Abstract
Soil microbial communities are vital for saline-alkaline ecosystem functioning; however, their succession during land degradation and their influence on phosphorus (P) transformation remain unclear. To address this gap, this study investigated the dynamics of soil microbial communities and P fractions along a degradation gradient from native grassland to Suaeda salsa vegetation and ultimately to bare land in the Songnen Plain, China. The results revealed that progressive saline-alkaline degradation significantly altered soil properties, increased the proportion of stable P fractions, and reduced microbial alpha diversity. Network analysis revealed that bacterial communities shifted from competition to cooperation along the salinity–alkalinity degradation gradient, indicating a cooperative strategy to cope with environmental stress. Fungal networks exhibit progressively reduced complexity and stability with increasing degradation. Partial least squares path modeling confirmed that soil pH and electrical conductivity directly and indirectly regulated P fractions by reshaping microbial communities, with bacteria exhibiting a stronger total effect than fungi. In conclusion, saline-alkaline degradation drives microbial community succession, which mediates the transformation of soil P into more stable forms and exacerbates P limitation. This study provides a scientific basis for targeted restoration and sustainable management of saline-alkaline ecosystems.
1. Introduction
The land degradation induced by salinization and sodification alters soil function, degrades soil health, and poses a serious threat to ecosystem stability, resource sustainability, and global agricultural productivity []. Under the influence of global climate change and unsustainable land use, natural and anthropogenic drivers have intensified primary and secondary salinization, significantly accelerating soil degradation [,]. According to the Food and Agriculture Organization of the United Nations (FAO) and the United Nations Educational, Scientific, and Cultural Organization (UNESCO), the global extent of saline-alkali land is approximately 1.38 billion hectares, accounting for approximately 10.7% of the Earth’s terrestrial surface []. With continued global warming, secondary salinization is expected to become increasingly severe worldwide []. The Songnen Plain, located in northeastern China, is renowned for its fertile black soils but is also one of the three global hotspots of saline-alkali soils. The area of saline-alkali land in the region has reached 3.4 × 106 hectares and is expanding at an annual rate of 1.4% []. Since the 1980s, cropland expansion and livestock grazing have degraded extensive grassland and farmland areas into saline-alkali land []. This makes the Songnen Plain a typical ecologically fragile agro-pastoral zone. Curbing the ongoing degradation of saline-alkali land has become a critical challenge for the conservation of black soils and the sustainable development of agriculture and animal husbandry in the Songnen Plain.
Soil conditions, which integrate the microbial community composition, soil structure, and physicochemical attributes, constitute key descriptors of ecosystem health and land degradation []. Phosphorus (P) is an essential macronutrient for all living organisms. However, in saline-alkaline ecosystems, elevated salinity often induces severe P deficiency. Under saline-alkaline conditions, high ionic strength suppresses P desorption, clay minerals and carbonates strongly fix P, and alkaline conditions significantly reduce the solubility of P-containing minerals. Collectively, these factors lead to a marked decrease in available soil P []. Based on plant availability, soil P is fractionated into labile P (H2O-Pi, NaHCO3-Pi, NaHCO3-Po), moderately labile P (NaOH-Pi, NaOH-Po, D.HCl-Pi), and stable P (C.HCl-Pi, C.HCl-Po, Residual-Pt) []. Labile P is classified as plant-available, whereas moderately labile P represents a potentially available P pool that serves as an important source for sustained P supply []. In contrast, stable P can persist in soils for decades without being available to organisms []. Under the high ionic strength of saline soils, P is rapidly converted into insoluble phosphates, resulting in low P availability.
Beneficial microorganisms can decompose and transport saline-alkali ions within the soil microbial community and their secondary metabolites can adsorb these ions []. These microorganisms also play critical roles in mediating ecosystem element cycles [] and function as biochemical engines that drive element cycling []. Soil microorganisms play a critical role in the P cycle, primarily influencing P bioavailability through four key processes: inorganic P solubilization, organic P mineralization, P starvation response, and P uptake and transport [,]. Halotolerant phosphate-solubilizing microorganisms (PSMs) isolated from saline-alkali soils can convert recalcitrant P pools into available forms under extreme conditions. In saline-alkaline environments, PSMs promote the dissolution of insoluble phosphate compounds by producing substantial amounts of organic acids and H+ through metabolic activities, which lower soil pH and alter the dissolution equilibrium of inorganic orthophosphates in the soil solution []. Additionally, protons and organic acid anions derived from microbial metabolism effectively complex with Ca2+, facilitating the dissolution and release of calcium phosphates []. However, some studies have indicated that salinity and pH can reduce the abundance and diversity of PSMs, thereby reshaping microbial community composition [,].
Research has revealed general microbial community responses to saline-alkaline stress and their implied roles in P transformation. However, a clear and systematic understanding of how microbial community structure directly influences P fraction dynamics is still lacking. For instance, Dey et al. (2021) reviewed the plant growth-promoting mechanisms of salt-tolerant PSMs in saline-alkaline agriculture, but their work primarily focused on functional validation under cultivation-based conditions, lacking in situ community-level evidence []. Recent research in the Songnen Plain demonstrated that electrical conductivity (EC) is a dominant factor shaping bacterial community structure [], but failed to elucidate how EC affects P speciation transformation. Similarly, Hu et al. (2024) reported that P fraction changes significantly influenced the fungal functional group composition in saline-alkaline soils under land use conversion, but could not differentiate the relative contributions of bacteria versus fungi to P cycling []. Notably, most existing studies rely on traditional correlation analyses, which fail to effectively decouple the direct versus indirect regulatory pathways of physicochemical factors, and rarely address how community interactions mediate phosphorus stability from the perspective of microbial co-occurrence networks.
In light of the limitations of existing research, soil samples representing three distinct soil types were collected along a secondary succession gradient (native grassland, Suaeda salsa vegetation, and bare land) in the saline-alkaline region of the Songnen Plain. We analyzed soil P fractions, physicochemical properties, and microbial (bacterial and fungal) community structures. The objectives were to (1) quantify the transformation of soil P fractions from labile to stable forms along a saline-alkaline degradation gradient; (2) analyze the differential effects of saline-alkaline degradation on the diversity, composition, and co-occurrence network topological features of soil bacterial and fungal communities, and to identify key responsive/tolerant taxa; (3) parse the direct effects of key soil saline-alkaline indicators (pH, EC) on P fraction transformation, along with the indirect effects mediated through alterations in bacterial and fungal community structure and network interactions, thereby quantifying the relative contributions and distinct regulatory pathways of microorganisms in this process. These findings are expected to provide a scientific basis for the bioremediation and sustainable utilization of saline-alkaline lands.
2. Materials and Methods
2.1. Study Site and Soil Sampling
The Songnen Plain is located in northeastern China (43°30′–48°40′ N, 121°40′–128°05′ E) and has a temperate semi-humid continental monsoon climate. The region is characterized by an average annual temperature of 4.7 °C, precipitation ranging from 350 to 500 mm, and evaporation between 1200 and 1800 mm []. The pronounced aridity and high evaporation rates contribute to its status as one of China’s primary saline-alkali soil distribution areas, where the dominant soil type is classified as soda saline-alkali soil.
Soil samples were collected in October 2018 from six representative sites in the Songnen Plain (Table A1). Three types of sample plots were selected based on the degree of land degradation: slightly degraded (grassland), moderately degraded (Suaeda salsa community), and severely degraded (saline-alkaline bare land). This gradient represents a retrogressive succession sequence driven primarily by natural soil salinization processes, historically influenced by livestock grazing. All sampling sites were located in native ecosystems without recent agricultural reclamation or active restoration, ensuring that the observed patterns reflect long-term natural degradation rather than recent anthropogenic land use change. A quadrat (10 m × 10 m) was placed at each sampling point. Within each plot, soil samples were collected from a depth of 0–20 cm using a five-point sampling method, with one sample taken from the center and four from the peripheral points of the plot. Each composite sample was divided into two portions: one was immediately snap-frozen in liquid nitrogen and stored at −80 °C for microbial analyses; the other was air-dried at room temperature for physicochemical and P fraction analyses. All treatments were performed in triplicate.
2.2. Soil Physicochemical Properties and P Fractionation Analysis
Soil pH and EC were measured using a pH meter and a conductivity meter at air-dried soil-to-water ratios of 1:2.5 and 1:5, respectively. Soil organic carbon (SOC) was quantified using the K2Cr2O7 oxidation method with external heating. Total nitrogen (TN) content was measured using the Kjeldahl nitrogen method. Alkali-hydrolyzable nitrogen (AN) content was assessed using the alkali diffusion method. Total P (TP), extracted by NaOH fusion, and available P (AP), extracted with NaHCO3, were both determined using molybdenum–antimony resistance colorimetry. Available potassium (AK) was extracted with ammonium acetate and measured using flame photometry.
Soil P fractions were determined using the Hedley fractionation procedure modified by Tiessen and Moir (2008) [] and classified into three major categories: (1) Labile P: H2O-P (water-soluble P extracted with deionized water), NaHCO3-Pi (exchangeable inorganic P adsorbed on soil surfaces), and NaHCO3-Po (organic P susceptible to mineralization) extracted with 0.5 mol L−1 NaHCO3; (2) Moderately labile P: NaOH-Pi and NaOH-Po (Fe/Al oxide-bound and carbonate-associated phosphate anions) extracted with 0.1 mol L−1 NaOH, and D.HCl-Pi (primary mineral P) released by 1 mol L−1 HCl; (3) Stable P: Occluded P (C.HCl-Po, C.HCl-Pi, Residual-Pt) extracted with concentrated HCl and mixed acids (concentrated sulfuric, nitric, and perchloric acids).
2.3. Soil Microbial Community Amplicon Sequencing
Microbial community DNA was extracted from 0.25 g of soil using the Soil DNA Kit (Omega Bio-tek, Norcross, GA, USA). The quality of the extracted DNA was assessed using 1% agarose gel electrophoresis, and DNA concentration and purity were measured using a NanoDrop 2000 spectrophotometer (Thermo Scientific, Waltham, MA, USA). The V3–V4 region of the bacterial 16S rRNA gene was amplified using primers 338F (5′-ACTCCTACGGGAGGCAGCA-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′), whereas the fungal ITS2 region was amplified using primers ITS1F (5′-CTTGGTCATTTAGAGGAAGTAA-3′) and ITS2R (5’-GCTGCGTTCTTCATCGATGC-3’). Following confirmation via agarose gel electrophoresis, the amplicons were purified and subjected to high-throughput sequencing (2 × 250 bp paired-end) on an Illumina MiSeq platform at Shanghai Personal Biotechnology Co., Ltd. Trimmomatic (version 0.32) and FLASH were used for quality filtering, trimming, and merging of raw reads to generate high-quality sequences. Non-redundant sequences were clustered into operational taxonomic units (OTUs) at a 97% similarity threshold using UPARSE software (version 7.0.1001). Taxonomic annotation of the OTUs was conducted using the RDP classifier (version 2.2) against the bacterial 16S rRNA SILVA database and the fungal ITS UNITE database.
2.4. Statistical Analysis
Differences in soil physicochemical properties were analyzed using a one-way analysis of variance (ANOVA) followed by Tukey’s HSD test in R version 4.5.0. The assumptions of normality and homogeneity of variances, verified by the Shapiro–Wilk and Levene’s tests, respectively, were met after log-transforming the data (p > 0.05). Microbial co-occurrence networks were constructed to quantify the dynamics of soil microbial communities across saline–alkaline degraded soils. Microbial networks were visualized using Gephi software (version 0.10.1) and key topological properties. These included edges, average degree, network density, modularity, average clustering coefficient, average path length, and proportions of positive/negative correlations. An online microbiome analysis platform (www.cloudtutu.com.cn, accessed on 5 September 2025) was used to calculate soil microbial α- and β-diversity indices (Shannon, Simpson, NMDS, and ANOSIM), and to conduct intergroup correlation analyses and Mantel tests between microbial communities and soil physicochemical properties or P fractions. The “plspm” package in R 4.5.0 was used to construct a PLS-PM model and explore the effects and relative contributions of soil physicochemical properties and microbial communities on soil P fractions. The PLS-PM model was constructed to examine the direct and indirect effects of pH and EC on P fractions. We hypothesized that soil pH and EC are the primary abiotic drivers that directly shape bacterial and fungal communities, which in turn function as the key biological pathways regulating the transformation of distinct soil phosphorus pools. In structural equation modeling, observed (manifest) variables are directly measured quantities, while latent variables represent underlying constructs inferred from multiple indicators. In the PLS-PM model, pH, EC, and P fractions were the observed variables, whereas bacterial and fungal communities were the latent variables, each represented by a composite of abundance and alpha-diversity indices. The PLS-PM model robustness was assessed via goodness-of-fit (GOF); R2 values represent the proportion of each variable’s explained variance. Mapping of soil P fractions was performed using Origin 2022 software.
3. Results
3.1. Basic Soil Properties
The results showed that the physicochemical properties of the soils varied significantly across different salinity-alkalinity degradation levels (Table 1). Across the three saline-alkaline soils, pH, EC, TN, AN, and AP exhibited significant differences (p < 0.05), whereas TP did not (p > 0.05). Compared with the lightly degraded (LD) soil, the moderately degraded (MD) and severely degraded (SD) soils showed EC increases of 382.69% and 827.88%, respectively. Soil pH increased progressively with intensifying salinity–alkalinity degradation, averaging 9.43 ± 0.12 in LD soils and exceeding 10 in both MD and SD soils. Soil nutrient contents (SOC and TN) declined as degradation intensified; however, the soil C/N ratio did not differ significantly among the three gradients (p > 0.05).

Table 1.
Physical and chemical properties of soils with different degradation gradients.
3.2. Characteristics of Soil P Fractions
Among the three saline-alkaline soils, labile P constituted the smallest proportion (3.87–8.95%). Moderately labile P and stable P accounted for 40.74–44.79% and 46.26–55.39%, respectively. The proportions of both labile P and moderately labile P were the highest in LD soils, whereas the stable P fraction was the lowest. Conversely, SD soils had the highest stable P content but the lowest labile P and moderately labile P contents. Inorganic P, comprising 53.58% of the total P, was more abundant than organic P (46.42%) across all soils. Among all P fractions, D. HCl-Pi (classified as moderately labile P) and Residual-Pt (classified as stable P) were dominant. The concentration of D. HCl-Pi decreased with increasing soil salinity and alkalinity, whereas that of Residual-Pt increased as soil degradation intensified (Figure 1).
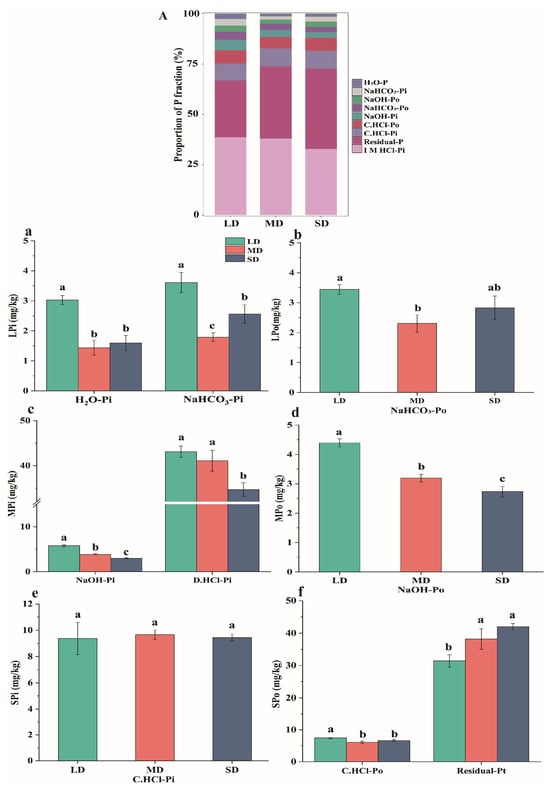
Figure 1.
Accumulation of P fractions in soils from different salt-affected lands (A). The contents of individual P fractions were: (a) Labile inorganic P (LPi), (b) labile organic P (LPo), (c) moderately labile inorganic P (MPi), (d) moderately labile organic P (MPo), (e) stable inorganic P (SPi), and (f) stable organic P (SPo). H2O-Pi, Deionized water-extracted inorganic P; NaHCO3-Pi/Po, bicarbonate-extracted inorganic/organic P; NaOH-Pi/Po, hydroxide-extracted inorganic/organic P; D.HCl-Pi, dilute HCl-extractable inorganic P; C.HCl-Pi/Po: concentrated hydrochloric acid-extracted inorganic/organic P; Residual-Pt: residual P. Different lowercase letters indicate significant differences among saline–alkali degraded soils (p < 0.05).
3.3. Microbial Community Composition
Across the three saline–alkaline soils with contrasting degradation levels, 4388 bacterial OTUs were recovered, and the bacterial assemblage was classified into 29 phyla, 71 classes, 133 orders, 196 families, and 329 genera (Figure 2). The nine dominant phyla—Pseudomonadota, Actinomycetota, Acidobacteriota, Bacteroidota, Bacillota, Chloroflexota, Gemmatimonadota, Cyanobacteriota, and Deinococcota collectively accounted for 97.98–99.27% of the sequences in each sample. Pseudomonadota and Actinomycetota were the most dominant, each with relative abundances > 30%. Along the salinization gradient, Acidobacteriota declined sharply from 9.13% under light degradation (LD) to 3.72% and 2.43% under moderate (MD) and severe degradation (SD), respectively, indicating high sensitivity to saline-alkaline stress. Conversely, the abundances of Bacteroidota, Bacillota, Gemmatimonadota, and Cyanobacteriota increased continuously with increasing stress; their proportions in SD soils were 2.95-, 2.05-, 1.76-, and 3.02-fold higher than those in LD soils, demonstrating typical halo-alkali-tolerant traits. Within the same degradation level, results from the six regional sampling sites exhibited consistent trends, indicating that the successional pattern was primarily driven by the degree of degradation.
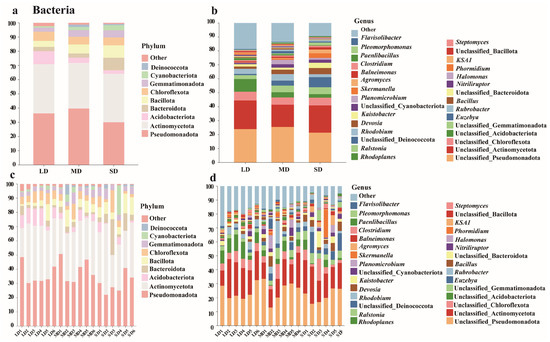
Figure 2.
Bacterial community composition in saline-alkali soils at the phylum (a) and genus (b) levels (averaged across sites for each land type), and across different sites at the phylum (c) and genus (d) levels.
A total of 946 fungal OTUs were obtained across the three saline–alkaline soils with contrasting degradation levels. Taxonomic annotation assigned the sequences to 350 species, 260 genera, 144 families, 77 orders, 35 classes, and 13 phyla (Figure 3). At the phylum level, Ascomycota, Basidiomycota, and Chytridiomycota were the dominant groups (mean relative abundance > 1%), jointly accounting for 77.07%, 93.32%, and 94.87% of all sequences in the lightly (LD), moderately (MD), and severely (SD) degraded soils, respectively. Ascomycota was the most abundant phylum, representing 67.70%, 85.38%, and 68.77% of reads under LD, MD, and SD conditions, respectively. Mortierellomycota maintained a relative abundance of 7.69% only in the low-salinity LD soils; however, its proportion dropped below 1% in both MD and SD soils, indicating high sensitivity to elevated salinity and alkalinity. Conversely, Chytridiomycota was markedly enriched in the severely degraded SD soils, reaching 20.42% of the total sequences, approximately 10-fold higher than in the LD and MD soils, making it the most indicative fungal phylum under extreme saline-alkaline stress.
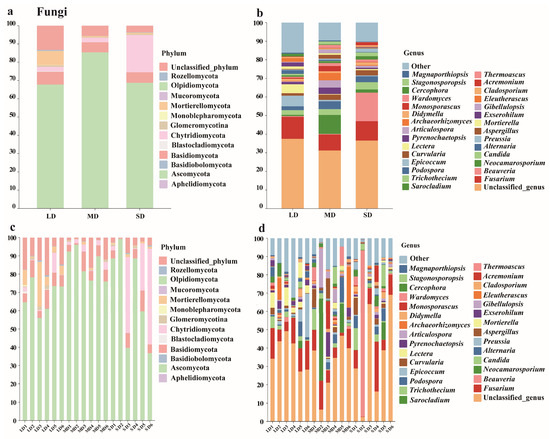
Figure 3.
Fungal community composition at the phylum (a) and genus (b) levels in saline-alkali soils (averaged across sites for each land type), and across different sites at the phylum (c) and genus (d) levels.
3.4. Alpha and Beta Diversity of Microbial Communities
The intensification of soil degradation exerted a significant impact on the α-diversity of both bacteria and fungi in the Songnen Plain saline-alkali soils (Figure 4). Bacterial diversity indices were higher than those of fungi. As saline-alkali degradation intensified, the Shannon and Simpson indices of both bacterial and fungal communities decreased significantly (p < 0.05). Compared to the LD grassland, the SD bare land exhibited decreases of 20.87% and 2.00% in bacterial Shannon and Simpson indices, respectively, and decreases of 27.46% and 21.28% in fungal Shannon and Simpson indices, respectively.
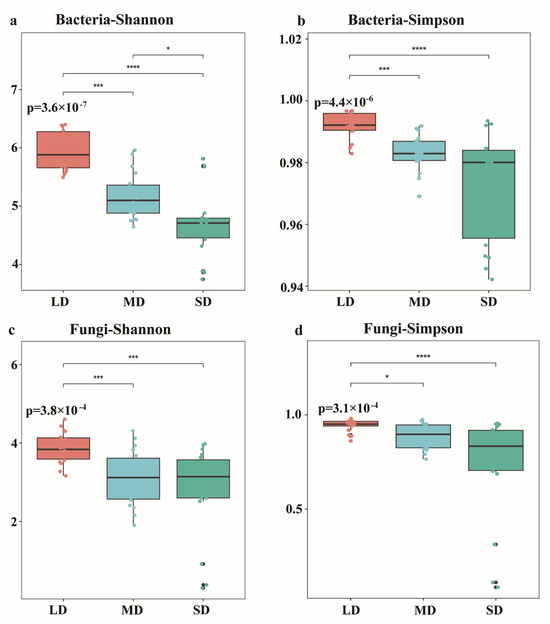
Figure 4.
The Shannon index (a) and the Simpson index (b) of bacteria in different salt-alkali land. The Shannon index (c) and the Simpson index (d) of fungi in different salt-alkali land. P-values on each panel represent overall significance among groups (p < 0.05). Lines with asterisks (*) denote pairwise comparisons between groups. A single asterisk indicates a significant difference (p < 0.05), with further significance levels defined as follows: * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001.
NMDS and ANOSIM analyses jointly demonstrated that the soil salinity gradient significantly influenced the divergence of microbial community structure (Figure 5). For bacteria, NMDS (stress = 0.104) showed a clear separation among the LD, MD, and SD salinity zones along the first axis, and ANOSIM further confirmed significant intergroup differences (R = 0.496, P = 0.001), indicating a strong salinity-induced filtering effect on bacterial communities. Fungal NMDS (stress = 0.1926) showed a more moderate response to salinity, with weaker separation than that observed in bacteria. Overall, salinity exerted a more pronounced influence on the bacterial community structure, whereas fungal communities displayed a weaker response, reflecting the distinct ecological adaptations of the two microbial groups under salt-stress conditions.
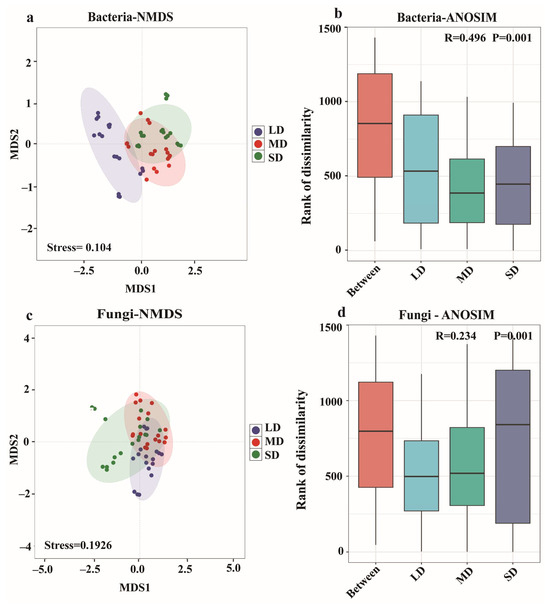
Figure 5.
Non-metric multidimensional scaling (NMDS) and analysis of similarities (ANOSIM) of bacterial (a,b) and fungal (c,d) communities in saline-alkali soils.
3.5. Co-Occurrence Network of Microbial Communities
Network topology analysis revealed significant structural differentiation in both bacterial and fungal co-occurrence networks along the gradient of saline-alkali land degradation (Figure 6, Table 2). The bacterial network at the LD site exhibited the highest complexity, with relatively high values for edge number, average degree, network density, and clustering coefficient, whereas the network at the MD site exhibited lower complexity. Betweenness centrality is a robust topological metric that identifies microbes acting as critical bridges for information or material flow, underscoring their pivotal role in maintaining community stability. Betweenness centrality of Actinobacteria and Pseudomonadota increased with salinization-alkalization degradation, while Gemmatimonadota remains relatively stable.
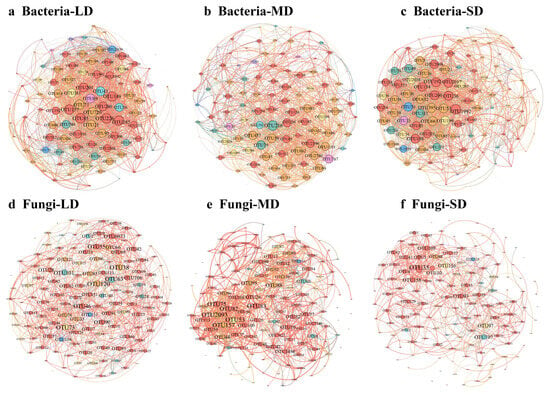
Figure 6.
Co-occurrence networks of bacterial and fungal community in saline-alkali soils with different degrees of degradation. (a–c) represent bacterial networks, and (d–f) represent fungal networks. Nodes represent OTUs, and edges represent correlations between OTUs with Spearman’s r > 0.8 and p < 0.05. Different node colors represent different phyla. The size of the nodes was proportional to the number of connected edges.

Table 2.
Network topological of bacterial and fungal community in saline-alkali soils with different degrees of degradation.
For fungal networks, edges, average degree, and network density consistently decreased with increasing degradation, indicating a decline in stability of the fungal network along the degradation gradient. The average path length remained relatively short and stable in bacterial networks (ranging between 1.91 and 2.11), whereas it increased markedly in fungal networks from 2.47 (LD) to 3.51 (SD). This suggested that fungal networks became progressively more dispersed and loosely connected under degradation. The proportion of positive correlations initially decreased and then increased in bacterial networks but increased consistently in fungal networks, reaching 88.17% under SD conditions. Negative correlations show the opposite pattern. Network topology analysis revealed that Ascomycota and Basidiomycota function as the highest connectivity hubs (with the highest betweenness centrality values) in the fungal network. The hub status of Mortierellomycota and Chytridiomycota changed significantly, with Mortierellomycota decreasing sharply from 792 (LD) to 290 (SD) and Chytridiomycota increasing markedly from 168 (LD) to 816 (SD).
3.6. Effects of Soil Physicochemical Properties and Microbial Communities on P Fractions
In the bacterial community, Bacteroidota, Bacillota, Gemmatimonadota, and Deinococcota were significantly and positively correlated with pH and EC (R = 0.27–0.44, p < 0.05). Among them, Bacteroidota and Bacillota were significantly negatively correlated with moderately labile P (R = −0.36–-0.33, p < 0.05). Acidobacteriota showed a significant negative correlation with pH and EC (R = −0.78, p < 0.001) and a significant positive correlation with MP, SOC, and TN (R = 0.32–0.49, p < 0.05). In the fungal community, Rozellomycota was significantly negatively correlated with pH (R = −0.63, p < 0.001), whereas Glomeromycotina was significantly negatively correlated with both pH and EC (R = −55–−0.67, p < 0.05).
Mantel tests showed that bacterial abundance was significantly positively correlated with EC, SOC, TN, AN, and AK (R = 0.10–0.29, p < 0.05). Bacterial α-diversity was also significantly positively correlated with pH, EC, SOC, and TN (R = 0.17–0.55, p < 0.001). Fungal abundance was significantly positively correlated with EC (R = 0.17, p < 0.01), whereas fungal α-diversity was significantly positively correlated with EC and AP (R = 0.17–0.20, p < 0.05). Additionally, pH was significantly negatively correlated with labile P, moderately labile P, SOC, TN, and AK (R = −0.317–−0.493, p < 0.05), and significantly positively correlated with stable P and EC (R = 0.31–0.56, p < 0.05). EC showed significant negative correlations with SOC, TN, and AK (R = −0.277–-0.585, p < 0.05), and significant positive correlations with AP (R = 0.352, p < 0.01).
To further investigate the interactions among soil pH, EC, microorganisms, and various P fractions, a partial least squares path model (PLS-PM) was constructed in this study (Figure 7). The overall goodness-of-fit of the model was 0.5253, and the results demonstrated that pH and EC influenced the transformation of soil P fractions both directly and indirectly through microbial mediation. Bacteria (R2 = 0.66) and fungi (R2 = 0.48) were identified as key microbial drivers, both exerting significant negative effects on soil labile P. Specifically, labile P was significantly negatively affected by pH (path coefficient: −0.60, p < 0.01). The total effects analysis revealed that the overall effect of bacteria on P fractions (0.92) was greater than that of fungi (−0.78). Among the different P components, moderately labile P and labile P were subject to relatively strong negative total effects (−1.21 and −1.096, respectively), whereas stable P had a relatively small positive total effect (0.746).
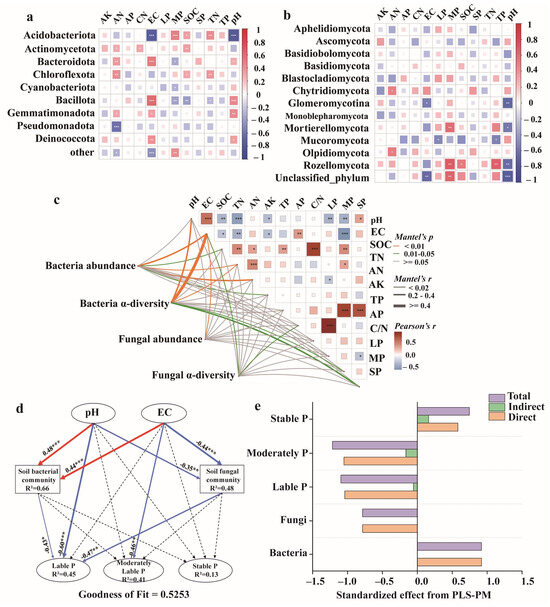
Figure 7.
Correlation heat map between soil physicochemical properties, P fractions and bacterial (a) and fungal (b) community (phyla level). Mantel test of soil physicochemical properties, P fractions, microbial abundance and alpha-diversity (c). The width of the connecting lines corresponds to the absolute value of Mantel’s r statistic, with orange, green, and gray lines indicating significance levels of p < 0.01, 0.01 ≤ p < 0.05, and p ≥ 0.05, respectively. Partial least squares path modeling (PLS-PM) with the direct and indirect effects of soil properties and microbial communities on soil P fractions (d). Standardized direct, indirect, and total effects of each factor on soil P fractions from PLS-PM (e). Path coefficients are represented by arrow thickness, with solid arrows indicating significant paths (p < 0.05) and dashed arrows indicating non-significant paths (p > 0.05). Arrow color denotes positive (red) and negative (blue) effects. ∗, ∗∗, and ∗∗∗ represent significance levels at p < 0.05, p < 0.01, and p < 0.001, respectively. R2 values indicate the proportion of variance explained.
4. Discussion
4.1. Effects of Saline-Alkaline Degradation on Soil Properties and P Availability
The progressive degradation gradient observed in the Songnen Plain, from grassland (LD) to Suaeda salsa (MD) and ultimately to bare land (SD), is driven by a combination of natural and anthropogenic factors. These include soil parent material, constrained hydrological conditions, overgrazing, and climate change, with increase aridity serving as a primary catalyst for the salinization, sodification, and desertification processes [,]. This shift in vegetation is closely associated with the deterioration of soil physicochemical properties. As degradation intensified, the EC of SD increased by 827.88% relative to LD, and soil pH rose correspondingly from 9.43 to 10.50. This pattern is consistent with observations from many salt-affected regions worldwide [], underscoring salt accumulation and alkalization as the defining characteristics of saline-sodic degradation. The degradation of saline-alkali land also leads to a reduction in soil nutrients. In our study, the LD site, covered by natural grassland, exhibited significantly higher SOC than the unvegetated SD site. SOC primarily originates from litter, roots, plant residues, and root exudates and is influenced by vegetation, land use, and species composition in management practices []. The reduction in vegetation cover shifts the dominant pathway of soil moisture loss from plant transpiration to surface evaporation, hereby promoting upward salt movement and surface crusting. Therefore, vegetation degradation in saline-alkali lands leads to fertility loss and initiates a positive feedback cycle in the ecosystem [].
Although TP concentrations did not differ significantly across the degradation gradient, AP, which is directly accessible to plants, was substantially lower in the MD and SD soils than in LD soils. During saline-alkaline soil degradation, soil P fractions shifted significantly from labile to stable forms, with the proportion of labile P decreasing from 8.95% under light degradation to 3.87% under severe degradation, whereas the stable P fraction increased by approximately 9%. Under saline-alkali stress, the capacity of vegetation to activate P through root-microbe interactions declines sharply along the degradation gradient. Healthy grasslands can enhance P availability through root secretion of organic acids and phosphatases, as well as the recruitment of symbiotic P-solubilizing microorganisms that directly mobilize fixed P []. As vegetation degrades, this positive biological regulation weakens and the soil P pool becomes increasingly governed by the physicochemical processes of the highly saline-alkaline environment. High pH promotes the precipitation of phosphate with Ca2+ and Mg2+, whereas the elevated ionic strength and competitive adsorption induced by salinity further reduce AP levels []. Consequently, although the TP reservoir remained relatively stable, its bioavailability was substantially lost during degradation.
4.2. Effects of Saline-Alkaline Degradation on Microbial Communities
Changes in soil microbial abundance and community structure are key biological indicators of saline-alkaline habitats [,]. In the present study, α-diversity of both bacteria and fungi declined significantly as salinization intensified, consistent with findings from other studies []. Salinity and pH are critical environmental filtering factors that eliminate a large number of microbial taxa sensitive to these conditions while selecting taxa with specific salt-alkali tolerance mechanisms [,]. Network topology analysis reveals that under intensifying saline-alkaline stress, the microbial community reorganizes its interaction network by elevating Actinobacteria and Pseudomonadota to keystone connectors, while depending on Gemmatimonadota to maintain topological stability. The abundance of Bacteroidota, Bacillota, and Gemmatimonadota increased significantly with saline-alkali degradation. These salt-tolerant bacteria typically possess more flexible cell membrane structures and osmoregulatory mechanisms to adapt to highly osmotic environments []. In contrast, the abundance of Acidobacteriota decreased sharply with increasing salinity, indicating high sensitivity to saline-alkali stress. Acidobacteriota and Bacillota are recognized as disease-suppressive microorganisms in the environment []. Our study showed that Bacillota have strong tolerance to saline-alkali conditions. The two most abundant genera, Bacillus and Paenibacillus, can survive and function under saline-alkali stress, highlighting their potential as bioinoculants for saline-alkaline soil improvement. Furthermore, bacterial co-occurrence networks reveal microbial survival strategies under stress. The increased proportion of positive correlations in bacterial networks under severe degradation indicates a stress-induced cooperative survival strategy. Such a strategy has been documented in various stressed ecosystems and is often interpreted as microorganisms sharing resources and engaging in cross-feeding to collectively cope with environmental stress, rather than performing specific nutrient cycling functions [,]. Our data support this interpretation, as the networks under intensified degradation were significantly enriched with halotolerant phyla such as Bacteroidota and Bacillota, which showed significant negative correlations (p < 0.05) with moderately labile phosphorus fractions. This suggests that microbial taxa selected by the stressful environment may adopt ecological strategies prioritizing survival—such as coping with high osmotic pressure—over traditional phosphorus mobilization functions.
In fungal communities, intensifying saline-alkaline degradation led to a marked functional attenuation and network simplification. Ascomycota, though dominant and recognized as the primary phylum with the highest abundance of the phosphate-solubilizing microbial gene phoD, maintains its prevalence through multifunctionality (decomposition, stress tolerance, and cooperation) rather than specialized phosphorus mobilization [,]. The relative abundance of Ascomycota peaked at 85.38% under moderate degradation but declined to 68.77% under extreme saline-alkaline stress, indicating that its metabolic functionality was also constrained by severe stress. Network topology analysis revealed that Ascomycota and Basidiomycota function as stable core hubs, maintaining the fundamental structure and function of the network. The replacement of Mortierellomycota by Chytridiomycota as key hubs, supported by abundance data, directly impaired the fungal community’s phosphorus transformation capacity. From an interaction perspective, topological metrics of fungal co-occurrence networks (e.g., edge number, average degree, network density) decrease continuously along the degradation gradient, whereas average path length increases significantly, reflecting looser network structures and reduced connectivity efficiency. Notably, while the overall fungal network tended to simplify, the proportion of positive correlations within it consistently increased from 70.67% to 88.17%. This enhanced cooperation does not represent functional optimization, but rather a passive and fragile survival response formed by stress-tolerant fungi during systemic degradation []. However, the cooperative strategy of fungi failed to reverse their overall functional decline. The sparsity and structural fragility of the network undermined the overall ecological functions of fungi, including their capacity for phosphorus mobilization, thereby exacerbating phosphorus availability limitations in the saline-alkaline ecosystem.
4.3. Microbial Community Driving P Dynamics in Saline-Alkaline Soils
Under saline-alkaline stress, the restructured microbial community structure directly influenced the processes of P mineralization, solubilization, and immobilization. Both Mantel tests and PLS-PM were used to reveal the relationships among soil properties, microorganisms, and P cycling. The Mantel test revealed complex relationships between environmental factors and microbial communities. The abundance and diversity of bacterial communities showed significant associations with a wide range of environmental factors (such as pH, EC, SOC, TN, AN, moderately active P, and stable P). In contrast, fungal communities were only significantly correlated with a limited number of factors (EC and AP), and these associations were weak (R < 0.2). These results indicate that the associations between bacterial communities and environmental factors are more extensive than those of fungi. Furthermore, the significant correlation of bacteria with multiple soil nutrient indicators suggests they may play a more important role than fungi in nutrient cycling within the studied saline-alkaline soils. The PLS-PM results indicated that the total standardized effect of bacteria on P fractions (0.92) exceeded that of fungi (−0.78), further emphasizing the dominant role of bacteria in driving P cycling in saline-alkaline soils. Previous studies have shown that the main microbial phyla contributing to soil P cycling include Acidobacteriota, Pseudomonadota, and Actinomycetota in bacteria, and Ascomycota, Basidiomycota, and Blastocladiomycota in fungi [,]. In our study, the abundance of both Acidobacteriota and Mortierellomycota, which were significantly positively correlated with MP, declined markedly with degradation.
Our results demonstrate that the shift in microbial community composition directly mediates the transformation of soil P towards more stable forms through several interconnected mechanisms. Firstly, the significant decline in the relative abundance of Acidobacteriota and Mortierellomycota—taxa frequently reported as key actors in organic P mineralization and inorganic P solubilization [,]—implies a substantial reduction in the microbial potential for P mobilization. The enrichment of halotolerant taxa (e.g., Bacteroidota, Bacillota), while advantageous for survival under stress, does not necessarily compensate for this functional loss, as these groups may prioritize osmoregulation over P-solubilizing metabolism. This functional shift is further evidenced by the significant negative correlations observed between these bacterial phyla and moderately labile phosphorus fractions.
The PLS-PM model indicated that both bacteria and fungi had negative effects on LP under saline-alkaline stress. Under these conditions, microorganisms widely adopt a ‘compatible solute strategy’ to balance osmotic pressure, allocating more energy to maintain cellular physicochemical integrity in response to salinity changes []. Although certain saline-alkaline-tolerant microbial taxa may survive or even become enriched, overall microbial biomass and metabolic activity are suppressed, resulting in a reduced capacity for organic P mineralization. Soil pH and EC are key regulators of microbial community distribution, influencing the dynamics and ecological processes of both bacteria and fungi across various saline-alkaline soils [,]. Chemically, high soil pH promotes the formation of insoluble phosphates through interactions between Na+, Ca2+, Mg2+, and phosphate ions, leading to the fixation of inorganic forms such as Na-P, Ca-P, and Mg-P, and a consequent reduction in the labile inorganic P pool [,]. Biologically, high EC suppresses microbial alkaline phosphatase activity and reduces the abundance of P-cycling microorganisms, retarding Po mineralization and leading to a relative accumulation of Po [,,]. Therefore, management strategies should focus on reducing soil pH and EC to break the chemical fixation of P and create favorable conditions for restoring bacterial communities. Since the dissolution of inorganic P and the mineralization of organic P are two key processes governing soil P bioavailability [], their simultaneous suppression under saline-alkaline stress leads to a synchronized decline in both chemical effectiveness and biological availability, ultimately resulting in an increase in the stable P pool. Furthermore, Residual-Pt, serving as an “inert P pool”, showed a marked increase in saline-alkaline soils, a trend that was further exacerbated in severely degraded bare lands due to the absence of mucilage and organic acids secreted by plant roots [,].
5. Conclusions
This study demonstrates that, during saline-alkaline degradation in the Songnen Plain, persistent saline-alkaline stress—manifested as increased pH and EC—directly enhances the chemical fixation of phosphorus in the soil. Simultaneously, it acts as an environmental filter that eliminates key phosphate-solubilizing microbial taxa such as Acidobacteriota and Mortierellomycota, thereby suppressing organic P mineralization and inorganic P solubilization capacity. This directly drives the transformation of the soil P pool from labile to stable forms, exacerbating P limitation. Microbial co-occurrence network analysis revealed that bacterial communities adopted a survival strategy shifting from competition to cooperation under stress, whereas fungal networks exhibited progressively reduced complexity and stability alongside significant functional attenuation as degradation intensified. The PLS-PM model and Mantel test results further confirmed that bacteria play a more critical and dominant role than fungi in driving phosphorus cycling.
The findings of this study provide clear implications for the remediation of saline-alkaline soils. Primarily, management strategies should focus on reducing soil pH and EC—for instance, through the application of amendments like phosphogypsum, organic fertilizers, or acidic conditioners—to directly neutralize alkalinity and displace sodium ions, thereby breaking the chemical fixation of P and creating favorable conditions for the restoration of bacterial communities. Secondly, during vegetation restoration, targeted inoculation with functional, salt-alkali-tolerant phosphate-solubilizing bacterial agents (e.g., Ascomycota, Pseudomonadota, and Actinomycetota) should be considered to directly enhance the P activation capacity of the soil ecosystem. This research offers a scientific basis for understanding the causes of P limitation in saline-alkaline ecosystems and developing targeted bioremediation strategies from the novel perspective of coupling microbial network evolution with P transformation.
Author Contributions
All authors conceived and designed the experiments; Z.T., L.T. and L.J. performed the experiments; Z.T., X.J., J.C. and C.C. analyzed the data and wrote the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by National Key Research and Development Program of China (E41AS207), Fundamental Research Program of Shanxi Province (20210302124154, 202303021222215, 202203021212178).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data and materials will be made available on from the corresponding author upon reasonable request.
Conflicts of Interest
All authors have no conflicts of interest with respect to any other parties.
Appendix A

Table A1.
Geographic location information of sampling points.
Table A1.
Geographic location information of sampling points.
| Sampling Site | Latitude (°) | Longitude (°) |
|---|---|---|
| Yujia | 45°25′33″ | 124°08′17″ |
| Qian’an | 45°19′46″ | 124°00′6″ |
| Dagangzi | 45°03′14″ | 123°56′56″ |
| San Tuanxiang | 44°34′57″ | 124°05′25″ |
| Chaganhua | 44°23′49″ | 123°43′20″ |
| Shuang Liao | 44°09′48″ | 123°54′44″ |
References
- Wang, J.; Ding, J.; Wang, Y.; Ge, X.; Lizaga, I.; Chen, X. Soil salinization in drylands: Measure, monitor, and manage. Ecol. Indic. 2025, 175, 113608. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2023: Synthesis Report. Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2003.
- Amin, A. Incubation time effect on releasing available phosphorus in saline sandy soil as a function of bone char application. Sci. Rep. 2025, 15, 29491. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. Global Status of Salt-Affected Soils—Main Report; Food and Agriculture Organization of the United Nations: Rome, Italy, 2004. [CrossRef]
- Hassani, A.; Azapagic, A.; Shokri, N. Global predictions of primary soil salinization under changing climate in the 21st century. Nat. Commun. 2021, 12, 6663. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Liu, Y.; Ferreira, J.F.; Wang, M.; Na, J.; Huang, J.; Liang, Z. Long-term combined effects of tillage and rice cultivation with phosphogypsum or farmyard manure on the concentration of salts, minerals, and heavy metals of saline-sodic paddy fields in Northeast China. Soil Tillage Res. 2022, 215, 105222. [Google Scholar] [CrossRef]
- Yang, F.; An, F.; Ma, H.; Wang, Z.; Zhou, X.; Liu, Z. Variations on soil salinity and sodicity and its driving factors analysis under Microtopography in different hydrological conditions. Water 2016, 8, 227. [Google Scholar] [CrossRef]
- Costantini, E.; Branquinho, C.; Nunes, A.; Schwilch, G.; Stavi, I.; Valdecantos, A.; Zucca, C. Soil indicators to assess the effectiveness of restoration strategies in dryland ecosystems. Solid Earth 2016, 7, 397–414. [Google Scholar] [CrossRef]
- Dey, G.; Banerjee, P.; Sharma, R.K.; Maity, J.P.; Etesami, H.; Shaw, A.K.; Huang, Y.; Huang, H.; Chen, C. Management of Phosphorus in Salinity-Stressed Agriculture for Sustainable Crop Production by Salt-Tolerant Phosphate-Solubilizing Bacteria—A Review. Agronomy 2021, 11, 1552. [Google Scholar] [CrossRef]
- Tiessen, H.; Moir, J.O. Characterization of Available P by Sequential Extraction. In Soil Sampling and Methods of Analysis; Canadian Society of Soil Science: Boca Raton, FL, USA, 2008. [Google Scholar]
- Maharjan, M.; Maranguit, D.; Kuzyakov, Y. Phosphorus fractions in subtropical soils depending on land use. Eur. J. Soil Biol. 2018, 87, 17–24. [Google Scholar] [CrossRef]
- Niederberger, J.; Kohler, M.; Bauhus, J. Distribution of phosphorus fractions with different plant availability in German forest soils and their relationship with common soil properties and foliar P contents. Soil 2019, 5, 189–204. [Google Scholar] [CrossRef]
- Zhao, X.; Gao, J.; Yu, X.; Borjigin, Q.; Qu, J.; Zhang, B.; Zhang, S.; Li, Q.; Guo, J.; Li, D. Evaluation of the microbial community in various saline alkaline-soils driven by soil factors of the Hetao Plain, Inner Mongolia. Sci. Rep. 2024, 14, 28931. [Google Scholar] [CrossRef]
- Hu, J.; Huang, Y.; Zhou, D. Phosphorus fractions affect fungal compositions and functions under land use conversions in saline-alkali soil in northeastern China. Chem. Biol. Technol. Agric. 2024, 11, 56. [Google Scholar] [CrossRef]
- Hallam, S.; Putnam, N.; Preston, C.; Detter, J.; Rokhsar, D.; Richardson, P.; DeLong, E. Reverse methanogenesis: Testing the hypothesis with environmental genomics. Science 2004, 305, 1457–1462. [Google Scholar] [CrossRef]
- Bergkemper, F.; Schöler, A.; Engel, M.; Lang, F.; Krüger, J.; Schloter, M.; Schulz, S. Phosphorus Depletion in Forest Soils Shapes Bacterial Communities Towards Phosphorus Recycling Systems. Environ. Microbiol. 2016, 8, 2767. [Google Scholar] [CrossRef]
- Dai, Z.; Liu, G.; Chen, H.; Chen, C.; Wang, J.; Ai, S.; Wei, D.; Li, D.; Ma, B.; Brookes, P. Long-Term Nutrient Inputs Shift Soil Microbial Functional Profiles of Phosphorus Cycling in Diverse Agroecosystems. ISME J. 2020, 14, 757–770. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Singh, B.; Singh, R. Effect of land rehabilitation on physicochemical and microbial properties of a sodic soil. Catena 2013, 109, 49–57. [Google Scholar] [CrossRef]
- Khan, A.; Jilani, G.; Akhtar, M.; Naqvi, S.; Rasheed, M. Phosphorus solubilizing bacteria: Occurrence, mechanisms and their role in crop production. J. Agric. Biol. Sci. 2009, 1, 48–58. [Google Scholar]
- Lozupone, C.; Knight, R. Global patterns in bacterial diversity. Proc. Natl. Acad. Sci. USA 2007, 104, 11436–11440. [Google Scholar] [CrossRef]
- Shen, W.; Ni, Y.; Gao, N.; Bian, B.; Zheng, S.; Lin, X.; Chu, H. Bacterial community composition is shaped by soil secondary salinization and acidification brought on by high nitrogen fertilization rates. Appl. Soil Ecol. 2016, 108, 76–83. [Google Scholar] [CrossRef]
- Wang, S.; Sun, L.; Ling, N.; Zhu, C.; Chi, F.; Li, W.; Hao, X.; Zhang, W.; Bian, J.; Chen, L.; et al. Exploring soil factors determining composition and structure of the bacterial communities in saline-alkali soils of Songnen Plain. Front. Microbiol. 2020, 10, 2902. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, S.; Li, Y.; Bu, K.; Zhang, Y.; Chang, L.; Zhang, Y. Dynamics of saline-alkali land and its ecological regionalization in western Songnen plain, China. Chin. Geogr. Sci. 2010, 20, 159–166. [Google Scholar] [CrossRef]
- Yu, H.; Wang, Z.; Mao, D.; Jia, M.; Chang, S.; Li, X. Spatiotemporal variations of soil salinization in China’s West Songnen Plain. Land Degrad. Dev. 2023, 34, 2366–2378. [Google Scholar] [CrossRef]
- Li, Q.; Yu, P.; Chen, X.; Zhou, D.; Zheng, W. Facilitative and inhibitory effect of litter on seedling emergence and early growth of six herbaceous species in an early successional old field ecosystem. Sci. World J. 2014, 2014, 101860. [Google Scholar] [CrossRef]
- Jia, Y.; Wu, J.; Cheng, M.; Xia, X. Global transfer of salinization on irrigated land: Complex network and endogenous structure. J. Environ. Manag. 2023, 336, 117592. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Shao, T.; Zhu, T.; Long, X.; Gao, X.; Liu, Z.; Shao, H.; Rengel, Z. Vegetation succession influences soil carbon sequestration in coastal alkali-saline soils in southeast China. Sci. Rep. 2018, 8, 9728. [Google Scholar] [CrossRef] [PubMed]
- Mazhar, S.; Pellegrini, E.; Contin, M.; Bravo, C.; De Nobili, M. Impacts of salinization caused by sea level rise on the biological processes of coastal soils-a review. Front. Environ. Sci. 2022, 10, 909415. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, X.; Zhou, J.; Zhou, W.; Zhou, S. Construction of Phosphate-Solubilizing Microbial Consortium and Its Effect on the Remediation of Saline-Alkali Soil. Microb. Ecol. 2025, 88, 11. [Google Scholar] [CrossRef]
- Cornish, P. Research directions: Improving plant uptake of soil phosphorus, and reducing dependency on input of phosphorus fertiliser. Crop Pasture Sci. 2009, 60, 190–196. [Google Scholar] [CrossRef]
- Hemkemeyer, M.; Schwalb, S.; Heinze, S.; Joergensen, R.; Wichern, F. Functions of elements in soil microorganisms. Microbiol. Res. 2021, 252, 126832. [Google Scholar] [CrossRef]
- Singh, K. Microbial and Enzyme Activities of Saline and Sodic Soils. Land Degrad. Dev. 2016, 27, 706–718. [Google Scholar] [CrossRef]
- Zhao, S.; van der Heijden, M.; Banerjee, S.; Liu, J.; Gu, H.; Zhou, N.; Yin, C.; Peng, B.; Liu, X.; Wang, B.; et al. The role of halophyte-induced saline fertile islands in soil microbial biogeochemical cycling across arid ecosystems. Commun. Biol. 2024, 7, 1061. [Google Scholar] [CrossRef]
- Varshney, S.; Bhattacharya, A.; Gupta, A. Halo-alkaliphilic microbes as an effective tool for heavy metal pollution abatement and resource recovery: Challenges and future prospects. 3 Biotech 2023, 13, 400. [Google Scholar] [CrossRef]
- Mokashe, N.; Chaudhari, B.; Patil, U. Operative utility of salt-stable proteases of halophilic and halotolerant bacteria in the biotechnology sector. Int. J. Biol. Macromol. 2018, 117, 493–522. [Google Scholar] [CrossRef]
- Shen, Z.; Ruan, Y.; Chao, X.; Zhang, J.; Li, R.; Shen, Q. Rhizosphere microbial community manipulated by 2 years of consecutive biofertilizer application associated with banana Fusarium wilt disease suppression. Biol. Fertil. Soils. 2015, 51, 553–562. [Google Scholar] [CrossRef]
- Li, Y.; Li, W.; Jiang, L.; Li, E.; Yang, X.; Yang, J. Salinity affects microbial function genes related to nutrient cycling in arid regions. Front. Microbiol. 2024, 15, 1407760. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Pan, X.; Yu, W.; Ye, X.; Erdenebileg, E.; Wang, C.; Ma, L.; Wang, R.; Huang, Z.; Indree, T.; et al. Aridity and decreasing soil heterogeneity reduce microbial network complexity and stability in the semi-arid grasslands. Ecol. Indic. 2023, 151, 110342. [Google Scholar] [CrossRef]
- Manici, L.; Caputo, F.; De Sabata, D.; Fornasier, F. The enzyme patterns of Ascomycota and Basidiomycota fungi reveal their different functions in soil. Appl. Soil Ecol. 2024, 196, 105323. [Google Scholar] [CrossRef]
- Ragot, S.; Kertesz, M.; Bünemann, E. phoD Alkaline Phosphatase Gene Diversity in Soil. Appl. Environ. Microbiol. 2015, 81, 72819. [Google Scholar] [CrossRef]
- Wang, M.; Chen, S.; Chen, L.; Wang, D. Responses of soil microbial communities and their network interactions to saline-alkaline stress in cd-contaminated soils. Environ. Pollut. 2019, 252, 1609–1621. [Google Scholar] [CrossRef]
- Ma, D.; Wang, J.; Chen, K.; Lan, W.; Ye, Y.; Ma, X.; Lin, K. Responses of Soil Phosphorus Cycling-Related Microbial Genes to Thinning Intensity in Cunninghamia lanceolata Plantations. Forests 2024, 15, 440. [Google Scholar] [CrossRef]
- Tripathi, B.; Stegen, J.; Kim, M.; Dong, K.; Adams, J.; Lee, Y. Soil pH mediates the balance between stochastic and deterministic assembly of bacteria. ISME J. 2018, 12, 1072–1083. [Google Scholar] [CrossRef]
- Yang, C.; Sun, J. Soil Salinity Drives the Distribution Patterns and Ecological Functions of Fungi in Saline-Alkali Land in the Yellow River Delta, China. Front. Microbiol. 2020, 11, 594284. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, A.; Pahari, A.; Mohapatra, S.; Mishra, B. Phosphate-Solubilizing Microorganisms in Sustainable Agriculture: Genetic Mechanism and Application. In Advances in Soil Microbiology: Recent Trends and Future Prospects; Springer: Singapore, 2017; pp. 81–97. [Google Scholar] [CrossRef]
- Rietz, D.; Haynes, R. Effects of irrigation-induced salinity and sodicity on soil microbial activity. Soil Biol. Biochem. 2003, 35, 845–854. [Google Scholar] [CrossRef]
- Zhang, Y.; Cui, Z.; Cao, C. Effects of Secondary Salinization on Soil Phosphorus Fractions and Microbial Communities Related to Phosphorus Transformation in a Meadow Grassland, Northeast China. Agronomy 2025, 15, 960. [Google Scholar] [CrossRef]
- Richardson, A.; Simpson, R. Soil microorganisms mediating phosphorus availability update on microbial phosphorus. Plant Physiol. 2011, 156, 989–996. [Google Scholar] [CrossRef]
- Chatterjee, S.; Singh, R.; Sharma, P. Response of Major Plant Nutrients to Salt Affected Environment. Int. J. Curr. Microbiol. App. Sci. 2019, 8, 2188–2203. [Google Scholar] [CrossRef]
- Shen, J.; Yuan, L.; Zhang, J.; Li, H.; Bai, Z.; Chen, X.; Zhang, W.; Zhang, F. Phosphorus dynamics: From soil to plant. Plant Physiol. 2011, 156, 997–1005. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).