure 4d) indicated that compared to the group without electro-Fenton treatment (PE-W filtrate), the group with electro-Fenton treatment (PE-E filtrate) produced significantly more dissolved organic carbon, suggesting that chain scission and reduction of PE MPs occurred during the electro-Fenton process, facilitating the release of organics.
The XPS spectral results further confirmed the changes in surface composition and functional groups during the electro-Fenton process. Figure 5 presents the XPS survey spectra of PE MPs and Fe3O4 before and after electro-Fenton treatment. The elemental analysis results are summarized in Table S1. It could be observed that after electro-Fenton treatment, there were significant changes in the number of C and O atoms on the surface of PE-E MPs, and the presence of the S element (originating from sodium persulfate) is also detected. The content of O increased, while the content of C decreased, resulting in a marked increase in the O/C atomic ratio. Meanwhile, in addition to the Fe and O elements, 88.6% of the C element was also present in Fe3O4-E.
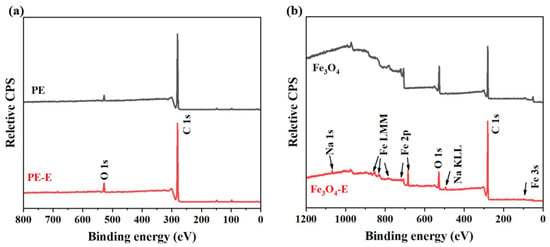
Figure 5.
Changes in the chemical properties of PE MPs and Fe3O4 after electro-Fenton treatment, (a) XPS survey spectra of original PE and PE MPs after electro-Fenton treatment, and (b) XPS survey spectra of Fe3O4. In the sample names, the absence of a suffix following PE or Fe3O4 indicates the original sample, while the suffix “-E” denotes the sample after electro-Fenton treatment.
To further elucidate the degradation of PE MPs by electro-Fenton treatment, high-resolution XPS analysis of C 1s and O 1s from the treated PE MPs was conducted. Figure 6a and Figure 6b show the high-resolution C 1s spectra of PE MPs and PE-E MPs, respectively. It could be observed that the abundance of C-O (285.52 eV) groups decreases, while the abundance of C-C (284.80 eV) groups increases, and new O-C=O (288.14 eV) groups were generated in PE-E MPs [58]. These results were consistent with the FTIR and Raman findings, further confirming the oxidation of PE MPs during the electro-Fenton process. Meanwhile, the high-resolution XPS spectra of O 1s (Figure 6c,d) confirmed the formation of O-H oxygen groups on the surface of PE MPs during electro-Fenton degradation, leading to a decrease in the content of C=O and C-O due to the formation of O-H [45,59]. The peak area of the O 1s spectrum represented the atomic concentration of oxygen, with a higher atomic composition of oxygen indicating a higher degree of oxidation [60]. In addition, the table of elemental composition and content data (Table S1) revealed that Fe3O4-E after electro-Fenton treatment contains an additional 88.6% of C element, with decreased percentage contents of O and Fe elements. This might be due to the adsorption of a large amount of organic material.
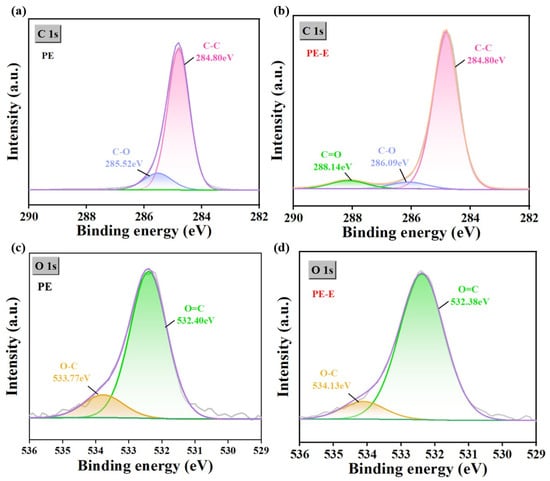
Figure 6.
Changes in the chemical properties of PE MPs after electro-Fenton treatment, (a,b) High-resolution X-ray photoelectron spectroscopy (XPS) of the C1s peak for original PE MPs and PE MPs after electro-Fenton treatment, and (c,d) high-resolution XPS of the O1s peak for PE MPs. In the sample names, PE MPs without a suffix represent the original microplastics, while the suffix “-E” denotes the microplastics after electro-Fenton treatment.
Figure 7 presents the XPS spectra of Fe 2p for Fe3O4 before and after electro-Fenton treatment. The main peaks at 711.59 eV (Fe 2p3/2) and 724.97 eV (Fe 2p1/2) indicated the +3 valence state of iron [61]. After electro-Fenton treatment, the ratio of Fe(II) to Fe(III) in Fe3O4-E decreased compared to the XPS spectrum of Fe 2p in the initial Fe3O4. Additionally, there were red shifts in the binding energy of Fe 2p, from 711.59 to 711.45 eV and from 724.97 to 724.82 eV, respectively. This might be due to the adsorption of PE MPs or SO4·− on the surface of Fe3O4, leading to an increase in negative charge on the Fe surface and subsequently a decrease in binding energy [62]. Based on the above characterization results and previous studies, a reaction pathway for the electro-Fenton treatment of PE MPs was proposed [49]. In the presence of ·OH and SO4·−, PE MPs were first oxidized to hydroperoxides, which further oxidized to form carbonyl groups and underwent chain scission reactions during the electro-Fenton process.

Figure 7.
Changes in the chemical properties of Fe3O4 after electro-Fenton treatment. (a,b) High-resolution X-ray photoelectron spectroscopy (XPS) of the Fe 2p peak for original Fe3O4 and Fe3O4 after electro-Fenton treatment. In the sample nomenclature, Fe3O4 without a suffix represents the original material, while the suffix “-E” denotes Fe3O4 after electro-Fenton treatment.
4. Conclusions
This study successfully established a green and sustainable magnetite-activated persulfate electro-Fenton (Mt-PS-EF) system for polyethylene microplastic (PE MP) degradation, integrating catalytic efficiency with circular economy principles. The Mt-PS-EF system achieved a 90.6% degradation rate under optimized conditions (60 mg/L PE, 40 mM persulfate, 150 mg Fe3O4, 20 h treatment), driven by hydroxyl (·OH) and sulfate (SO4·⁻) radicals that induce chain scission and mineralization of PE MPs. Crucially, the magnetic recovery and reuse of Fe3O4 reduced catalyst waste and operational costs, aligning with sustainable resource management goals. This innovation directly contributes to UN Sustainable Development Goals (SDGs) 6 (Clean Water) and 14 (Life Below Water) by addressing microplastic pollution in aquatic ecosystems while minimizing secondary environmental impacts. The system’s integration of heterogeneous catalysis, persulfate activation, and electrochemical processes exemplifies a scalable and eco-friendly approach to wastewater treatment, bridging the gap between advanced oxidation technologies and practical sustainability. By prioritizing catalyst recyclability and energy-efficient design, the Mt-PS-EF system advances the transition toward circular economy frameworks in plastic waste management. However, challenges remain in translating laboratory-scale success to real-world applications. Future work must address energy consumption reduction through renewable energy integration (e.g., solar-driven systems) and optimize Fe3O4 stability under dynamic environmental conditions (e.g., pH fluctuations, coexisting pollutants). Additionally, scaling this technology to treat complex microplastic mixtures in natural water systems will require interdisciplinary collaboration and policy support to ensure alignment with global sustainability agendas. By harmonizing technical innovation with environmental and economic sustainability, this research provides a foundational roadmap for developing hybrid technologies that mitigate plastic pollution while conserving resources—a critical step toward achieving planetary health and sustainable industrial practices.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/su17083559/s1, Figure S1: Scanning Electron Microscope (SEM) Image of the Original Nickel Mesh; Figure S2: Scanning Electron Microscope (SEM) Image of Nickel Mesh after Electro-Fenton Treatment; Figure S3: Scanning Electron Microscope (SEM) Image of Original PE MPs; Figure S4: Scanning Electron Microscope (SEM) Image of PE MPs after Electro-Fenton Treatment; Table S1: Elemental Composition and Percentage Content of the Sample.
Author Contributions
W.G.: Methodology, data curation, visualization, formal analysis, writing—original draft; T.T.: Writing—review and editing, validation, supervision; X.C.: Writing—review and editing, validation, supervision; D.Z.: Funding acquisition, validation, writing—review and editing; L.Y.: Formal analysis, investigation. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (52479064), the Science and Technology Innovation Program from Water resources of Guangdong Province (2023-06), the Basic and Applied Basic Research Fund of Guangdong Province (2024A1515011047), the Southern Marine Science and Engineering Guangdong Laboratory (Zhuhai) (SML2021SP202).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data will be made available on request.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Tomat, R.; Rigo, A. Electrochemical production of OH· radicals and their reaction with toluene. J. Appl. Electrochem. 1976, 6, 257–261. [Google Scholar] [CrossRef]
- Tzedakis, T.; Savall, A.; Clifton, M.J. The electrochemical regeneration of Fenton’s reagent in the hydroxylation of aromatic substrates: Batch and continuous processe. J. Appl. Electrochem. 1989, 19, 911–921. [Google Scholar] [CrossRef]
- Oturan, M.A.; Peiroten, J.; Chartrin, P.; Acher, A.J. Complete destruction of p-nitrophenol in aqueous medium by electro-Fenton method. Environ. Sci. Technol. 2000, 34, 3474–3479. [Google Scholar] [CrossRef]
- Oturan, M.A.; Pinson, J.; Bizot, J.; Deprez, D.; Terlain, B. Reaction of inflammation inhibitors with chemically and electrochemically generated hydroxyl radicals. J. Electroanal. Chem. 1992, 334, 103–109. [Google Scholar] [CrossRef]
- Brillas, E.; Mur, E.; Casado, J. Iron (II) catalysis of the mineralization of aniline using a Carbon-PTFE O2-Fed cathode. J. Electrochem. Soc. 1996, 143, L49. [Google Scholar] [CrossRef]
- Lin, Y.; Huo, P.; Li, F.; Chen, X.; Yang, L.; Jiang, Y.; Zhang, Y.; Ni, B.-J.; Zhou, M. A critical review on cathode modification methods for efficient Electro-Fenton degradation of persistent organic pollutants. Chem. Eng. J. 2022, 450, 137948. [Google Scholar] [CrossRef]
- Lei, J.; Duan, P.; Liu, W.; Sun, Z.; Hu, X. Degradation of aqueous cefotaxime in electro-oxidation—Electro-Fenton—Persulfate system with Ti/CNT/SnO2–Sb–Er anode and Ni@NCNT cathode. Chemosphere 2020, 250, 126163. [Google Scholar] [CrossRef]
- Ganiyu, S.O.; Zhou, M.; Martínez-Huitle, C.A. Heterogeneous electro-Fenton and photoelectro-Fenton processes: A critical review of fundamental principles and application for water/wastewater treatment. Appl. Catal. B Environ. 2018, 235, 103–129. [Google Scholar] [CrossRef]
- Geng, J.; Zhang, H.; Zhang, Z.; Gao, J.; Wang, S.; Hu, X.; Li, J. Enhanced electro-Fenton oxidation by introducing three-phase interface with simultaneous optimization of O2 and pollutant transfer for effective tetracycline hydrochloride removal. Chem. Eng. J. 2022, 450, 137891. [Google Scholar] [CrossRef]
- Huang, H.; Zou, X.; Ji, R.; Zhang, J.; Yuan, Z.; Zhao, M.; Zhang, H.; Geng, J.; Li, J. Inducing three-phase interface to enhance hydroxyl radical production via green atomic H*-mediated electro-Fenton process for highly-efficient tetracycline degradation. Colloids Surf. A Physicochem. Eng. Asp. 2024, 698, 134577. [Google Scholar] [CrossRef]
- Ning, Z.; Duan, X.; Li, Y.; Zhao, X.; Chang, L. Degradation of polyvinyl chloride microplastics via electrochemical oxidation with a CeO2–PbO2 anode. J. Clean. Prod. 2023, 432, 139668. [Google Scholar] [CrossRef]
- Dong, G.; Chen, B.; Liu, B.; Hounjet, L.J.; Cao, Y.; Stoyanov, S.R.; Yang, M.; Zhang, B. Advanced oxidation processes in microreactors for water and wastewater treatment: Development, challenges, and opportunities. Water Res. 2022, 211, 118047. [Google Scholar] [CrossRef]
- Zhong, S.; Zhu, Z.-S.; Duan, X.; Wang, S. Electro-Fenton-Based Membrane System for Organic Micropollutant Removal: New Trend and Prospect. ACS EST Eng. 2023, 3, 2147–2160. [Google Scholar] [CrossRef]
- Chu, Y.; Su, H.; Liu, C.; Zheng, X. Fabrication of sandwich-like super-hydrophobic cathode for the electro-Fenton degradation of cefepime: H2O2 electro-generation, degradation performance, pathway and biodegradability improvement. Chemosphere 2022, 286, 131669. [Google Scholar] [CrossRef]
- Plakas, K.V.; Sklari, S.D.; Yiankakis, D.A.; Sideropoulos, G.T.; Zaspalis, V.T.; Karabelas, A.J. Removal of organic micropollutants from drinking water by a novel electro-Fenton filter: Pilot-scale studies. Water Res. 2016, 91, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Miao, F.; Liu, Y.; Gao, M.; Yu, X.; Xiao, P.; Wang, M.; Wang, S.; Wang, X. Degradation of polyvinyl chloride microplastics via an electro-Fenton-like system with a TiO2/graphite cathode. J. Hazard. Mater. 2020, 399, 123023. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, L.; Wang, Y. A Critical Review on Removal of Gaseous Pollutants Using Sulfate Radical-based Advanced Oxidation Technologies. Environ. Sci. Technol. 2021, 55, 9691–9710. [Google Scholar] [CrossRef] [PubMed]
- Ushani, U.; Lu, X.; Wang, J.; Zhang, Z.; Dai, J.; Tan, Y.; Wang, S.; Li, W.; Niu, C.; Cai, T.; et al. Sulfate radicals-based advanced oxidation technology in various environmental remediation: A state-of-the–art review. Chem. Eng. J. 2020, 402, 126232. [Google Scholar] [CrossRef]
- Honarmandrad, Z.; Sun, X.; Wang, Z.; Naushad, M.; Boczkaj, G. Activated persulfate and peroxymonosulfate based advanced oxidation processes (AOPs) for antibiotics degradation—A review. Water Resour. Ind. 2023, 29, 100194. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Activation of persulfate (PS) and peroxymonosulfate (PMS) and application for the degradation of emerging contaminants. Chem. Eng. J. 2018, 334, 1502–1517. [Google Scholar] [CrossRef]
- Doumbi, R.T.; Bertrand Noumi, G.; Ngobtchok, B. Domga Tannery wastewater treatment by electro-Fenton and electro-persulfate processes using graphite from used batteries as free-cost electrode materials. Case Stud. Chem. Environ. Eng. 2022, 5, 100190. [Google Scholar] [CrossRef]
- Ganiyu, S.O.; Hussain, N.A.S.; Stafford, J.L.; Gamal El-Din, M. Electrocatalytic activation of peroxomonosulfate (PMS) under aerobic condition for the remediation of oil sands process water: Insight into one-pot synergistic coupling of PMS electro-activation and heterogeneous electro-Fenton processes. Chem. Eng. J. 2024, 480, 147737. [Google Scholar] [CrossRef]
- Okoye, C.O.; Addey, C.I.; Oderinde, O.; Okoro, J.O.; Uwamungu, J.Y.; Ikechukwu, C.K.; Okeke, E.S.; Ejeromedoghene, O.; Odii, E.C. Toxic Chemicals and Persistent Organic Pollutants Associated with Micro-and Nanoplastics Pollution. Chem. Eng. J. Adv. 2022, 11, 100310. [Google Scholar] [CrossRef]
- Liu, L.; Xu, M.; Ye, Y.; Zhang, B. On the degradation of (micro)plastics: Degradation methods, influencing factors, environmental impacts. Sci. Total Environ. 2022, 806, 151312. [Google Scholar] [CrossRef]
- Zuo, S.; Wang, Y.; Wan, J.; Ma, Y.; Yan, Z.; Tang, M. Rethinking strategies to attenuate organic pollutants: Mechanisms and challenges of catalytic pollutants polymerization. Crit. Rev. Environ. Sci. Technol. 2024, 55, 169–189. [Google Scholar] [CrossRef]
- Munoz, M.; de Pedro, Z.M.; Casas, J.A.; Rodriguez, J.J. Preparation of magnetite-based catalysts and their application in heterogeneous Fenton oxidation—A review. Appl. Catal. B Environ. 2015, 176–177, 249–265. [Google Scholar] [CrossRef]
- Han, X.; Wang, F.; Zhao, Y.; Meng, J.; Tian, G.; Wang, L.; Liang, J. Recycling of iron ore tailings into magnetic nanoparticles and nanoporous materials for the remediation of water, air and soil: A review. Environ. Chem. Lett. 2022, 21, 1005–1028. [Google Scholar] [CrossRef]
- Casado, J. Minerals as catalysts of heterogeneous Electro-Fenton and derived processes for wastewater treatment: A review. Environ. Sci. Pollut. Res. 2023, 30, 76405–76420. [Google Scholar] [CrossRef]
- Wu, G.; Kong, W.; Gao, Y.; Kong, Y.; Dai, Z.; Dan, H.; Shang, Y.; Wang, S.; Yin, F.; Yue, Q.; et al. Removal of chloramphenicol by sulfide-modified nanoscale zero-valent iron activated persulfate: Performance, salt resistance, and reaction mechanisms. Chemosphere 2022, 286, 131876. [Google Scholar] [CrossRef]
- Lin, Z.; Jin, T.; Zou, T.; Xu, L.; Xi, B.; Xu, D.; He, J.; Xiong, L.; Tang, C.; Peng, J.; et al. Current progress on plastic/microplastic degradation: Fact influences and mechanism. Environ. Pollut. 2022, 304, 119159. [Google Scholar] [CrossRef]
- Qiu, Y.; Zhang, T.; Zhang, P. Fate and environmental behaviors of microplastics through the lens of free radical. J. Hazard. Mater. 2023, 453, 131401. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chen, J.; Chen, Z.; Yu, Z.; Xue, J.; Luan, T.; Chen, S.; Zhou, S. Mechanisms of polystyrene microplastic degradation by the microbially driven Fenton reaction. Water Res. 2022, 223, 118979. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Yin, X.; Xi, X.; Guan, D.; Sun, H.; Wang, N. Effect of surfactants on the transport of polyethylene and polypropylene microplastics in porous media. Water Res. 2021, 196, 117016. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Mo, W.Y.; Luukkonen, T. Adsorption behaviour and interaction of organic micropollutants with nano and microplastics—A review. Sci. Total Environ. 2021, 797, 149140. [Google Scholar] [CrossRef]
- Cheng, M.; Zeng, G.; Huang, D.; Lai, C.; Xu, P.; Zhang, C.; Liu, Y. Hydroxyl radicals based advanced oxidation processes (AOPs) for remediation of soils contaminated with organic compounds: A review. Chem. Eng. J. 2016, 284, 582–598. [Google Scholar] [CrossRef]
- Han, Z.; Li, S.; Yue, Y.; Tian, Y.; Wang, S.; Qin, Z.; Ji, L.; Han, D.; Jiao, W. Enhancing remediation of PAH-contaminated soil through coupling electrical resistance heating using Na2S2O8. Environ. Res. 2021, 198, 110457. [Google Scholar] [CrossRef]
- Cai, J.; Niu, T.; Shi, P.; Zhao, G. Boron-Doped Diamond for Hydroxyl Radical and Sulfate Radical Anion Electrogeneration, Transformation, and Voltage-Free Sustainable Oxidation. Small 2019, 15, 1900153. [Google Scholar] [CrossRef] [PubMed]
- Ashrafmansouri, S.-S.; Nasr Esfahany, M. Mass transfer in nanofluids: A review. Int. J. Therm. Sci. 2014, 82, 84–99. [Google Scholar] [CrossRef]
- Dalvand, A.; Gholami, M.; Joneidi, A.; Mahmoodi, N.M. Dye Removal, Energy Consumption and Operating Cost of Electrocoagulation of Textile Wastewater as a Clean Process. CLEAN—Soil Air Water 2011, 39, 665–672. [Google Scholar] [CrossRef]
- Wang, L.; Huang, X.; Wang, C.; Tian, X.; Chang, X.; Ren, Y.; Yu, S. Applications of surface functionalized Fe3O4 NPs-based detection methods in food safety. Food Chem. 2021, 342, 128343. [Google Scholar] [CrossRef]
- Zhao, J.; Lian, J.; Zhao, Z.; Wang, X.; Zhang, J. A Review of In-Situ Techniques for Probing Active Sites and Mechanisms of Electrocatalytic Oxygen Reduction Reactions. Nano-Micro Lett. 2022, 15, 19. [Google Scholar] [CrossRef]
- Muzenda, C.; Nkwachukwu, O.V.; Jayeola, K.D.; Zinyemba, O.; Zhou, M.; Arotiba, O.A. Heterogenous electro-Fenton degradation of sulfamethoxazole on a polyethylene glycol-coated magnetite nanoparticles catalyst. Chemosphere 2023, 339, 139698. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Xu, M.; Jin, B.; Wünsch, U.J.; Su, Y.; Zhang, Y. Electrochemical and microbiological response of exoelectrogenic biofilm to polyethylene microplastics in water. Water Res. 2022, 211, 118046. [Google Scholar] [CrossRef] [PubMed]
- Kiendrebeogo, M.; Karimi Estahbanati, M.R.; Ouarda, Y.; Drogui, P.; Tyagi, R.D. Electrochemical degradation of nanoplastics in water: Analysis of the role of reactive oxygen species. Sci. Total Environ. 2022, 808, 151897. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, N.d.O.; Busquets, R.; Campos, L.C. Insights into the removal of microplastics and microfibres by Advanced Oxidation Processes. Sci. Total Environ. 2023, 861, 160665. [Google Scholar] [CrossRef]
- Kida, M.; Ziembowicz, S.; Koszelnik, P. Decomposition of microplastics: Emission of harmful substances and greenhouse gases in the environment. J. Environ. Chem. Eng. 2023, 11, 109047. [Google Scholar] [CrossRef]
- Chen, J.; Wu, J.; Sherrell, P.C.; Chen, J.; Wang, H.; Zhang, W.X.; Yang, J. How to Build a Microplastics-Free Environment: Strategies for Microplastics Degradation and Plastics Recycling. Adv. Sci. 2022, 9, 2103764. [Google Scholar] [CrossRef]
- Bule Možar, K.; Miloloža, M.; Martinjak, V.; Radovanović-Perić, F.; Bafti, A.; Ujević Bošnjak, M.; Markić, M.; Bolanča, T.; Cvetnić, M.; Kučić Grgić, D.; et al. Evaluation of Fenton, Photo-Fenton and Fenton-like Processes in Degradation of PE, PP, and PVC Microplastics. Water 2024, 16, 673. [Google Scholar] [CrossRef]
- Du, T.; Qian, L.; Shao, S.; Xing, T.; Li, T.; Wu, L. Comparison of sulfide-induced transformation of biodegradable and conventional microplastics: Mechanism and environmental fate. Water Res. 2024, 253, 121295. [Google Scholar] [CrossRef]
- Gallo-Cordova, A.; Corrales-Pérez, B.; Cabrero, P.; Force, C.; Veintemillas-Verdaguer, S.; Ovejero, J.G.; Morales, M.d.P. Magnetic Harvesting and Degradation of Microplastics using Iron Oxide Nanoflowers prepared by a Scaled-up Procedure. Chem. Eng. J. 2024, 490, 151725. [Google Scholar] [CrossRef]
- Bredács, M.; Barretta, C.; Castillon, L.F.; Frank, A.; Oreski, G.; Pinter, G.; Gergely, S. Prediction of polyethylene density from FTIR and Raman spectroscopy using multivariate data analysis. Polym. Test. 2021, 104, 107406. [Google Scholar] [CrossRef]
- Neo, E.R.K.; Low, J.S.C.; Goodship, V.; Debattista, K. Deep learning for chemometric analysis of plastic spectral data from infrared and Raman databases. Resour. Conserv. Recycl. 2023, 188, 106718. [Google Scholar] [CrossRef]
- Montano, L.; Giorgini, E.; Notarstefano, V.; Notari, T.; Ricciardi, M.; Piscopo, M.; Motta, O. Raman Microspectroscopy evidence of microplastics in human semen. Sci. Total Environ. 2023, 901, 165922. [Google Scholar] [CrossRef]
- Pei, W.; Hu, R.; Liu, H.; Wang, L.; Lai, Y. Advanced Raman spectroscopy for nanoplastics analysis: Progress and perspective. TrAC Trends Anal. Chem. 2023, 166, 117188. [Google Scholar] [CrossRef]
- Cheng, F. Characterization of aqueous dispersions of Fe3O4 nanoparticles and their biomedical applications. Biomaterials 2005, 26, 729–738. [Google Scholar] [CrossRef]
- Chu, M.; Shao, Y.; Peng, J.; Dai, X.; Li, H.; Wu, Q.; Shi, D. Near-infrared laser light mediated cancer therapy by photothermal effect of Fe3O4 magnetic nanoparticles. Biomaterials 2013, 34, 4078–4088. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Liu, S.; Zhang, W.; Yi, K.; Zhang, C.; Pang, H.; Huang, D.; Huang, J.; Li, X. Recent advances on microplastic aging: Identification, mechanism, influence factors, and additives release. Sci. Total Environ. 2023, 889, 164035. [Google Scholar] [CrossRef]
- Ma, J.; Cao, Y.; Fan, L.; Xie, Y.; Zhou, X.; Ren, Q.; Yang, X.; Gao, X.; Feng, Y. Degradation characteristics of polybutylene adipate terephthalic acid (PBAT) and its effect on soil physicochemical properties: A comparative study with several polyethylene (PE) mulch films. J. Hazard. Mater. 2023, 456, 131661. [Google Scholar] [CrossRef]
- Golmohammadi, M.; Fatemeh Musavi, S.; Habibi, M.; Maleki, R.; Golgoli, M.; Zargar, M.; Dumée, L.F.; Baroutian, S.; Razmjou, A. Molecular mechanisms of microplastics degradation: A review. Sep. Purif. Technol. 2023, 309, 122906. [Google Scholar] [CrossRef]
- Kiendrebeogo, M.; Karimi Estahbanati, M.R.; Khosravanipour Mostafazadeh, A.; Drogui, P.; Tyagi, R.D. Treatment of microplastics in water by anodic oxidation: A case study for polystyrene. Environ. Pollut. 2021, 269, 116168. [Google Scholar] [CrossRef]
- Atrei, A.; Lesiak-Orlowska, B.; Tóth, J. Magnetite nanoparticles functionalized with citrate: A surface science study by XPS and ToF-SIMS. Appl. Surf. Sci. 2022, 602, 154366. [Google Scholar] [CrossRef]
- Gao, Y.; Luo, Y.; Pan, Z.; Zeng, Z.; Fan, W.; Hu, J.; Zhang, Z.; Ma, J.; Zhou, Y.; Ma, J. Comparative study of Fe(II)/sulfite, Fe(II)/PDS and Fe(II)/PMS for p-arsanilic acid treatment: Efficient organic arsenic degradation and contrasting total arsenic removal. Water Res. 2024, 249, 120967. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).