Abstract
This study systematically investigates the influence of CaO reactivity on the fresh-state rheological behavior and long-term mechanical performance of GGBFS binders. Four types of industrial quicklime, characterized by distinct slaking kinetics (tmax ranging from 9 to 36 min), were used as primary activators, with dosages of 5%, 9%, and 13% by binder mass. The results reveal that CaO reactivity significantly affects water demand, yield stress, and setting times, with extremely reactive quicklime inducing the greatest increases in water demand and plastic viscosity. Setting behavior was strongly dependent on both activator content and curing environment, with less reactive limes failing to induce setting under water immersion. Compressive strength development was largely unaffected by CaO reactivity up to 120 days, but at 720 days, higher CaO contents (≥9%) contributed to significant strength gains, likely due to carbonation-induced matrix densification. These findings demonstrate that controlling CaO reactivity and dosage is essential for optimizing the workability and durability of CaO-activated slag binders.
1. Introduction
The urgent need to reduce global greenhouse gas emissions and mitigate climate change has intensified efforts to develop sustainable alternatives to ordinary Portland cement (OPC), which remains one of the most widely used material in the construction industry. The cement industry is currently responsible for approximately 5–7% of total anthropogenic CO2 emissions and consumes 12–15% of the global industrial electricity supply each year [1]. These figures underscore the significant environmental burden associated with cement production, primarily stemming from the calcination of limestone and the high energy demands of clinker manufacturing. Therefore, identifying and implementing alternative binders with a substantially lower carbon footprint has become a critical priority for both the construction industry and environmental policy. Among various proposed solutions, alkali-activated materials (AAMs) represent a promising class of binders, offering a pathway to valorize industrial by-products such as ground granulated blast furnace slag (GGBFS) or fly ash (FA).
Ground granulated blast furnace slag (GGBFS), a by-product of the steel industry, is one of the most commonly employed precursors for alkali-activated binders owing to its high calcium and aluminosilicate content, resembling the chemical composition of Portland clinker. Activation of GGBFS is typically achieved using various alkaline compounds, including sodium hydroxide (NaOH), potassium hydroxide (KOH), sodium silicate (Na2O·rSiO2), sodium carbonate (Na2CO3), and calcium hydroxide (Ca(OH)2) [2,3]. Among these, NaOH and Na2O·rSiO2 solutions are regarded as highly effective activators, yielding binders with excellent mechanical performance and durability [4,5,6]. However, their practical application is hindered by significant drawbacks, such as high cost, handling and safety concerns, and the tendency to induce rapid setting, complicating workability and placement [7,8,9,10].
In contrast, calcium-based activators, such as calcium hydroxide, present an economically attractive and more user-friendly option. Nonetheless, GGBFS binders activated with Ca(OH)2 are often characterized by prolonged setting times, low early-age strength, and deterioration of reactivity over storage, which limits their practical use in construction applications [11,12].
In this context, CaO activation has emerged as a promising approach to overcome the limitations associated with Ca(OH)2 activation [13], by enhancing the hydration kinetics of GGBFS [14] and providing higher compressive strength [15,16], while remaining a cost-effective and sustainable alternative to conventional alkali activators such as NaOH and Na2O·rSiO2 solutions.
According to the most recent industrial assessments [17], the total cradle-to-gate greenhouse-gas emissions associated with quicklime production amount to approximately 0.95–1.10 t CO2 per t CaO, combining ≈ 0.79 t CO2 t−1 from limestone calcination and ≈ 0.20–0.30 t CO2 t−1 from fuel combustion. During service and end-of-life stages, lime products progressively reabsorb atmospheric CO2 through natural carbonation. A comprehensive meta-analysis by Campo et al. [18] indicates that, under typical exposure conditions, 23–33% of calcination emissions are reabsorbed during the life cycle of lime applications, with most uptake occurring within the first decade of use. Combining these data yields a net life-cycle footprint of approximately 0.70–0.80 t CO2 eq t−1 CaO, confirming the partial carbon-closing potential of the lime cycle.
The EuLA decarbonization roadmap [17] further identifies significant opportunities to reduce this footprint through technological innovation. The replacement of fossil-fuel-fired kilns with electric kilns powered by renewable energy could virtually eliminate combustion-related CO2 emissions, reducing the total footprint by about 20–30%. In parallel, the integration of carbon capture, utilization, and storage (CCUS) technologies is expected to address the process-related emissions from limestone calcination, which account for nearly 70–80% of the sector’s total CO2 output. Combined implementation of renewable electrification and CCUS could reduce the effective cradle-to-gate emissions of quicklime to below 0.2 t CO2 eq t−1 CaO, or potentially achieve net-zero performance when coupled with natural and enhanced recarbonation during the service life of lime-based products [17].
From an energy standpoint, the embodied energy of quicklime is relatively low, approximately 3.2–5.2 MJ kg−1 [19], which results from the thermal demand of limestone calcination and the efficiency of modern kiln systems. In contrast, NaOH requires substantially higher energy inputs of 20–25 MJ kg−1 [20], while Na2O·rSiO2 typically requires 11–13 MJ kg−1 [21].
Economically, CaO remains the most affordable alkali activator, with market prices of 150 EUR t−1 [22], compared with 250 EUR t−1 [23] for NaOH and 240 EUR t−1 for Na2O·rSiO2 [24]. In addition, CaO and Ca(OH)2 are easier to handle and less hazardous than concentrated sodium activators, while enabling partial CO2 reabsorption during the service life of the binder. These combined features, moderate energy demand, comparatively low cost, manageable alkalinity, and in-use carbonation potential, support the suitability of CaO activation as a technically and environmentally balanced route for producing sustainable alkali-activated GGBFS binders.
To date, research on CaO activation of GGBFS has primarily focused on evaluating the effect of auxiliary activators on the properties of CaO-activated GGBFS composites [14,25,26,27,28,29], as well as on investigating the influence of GGBFS characteristics on the performance of these composites [30,31]. However, the influence of quicklime characteristics themselves on the properties of CaO-activated GGBFS binders remains largely unexplored.
The physical and chemical properties of quicklime are inherently linked to both its chemical composition and the specific conditions applied during the calcination process. Among these, calcination temperature plays a critical role in determining key physical attributes, including particle size distribution, morphology, bulk density, specific surface area, and overall reactivity. As calcination temperature increases, several notable transformations occur: CaO crystallites enlarge, particle bulk density rises, while specific surface area and porosity are progressively reduced (Figure 1). These structural changes are often accompanied by visible alterations such as color shifts. Consequently, elevated calcination temperatures tend to diminish the reactivity of quicklime, which is essential in various binder activation processes. Depending on the temperature regime applied during production, quicklime is typically classified into four categories—soft-burnt, medium-burnt, hard-burnt, and dead-burnt—each characterized by distinct physical and reactive properties.
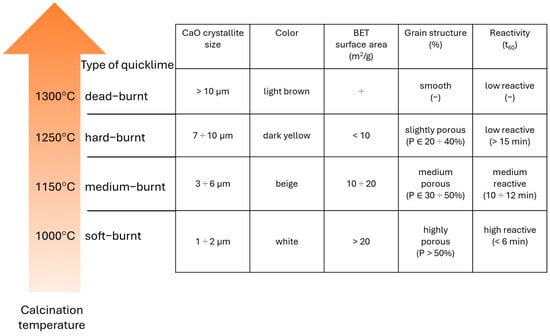
Figure 1.
The effect of calcination temperature of calcium lime on its physical properties. Specific surface area using the BET method based on [32], reactivity based on experimental results, and other properties based on [33,34].
On the European market, quicklime with varying properties is used, ranging from soft burned quicklime to medium and dead-burned. These types of quicklime are characterized by significantly different slaking reaction kinetics, allowing for different peak temperatures to be reached at different times. This study aims to investigate the impact of lime reactivity on both the fresh-state properties, including rheological and setting behavior, and the long-term strength development of CaO-activated composites.
The present investigation forms part of a broader research programme devoted to understanding the influence of CaO reactivity on the behavior of GGBFS-based binders. In addition to the rheological, setting and long-term strength development parameters discussed in this paper, the programme also examined early-age volumetric stability and microstructural development of pastes incorporating CaO of varying reactivity [35]. These studies confirmed that all tested formulations (5, 9, 13% CaO) fulfilled the volumetric stability requirements for cementitious binders and that deformation could be effectively regulated by adjusting the CaO reactivity and dosage. Microstructural observations revealed a predominantly amorphous C–S–H matrix containing numerous fine portlandite crystallites uniformly distributed within the binding phase. The morphology and chemical composition of the C–S–H phase were largely unaffected by CaO reactivity, with Ca/Si ratios typically ranging between 0.9 and 1.5.
2. Experimental Framework
The aim of the conducted research was to establish the relationship between the hydration reactivity of quicklime and its content in the binder, and the fresh properties and long-term strength development of CaO-activated GGBFS composites. Additionally, the goal was to determine the differences and similarities between the process and effects of ground granulate blast furnace slag (GGBFS) activation using quicklime, compared to hydrated lime (CL 90-S according to PN-EN 459-1 [36]) and Portland cement (CEM I 42.5R according to PN-EN 197-1 [37]), which were adopted as reference alkaline activators for GGBFS.
The experimental program (Figure 2) was designed based on two independent variables: quicklime reactivity (four distinct types) and its dosage within the binder (three levels). The reactivity of quicklime was characterized by the time required to reach the maximum temperature (tₘₐₓ), determined in accordance with the procedure specified in PN-EN 459-2 [38]. The quicklime content was set at 5%, 9%, and 13% by mass of the binder.
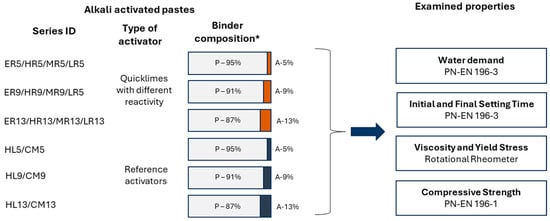
Figure 2.
Experimental framework (Acronyms: ER—extremely reactive, HR—highly reactive, MR—moderately reactive, LR—low reactive, HL—Hydrated Lime, CM—Portland Cement) * P—Precursor, A—Activator.
This study examined the fresh and hardened properties of CaO-activated GGBFS binders, including water demand, initial and final setting times, rheological behavior of fresh pastes, and the evolution of compressive strength up to 720 days after casting. The characterization of binder properties was conducted using pastes prepared to achieve standard consistency, following the methodology outlined in PN-EN 196-3 [39], with the water-to-binder ratio adjusted according to the determined water demand. In contrast, rheological measurements and strength development assessments were performed on pastes with a fixed water-to-binder ratio of 0.35, ensuring comparability across different compositions.
3. Methods and Materials
3.1. Methods
The chemical composition of ground granulated blast furnace slag (GGBFS) and Portland cement, employing an Axios Minerals (Malvern Panalytical B.V, Almelo, The Netherlands) spectrometer equipped with a rhodium (Rh) anode tube. Quantification of oxide content was carried out using IQ+ analytical software (v1), covering elements ranging from oxygen (O) to uranium (U) in the measurement spectrum.
The particle size distribution of both precursor (GGBFS) and activators was assessed using laser diffraction analysis (HORIBA LA-300, HORIBA, Ltd., Japan, Kyoto), operating over a particle diameter range of 0.1 to 600 μm. For accurate dispersion during analysis, quicklime samples were suspended in 96% ethanol, while GGBFS and cement samples were dispersed in aqueous solutions of PEO/PPO copolymers. All suspensions underwent ultrasonic treatment for 60 s to ensure homogeneity prior to measurement. The specific surface area of GGBFS, cement, and quicklime was determined according to the Blaine air permeability method, utilizing a manual Blaine apparatus, following standardized procedures. To determine the phase composition of GGBFS, X-ray diffraction (XRD) analysis was carried out using a Bruker-AXS D8 DAVINCI (Bruker AXS SE, Karlsruhe, Germany) diffractometer operating in Bragg–Brentano geometry and equipped with a copper anode X-ray tube (Cu Kα radiation, λ = 1.5406 Å). Diffraction patterns were recorded over the 2θ range of 5–120°, with a step size of 0.01° and a counting time of 2 s per step. The optical system consisted of a 0.3° divergence slit, a 1.5° anti-scatter slit, two 2.5° soller slits, a nickel filter, and a LynxEye strip detector with a field of view of 2.94°. Phase identification was performed by comparing the recorded diffraction patterns with reference data from the ICDD PDF-2 and PDF-4+ (2016) databases using the DIFFRACplus EVA-SEARCH software (V7, Bruker AXS SE, Karlsruhe, Germany).
The reactivity of quicklime was evaluated following the standard procedure described in PN-EN 459-2 [38]. This method involves mixing a specified quantity of quicklime with deionized water and recording the temperature evolution under adiabatic conditions to assess hydration kinetics. The test was conducted in a thermally insulated vessel to prevent heat loss. Three key parameters were used to characterize quicklime reactivity:
t60—the time (in minutes) required for the temperature to rise by 60 °C from the initial water temperature, indicating the initial rate of reaction (lower values correspond to higher reactivity);
tmax—the time (in minutes) to reach the peak temperature during the reaction, reflecting the overall kinetics of the hydration process;
Tmax—the maximum temperature (in °C) attained during the test, related to the total exothermic effect of lime hydration.
Both t60 and tmax provide insights into the rate of reaction, while Tmax reflects the magnitude of heat released, which correlates with quicklime’s reactivity. Additionally, the chemical composition of quicklime samples was determined in accordance with PN-EN 459-2 [38], ensuring consistency between compositional and reactivity assessments.
The water demand of binders was expressed as the ratio of the amount of water required to obtain a paste of standard consistency, determined in accordance with PN-EN 196-3 [39], to the total binder mass. The consistency of each paste was assessed using the Vicat apparatus, in which the plunger was freely released into the paste. Standard consistency was defined as the condition where the distance between the lower end of the plunger and the base plate is 6 ± 2 mm.
The initial and final setting times of the binders were determined using the Vicat method, according to the standard procedure described in PN-EN 196-3 [39], using the automatic Vicat apparatus CONTROLS Vicamatic 2 (CONTROLS S.p.A., Liscate, Italy). The initial setting time was defined as the time from adding water to the binder until the distance between the needle and the plate surface was within the range of 3 +/− 2 mm. The final setting time was defined as the time from adding water to the binder until the penetration of the needle used for the initial setting time determination was no more than 0.5 mm. The initial and final setting times were measured in water at a temperature of 20 +/− 1 °C and in air at a temperature of 20 +/− 1 °C and RH = 50 +/− 1%, allowing for a comparison of the binder’s setting properties in these two environments.
Rheological properties were tested using an Anton Paar RheolabQC (Anton Paar GmbH, Graz, Austria) rotational rheometer with a sample thermostat attachment. The measurements were performed at 20 °C in CSR mode. A relative measurement geometry was used, with a CC27-SN measuring cup (Di = 26.660 mm; L = 40.000 mm; De = 28.920 mm) and an ST24-2D/2V/2V-30-109 vane spindle. The testing procedure included the following steps:
- Preparation of the alkali activated paste.
- Determination of the flow curve.
The prepared sample was placed in the measuring container, which, along with the spindle, was mounted in the rheometer. The measuring container remained inside the sample thermostat unit at 20 °C throughout the test. 2 min after mounting the container in the rheometer, pre-shear of the paste began at a shear rate of 100 s−1. After 30 s of pre-shear, the main test began, consisting of 15 measurements of shear stress at 1-s intervals for the following shear rates: 100; 80; 60; 40; 20; 10; 8; 6; 4; 2; 1; 0.8; 0.6; 0.4; 0.2; 0.1 s−1. In this way, the shear rate vs. shear stress relationship was established for each paste, forming the basis for the flow curve determination.
The shear sequence is shown in Figure 3.
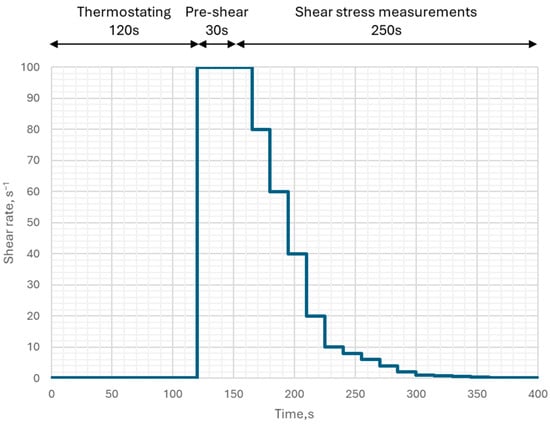
Figure 3.
The shear sequence for the alkali-activated pastes.
The flow curve was determined using the least-squares method by approximating the measurement points (shear rate, average shear stress) from the last two or three measurement points (depending on measurement stability) for each shear rate. The Bingham rheological model (1) was then fitted to the experimental data points.
where
- τ—shear stress, Pa.
- —yield stress, Pa.
- —plastic viscosity, Pa·s.
- —shear rate, 1/s.
The compressive strength of the paste samples was measured following the procedure outlined in the PN-EN 196-1 standard [40], using cubic samples with nominal dimensions of 40 × 40 × 40 mm, which were cut from larger specimens measuring 40 × 40 × 160 mm. Two cubic samples were prepared from each prismatic specimen. The tests were carried out using a CONTROLS Automax Multitest testing machine (CONTROLS S.p.A., Liscate, Italy).
Paste samples were prepared using tap water and a planetary mixer, following the procedure specified in PN-EN 196-3 [39]. Initially, GGBFS and the alkali activator were blended in the dry state for 2 min at low speed, with periodic pauses to scrape any adhering material from the walls and blades of the mixer to ensure uniform dispersion. After achieving a homogeneous dry mixture, the required amount of tap water was added, and the pastes were subjected to a three-stage mixing process, consisting of two cycles of 90 s at 140 RPM, each interrupted by a 15-s pause to collect any residual paste from the sides of the mixing bowl and ensure proper homogenization.
Following mixing, the fresh pastes were cast into molds and kept under controlled laboratory conditions (temperature: 20 ± 2 °C; relative humidity: 50 ± 20%), covered with vapor-proof foil to prevent moisture loss. After 24 h, the samples were demolded and transferred to tap water at 20 ± 2 °C for curing. For specimens tested at early ages (up to 120 days), water curing was maintained until the day of testing. Samples intended for long-term evaluation (beyond 120 days) were cured in water for 119 days, after which they were stored in laboratory conditions to allow natural carbonation until the testing date. To ensure moisture equilibration prior to mechanical testing, all air-stored specimens were submerged in water for 24 h before testing.
Compressive strength measurements were conducted at 7, 28, 120, and 720 days after casting, enabling an evaluation of both early-age and long-term mechanical performance.
3.2. Materials
3.2.1. Precursor
Ground granulated blast furnace slag (GGBFS) used in this study was obtained from the steelworks located in Dąbrowa Górnicza, Poland. The chemical composition of the slag was characterized by a CaO/SiO2 mass ratio of 1.23 and an Al2O3/SiO2 mass ratio of 0.15 (Table 1). According to the criteria outlined by Provis [41], slags suitable for alkali activation typically exhibit CaO/SiO2 ratios ranging from 0.5 to 2.0 and Al2O3/SiO2 ratios between 0.1 and 0.6. Based on these parameters, the GGBFS sourced from Dąbrowa Górnicza satisfied the compositional requirements for alkali activation, confirming its suitability as a precursor for alkali-activated slag binders.

Table 1.
The chemical composition (XRF) of GGBFS.
The phase composition of the slag was predominantly glassy, comprising 99% of the sample. This surpasses the optimal range of ≥85–90% recommended in the literature, including studies by Provis [42] and Shi and Krivienko [43], for effective alkali activation. The average particle size of the GGBFS was 22.2 μm (Figure 4), with a specific surface area of 3610 cm2/g, as determined by the Blaine method.
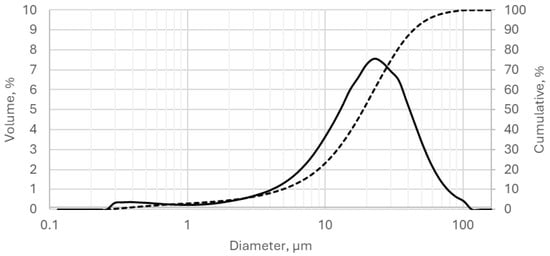
Figure 4.
Granulation curve of GGBFS. The solid line represents the particle size distribution expressed as volume percentage, while the dashed line indicates the corresponding cumulative particle size distribution.
3.2.2. Activators
In this study, four industrial quicklimes supplied by Trzuskawica, Poland, and produced under different calcination conditions, were employed as primary activators for GGBFS. These quicklimes exhibited distinct hydration reactivities, reflecting variations in their thermal processing. The extremely reactive (ER) quicklime displayed the highest reactivity, reaching a 60 °C temperature increase (t60) within 1.5 min and achieving the maximum temperature (tmax) after 9.20 min. The highly reactive (HR) quicklime exhibited slightly slower kinetics, with a t60 of 4.7 min and a tmax of 18.9 min. In contrast, the moderately reactive (MR) and low-reactive (LR) quicklimes were characterized by significantly longer hydration times, with t60 values exceeding 12 min for MR and 16 min for LR, while their tmax values were approximately 31 min and 36 min, respectively. Despite notable differences in reaction rates, all quicklime samples reached comparable maximum temperatures (Tmax) during the reactivity test, ranging from 69.6 °C to 72.0 °C (Figure 5).
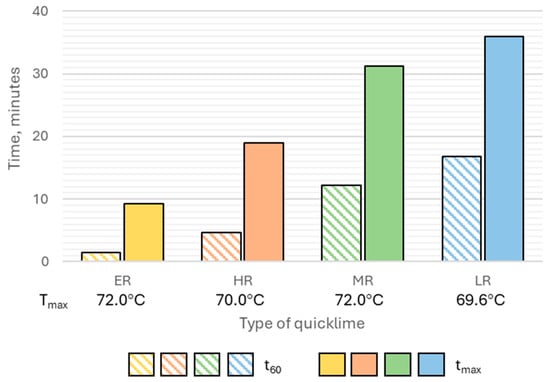
Figure 5.
The hydration reactivity of the quicklimes selected for this study, characterized by: t60, tmax and Tmax (Acronyms: ER—extremely reactive, HR—highly reactive, MR—moderately reactive, LR—low reactive).
All quicklime samples contained over 90% calcium oxide (CaO) as the principal component. Magnesium oxide (MgO) content exceeded 1% only in the extremely reactive (ER) quicklime, while remaining below this level in the other samples. Furthermore, the total content of carbonaceous compounds in all tested quicklimes was less than 2% by mass (Table 2).

Table 2.
The chemical composition of quicklimes.
The activators exhibited notable differences in particle size distribution and specific surface area (Figure 6). With the exception of the low-reactive (LR) quicklime, all quicklime samples showed a bimodal distribution, with peaks near 0.3–0.4 μm and 15–20 μm. The LR quicklime was markedly coarser, with an average particle size of 362.1 μm, while hydrated lime displayed a similar but much finer distribution (average 28.8 μm). Portland cement also showed a bimodal distribution with maxima at 0.3 μm and 22.0 μm.
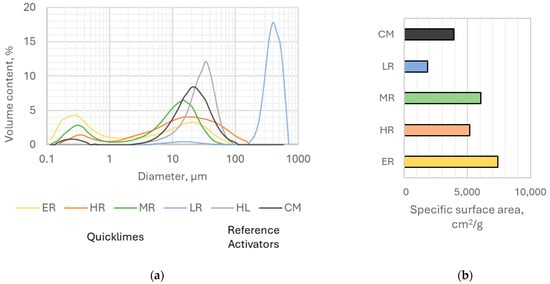
Figure 6.
(a) Granulation curve (b) Specific surface area (Blaine) of activators used in the study (Acronyms: Quicklimes: ER—extremely reactive, HR—highly reactive, MR—moderately reactive, LR—low reactive, Reference activators: Portland Cement—CM, Hydrated Lime—HL).
The specific surface area (SSA) varied consistently with reactivity: the extremely reactive (ER) quicklime had the highest SSA (7420 cm2/g), followed by moderately reactive (MR) (6050 cm2/g) and highly reactive (HR) (5160 cm2/g) samples, whereas the low-reactive (LR) quicklime exhibited the lowest value (1860 cm2/g). For comparison, Portland cement had an SSA of 3920 cm2/g.
4. Results and Discussion
4.1. Viscosity and Yield Stress
The plastic viscosity of the pastes ranged from 6.0 to 25.0 Pa·s (Figure 7a). The highest measured plastic viscosity was observed for the paste containing 9% extremely reactive lime, while the lowest was for the paste with 13% Portland cement; however, this paste was not the least fluid. The fluidity of the paste with 13% extremely reactive lime was so low that a reliable measurement could not be obtained for this case. The plastic viscosity of this paste exceeded the measurement range of the rheometer.
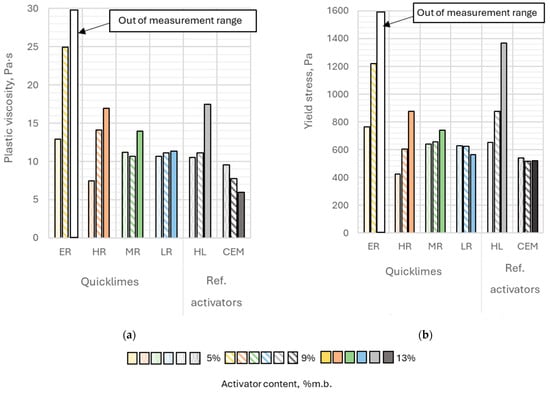
Figure 7.
(a) Plastic viscosity and (b) Yield stress of alkali-activated pastes as a function of the activator type and its content in the binder. Color code representing the activator content in the binder.
The yield stress of the analyzed pastes ranged between 426 and 1366 Pa (Figure 7b), indicating high variability within the examined set of results. The highest value (1366 Pa) was recorded for the binder containing 13% hydrated lime by binder mass. Similar to the plastic viscosity, the yield stress of the paste with the highest content of extremely reactive lime exceeded the measurement range of the viscometer. The lowest yield stress (426 Pa) was observed for the paste with 5% highly reactive lime content, which was more than three times lower than the yield stress of the paste containing 13% hydrated lime by binder mass.
Alkaline pastes with Portland cement exhibited a similar yield stress regardless of the amount of activator used. In contrast, binders activated with calcium hydroxide showed a significant increase in yield stress as the activator content in the binder increased. Increasing the content of this activator from 5% to 13% by binder mass resulted in more than a twofold rise in the yield stress value. A similar trend was observed when using highly reactive quicklime and lightly burned lime as activators.
The content of extremely reactive lime in the binder exhibited the strongest influence on both the plastic viscosity and yield stress of the analyzed pastes. Increasing the content of this activator from 5% to 9% by binder mass resulted in nearly a twofold increase in both yield stress and plastic viscosity. A similarly strong effect on yield stress was observed with changes in the content of hydrated lime. Increasing the content of hydrated lime led to yield stress increases of 34% (from 5% to 9% by mass) and 109% (from 5% to 13% by mass). A comparable trend was noted for highly reactive lime, with yield stress increasing by 42% (from 5% to 9% by mass) and 105% (from 5% to 13% by mass).
In contrast, plastic viscosity increased more rapidly with the addition of highly reactive lime compared to hydrated lime. The pronounced impact of ER and HR lime on the rheological properties of the pastes could be attributed to their high reactivity among the considered types of quicklime and their relatively large specific surface area.
Interestingly, hydrated lime showed a surprisingly strong influence, despite its lower specific surface area compared to other activators. This could be explained by its significantly lower bulk density compared to other activators, resulting in the largest volume for hydrated lime at the same mass.
Changes in the content of MR and LR lime had only a minor effect on the plastic viscosity and yield stress of the pastes containing these activators. For low reactive lime, even a slight decrease in yield stress (by approximately 10%) was observed when its content increased from 9% to 13% by binder mass.
A clear correlation was observed between the chemical reactivity of CaO and the rheological behavior of the activated slag pastes. Mixtures containing extremely reactive quicklime exhibited the greatest plastic viscosity and yield stress, both increasing sharply with CaO content. This behavior can be attributed to the rapid hydration of CaO, which not only accelerates the formation of Ca(OH)2 and early Ca-rich hydrates but also leads to intensive water absorption during the exothermic hydration process. The resulting reduction in free water and the increase in solid volume fraction enhance interparticle cohesion and friction, thereby increasing viscosity and yield stress. In contrast, pastes prepared with moderately or low-reactive quicklime showed a slower rise in rheological parameters or remained relatively stable, maintaining higher workability.
These observations indicate that the reactivity of CaO affects not only the kinetics of hydration but also the structure of the liquid phase and the intensity of particle–particle interactions. The rapid consumption of mixing water by extremely and highly reactive quicklimes significantly reduces the volume of the free liquid phase, increasing the solid concentration and limiting the hydrodynamic separation between particles. As a result, interparticle contacts become more frequent and frictional resistance rises, leading to the formation of a semi-rigid, cohesive network within the suspension. This interpretation is supported by the rheological measurements, where the most pronounced increases in plastic viscosity and yield stress were recorded for pastes containing the most reactive quicklimes, confirming that water depletion and intensified particle interactions are key factors controlling the flow behavior of CaO-activated slag binders.
4.2. Water Demand
The water demand of binders depended on the type and content of the activator used. Except for Portland cement, the water demand of slag–alkaline binders increased with a higher activator content. This indicates that all types of quicklime and hydrated lime used exhibited higher water demand than the ground granulated blast furnace slag (GGBFS). The activator with the highest water demand was the extremely reactive quicklime, which could be attributed to its largest specific surface area and highest reactivity. The water required to achieve standard consistency for binders with extremely reactive lime ranged from 32.9% to 38.0% (Figure 8).
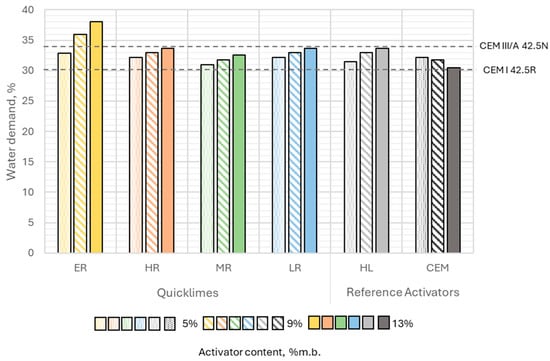
Figure 8.
Water demand of slag–alkali binders as a function of activator content and type, compared to the water demand of selected general-purpose cements (Acronyms: ER—extremely reactive, HR—highly reactive, MR—moderately reactive, LR—low reactive, HL—hydrated lime, CEM—cement).
Binders with other activators had similar water requirements despite differences in particle size distribution and morphology. For these, the water content needed to achieve standard consistency ranged from 30.4% to 33.7% relative to the binder mass. The activator with the lowest water demand was Portland cement, where a slight decrease in water demand was observed as its content in the binder increased (Figure 8).
The water demand of binders with quicklime increased proportionally with the increasing lime content. This could be attributed to the higher specific surface area of these activators and their significantly greater reactivity with water compared to ground granulated blast furnace slag (GGBFS).
Using quicklime with lower reactivity resulted in a reduced water demand for the binder, although this relationship was not linear. Changes in the tmax time, ranging from 10 to 20 min (for extremely and highly reactive), led to significant variations in the binder’s water demand. However, once the tmax exceeded 20 min (for moderately reactive and low reactive quicklime), the reactivity of the lime no longer had a significant effect on the binder’s water demand. This could be attributed to a change in grains morphology associated with increasing calcination temperature. Quicklimes calcined at higher temperatures, such as LR and MR, exhibit grains with a dense, glassy, and non-porous morphology, in contrast to the more porous structure observed in quicklimes calcined at lower temperatures, such as ER and HR.
4.3. Initial and Final Setting Time
The setting process of CaO-activated binders was highly influenced by the environment in which it occurred—whether in water or in air. Samples containing moderately or low reactive lime did not exhibit setting properties in water within 24 h of preparation, even when the lime content relative to the binder mass was minimized. Instead, these samples demonstrated pronounced and uncontrolled expansion of the paste, driven by the hydration of calcium oxide due to abundant water availability from the surroundings.
When the deformation of the paste was constrained using a Vicat ring, significant internal stresses developed in the forming microstructure of the material, leading to partial damage. This was evident in the cracks observed on the sample surfaces, particularly near the walls of the Vicat ring (Figure 9). The expansion was so intense that it prevented the development of a stable material structure within the first 24 h after sample preparation.
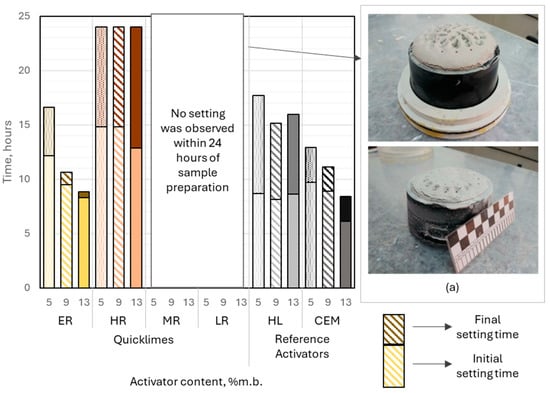
Figure 9.
Left: Initial and final setting times of slag–alkali pastes immersed in water, influenced by the type and dosage of the alkali activator. Right: Volume expansion of slag–alkali pastes caused by calcium oxide hydration in binders containing moderately or low-reactive quicklime. Each color represents a different activator and dosage level.
Extremely reactive CaO-activated binders demonstrated setting behavior under water curing conditions. The initial setting time ranged from approximately 8.2 to 12.1 h, with activator dosages of 9% and 13% (by binder mass) yielding results comparable to those of binders activated with hydrated lime. A significant reduction in setting time was observed as the activator content increased, dropping from 4.5 h at 5% dosage to 30 min at 13% dosage. Importantly, no measurable expansion of the paste was noted during water curing, indicating stable volumetric behavior of the binder.
Binders activated with highly reactive lime demonstrated initial setting times that were relatively insensitive to variations in activator dosage, ranging from 13 to 15 h. The final setting time of these binders, under water curing conditions, was not achieved within 24 h of sample preparation. Minor paste expansion was observed at activator dosages of 9% and 13% (relative to binder mass), which likely extended the time to initial setting and prevented the attainment of final setting within 24 h.
All binders incorporating reference activators exhibited successful setting behavior in water. The quantity of hydrated lime had no significant impact on the initial or final setting times. The initial setting time of these binders ranged from 8 to 9 h, with the setting times, understood as the difference between the initial and final setting times, between 7 and 9 h. Slag–alkali binders incorporating Portland cement at 5% and 9% (binder mass) showed initial setting times similar to those of hydrated lime-activated systems, at approximately 10 and 9 h, respectively. Increasing the cement dosage to 13% significantly shortened the initial setting time to 6.1 h. The setting time of slag-cement binders was less influenced by the activator dosage, ranging between 2 and 3.5 h.
The analyzed slag–alkali binders were characterized by a longer initial setting time compared to commonly used general-purpose cements. Considering their composition, the analyzed binders can be compared to blast-furnace cements CEM III/B and CEM III/C, in which blast-furnace slag may constitute 66% to 95% of the binder’s mass. Currently, the European cement market offers only CEM III/B cements. According to data published by cement manufacturers, the initial setting time of CEM III/B cements typically ranges from 240 to 300 min. In comparison, the binder with the shortest initial setting time among those analyzed contained 13% Portland cement, with an initial setting time of 366 min. Based on its composition, this binder could be classified as a blast-furnace cement CEM III/C.
Adjusting the curing conditions had a significant impact on the setting kinetics of alkali-activated slag binders, particularly for binders incorporating calcined lime. The initial setting time of these binders exhibited strong sensitivity to the activator dosage, ranging from 1.6 to 9 h. Similarly, the setting time was markedly influenced by the activator content, spanning from 2 to over 11 h, demonstrating the critical role of activator content in controlling the setting behavior of these binders.
For all pastes containing quicklimes, setting times significantly decreased with increasing activator dosage. The most pronounced reductions were observed in pastes containing highly reactive (HR), moderately reactive (MR), and low-reactive (LR) limes. For MR lime, the initial setting time decreased sharply from approximately 8.5 h at 5% activator content to around 2 h at 13%, while the final setting time dropped from over 10 h to approximately 2 h. Similarly, LR lime pastes showed a substantial reduction, with the initial setting time decreasing from 9 h at 5% to 2 h at 13%, and the final setting time dropping from over 11 h to slightly above 2 h. The HR samples followed a similar trend, with initial setting times decreasing from approximately 12 h at 5% activator content to 2 h at 13% (Figure 10).
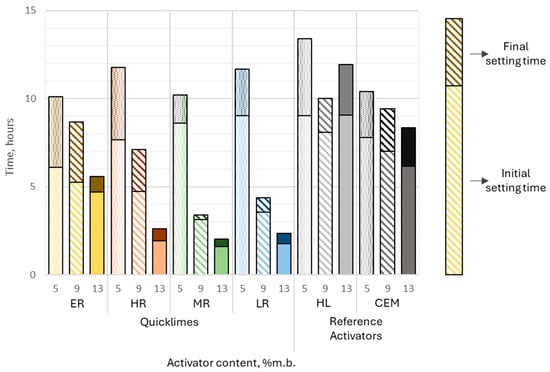
Figure 10.
The initial and final setting times of slag–alkali pastes immersed in air, as influenced by the type and dosage of the alkali activator. Each color represents a different activator and dosage level.
In contrast, pastes activated with extremely reactive lime (ER) exhibited smaller reductions in setting times. For ER lime, the initial setting time decreased slightly from approximately 6 h at 5% to 5 h at 13%, while the final setting time dropped from 10 h to 5.5 h.
Reference activators behaved differently. Hydrated lime (HL) produced consistent setting times, with initial times ranging from 8 to 9 h and final times from 10 to 13 h. Samples containing Portland cement (CEM), however, displayed a linear decrease in both initial and final setting times as activator content increased from 5% to 13%. Initial setting times decreased from approximately 8 h to 6 h, while final setting times dropped from 10 h to 8 h.
Tian et al. [14] reported that CaO-activated pastes with 8% CaO (by mass) exhibited an initial setting time of approximately 6–7 h and a final setting time of 8–9 h under the same testing conditions. These values varied depending on the source of GGBFS; however, no details were provided regarding the reactivity of the quicklime or the specific surface area of the GGBFS used. The results obtained by Tian et al. align with those observed in this study for samples containing 9% highly reactive lime, despite Tian et al. employing a significantly higher water-to-binder ratio of 0.5.
For comparison, Yum et al. [27] reported an initial setting time of approximately 9 h and a final setting time of 19 h for pastes with 4% CaO and a GGBFS specific surface area of 2970 cm2/g. When comparing these findings with the results from this study and Tian et al. [14], it can be concluded that the specific surface area of GGBFS plays a crucial role in influencing the hydration kinetics of CaO-activated GGBFS binders.
As mentioned, increasing the content of HR, MR, and ER limes in the binder from 5% to 13% resulted in more than a fivefold reduction in the time to the initial setting of the binders. This could be attributed to the acceleration of the hydration process of blast-furnace slag. However, this effect was not observed in binders containing highly reactive lime, which served as the basis for formulating an alternative hypothesis.
The slaking reaction of quicklime was accompanied by a noticeable decrease in paste fluidity, as free water in the paste was consumed during calcium oxide hydration. According to the slaking reaction Equation (2), hydrating 1 g of CaO required approximately 0.3 g of water. At lime contents of 9% and 13% by binder mass, this corresponded to 12 g and 18 g of water, respectively, accounting for 8% to 15% of the initial water content in the analyzed pastes.
The results presented in Section 3.2.2 regarding the reactivity of highly reactive lime indicated that pastes containing this quicklime should reached their maximum temperature within 10 min of mixing. This suggested that a significant portion of calcium oxide hydrated during mixing, before the paste was placed in the Vicat ring. The resulting decrease in fluidity was observed during the determination of the binder’s water demand (Section 4.2) using the Vicat apparatus and was offset by increasing the water content. Consequently, binders containing ER quicklime at 9% and 13% by binder mass exhibited the highest water demand among the analyzed pastes.
In contrast, pastes with other types of quicklime behaved differently. According to reactivity tests, the hydration of these limes entered its critical phase much later. This delayed hydration was not accounted for during the determination of the water required to achieve standard consistency, as this test was typically completed within 5 to 6 min of mixing.
As a result, the observed acceleration in the setting process of binders activated with quicklimes, other than extremely reactive lime, was likely due not only to changes in the hydration kinetics of GGBFS but also to the extended slaking reaction of burnt lime. This reaction, occurring in the presence of limited free water, caused a gradual loss of paste fluidity over time, which in turn accelerated stiffening.
This hypothesis was supported by the observation that binders with reference activators, as well as those with highly reactive lime, exhibited a less pronounced effect of activator content on setting times and the time to initial setting. Additionally, the initial setting times of binders containing hydrated lime, determined in air, were comparable to those determined in water, with differences ranging from 1.9% to 4.5%. In contrast, binders with Portland cement showed more significant differences between initial setting times determined in air and in water. These binders began setting 4% to 27% faster in air than in water.
4.4. Long-Term Strength Development
The average compressive strength of alkali-activated paste samples with quicklime ranged from 8.4 to 11.1 MPa at 7 days of curing, 11.4 to 17.2 MPa at 28 days, 25.7 to 29.7 MPa at 120 days, and 26.1 to 36.3 MPa at 720 days of curing (Figure 11).
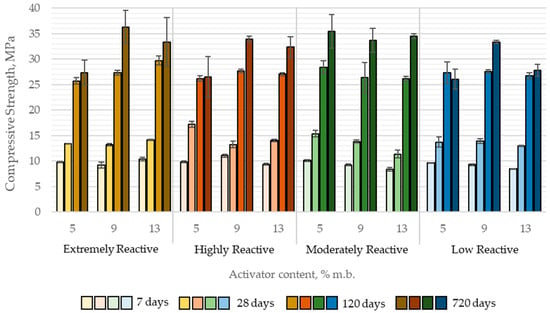
Figure 11.
Compressive strength development of paste samples activated with quicklimes over time as a function of quicklime reactivity and its content in the binder.
Given the extensive dataset comprising 192 individual measurements, a comprehensive statistical analysis was carried out to assess the influence of lime reactivity on binder properties. Initially, a multivariate analysis of variance (MANOVA) was applied, with tₘₐₓ (time to reach maximum temperature) selected as the quantitative descriptor of quicklime reactivity. To facilitate group comparisons, tmax values were rounded to the nearest whole minute, yielding representative reactivity classes: 9 min for extremely reactive (ER), 19 min for highly reactive (HR), 31 min for moderately reactive (MR), and 36 min for low-reactive (LR) quicklimes. The primary goal of MANOVA was to determine whether statistically significant differences existed among these groups. Following MANOVA, a Scheffé’s post hoc test was conducted to identify homogeneous subsets and to clarify which groups exhibited statistically significant differences in their performance.
The results of the MANOVA analysis demonstrated that both quicklime reactivity and its content in the binder had a statistically significant influence on the compressive strength of CaO-activated pastes in three out of four tested time intervals, specifically at 7, 28, and 720 days. Notably, at 120 days, neither the reactivity of the quicklime nor its content showed a statistically significant effect on compressive strength.
The direction and magnitude of these effects varied with curing time. At early ages (7 and 28 days), an increase in quicklime content and a decrease in reactivity (higher tₘₐₓ values beyond those for highly reactive lime) were generally associated with a slight reduction in compressive strength. However, the magnitude of these differences was negligible from a practical perspective. For instance, increasing the quicklime content from 5% to 13% (by binder mass) led to a compressive strength decrease of 0.68 MPa at 7 days and 1.80 MPa at 28 days.
Interestingly, this trend did not persist at later curing stages. At 120 days, compressive strength was independent of both quicklime reactivity and content, while at 720 days, pastes containing higher quicklime content exhibited significantly greater compressive strength. This may be attributed to long-term processes such as carbonation, which could enhance the mechanical properties of alkali-activated slag binders containing CaO. Furthermore, at 720 days, no strength reduction was observed in relation to decreasing quicklime reactivity; in fact, the highest compressive strength was recorded for pastes activated with moderately reactive (MR) quicklime (Figure 12).
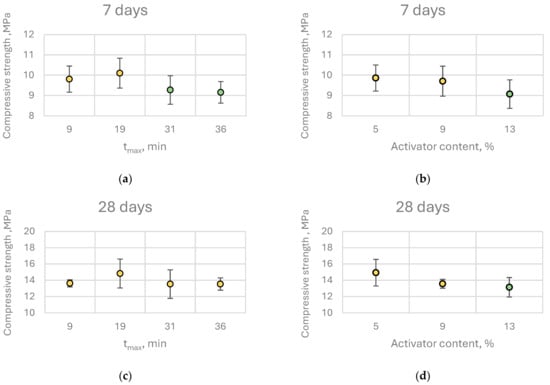
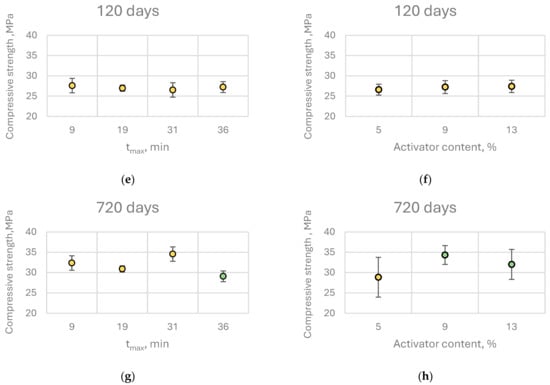
Figure 12.
Effect of (a,c,e,g) tmax and (b,d,f,h) activator content on the compressive strength of CaO-activated pastes at different curing ages. Identical colors indicate statistically homogeneous groups as identified by Scheffé’s post hoc test.
Post hoc analysis (Scheffé’s test) revealed that increasing quicklime content beyond 9% by binder mass did not result in statistically significant improvements in compressive strength at any curing age. The same analysis indicated that for early-age (7 days) and 28-day strength, it is preferable to use highly reactive quicklime at the lowest investigated dosage, although the differences among groups were minor. Consequently, from a practical engineering standpoint, within the studied range of quicklime reactivity and content, these parameters do not exert a substantial influence on the early compressive strength of the binder.
In contrast, when considering the long-term compressive strength (720 days), samples containing LR quicklime exhibited significantly lower compressive strength compared to those activated with other types of quicklime. Conversely, the results also indicated that increasing the quicklime content from 5% to 9% of the binder mass was beneficial for the mechanical properties of CaO-activated composites, leading to a substantial increase in average compressive strength—nearly 20% at 720 days.
This finding indicates that curing conditions strongly influenced the microstructural evolution and long-term strength of CaO-activated composites. After 120 days of curing under water, the samples were transferred to air and stored under laboratory conditions, where exposure to atmospheric CO2 enabled gradual carbonation. The two-stage curing regime therefore facilitated both initial hydration under saturated conditions and subsequent carbonation during air storage. In binders containing higher CaO content, the larger amount of residual Ca(OH)2 in the matrix provided an additional source of reactive calcium for carbonation. As reported previously [44], the formation of CaCO3 from Ca(OH)2 leads to a reduction in porosity, as the reaction product occupies a greater solid volume. A similar densification process likely occurred in the present study, where controlled carbonation during the air-curing stage improved the microstructural compactness and, consequently, enhanced the compressive strength of the pastes.
On the microstructural level, carbonation likely induced partial decalcification of the C–S–H phase and the precipitation of finely crystalline CaCO3, mainly calcite and vaterite, within gel pores and interparticle voids. These reaction products filled capillary spaces and refined the pore network, leading to a more compact and continuous matrix. The formation of CaCO3 at the C–S–H interfaces also contributed to improved particle bonding and reduced permeability. Such controlled carbonation resulted in a denser, more cohesive microstructure, which explains the observed long-term increase in compressive strength.
The influence of carbonation on the mechanical properties of CaO-activated composites is strongly time-dependent. At early stages of curing, when the pore structure remains relatively open, the system is particularly susceptible to CO2 ingress, which facilitates rapid carbonation of Ca(OH)2 and the formation of finely dispersed CaCO3. This early carbonation can significantly enhance the compactness of the microstructure and contribute to the rapid development of mechanical strength. Recent findings by Jeon et al. [45] confirmed that the introduction of NaHCO3 as an in situ carbonation agent in CaO-activated slag systems more than doubled the 3-day compressive strength, demonstrating the potential of controlled early carbonation to accelerate hardening.
As hydration and carbonation progress, CaCO3 progressively fills capillary pores, and the matrix becomes denser and less permeable, thereby reducing the rate and extent of subsequent carbonation. At later ages, the ongoing but slower carbonation process continues to refine the microstructure, though its effect on strength becomes less pronounced. Thus, carbonation exerts its greatest influence at early curing stages and remains a secondary, yet beneficial, mechanism contributing to the long-term densification and strength evolution of CaO-activated GGBFS binders.
However, further increasing the CaO content to 13% of the binder mass did not produce any additional improvement in compressive strength, indicating an upper limit to the beneficial effect of quicklime dosage in these composites. At early ages, this plateau results from physicochemical constraints of slag activation under high Ca2+ concentrations. At elevated CaO contents, the pore solution rapidly becomes saturated with Ca2+ and OH− ions, and further additions of CaO do not increase the pH of the system, providing no additional benefit in promoting GGBFS dissolution. Simultaneously, rapid CaO hydration consumes a significant portion of the available mixing water, while the early precipitation of a dense Ca-rich layer on slag particle surfaces restricts ion diffusion and limits further reaction progress. This limitation could potentially be mitigated under accelerated or in situ carbonation conditions, where the excess Ca(OH)2 present in high-lime mixtures might contribute to early strength improvement through controlled CaCO3 formation.
At extended curing times (up to 720 days), the strength plateau likely results from the restricted carbonation of residual Ca(OH)2 within the hardened matrix. As the composite becomes progressively denser and less permeable, the ingress of CO2 is hindered, limiting further CaCO3 precipitation and microstructural densification. Consequently, despite the higher CaO content, no measurable long-term strength enhancement was observed, as the combined effects of Ca2+ saturation, early water depletion, and reduced permeability prevented additional C–S–H or carbonation-induced strengthening.
The compressive strength of pastes activated with quicklime increased gradually over time. On the 7th day of curing, the compressive strength of the tested samples ranged from approximately 24% to 37% of the 720-day strength, which was considered the reference level for further analysis (relative compressive strength). At 28 days, the relative compressive strength of the samples was mostly within the range of 33% to 52%. The only exception were the samples with highly reactive lime in the lowest content (5% of binder mass), where the relative compressive strength was higher than 60%. However, the most significant increase in mechanical properties was observed between 28 and 120 days of curing. After 120 days of curing, the strength gain slowed considerably. At this stage, the relative compressive strength of the samples ranged from 75% to 100% (Figure 13).
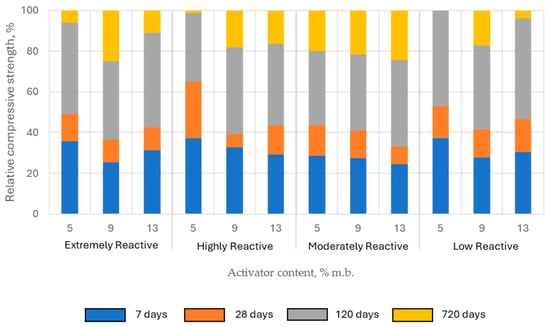
Figure 13.
The relative compressive strength of alkali-activated paste samples with quicklime, depending on lime reactivity and its content in the binder, expressed as the ratio of average compressive strength at a given age to the average compressive strength at 720 days.
Lower activator content favored higher early-age relative compressive strength (7 and 28 days), while increasing the lime content in the binder resulted in greater compressive strength gains over longer periods. This observed trend aligns with the hypothesis that carbonation plays a significant role in shaping the mechanical properties of CaO-activated composites.
The average compressive strength of samples containing hydrated lime and Portland cement ranged from 9.7 to 26.7 MPa at 7 days of curing, 11.1 to 39.0 MPa at 28 days, 26.4 to 59.8 MPa at 120 days, and 26.5 to 63.9 MPa at 720 days. The highest strengths were achieved when Portland cement was used as the activator.
The compressive strength of paste samples activated with Portland cement was significantly higher compared to those activated with hydrated lime. At 7 days, the strength of cement-activated pastes exceeded that of hydrated lime-activated samples by 76% to 141%, while at 28 days, this difference ranged from 51% to 196%. Although the gap between the two groups narrowed slightly with extended curing time, it remained substantial. After 120 days, the compressive strength of Portland cement-activated samples was still 28% to 109% higher, and at 720 days, the difference ranged from 49% to 100%. Moreover, the magnitude of these differences increased proportionally with higher activator content in the binder (Figure 14).
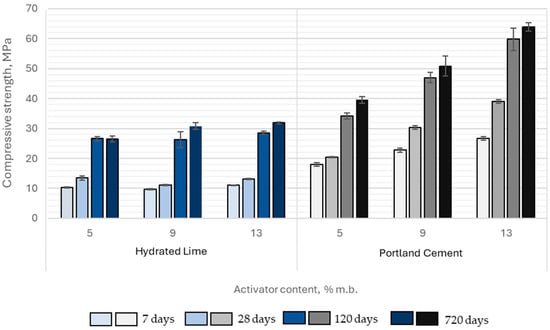
Figure 14.
Compressive strength development of paste samples activated with hydrated lime and portlandite cement over time as a function of activator content in the binder.
The compressive strength of samples activated with Portland cement increased proportionally with its content in the binder. In contrast, increasing the hydrated lime content above 5% by binder mass did not produce any significant improvement in compressive strength. This behavior can be explained by both chemical and microstructural constraints governing slag activation. At around 5% Ca(OH)2, the pore solution becomes saturated with Ca2+ and OH− ions released from the activator and early slag dissolution. Further additions increase ionic strength without substantially elevating pH, which remains lower than in Na-based activators and therefore does not further accelerate slag dissolution. The excess Ca(OH)2 tends to remain undissolved and may become incorporated into the developing C–S–H structure. In addition, surplus Ca2+ promotes the early formation of Ca-rich surface layers on slag particles, which limit ion diffusion and hinder continued reaction and densification of the binding phase. As a result, a practical upper limit of approximately 5% Ca(OH)2 can be considered optimal for strength development under the tested mix design and curing conditions. Similar observations were reported by Tang et al. [46] and Lei et al. [47], who found negligible improvements in compressive strength when increasing Ca(OH)2 dosage beyond 3–5% of binder mass in alkali-activated slag mortars.
A similar mechanism was also observed in the case of CaO activation, although it occurred at a different activator dosage, as discussed earlier. In CaO-activated systems, the plateau in compressive strength appeared at around 9% CaO, which corresponds to a comparable degree of Ca2+ saturation in the pore solution. Despite the different hydration kinetics between Ca(OH)2 and CaO, both systems exhibited a similar physicochemical limitation: excess calcium availability and rapid hydroxyl ion release promoted surface passivation and reduced slag dissolution, thereby constraining further C–S–H formation and limiting the potential for additional strength development.
The compressive strength of pastes containing quicklime was comparable to that of hydrated lime-activated samples at 7, 28, and 120 days of curing, with no statistically significant differences observed. However, after approximately two years (720 days), specimens containing 9% and 13% quicklime demonstrated approximately 15% higher compressive strength relative to those activated with hydrated lime (Figure 15). Overall, the substitution of hydrated lime with quicklime did not lead to a substantial enhancement in compressive strength of the alkali-activated slag pastes, consistent with observations reported by Kim et al. [13].
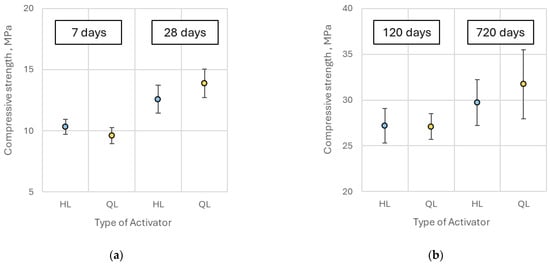
Figure 15.
Comparison of the average compressive strength of alkali-activated pastes containing hydrated lime (HL) and quicklime (QL) at (a) 7 and 28 days and (b) 120 and 720 days.
The 28-day compressive strength obtained in this study was approximately 50% lower than the values reported in the literature for pastes with the same water-to-binder (w/b) ratio, but cured under air conditions (T = 23 °C, RH > 99%). Specifically, Yum et al. [15] reported an average compressive strength of 27.3 MPa, while Sim et al. [26] observed a mean value of 25 MPa. In comparison, the average compressive strength achieved in this research was only 13.9 MPa.
This significant discrepancy may be attributed primarily to differences in the particle size distribution of GGBFS and the applied curing conditions. Although neither Yum nor Sim provided data on the specific surface area of the slag used, they reported mean particle sizes of 15 μm and 9.7 μm, respectively. In contrast, the GGBFS employed in this study had a considerably coarser mean particle size of 22.3 μm, which likely contributed to its reduced reactivity and lower mechanical performance. The limited surface area restricts slag dissolution, reducing ion availability for C–S–H formation and slowing early hydration kinetics. Similar effects were reported by Burciaga-Díaz [31], who demonstrated that increasing the Blaine fineness of GGBFS from 360 to 450 m2 kg−1 significantly enhanced dissolution, produced denser reaction products, and improved 90-day compressive strength.
Moreover, as discussed previously, the effect of carbonation on the mechanical properties of CaO-activated slag binders must also be considered. Samples cured under air conditions, as in the studies by Yum and Sim, were exposed to natural carbonation, which may have enhanced their strength. Conversely, samples in this study were water-cured for 120 days, preventing carbonation and possibly limiting strength development.
5. Conclusions
The search for environmentally friendly and resource-efficient binders has become a key challenge in reducing the carbon footprint of the construction industry. One promising solution involves the alkali activation of industrial by-products, such as ground granulated blast furnace slag (GGBFS), using calcium-based activators. Unlike conventional alkali activators, such as sodium hydroxide or sodium silicate, calcium-based activators offer a lower-cost and potentially less hazardous alternative, while enabling the utilization of waste materials. This strategy not only promotes circular economy principles but also contributes to lowering greenhouse gas emissions associated with traditional cement production.
This study investigated the influence of quicklime reactivity on both the fresh-state properties and long-term compressive strength development of CaO-activated GGBFS composites. Quicklime reactivity, expressed as the time to reach maximum temperature (tmax) during slaking, was found to have a pronounced effect on the rheological behavior and setting times of alkali-activated pastes. However, its influence on compressive strength development was comparatively limited. Additionally, the results highlighted the significant role of carbonation in enhancing the mechanical performance of CaO-activated composites, primarily through earlier reported pore structure refinement and matrix densification.
Based on the experimental results, the following conclusions can be drawn:
- The water demand of CaO-activated slag binders was significantly influenced by the reactivity and content of quicklime used as an activator. Binders containing extremelyreactive CaO exhibited the greatest water demand (32.8–38.1%), exceeding that of the reference systems with hydrated lime and Portland cement. In contrast, mixtures with less reactive quicklimes showed relatively constant water requirements (31.0–33.7%). Increasing the quicklime content from 5% to 13% by binder mass led to a rise in water demand of up to five percentage points, particularly in systems incorporating extremely reactive lime.
- The rheological behavior of the pastes followed the same trend as water demand. Mixtures with extremely reactive quicklime showed the greatest plastic viscosity (12.9–25.0 Pa·s) and yield stress (764–1219 Pa), both increasing sharply with activator content; at 13% CaO, the paste became too stiff for rheological testing. In comparison, pastes with moderately reactive lime exhibited a near-linear rise in viscosity (7.5–16.9 Pa·s) and yield stress (426–874 Pa) with increasing CaO content, while the other quicklime types caused only minor or negligible changes.
- The setting behavior of CaO-activated slag binders was strongly affected by quicklime content. The initial and final setting times ranged from 1.6–9.0 h and 2.0–11.8 h, respectively, both decreasing nearly linearly with higher CaO dosage. Increasing the content of reactive quicklimes from 5% to 13% reduced the initial setting time more than fivefold, whereas pastes with moderately and low reactive quicklime showed no setting when cured under water.
- The compressive strength of CaO-activated binders showed only minor variation with quicklime reactivity or content during the first 120 days, with the most pronounced strength gains occurring between 28 and 120 days. After 7 days, samples reached less than 40% of their 720-day strength, confirming the slow development of mechanical properties. Long-term testing (720 days) revealed that binders with 9% CaO achieved approximately 20% higher compressive strength than those with 5%, while further increases in CaO content produced no additional improvement, emphasizing the role of gradual carbonation in long-term strength enhancement.
The amount and reactivity of CaO in the binder significantly affected both the fresh and early-age properties of the material. Among the tested compositions, the mixture containing 9% CaO by binder mass provided the most balanced performance, combining manageable plastic viscosity and yield stress with satisfactory reaction kinetics, as reflected by moderate initial and final setting times. Lower CaO content (5%) resulted in high workability but excessively long setting times and limited early strength, while higher dosage (13%) caused a marked increase in viscosity and rapid stiffening without further strength improvement. These findings indicate that moderately to highly reactive quicklime, used at approximately 9% of the binder mass, provides an optimal compromise between fresh-state workability, reaction efficiency, and long-term mechanical performance.
Highly reactive quicklime produced at lower calcination temperatures (≈1000 °C) requires less energy and emits less CO2 than less reactive grades fired at 1200–1300 °C, thereby offering a more sustainable option. Moreover, the applied two-stage curing process—initial hydration under water followed by air exposure—enhanced long-term strength through gradual carbonation and microstructural densification. This combination of optimized CaO reactivity, dosage, and curing conditions ensures favorable rheological and mechanical properties while contributing to the reduction in the overall carbon footprint of CaO-activated GGBFS binders.
Author Contributions
Conceptualization, K.C., P.W. and B.J.; methodology, K.C. and P.W.; software, K.C.; validation, K.C. and P.W.; formal analysis, K.C., P.W. and B.J.; investigation, K.C.; resources, K.C.; data curation, K.C.; writing—original draft preparation, K.C.; writing—review and editing, P.W. and B.J.; visualization, K.C.; supervision, P.W. and B.J.; project administration, P.W.; funding acquisition, K.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data are included in the paper and are available on request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Global Cement and Concrete Association. Cement Industry Net Zero Progress Report 2024/25; GCCA: London, UK, 2024. [Google Scholar]
- Biricik, H.; Kırgız, M.S.; de Sousa Galdino, A.G. Activation of slag through a combination of NaOH/Na2SiO3 alkali for transforming it into geopolymer slag binder mortar—Assessment of the effects of two different alkalis. J. Mater. Res. Technol. 2021, 15, 512–525. [Google Scholar] [CrossRef]
- Cheah, C.B.; Tan, L.E.; Ramli, M. The engineering properties and microstructure of sodium carbonate activated fly ash/slag blended mortars with silica fume. Compos. Part. B Eng. 2019, 160, 558–572. [Google Scholar] [CrossRef]
- Biricik, H.; Kırgız, M.S.; de Sousa Galdino, A.G.; Kenai, S.; Mirza, J.; Kinuthia, J.; Khatib, J. Activation of slag through a combination of NaOH/Na2S alkali for transforming it into geopolymer slag binder mortar—Assessment of the effects of two different Blaine fines and three different curing conditions. J. Mater. Res. Technol. 2021, 14, 1569–1584. [Google Scholar]
- Abed, F.H.; Zareei, S.A.; Kurdi, N.H.; Emami, A. Enhancing geopolymer binder reactivity and performance via mechanochemical activation: A comprehensive study of rheological, mechanical, and microstructural properties. Constr. Build. Mater. 2024, 430, 136456. [Google Scholar] [CrossRef]
- Dai, X.; Aydin, S.; Yardimci, M.Y.; De Schutter, G. Rheology and structural build-up of sodium silicate- and sodium hydroxide-activated GGBFS mixtures. Cem. Concr. Compos. 2022, 131, 104570. [Google Scholar] [CrossRef]
- Awoyera, P.O.; Adesina, A.; Sivakrishna, A.; Gobinath, R.; Kumar, K.R.; Srinivas, A. Alkali activated binders: Challenges and opportunities. Mater. Today Proc. 2020, 27, 40–43. [Google Scholar] [CrossRef]
- Matsimbe, J.; Dinka, M.; Olukanni, D.; Musonda, I. Geopolymer: A systematic review of methodologies. Materials 2022, 15, 6852. [Google Scholar] [CrossRef]
- Zhao, J.; Tong, L.; Li, B.; Chen, T.; Wang, C.; Yang, G.; Zheng, Y. Eco-friendly geopolymer materials: A review of performance improvement, potential application and sustainability assessment. J. Clean. Prod. 2021, 307, 127085. [Google Scholar] [CrossRef]
- Danish, A.; Ozbakkaloglu, T.; Mosaberpanah, M.A.; Salim, M.U.; Bayram, M.; Yeon, J.H.; Jafar, K. Sustainability benefits and commercialization challenges and strategies of geopolymer concrete: A review. J. Build. Eng. 2022, 58, 105005. [Google Scholar] [CrossRef]
- Zhai, Q.; Kurumisawa, K. Mechanisms of inorganic salts on Ca(OH)2-activated ground granulated blast-furnace slag curing under different temperatures. Constr. Build. Mater. 2022, 338, 127637. [Google Scholar] [CrossRef]
- Zhai, Q.; Kurumisawa, K. Effects of cation in sulfate, chloride and nitrite on Ca(OH)2-activated ground granulated blast-furnace slag. Cem. Concr. Compos. 2022, 133, 104648. [Google Scholar] [CrossRef]
- Kim, M.S.; Jun, Y.; Lee, C.; Oh, J.E. Use of CaO as an activator for producing a price-competitive non-cement structural binder using ground granulated blast furnace slag. Cem. Concr. Res. 2013, 54, 208–214. [Google Scholar] [CrossRef]
- Tian, Y.; Xing, J.; Zhao, Y.; Sun, X.; Wu, P.; Qiu, J. Influence of aluminum sulfate on strength of CaO-activated slag system. Constr. Build. Mater. 2021, 306, 124895. [Google Scholar] [CrossRef]
- Yum, W.S.; Suh, J.I.; Jeon, D.; Oh, J.E. Strength enhancement of CaO-activated slag system through addition of calcium formate as a new auxiliary activator. Cem. Concr. Compos. 2020, 109, 103572. [Google Scholar] [CrossRef]
- Yoon, S.; Park, H.; Yum, W.S.; Suh, J.I.; Oh, J.E. Influence of calcium sulfate type on evolution of reaction products and strength in NaOH- and CaO-activated ground granulated blast-furnace slag. Appl. Sci. 2018, 8, 2500. [Google Scholar] [CrossRef]
- European Lime Association (EuLA); South Pole. Carbon Accounting of Recarbonation in Lime Products; EuLA: Brussels, Belgium, 2024. [Google Scholar]
- Campo, F.P.; Tua, C.; Biganzoli, L.; Pantini, S.; Grosso, M. Natural and Enhanced Carbonation of Lime in Its Different Applications: A Review. Environ. Technol. Rev. 2021, 10, 224–237. [Google Scholar] [CrossRef]
- Mitraki, R. Transition Pathways for the Belgian Industry: Application to the Case of the Lime Sector. Master’s Thesis, University of Liège, Liège, Belgium, 2024. Available online: http://hdl.handle.net/2268.2/20254 (accessed on 22 October 2025).
- Rodríguez, E.D.; Bernal, S.A.; Provis, J.L.; Paya, J.; Monzo, J.M.; Borrachero, M.V. Effect of nanosilica-based activators on the performance of an alkali-activated fly ash binder. Cem. Concr. Compos. 2013, 35, 1–11. [Google Scholar] [CrossRef]
- Andreola, F.; Barbieri, L.; Lancellotti, I. The Environmental Friendly Route to Obtain Sodium Silicate Solution from Rice Husk Ash: A Comparative Study with Commercial Silicates Deflocculating Agents. Waste Biomass Valor 2020, 11, 6295–6305. [Google Scholar] [CrossRef]
- IndexBox. EU—Quicklime—Market Analysis, Forecast, Size, Trends and Insights. Available online: https://www.indexbox.io/store/eu-quicklime-market-analysis-forecast-size-trends-and-insights/ (accessed on 23 October 2025).
- BusinessAnalytiq. Sodium Hydroxide Price Index—Europe: US $0.27 kg−1. Available online: https://businessanalytiq.com/procurementanalytics/index/sodium-hydroxide-price-index/ (accessed on 23 October 2025).
- ChemAnalyst. Sodium Silicate Price Trend and Forecast. Available online: https://www.chemanalyst.com/Pricing-data/sodium-silicate-1340 (accessed on 23 October 2025).
- Liu, Q.; Yang, C.; Lyu, X.; Chen, P.; You, X.; Li, L.; Wang, J. Evolution of ettringite content and its effects on hydration properties of CaO/fluorgypsum-activated granulated blast furnace slag binders. Adv. Compos. Hybrid Mater. 2021, 4, 350–359. [Google Scholar] [CrossRef]
- Sim, S.; Lee, H.; Jeon, D.; Song, H.; Yum, W.S.; Kim, D.; Oh, J.E. Gypsum-dependent effect of NaCl on strength enhancement of CaO-activated slag binders. Appl. Sci. 2018, 8, 2515. [Google Scholar] [CrossRef]
- Yum, W.S.; Suh, J.I.; Sim, S.; Yoon, S.; Jun, Y.; Oh, J.E. Influence of calcium and sodium nitrate on the strength and reaction products of the CaO-activated GGBFS system. Constr. Build. Mater. 2019, 215, 839–848. [Google Scholar] [CrossRef]
- Zhang, S.; Ren, F.; Ding, H.; Qiu, J.; Tian, Y.; Liu, N. Recycling aluminum dross as a mineral admixture in CaO-activated superfine slag. Constr. Build. Mater. 2021, 279, 122434. [Google Scholar] [CrossRef]
- Park, H.; Jeong, Y.; Jun, Y.; Jeong, J.H.; Oh, J.E. Strength enhancement and pore-size refinement in clinker-free CaO-activated GGBFS systems through substitution with gypsum. Cem. Concr. Compos. 2016, 68, 57–65. [Google Scholar] [CrossRef]
- Jeong, Y.; Park, H.; Jun, Y.; Jeong, J.H.; Oh, J.E. Influence of slag characteristics on strength development and reaction products in a CaO-activated slag system. Cem. Concr. Compos. 2016, 72, 155–167. [Google Scholar] [CrossRef]
- Burciaga-Díaz, O. Parameters affecting the properties and microstructure of quicklime (CaO)-activated slag cement pastes. Cem. Concr. Compos. 2019, 103, 104–111. [Google Scholar] [CrossRef]
- Moropoulou, A.; Bakolas, A.; Aggelakopoulou, E. The effects of limestone characteristics and calcination temperature to the reactivity of the quicklime. Cem. Concr. Res. 2001, 31, 633–639. [Google Scholar] [CrossRef]
- Oates, J.A.H. Lime and Limestone: Chemistry and Technology, Production and Uses; Wiley-VCH: Weinheim, Germany, 2008. [Google Scholar]
- Osiecka, E. Wapno w Budownictwie—Tradycja i Nowoczesność; Stowarzyszenie Przemysłu Wapienniczego: Kraków, Poland, 2006. [Google Scholar]
- Chilmon, K.; Woyciechowski, P.; Jaworska, B. The Effect of CaO Properties on the Early Volume Change Behavior, Compressive Strength and Microstructure of CaO-Activated GGBFS Composites. Case Stud. Constr. Mater. 2025, 23, e05467. [Google Scholar] [CrossRef]
- PN-EN 459-1:2015-06; Building Lime—Part 1: Definitions, Requirements and Conformity Criteria. Polish Committee for Standardization: Warsaw, Poland, 2015.
- PN-EN 197-1:2012; Cement—Part 1: Composition, Requirements and Conformity Criteria for Cements for General Use. Polish Committee for Standardization: Warsaw, Poland, 2012.
- PN-EN 459-2:2021-12; Building Lime—Part 2: Test Methods. Polish Committee for Standardization: Warsaw, Poland, 2021.
- PN-EN 196-3:2016-12; Test Methods for Cement—Part 3: Determination of Setting Times and Volume Constancy. Polish Committee for Standardization: Warsaw, Poland, 2016.
- PN-EN 196-1:2016-07; Methods of Testing Cement—Part 1: Determination of Strength. Polish Committee for Standardization: Warsaw, Poland, 2016.
- Provis, J.L.; van Deventer, J.S.J. Alkali Activated Materials: State-of-the-Art Report; RILEM TC 224-AAM; Springer: Dordrecht, The Netherlands, 2013. [Google Scholar]
- Provis, J.L.; van Deventer, J.S.J. Geopolymers: Structures, Processing, Properties and Industrial Applications; Elsevier: Oxford, UK, 2009. [Google Scholar]
- Shi, C.; Roy, D.; Krivenko, P. Alkali-Activated Cements and Concretes; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar]
- Woyciechowski, P. Influence of mineral additives on concrete carbonation. In Brittle Matrix Composites 10; Brandt, A.M., Ed.; Woodhead Publishing: Cambridge, UK, 2012; pp. 115–124. [Google Scholar]
- Jeon, D.; Moon, J. NaHCO3-Induced Carbonation Reaction for Enhancing the Properties of CaO-Activated GGBFS. Constr. Build. Mater. 2024, 438, 137076. [Google Scholar] [CrossRef]
- Tang, D.; Yang, C.; Li, X.; Zhu, X.; Yang, K.; Yu, L. Mitigation of Efflorescence of Alkali-Activated Slag Mortars by Incorporating Calcium Hydroxide. Constr. Build. Mater. 2021, 298, 123873. [Google Scholar] [CrossRef]
- Lei, J.; Law, W.W.; Yang, E.H. Effect of Calcium Hydroxide on the Alkali–Silica Reaction of Alkali-Activated Slag Mortars Activated by Sodium Hydroxide. Constr. Build. Mater. 2021, 272, 121868. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).