β-Cyclodextrin/Graphene Oxide Multilayer Composite Membrane: A Novel Sustainable Strategy for High-Efficiency Removal of Pharmaceuticals and Personal Care Products
Abstract
1. Introduction
2. Materials and Methods
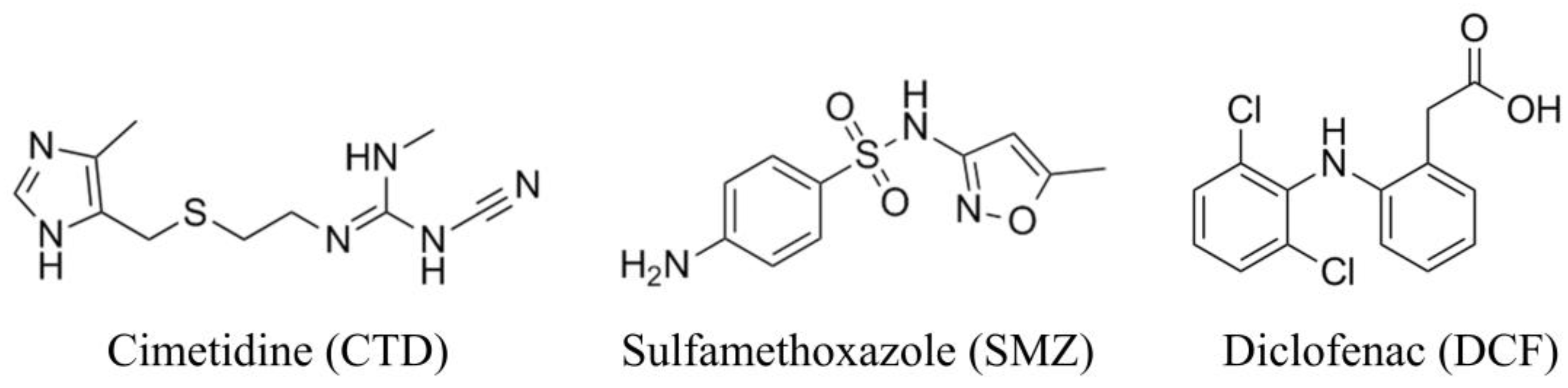
2.1. Materials
2.2. Synthesis of Adsorbents
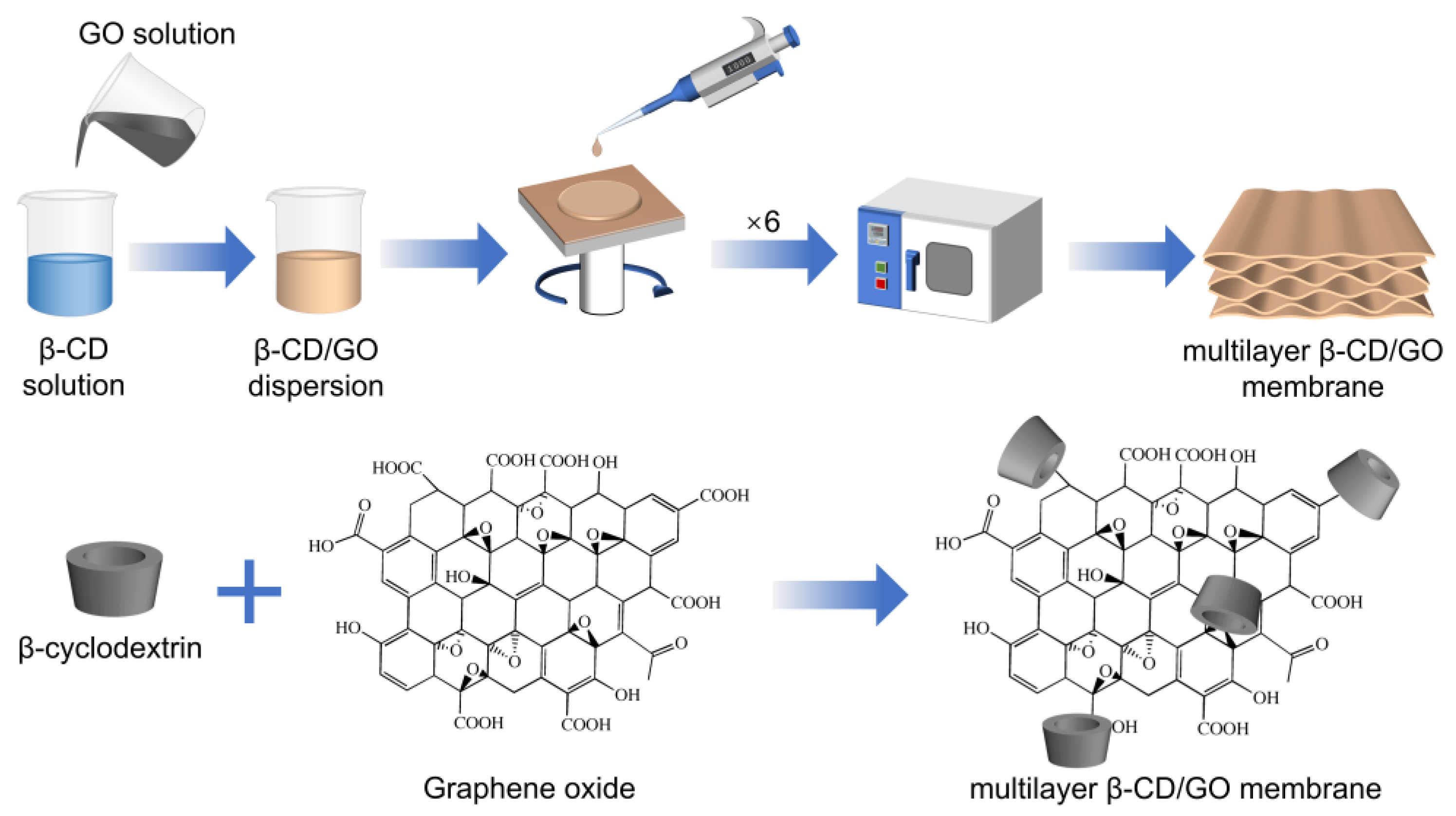
2.2.1. Preparation of GO
2.2.2. Preparation of Multilayer β-CD/GO Membrane
2.3. Characterization
2.4. Adsorption Experiments
3. Results and Discussion
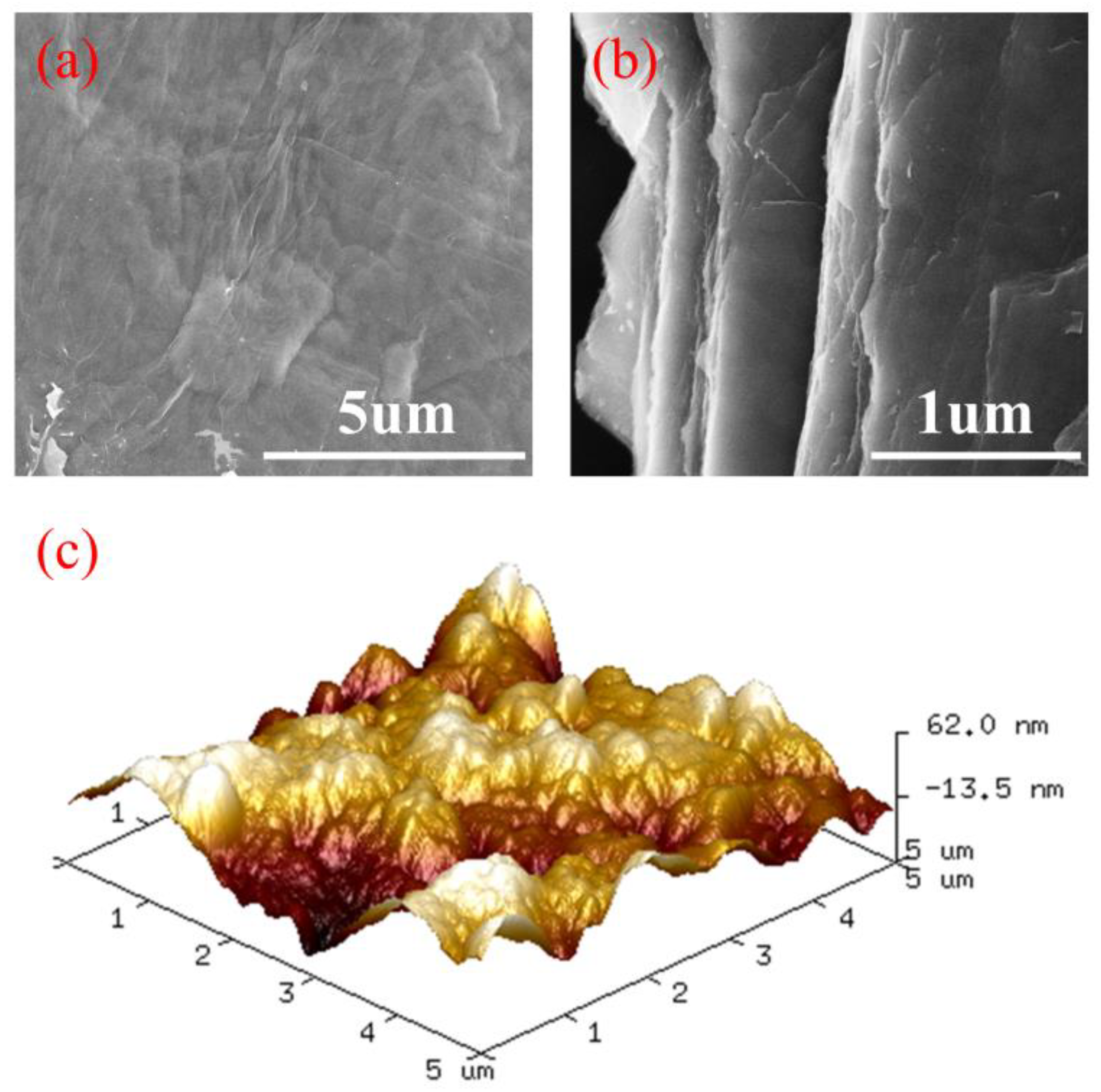
3.1. Characterization of Multilayer β-CD/GO Membrane
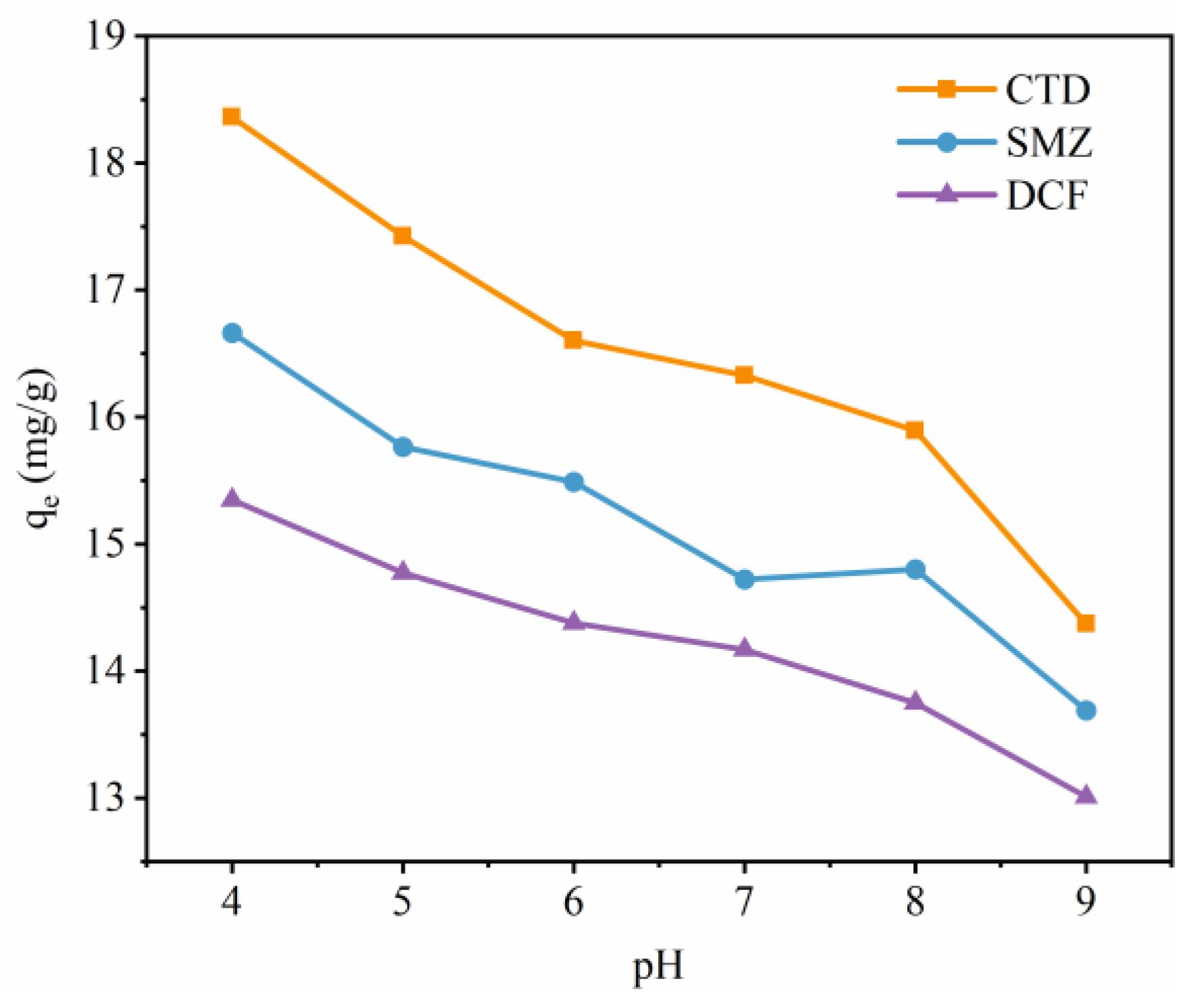
3.2. Effect of pH Values
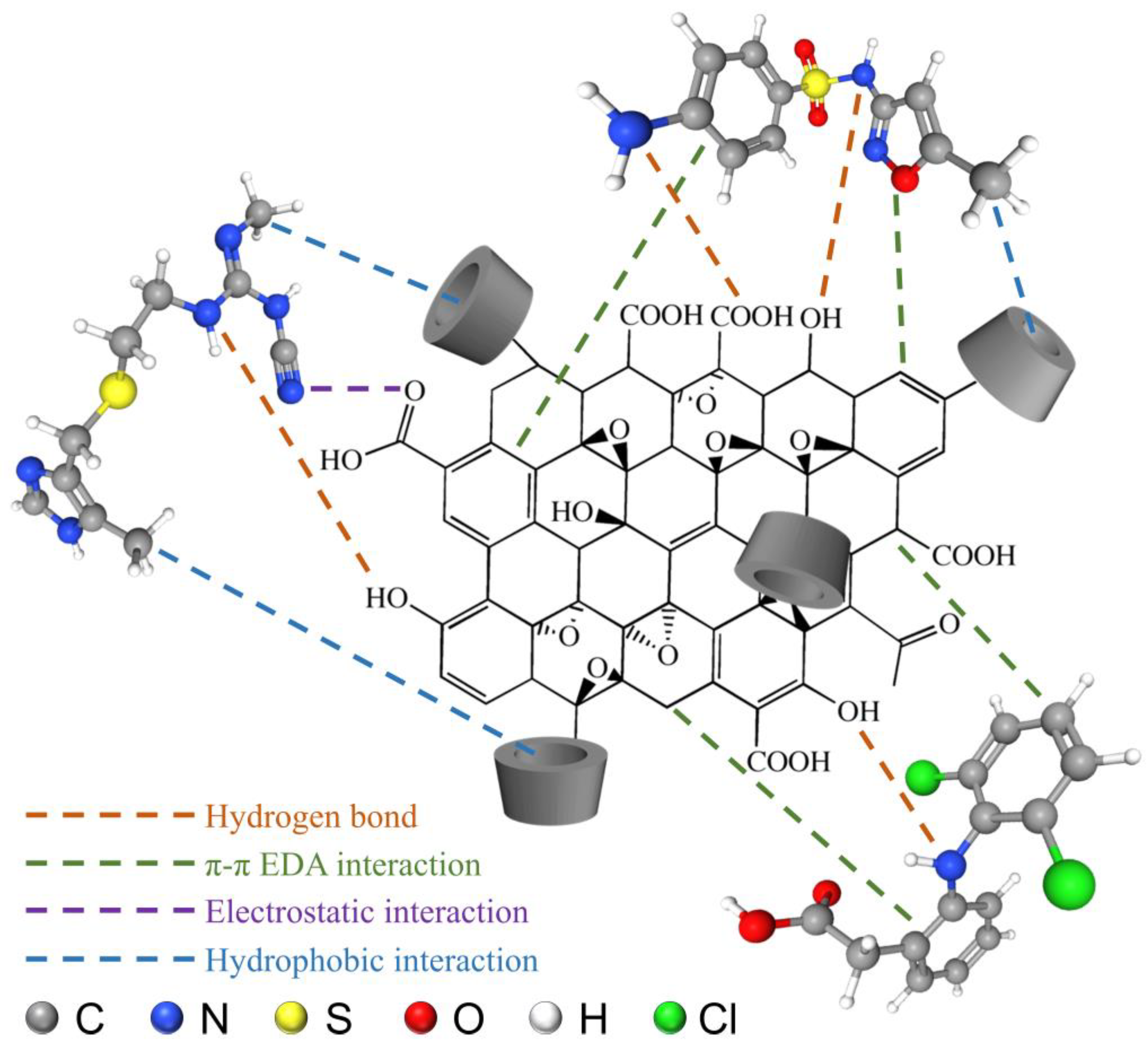
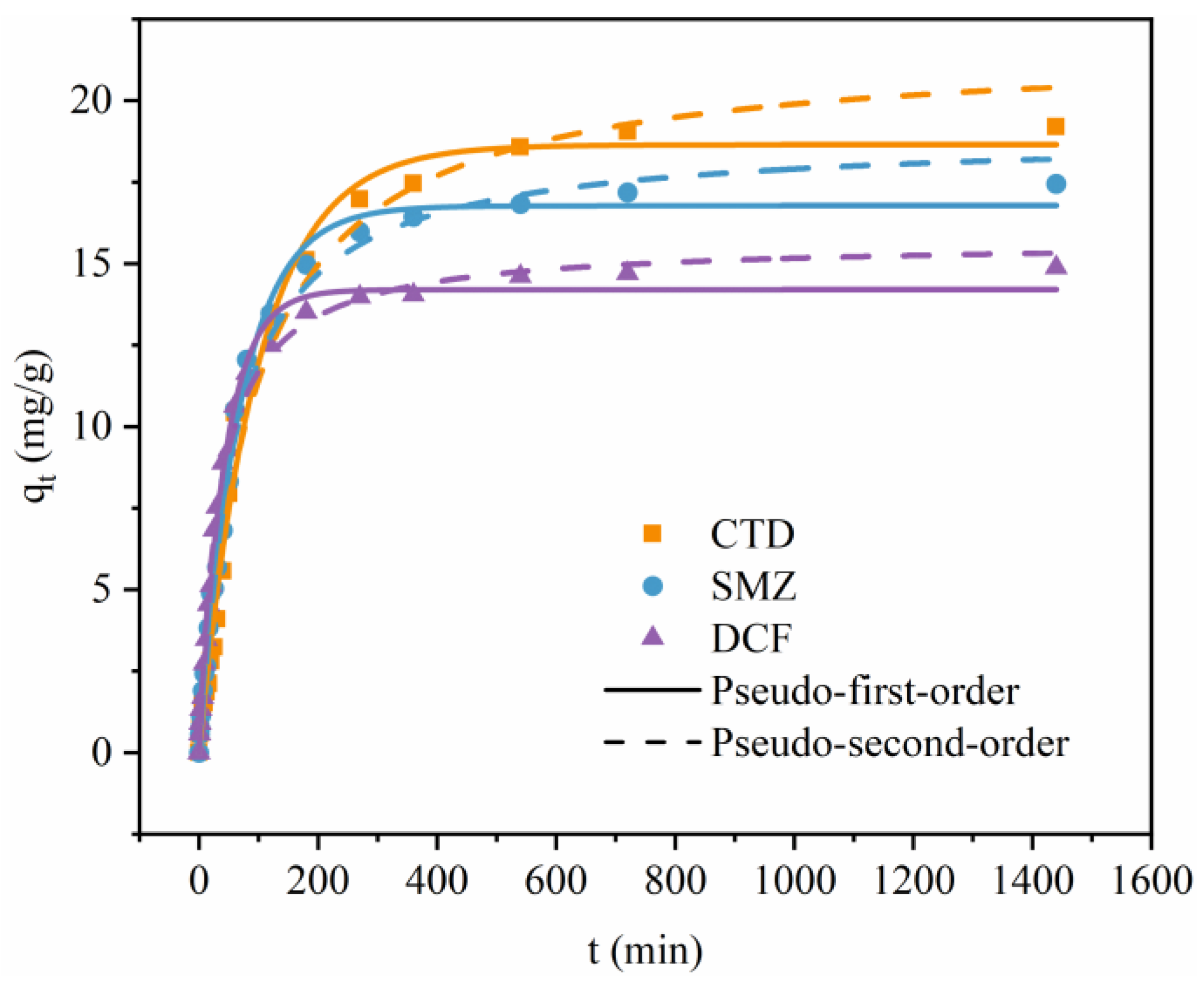
3.3. Adsorption Kinetics
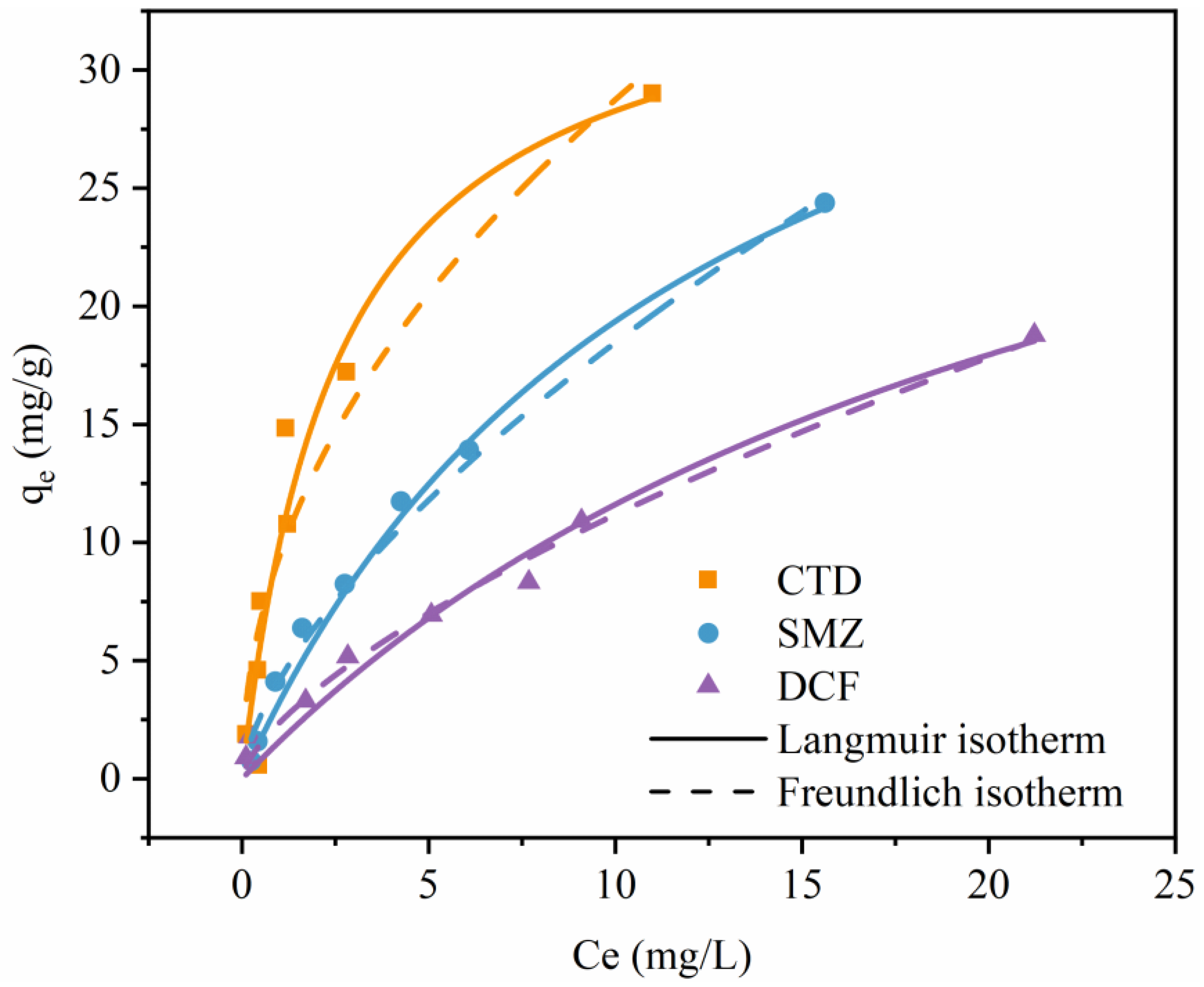
3.4. Adsorption Isotherms
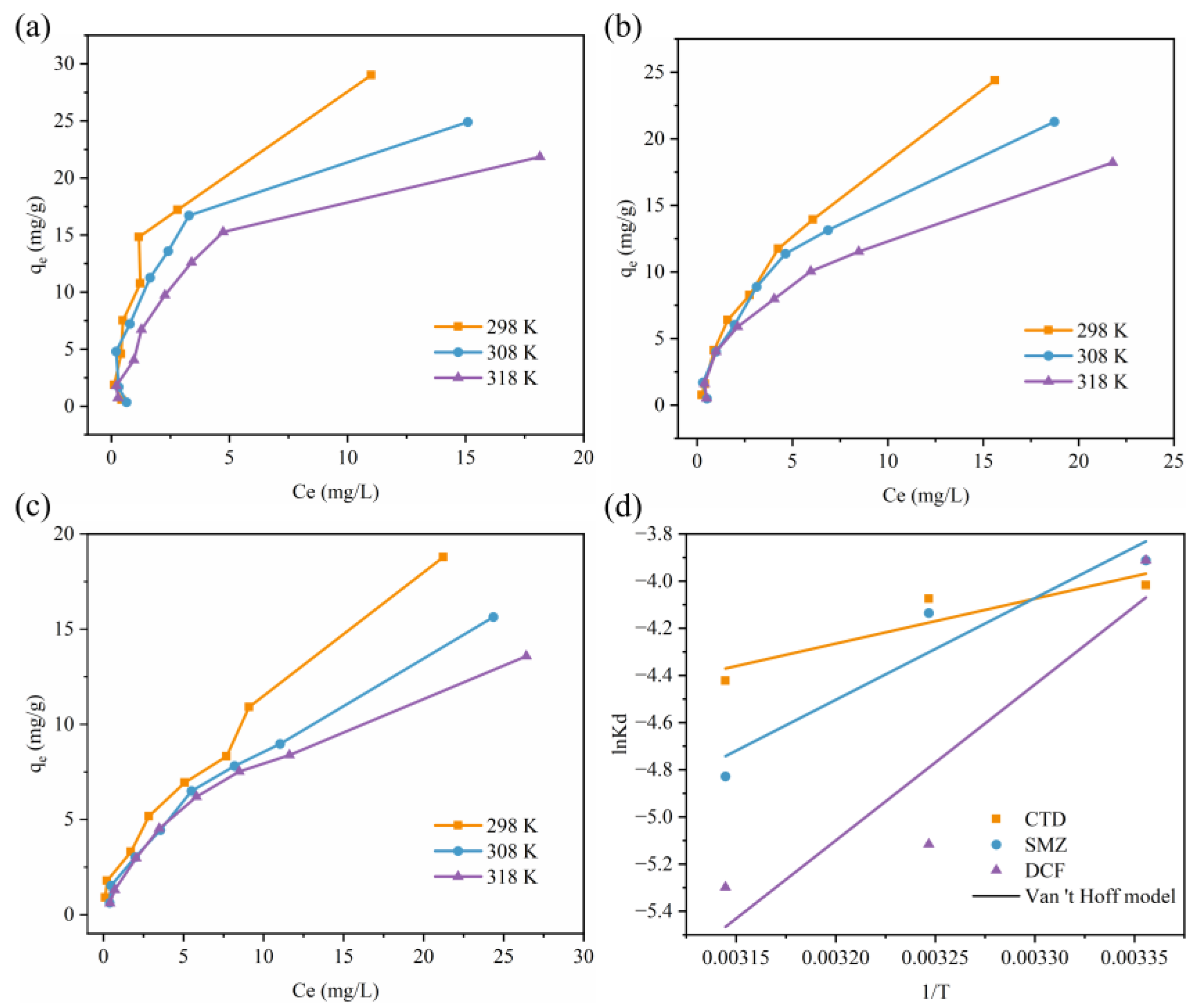
3.5. Adsorption Thermodynamics
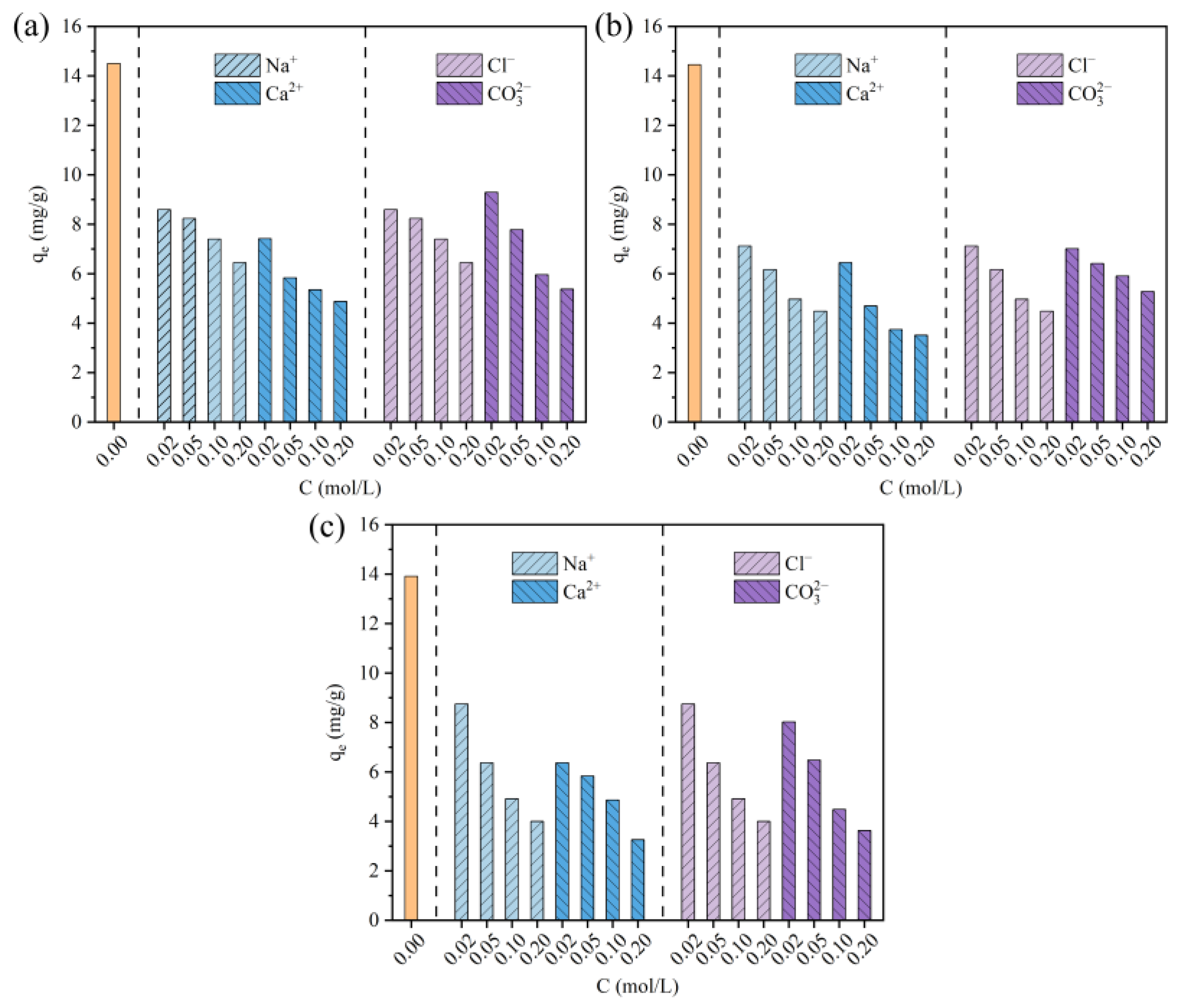
3.6. Effect of Coexisting Ions
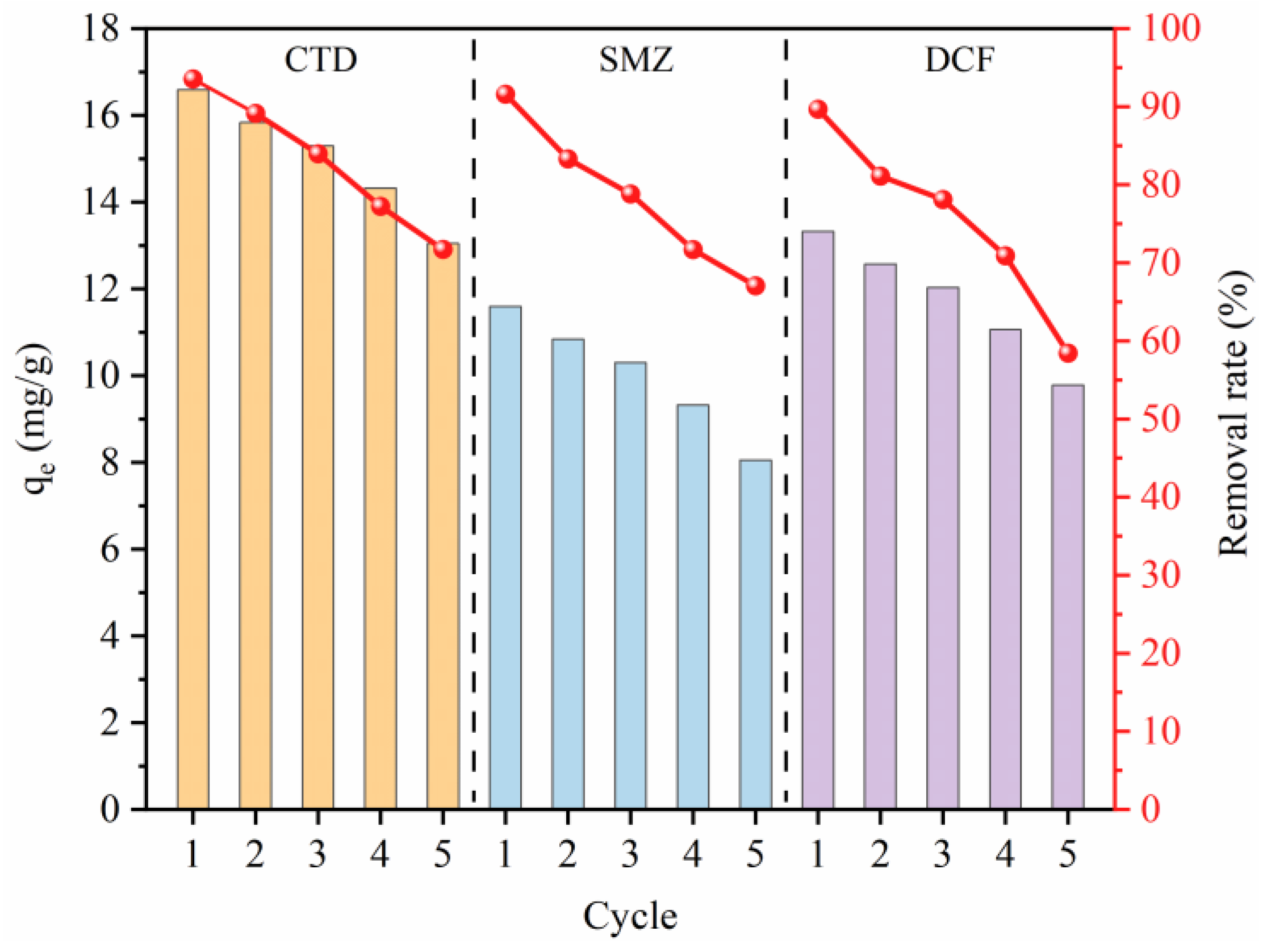
3.7. Regeneration of Multilayer β-CD/GO Membrane
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gogoi, A.; Mazumder, P.; Tyagi, V.K.; Tushara Chaminda, G.G.; An, A.K.; Kumar, M. Occurrence and fate of emerging contaminants in water environment: A review. Groundw. Sustain. Dev. 2018, 6, 169–180. [Google Scholar] [CrossRef]
- Subedi, B.; Balakrishna, K.; Joshua, D.I.; Kannan, K. Mass loading and removal of pharmaceuticals and personal care products including psychoactives, antihypertensives, and antibiotics in two sewage treatment plants in southern India. Chemosphere 2017, 167, 429–437. [Google Scholar] [CrossRef]
- Ebele, A.J.; Abou-Elwafa Abdallah, M.; Harrad, S. Pharmaceuticals and personal care products (PPCPs) in the freshwater aquatic environment. Emerg. Contam. 2017, 3, 1–16. [Google Scholar] [CrossRef]
- Kovalakova, P.; Cizmas, L.; McDonald, T.J.; Marsalek, B.; Feng, M.; Sharma, V.K. Occurrence and toxicity of antibiotics in the aquatic environment: A review. Chemosphere 2020, 251, 126351. [Google Scholar] [CrossRef]
- Chen, W.; Xu, J.; Lu, S.; Jiao, W.; Wu, L.; Chang, A.C. Fates and transport of PPCPs in soil receiving reclaimed water irrigation. Chemosphere 2013, 93, 2621–2630. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Hao, H.; Xu, N.; Liang, X.; Gao, D.; Xu, Y.; Gao, Y.; Tao, H.; Wong, M. Pharmaceuticals and personal care products in water, sediments, aquatic organisms, and fish feeds in the Pearl River Delta: Occurrence, distribution, potential sources, and health risk assessment. Sci. Total Environ. 2019, 659, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Jin, X.; Feng, C.; Wang, Z.; Wu, F.; Johnson, A.C.; Xiao, H.; Hollert, H.; Giesy, J.P. Ecological risk assessment of fifty pharmaceuticals and personal care products (PPCPs) in Chinese surface waters: A proposed multiple-level system. Environ. Int. 2020, 136, 105454. [Google Scholar] [CrossRef]
- Tang, K.; Ooi, G.T.; Torresi, E.; Kaarsholm, K.M.; Hambly, A.; Sundmark, K.; Lindholst, S.; Sund, C.; Kragelund, C.; Christensson, M.; et al. Municipal wastewater treatment targeting pharmaceuticals by a pilot-scale hybrid attached biofilm and activated sludge system (Hybas™). Chemosphere 2020, 259, 127397. [Google Scholar] [CrossRef]
- Adeoye, J.B.; Tan, Y.H.; Lau, S.Y.; Tan, Y.Y.; Chiong, T.; Mubarak, N.M.; Khalid, M. Advanced oxidation and biological integrated processes for pharmaceutical wastewater treatment: A review. J. Environ. Manag. 2024, 353, 120170. [Google Scholar] [CrossRef]
- Xue, Y.; Sun, W.; Shao, P.; Yuan, Y.; Cui, F.; Shi, W. Degradation of contaminants of PPCPs by photocatalysis for water purification: Kinetics, mechanisms, and cytotoxicity analysis. Chem. Eng. J. 2023, 454, 140505. [Google Scholar] [CrossRef]
- Tu, Y.; Han, L.; Ding, X.; Zhu, X.; Xu, G.; Xie, X.; Zhou, Q.; Shuang, C.; Li, A. Degradation of pharmaceuticals and personal care products (PPCPs) in municipal wastewater by white rot fungi: Neutral environment exploitation and degradation mechanisms. Chem. Eng. J. 2025, 507, 160794. [Google Scholar] [CrossRef]
- Yin, Y.; Shi, M.; Ren, Y.; Wang, S.; Hua, M.; Lu, J.; Zhang, W.; Lv, L. Wrinkle structure on multifunctional MOFs to facilitate PPCPs adsorption in wastewater. Chem. Eng. J. 2020, 387, 124196. [Google Scholar] [CrossRef]
- Fang, C.; Wang, S.; Xu, H.; Huang, Q. Degradation of tetracycline by atmospheric-pressure non-thermal plasma: Enhanced performance, degradation mechanism, and toxicity evaluation. Sci. Total Environ. 2022, 812, 152455. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Chen, D.; Wu, J.; Liu, J.; Shi, S.; Zhang, J.; Feng, Y. Construction of a novel integrated electrochemical oxidation-coagulation system for simultaneous removal of suspended solids and antibiotics. Chem. Eng. J. 2022, 447, 137505. [Google Scholar] [CrossRef]
- Zhu, X.; He, M.; Sun, Y.; Xu, Z.; Wan, Z.; Hou, D.; Alessi, D.S.; Tsang, D.C. Insights into the adsorption of pharmaceuticals and personal care products (PPCPs) on biochar and activated carbon with the aid of machine learning. J. Hazard. Mater. 2022, 423, 127060. [Google Scholar] [CrossRef]
- Zhang, H.; Huang, H.; Chen, W.; Zhang, Z.; Li, H.; Li, J. Mechanistic insight into efficient PPCPs removal on waste biological activated carbon for low-carbon emission reutilization. Chem. Eng. J. 2024, 487, 150759. [Google Scholar] [CrossRef]
- Kumari, S.; Chowdhry, J.; Kumar, M.; Garg, M.C. Zeolites in wastewater treatment: A comprehensive review on scientometric analysis, adsorption mechanisms, and future prospects. Environ. Res. 2024, 260, 119782. [Google Scholar] [CrossRef]
- Doan, T.H.Y.; Hoang, T.H.; Le, V.A.; Vu, D.N.; Vu, T.N.; Srivastav, A.L.; Pham, T.D. Adsorption and transformation of tetracyclines on alpha alumina particles with surface modification by anionic surfactant. Environ. Res. 2023, 216, 114618. [Google Scholar] [CrossRef]
- Guo, L.; Xu, X.; Niu, C.; Wang, Q.; Park, J.; Zhou, L.; Lei, H.; Wang, X.; Yuan, X. Machine learning-based prediction and experimental validation of heavy metal adsorption capacity of bentonite. Sci. Total Environ. 2024, 926, 171986. [Google Scholar] [CrossRef]
- Rahman, N.; Raheem, A. Adsorption of Cd(II) ions on magnetic graphene oxide/cellulose modified with β-cyclodextrin: Analytical interpretation via statistical physics modeling and fractal like kinetic approach. Environ. Res. 2024, 243, 117868. [Google Scholar] [CrossRef]
- Jemli, S.; Pinto, D.; Kanhounnon, W.G.; Ben Amara, F.; Sellaoui, L.; Bonilla-Petriciolet, A.; Dhaouadi, F.; Ameri, R.; Silva, L.F.; Bejar, S.; et al. Green β-cyclodextrin nanosponges for the efficient adsorption of light rare earth elements: Cerium and lanthanum. Chem. Eng. J. 2023, 466, 143108. [Google Scholar] [CrossRef]
- Mallard, I.; Städe, L.W.; Ruellan, S.; Jacobsen, P.A.L.; Larsen, K.L.; Fourmentin, S. Synthesis, characterization and sorption capacities toward organic pollutants of new β-cyclodextrin modified zeolite derivatives. Colloids Surf. A Physicochem. Eng. Asp. 2015, 482, 50–57. [Google Scholar] [CrossRef]
- Huang, W.; Hu, Y.; Li, Y.; Zhou, Y.; Niu, D.; Lei, Z.; Zhang, Z. Citric acid-crosslinked β-cyclodextrin for simultaneous removal of bisphenol A, methylene blue and copper: The roles of cavity and surface functional groups. J. Taiwan Inst. Chem. Eng. 2018, 82, 189–197. [Google Scholar] [CrossRef]
- Smith, A.T.; LaChance, A.M.; Zeng, S.; Liu, B.; Sun, L. Synthesis, properties, and applications of graphene oxide/reduced graphene oxide and their nanocomposites. Nano Mater. Sci. 2019, 1, 31–47. [Google Scholar] [CrossRef]
- Sun, M.; Li, J. Graphene oxide membranes: Functional structures, preparation and environmental applications. Nano Today 2018, 20, 121–137. [Google Scholar] [CrossRef]
- Peng, G.; Zhang, M.; Deng, S.; Shan, D.; He, Q.; Yu, G. Adsorption and catalytic oxidation of pharmaceuticals by nitrogen-doped reduced graphene oxide/Fe3O4 nanocomposite. Chem. Eng. J. 2018, 341, 361–370. [Google Scholar] [CrossRef]
- Ersan, G.; Apul, O.G.; Perreault, F.; Karanfil, T. Adsorption of organic contaminants by graphene nanosheets: A review. Water Res. 2017, 126, 385–398. [Google Scholar] [CrossRef]
- Chen, J.; Yao, B.; Li, C.; Shi, G. An improved Hummers method for eco-friendly synthesis of graphene oxide. Carbon 2013, 64, 225–229. [Google Scholar] [CrossRef]
- Jiang, S.; Wang, Q.; Wang, Z.; Borjigin, G.; Sun, J.; Zhao, Y.; Li, Q.; Shi, X.; Shah, S.F.A.; Wang, X.; et al. Ultrasound-assisted polysaccharide extraction from Fritillaria ussuriensis Maxim. and its structural characterization, antioxidant and immunological activity. Ultrason. Sonochem. 2024, 103, 106800. [Google Scholar] [CrossRef]
- Zhou, W.; Zhang, W.; Cai, Y. Enzyme-enhanced adsorption of laccase immobilized graphene oxide for micro-pollutant removal. Sep. Purif. Technol. 2022, 294, 121178. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, W.; Liu, F.; Chen, H.; Zhang, X.; Tan, C.; Gong, Y. Removal Efficiency and Mechanism of Typical PPCPs onto Novel Cyclodextrin–Graphene Oxide Composite Adsorbent in Aqueous Solutions. Water 2025, 17, 590. [Google Scholar] [CrossRef]
- Maeng, S.K.; Cho, K.; Jeong, B.; Lee, J.; Lee, Y.; Lee, C.; Choi, K.J.; Hong, S.W. Substrate-immobilized electrospun TiO2 nanofibers for photocatalytic degradation of pharmaceuticals: The effects of pH and dissolved organic matter characteristics. Water Res. 2015, 86, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W.; Johir, M.A.H.; Belhaj, D. Competitive sorption affinity of sulfonamides and chloramphenicol antibiotics toward functionalized biochar for water and wastewater treatment. Bioresour. Technol. 2017, 238, 306–312. [Google Scholar] [CrossRef]
- Reguyal, F.; Sarmah, A.K.; Gao, W. Synthesis of magnetic biochar from pine sawdust via oxidative hydrolysis of FeCl2 for the removal sulfamethoxazole from aqueous solution. J. Hazard. Mater. 2017, 321, 868–878. [Google Scholar] [CrossRef]
- Zeng, S.; Choi, Y.-K.; Kan, E. Iron-activated bermudagrass-derived biochar for adsorption of aqueous sulfamethoxazole: Effects of iron impregnation ratio on biochar properties, adsorption, and regeneration. Sci. Total Environ. 2021, 750, 141691. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.-K.; Kan, E. Effects of pyrolysis temperature on the physicochemical properties of alfalfa-derived biochar for the adsorption of bisphenol A and sulfamethoxazole in water. Chemosphere 2019, 218, 741–748. [Google Scholar] [CrossRef] [PubMed]
- Keiluweit, M.; Kleber, M. Molecular-Level Interactions in Soils and Sediments: The Role of Aromatic π-Systems. Environ. Sci. Technol. 2009, 43, 3421–3429. [Google Scholar] [CrossRef]
- Pan, B.; Xing, B. Adsorption Mechanisms of Organic Chemicals on Carbon Nanotubes. Environ. Sci. Technol. 2008, 42, 9005–9013. [Google Scholar] [CrossRef]
- Peiris, C.; Gunatilake, S.R.; Mlsna, T.E.; Mohan, D.; Vithanage, M. Biochar based removal of antibiotic sulfonamides and tetracyclines in aquatic environments: A critical review. Bioresour. Technol. 2017, 246, 150–159. [Google Scholar] [CrossRef]
- Wang, Y.; Jiao, W.-B.; Wang, J.-T.; Liu, G.-F.; Cao, H.-L.; Lü, J. Amino-functionalized biomass-derived porous carbons with enhanced aqueous adsorption affinity and sensitivity of sulfonamide antibiotics. Bioresour. Technol. 2019, 277, 128–135. [Google Scholar] [CrossRef]
- Lv, M.; Li, D.; Zhang, Z.; Logan, B.E.; Liu, G.; Sun, M.; Dai, C.; Feng, Y. Unveiling the correlation of Fe3O4 fractions upon the adsorption behavior of sulfamethoxazole on magnetic activated carbon. Sci. Total Environ. 2021, 757, 143717. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Zhang, Y.; Gao, B.; Chen, R.; Wu, F. Removal of sulfamethoxazole (SMX) and sulfapyridine (SPY) from aqueous solutions by biochars derived from anaerobically digested bagasse. Environ. Sci. Pollut. Res. 2018, 25, 25659–25667. [Google Scholar] [CrossRef]
- Xie, M.; Chen, W.; Xu, Z.; Zheng, S.; Zhu, D. Adsorption of sulfonamides to demineralized pine wood biochars prepared under different thermochemical conditions. Environ. Pollut. 2014, 186, 187–194. [Google Scholar] [CrossRef]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W.; Johir, M.A.H.; Sornalingam, K. Single and competitive sorption properties and mechanism of functionalized biochar for removing sulfonamide antibiotics from water. Chem. Eng. J. 2017, 311, 348–358. [Google Scholar] [CrossRef]
- Zheng, H.; Wang, Z.; Zhao, J.; Herbert, S.; Xing, B. Sorption of antibiotic sulfamethoxazole varies with biochars produced at different temperatures. Environ. Pollut. 2013, 181, 60–67. [Google Scholar] [CrossRef]
- Rahman, N.; Raheem, A.; Nasir, M.; Franco, D.S. Fractal-like kinetic modelling for sorption of diclofenac onto graphene oxide/polypyrrole composite: Mechanism analysis and response surface methodology for optimization. Diam. Relat. Mater. 2023, 139, 110328. [Google Scholar] [CrossRef]
- Hu, C.; Jiang, J.; An, Y.; Jiang, X.; Sun, Q.; Zheng, H.; Li, H. A novel self-floating silica adsorbent for antibiotic ciprofloxacin and nickel (II) ion. Chem. Eng. J. 2022, 429, 132227. [Google Scholar] [CrossRef]
- Wang, R.; Yao, C.; Peng, C.; Qiu, J.; Wang, Q.; Liu, X.; Meng, J.; Wang, W. Ultra-strong adsorption of organic dyes and antibiotic onto the alk-MXene/ZIF adsorbents with a specific intercalation structure. Chem. Eng. J. 2024, 485, 149916. [Google Scholar] [CrossRef]
- Yadav, S.; Asthana, A.; Singh, A.K.; Chakraborty, R.; Vidya, S.S.; Susan, A.B.H.; Carabineiro, S.A. Adsorption of cationic dyes, drugs and metal from aqueous solutions using a polymer composite of magnetic/β-cyclodextrin/activated charcoal/Na alginate: Isotherm, kinetics and regeneration studies. J. Hazard. Mater. 2021, 409, 124840. [Google Scholar] [CrossRef]
- Liu, B.; Chen, T.; Wang, B.; Zhou, S.; Zhang, Z.; Li, Y.; Pan, X.; Wang, N. Enhanced removal of Cd2+ from water by AHP-pretreated biochar: Adsorption performance and mechanism. J. Hazard. Mater. 2022, 438, 129467. [Google Scholar] [CrossRef]
- Huang, Y.; Zheng, H.; Li, H.; Zhang, Z.; Zhao, C.; Gou, Q.; Liu, Y. Highly effective and selective adsorption of thorium(Ⅳ) from aqueous solution using mesoporous graphite carbon nitride prepared by sol–gel template method. Chem. Eng. J. 2021, 410, 128321. [Google Scholar] [CrossRef]
- Yao, N.; Li, C.; Yu, J.; Xu, Q.; Wei, S.; Tian, Z.; Yang, Z.; Yang, W.; Shen, J. Insight into adsorption of combined antibiotic-heavy metal contaminants on graphene oxide in water. Sep. Purif. Technol. 2020, 236, 116278. [Google Scholar] [CrossRef]
- Hamza, M.F.; Hamad, D.M.; Hamad, N.A.; Abdel-Rahman, A.A.-H.; Fouda, A.; Wei, Y.; Guibal, E.; El-Etrawy, A.-A.S. Functionalization of magnetic chitosan microparticles for high-performance removal of chromate from aqueous solutions and tannery effluent. Chem. Eng. J. 2022, 428, 131775. [Google Scholar] [CrossRef]
- Jiao, C.; Wang, Y.; Li, M.; Wu, Q.; Wang, C.; Wang, Z. Synthesis of magnetic nanoporous carbon from metal-organic framework for the fast removal of organic dye from aqueous solution. J. Magn. Magn. Mater. 2016, 407, 24–30. [Google Scholar] [CrossRef]
- Yao, M.; Zhang, G.; Shao, D.; Ding, S.; Li, L.; Li, H.; Zhou, C.; Luo, B.; Lu, L. Preparation of chitin/MXene/poly(L-arginine) composite aerogel spheres for specific adsorption of bilirubin. Int. J. Biol. Macromol. 2023, 243, 125140. [Google Scholar] [CrossRef]
- Fan, W.; Xin, Q.; Dai, Y.; Chen, Y.; Liu, S.; Zhang, X.; Yang, Y.; Gao, X. Competitive transport and adsorption of CO2/H2O in the graphene nano-slit pore: A molecular dynamics simulation study. Sep. Purif. Technol. 2025, 353, 128394. [Google Scholar] [CrossRef]
- Park, S.; Kim, S.; Yea, Y.; Saravanakumar, K.; Lee, E.; Yoon, Y.; Park, C.M. Adsorptive and photocatalytic performance of cobalt-doped ZnTiO3/Ti3C2Tx MXene nanohybrids towards tetracycline: Kinetics and mechanistic insight. J. Hazard. Mater. 2023, 443, 130165. [Google Scholar] [CrossRef]
- Reguyal, F.; Sarmah, A.K. Adsorption of sulfamethoxazole by magnetic biochar: Effects of pH, ionic strength, natural organic matter and 17α-ethinylestradiol. Sci. Total Environ. 2018, 628–629, 722–730. [Google Scholar] [CrossRef]
- Gil, A.; Santamaría, L.; Korili, S. Removal of Caffeine and Diclofenac from Aqueous Solution by Adsorption on Multiwalled Carbon Nanotubes. Colloid Interface Sci. Commun. 2018, 22, 25–28. [Google Scholar] [CrossRef]
- Lonappan, L.; Rouissi, T.; Brar, S.K.; Verma, M.; Surampalli, R.Y. An insight into the adsorption of diclofenac on different biochars: Mechanisms, surface chemistry, and thermodynamics. Bioresour. Technol. 2018, 249, 386–394. [Google Scholar] [CrossRef]
- Sun, M.; Sun, Q.; Zhao, C.; Huang, Y.; Jiang, J.; Ding, W.; Zheng, H. Degradation of diclofenac sodium with low concentration from aqueous milieu through polydopamine-chitosan modified magnetic adsorbent-assisted photo-Fenton process. Sep. Purif. Technol. 2022, 289, 120771. [Google Scholar] [CrossRef]
- Azqandi, M.; Ramavandi, B.; Nasseh, N.; Zaarei, D.; Fanaei, F. Green synthesis of manganese ferrite magnetic nanoparticle and its modification with metallic-organic frameworks for the tetracycline adsorption from aqueous solutions: A mathematical study of kinetics, isotherms, and thermodynamics. Environ. Res. 2024, 256, 118957. [Google Scholar] [CrossRef]
- Li, C.; Wang, F.; Xu, X.; Shi, Y.; Liang, J.; Yang, R.; Liu, J.; Zhao, Z. A high-capacity malleable cellulose aerogel with layered double hydroxide decorating ZIF-8 for efficient adsorption of ciprofloxacin. Chem. Eng. J. 2023, 455, 140841. [Google Scholar] [CrossRef]
- Tang, M.; Jia, R.; Kan, H.; Liu, Z.; Yang, S.; Sun, L.; Yang, Y. Kinetic, isotherm, and thermodynamic studies of the adsorption of dye from aqueous solution by propylene glycol adipate-modified cellulose aerogel. Colloids Surf. A Physicochem. Eng. Asp. 2020, 602, 125009. [Google Scholar] [CrossRef]
- Du, R.; Cao, H.; Wang, G.; Dou, K.; Tsidaeva, N.; Wang, W. PVP modified rGO/CoFe2O4 magnetic adsorbents with a unique sandwich structure and superior adsorption performance for anionic and cationic dyes. Sep. Purif. Technol. 2022, 286, 120484. [Google Scholar] [CrossRef]
- Kong, X.; Feng, S.; Zhang, X.; Li, Y. Effects of bile salts and divalent cations on the adsorption of norfloxacin by agricultural soils. J. Environ. Sci. 2014, 26, 846–854. [Google Scholar] [CrossRef]
- Wu, G.; Ma, J.; Li, S.; Guan, J.; Jiang, B.; Wang, L.; Li, J.; Wang, X.; Chen, L. Magnetic copper-based metal organic framework as an effective and recyclable adsorbent for removal of two fluoroquinolone antibiotics from aqueous solutions. J. Colloid Interface Sci. 2018, 528, 360–371. [Google Scholar] [CrossRef]
- Zhao, Y.; Geng, J.; Wang, X.; Gu, X.; Gao, S. Adsorption of tetracycline onto goethite in the presence of metal cations and humic substances. J. Colloid Interface Sci. 2011, 361, 247–251. [Google Scholar] [CrossRef]
- Xie, J.; Liu, M.; He, M.; Liu, Y.; Li, J.; Yu, F.; Lv, Y.; Lin, C.; Ye, X. Ultra-efficient adsorption of diclofenac sodium on fish-scale biochar functionalized with H3PO4 via synergistic mechanisms. Environ. Pollut. 2023, 322, 121226. [Google Scholar] [CrossRef]
- Dou, S.; Ke, X.-X.; Shao, Z.-D.; Zhong, L.-B.; Zhao, Q.-B.; Zheng, Y.-M. Fish scale-based biochar with defined pore size and ultrahigh specific surface area for highly efficient adsorption of ciprofloxacin. Chemosphere 2022, 287, 131962. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, C.; Liu, F.; Yuan, Y.; Wu, H.; Li, A. Effects of ionic strength on removal of toxic pollutants from aqueous media with multifarious adsorbents: A review. Sci. Total Environ. 2019, 646, 265–279. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Lu, Y.; Zheng, F.; Xue, X.; Li, N.; Liu, D. Adsorption behavior and mechanisms of norfloxacin onto porous resins and carbon nanotube. Chem. Eng. J. 2012, 179, 112–118. [Google Scholar] [CrossRef]
- Jiang, L.; Liu, Y.; Liu, S.; Hu, X.; Zeng, G.; Hu, X.; Liu, S.; Liu, S.; Huang, B.; Li, M. Fabrication of β-cyclodextrin/poly (l-glutamic acid) supported magnetic graphene oxide and its adsorption behavior for 17β-estradiol. Chem. Eng. J. 2017, 308, 597–605. [Google Scholar] [CrossRef]
- Roy, N.; Kannabiran, K.; Mukherjee, A. Integrated adsorption and photocatalytic degradation based removal of ciprofloxacin and sulfamethoxazole antibiotics using Fc@rGO-ZnO nanocomposite in aqueous systems. Chemosphere 2023, 333, 138912. [Google Scholar] [CrossRef] [PubMed]
| Adsorption Kinetic Models | Parameters | PPCPs | ||
|---|---|---|---|---|
| CTD | SMZ | DCF | ||
| Experimental data | qe,exp (mg/g) | 19.06 | 17.19 | 14.70 |
| Pseudo-first-order | qe,cal (mg/g) | 18.64 | 16.77 | 14.21 |
| k1 (1/min) | 0.618 | 0.883 | 1.395 | |
| R2 | 0.990 | 0.995 | 0.995 | |
| Pseudo-second-order | qe,cal (mg/g) | 21.67 | 18.94 | 15.68 |
| k2 (g/mg⋅min) | 0.031 | 0.055 | 0.112 | |
| R2 | 0.984 | 0.992 | 0.994 | |
| Adsorption Kinetic Models | Parameters | PPCPs | ||
|---|---|---|---|---|
| CTD | SMZ | DCF | ||
| Langmuir | qmax (mg/g) | 35.56 | 43.29 | 39.49 |
| KL | 0.388 | 0.081 | 0.042 | |
| R2 | 0.923 | 0.980 | 0.972 | |
| Freundlich | 1/n | 0.49 | 0.65 | 0.67 |
| KF | 9.404 | 4.170 | 2.368 | |
| R2 | 0.879 | 0.984 | 0.988 | |
| Adsorbents | qmax (mg/g) | Reference |
|---|---|---|
| Magnetic biochar | 13.83 | [34] |
| Magnetic biochar | 9.42 | [58] |
| Multiwalled carbon nanotubes | 7.26 | [59] |
| Pinewood biochar | 0.53 | [60] |
| Polydopamine–chitosan modified adsorbent | 11.11 | [61] |
| PPCPs | Temperature (K) | (kJ/mol) | (kJ/mol) | (J/mol/K) |
|---|---|---|---|---|
| CTD | 298 | 9.84 | −15.84 | −86.16 |
| 308 | 10.70 | |||
| 318 | 11.56 | |||
| SMZ | 298 | 9.50 | −35.88 | −152.27 |
| 308 | 11.02 | |||
| 318 | 12.54 | |||
| DCF | 298 | 10.09 | −55.02 | −218.49 |
| 308 | 12.27 | |||
| 318 | 14.46 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Yang, Y.; Tang, Z.; Liu, F.; Chen, H. β-Cyclodextrin/Graphene Oxide Multilayer Composite Membrane: A Novel Sustainable Strategy for High-Efficiency Removal of Pharmaceuticals and Personal Care Products. Sustainability 2025, 17, 3322. https://doi.org/10.3390/su17083322
Zhang Z, Yang Y, Tang Z, Liu F, Chen H. β-Cyclodextrin/Graphene Oxide Multilayer Composite Membrane: A Novel Sustainable Strategy for High-Efficiency Removal of Pharmaceuticals and Personal Care Products. Sustainability. 2025; 17(8):3322. https://doi.org/10.3390/su17083322
Chicago/Turabian StyleZhang, Ziyang, Ying Yang, Zibo Tang, Fangyuan Liu, and Hongrui Chen. 2025. "β-Cyclodextrin/Graphene Oxide Multilayer Composite Membrane: A Novel Sustainable Strategy for High-Efficiency Removal of Pharmaceuticals and Personal Care Products" Sustainability 17, no. 8: 3322. https://doi.org/10.3390/su17083322
APA StyleZhang, Z., Yang, Y., Tang, Z., Liu, F., & Chen, H. (2025). β-Cyclodextrin/Graphene Oxide Multilayer Composite Membrane: A Novel Sustainable Strategy for High-Efficiency Removal of Pharmaceuticals and Personal Care Products. Sustainability, 17(8), 3322. https://doi.org/10.3390/su17083322






