Stabilization Effect of Combined Stabilizing Agent on Heavy Metals in Hazardous Waste Incineration Fly Ash and Effect on Solidification Volume
Abstract
1. Introduction
2. Materials and Methods
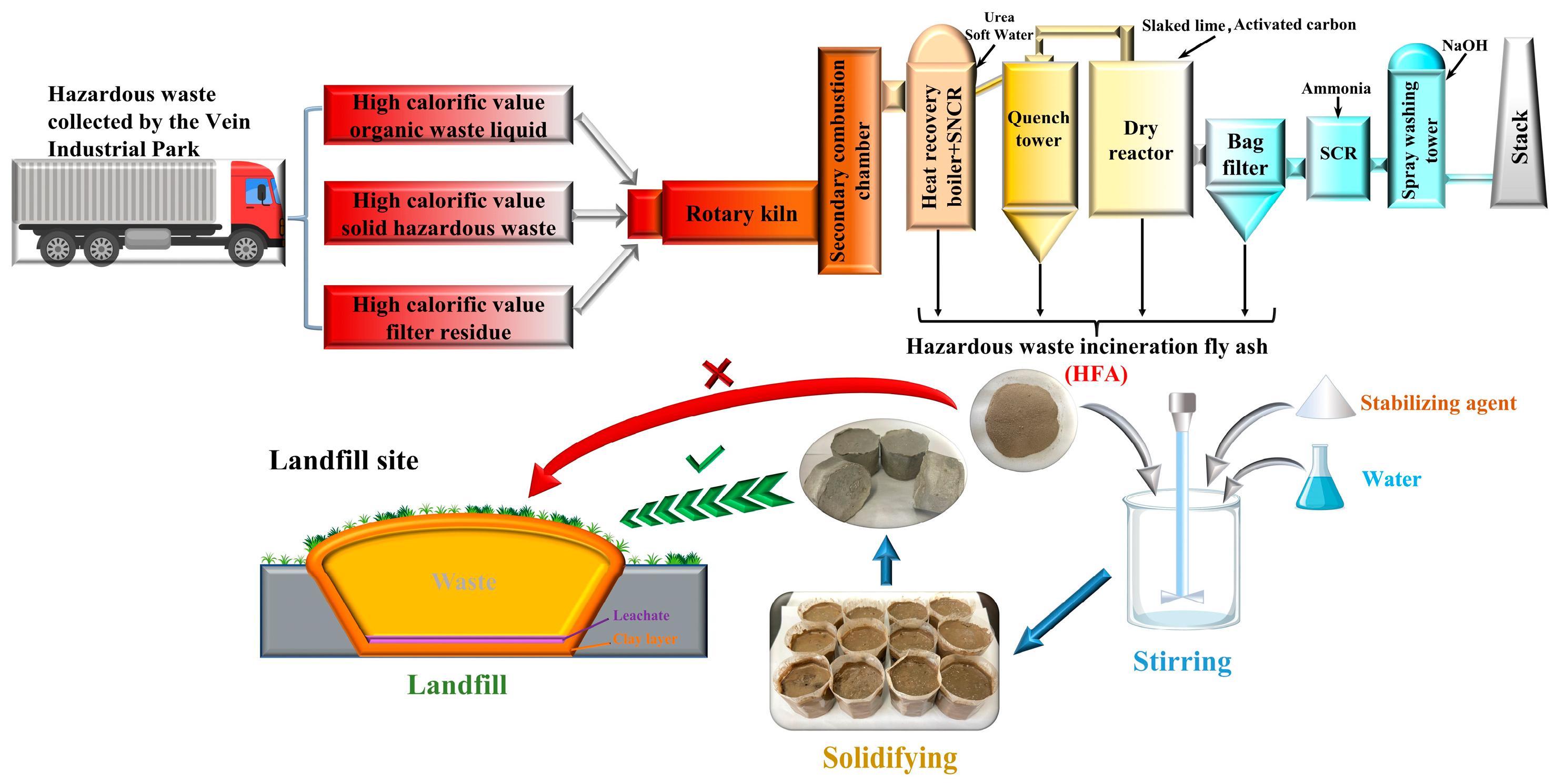
2.1. Materials
2.2. Methods
2.2.1. Characterization of Materials
2.2.2. Solidification and Stabilization Experiment
2.2.3. Heavy Metal Ablation and Heavy Metal Leaching Tests
2.2.4. Solidification Volume Expansion Ratio Detection
- (1)
- The quartz sand was first sieved to a particle size smaller than 60 mesh to ensure uniformity.
- (2)
- A standard mold with a volume of 0.001 m3 was used to contain a known volume of quartz sand, from which the bulk density of the sand was determined to be 1397.5 kg/m3.
- (3)
- Two identical 250 mL molds of cuboid shape (labeled as mold 1 and mold 2) were used. Mold 1 was completely filled with quartz sand, ensuring the sand was heaped above the rim before surface-leveling. The volume and mass are labeled as V1 and m1.
- (4)
- Approximately one-third of the sand from beaker 1 was transferred to the empty mold 2. The solidified specimen was then placed into mold 2, and additional quartz sand in mold 1 was poured in until mold 2 was full with surface leveling. The quartz sand remaining in mold 1 and the excess sand scraped off represent the volume of the solidified HFA product (the volume and mass are labeled as V and m2).
- (5)
- The mass of the remaining quartz sand was measured. The volume of the solidified specimen was calculated using the density formula and the bulk density value obtained in Step 2.
3. Results
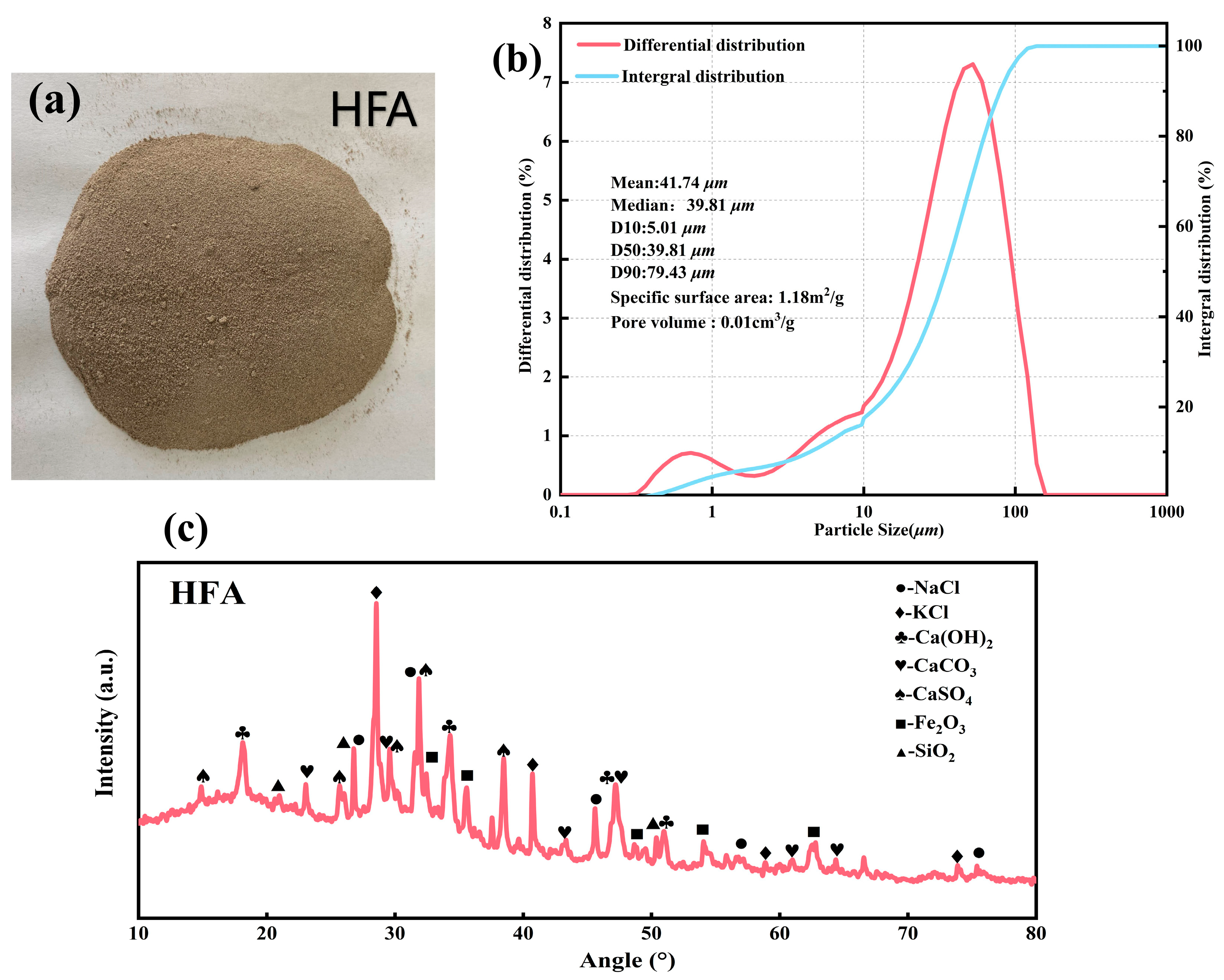
3.1. Original HFA Properties
3.1.1. Appearance and Particle Size Distribution of HFA
3.1.2. XRD Analysis of Original Fly Ash
3.1.3. XRF Analysis of HFA
3.1.4. Heavy Metal Content and Leaching Analysis
3.2. Analysis of Heavy Metal Stabilization Effect and Mechanism of Different Stabilizing Agents
3.2.1. Heavy Metal Stabilization by Stabilizing Agent Alone
3.2.2. Synergistic Solidification Effect of the Combined Stabilizing Agent on Heavy Metals
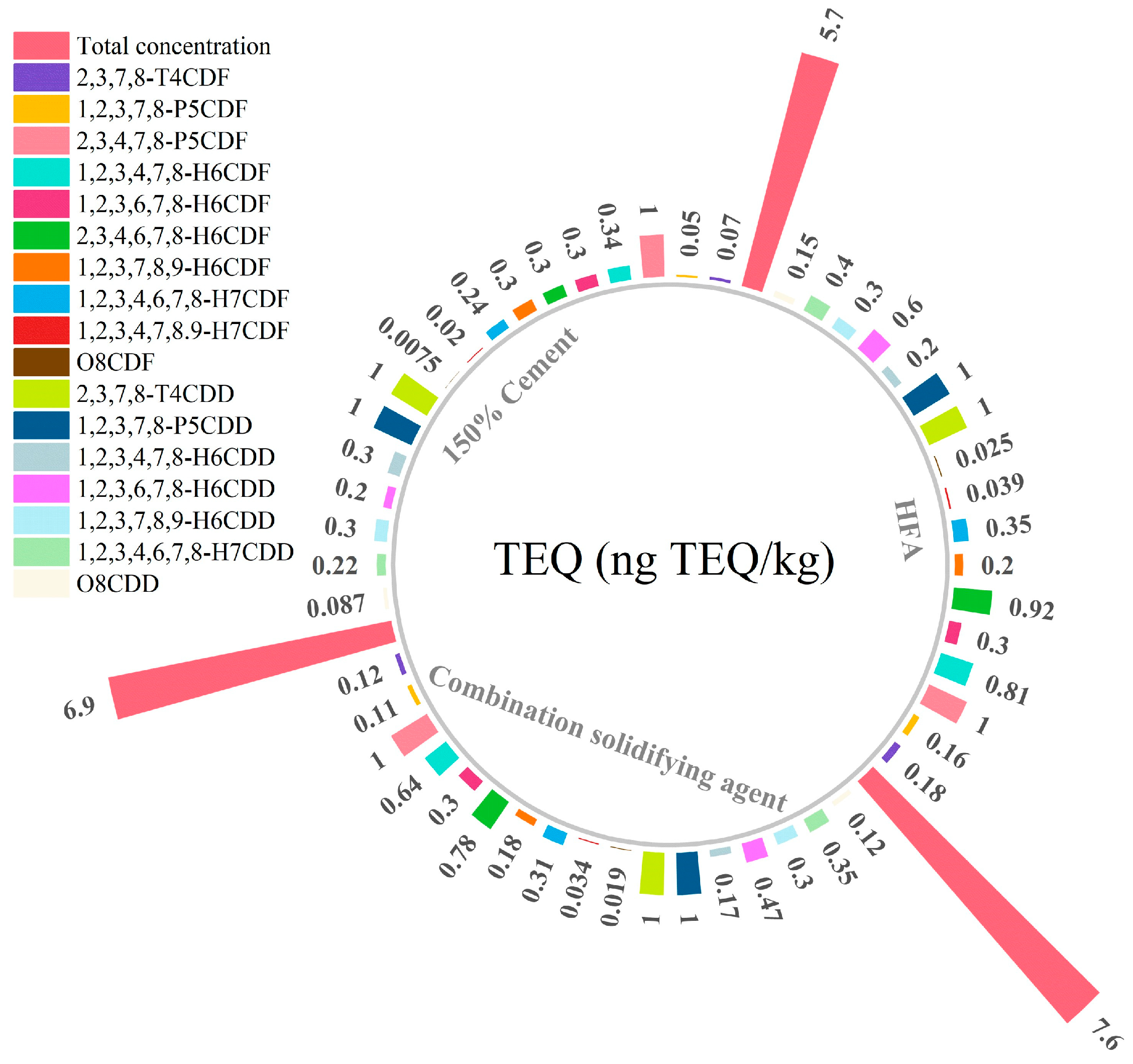
3.3. Change in Dioxin Content
3.4. Solidification Product Analysis
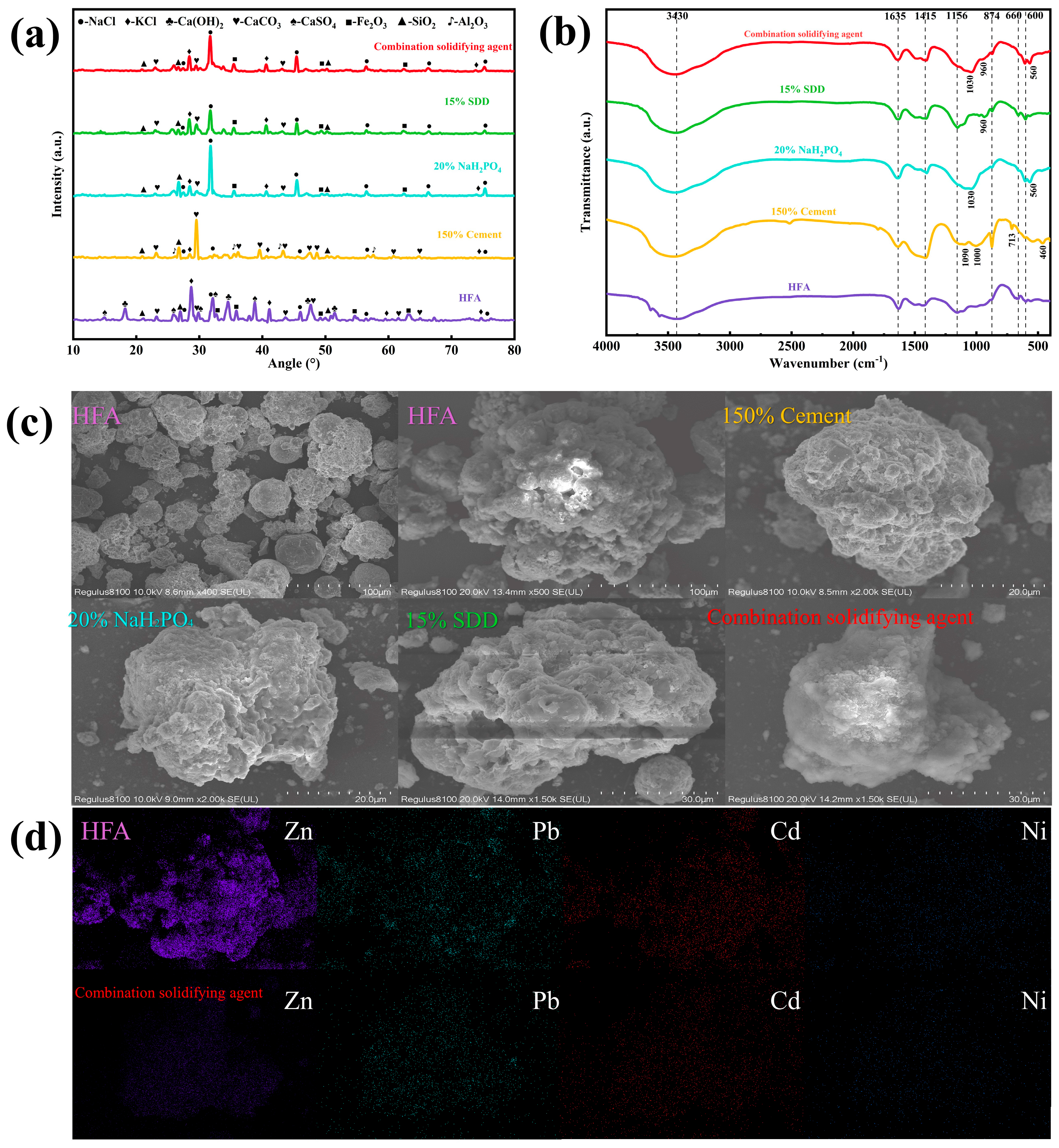
3.4.1. XRD Analysis
3.4.2. FTIR Analysis
3.4.3. Micromorphology and Element Distribution
4. Conclusions
- (1)
- The hazardous waste incineration fly ash produced through the new co-disposal process exhibits differences in appearance, chemical composition, and dioxin content compared to traditional fly ash, primarily due to distinct generation conditions. Notably, the heavy metal leachate from HFA surpasses the pollution control standards established for municipal landfills in China concerning the content of zinc, lead, cadmium, and nickel. Therefore, effective stabilization is imperative before landfill disposal.
- (2)
- Cement was found to exert a commendable stabilizing influence on Pb and Cd, achieving compliance with pollution control requirements at a cement addition rate of 150%. Nevertheless, it should be noted that this approach is associated with a relatively high volume change ratio, amounting to 2.7%.
- (3)
- Sodium dihydrogen phosphate can complement the deficiency of SDD in stabilizing Pb and reduce the quantity of SDD required. Simultaneously, cement can enhance the solidifying strength to a certain extent. When employing a combined stabilizing agent consisting of 5% cement, 15% SDD, and 10% NaH2PO4, the heavy metal concentration in the leachate from HFA falls below the Chinese municipal waste landfill pollution control standard. Furthermore, this combination ensures compliance with dioxin content disposal requirements. Importantly, it leads to a lower volume change ratio of approximately 1.31, rendering it a cost-effective and space-saving solution for landfill disposal.
- (4)
- The combined stabilizing agent demonstrates a strong binding capacity and stability when interacting with the target heavy metals. Analysis of XRD, FTIR, and SEM data reveals that the combined stabilizing agent can effectuate the transformation of heavy metals from unstable to stable states through precipitation, adsorption, and chelation reactions with HFA. Additionally, it partially adheres to the surface of HFA particles, thereby densifying the surface structure of the particles and substantially decreasing the potential for heavy metal leaching from HFA.
- (5)
- The findings of this study demonstrate a significant step forward in the sustainable management of hazardous waste. The proposed combined S/S system offers a multi-faceted sustainability benefit: (1) Environmental Sustainability: It ensures the long-term immobilization of heavy metals, preventing ecosystem pollution and protecting human health. (2) Technical and Economic Sustainability: By significantly reducing the dosage of cement and expensive chelators, it lowers the treatment cost and energy consumption associated with material production. More importantly, the controlled volume expansion (~1.31 times) directly translates to a substantial reduction in the demand for precious landfill space, which is a critical economic and environmental factor in urban areas.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shapiro-Bengtsen, S.; Andersen, F.M.; Münster, M.; Zou, L. Municipal solid waste available to the Chinese energy sector—Provincial projections to 2050. Waste Manag. 2020, 112, 52–65. [Google Scholar] [CrossRef]
- Tang, P.; Chen, W.; Xuan, D.; Cheng, H.; Poon, C.S.; Tsang, D.C.W. Immobilization of hazardous municipal solid waste incineration fly ash by novel alternative binders derived from cementitious waste. J. Hazard. Mater. 2020, 393, 122386. [Google Scholar] [CrossRef]
- Huber, F.; Blasenbauer, D.; Aschenbrenner, P.; Fellner, J. Chemical composition and leachability of differently sized material fractions of municipal solid waste incineration bottom ash. Waste Manag. 2019, 95, 593–603. [Google Scholar] [CrossRef]
- Liu, H.M.; Li, S.; Guo, G.Z.; Gong, L.F.; Zhang, L.Q.; Qie, Y.N.; Hu, H.Y.; Yao, H. Ash formation and the inherent heavy metal partitioning behavior in a 100 t/d hazardous waste incineration plant. Sci. Total Environ. 2022, 814, 151938. [Google Scholar] [CrossRef]
- Chen, L.; Wang, Y.S.; Wang, L.; Zhang, Y.; Li, J.; Tong, L.; Hu, Q.; Dai, J.G.; Tsang, D.C.W. Stabilisation/solidification of municipal solid waste incineration fly ash by phosphate-enhanced calcium aluminate cement. J. Hazard. Mater. 2021, 408, 124404. [Google Scholar] [CrossRef]
- Fan, C.C.; Wang, B.M.; Ai, H.M.; Qi, Y.; Liu, Z. A comparative study on solidification/stabilization characteristics of coal fly ash-based geopolymer and Portland cement on heavy metals in MSWI fly ash. J. Cleaner Prod. 2021, 319, 128790. [Google Scholar] [CrossRef]
- Fan, C.C.; Wang, B.M.; Zhang, T.T. Review on Cement Stabilization/Solidification of Municipal Solid Waste Incineration Fly Ash. Adv. Mater. Sci. Eng. 2018, 2018, 5120649. [Google Scholar] [CrossRef]
- Ji, Z.H.; Pei, Y.S. Geopolymers produced from drinking water treatment residue and bottom ash for the immobilization of heavy metals. Chemosphere 2019, 225, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.; Lyu, Y.; Wang, D.H.; Ju, Y.Z.; Shang, X.Y.; Li, L.K. Application of Fly Ash and Slag Generated by Incineration of Municipal Solid Waste in Concrete. Adv. Mater. Sci. Eng. 2020, 2020, 7802103. [Google Scholar] [CrossRef]
- Bie, R.S.; Chen, P.; Song, X.F.; Ji, X.Y. Characteristics of municipal solid waste incineration fly ash with cement solidification treatment. J. Energy Inst. 2016, 89, 704–712. [Google Scholar] [CrossRef]
- Wang, Y.S.; Dai, J.G.; Wang, L.; Tsang, D.C.W.; Poon, C.S. Influence of lead on stabilization/solidification by ordinary Portland cement and magnesium phosphate cement. Chemosphere 2018, 190, 90–96. [Google Scholar] [CrossRef]
- Li, T.R.; Wang, B.M.; Zhang, X.; Han, X.; Xing, Y.Q.; Fan, C.C.; Liu, Z. A novel method for solidification/stabilization of MSWI fly ash by graphene nanoplatelets synergistic alkali-activated technology. J. Environ. Chem. Eng. 2023, 11, 110589. [Google Scholar] [CrossRef]
- Ma, X.D.; He, T.S.; Da, Y.Q.; Xu, Y.D.; Luo, R.Y.; Yang, R.H. Improve toxicity leaching, physicochemical properties of incineration fly ash and performance as admixture by water washing. Constr. Build. Mater. 2023, 386, 131568. [Google Scholar] [CrossRef]
- Peng, Y.Q.; Lu, S.Y.; Li, X.D.; Yan, J.H.; Cen, K.F. Formation, Measurement, and Control of Dioxins from the Incineration of Municipal Solid Wastes: Recent Advances and Perspectives. Energ Fuel. 2020, 34, 13247–13267. [Google Scholar] [CrossRef]
- Pesonen, J.; Yliniemi, J.; Illikainen, M.; Kuokkanen, T.; Lassi, U. Stabilization/solidification of fly ash from fluidized bed combustion of recovered fuel and biofuel using alkali activation and cement addition. J. Environ. Chem. Eng. 2016, 4, 1759–1768. [Google Scholar] [CrossRef]
- Du, B.; Li, J.T.; Fang, W.; Liu, J.G. Comparison of long-term stability under natural ageing between cement solidified and chelator-stabilised MSWI fly ash. Environ. Pollut. 2019, 250, 68–78. [Google Scholar] [CrossRef]
- Sun, Y.Y.; Xu, C.B.; Yang, W.J.; Ma, L.K.; Tian, X.; Lin, A.J. Evaluation of a mixed chelator as heavy metal stabilizer for municipal solid-waste incineration fly ash: Behaviors and mechanisms. J. Chin. Chem. Soc. 2019, 66, 188–196. [Google Scholar] [CrossRef]
- Chen, W.M.; Wang, F.; Li, Z.; Li, Q.B. A comprehensive evaluation of the treatment of lead in MSWI fly ash by the combined cement solidification and phosphate stabilization process. Waste Manag. 2020, 114, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Liu, X.B.; Lin, Z.; Khalid, Z.; Long, L.; Jiang, X.G. MgO-based binders with different formulations for solidifying Pb and Cd in MSWI fly ash: Solidification effect and related mechanisms. Process Saf. Environ. 2023, 175, 160–167. [Google Scholar] [CrossRef]
- Vavva, C.; Voutsas, E.; Magoulas, K. Process development for chemical stabilization of fly ash from municipal solid waste incineration. Chem. Eng. Res. Des. 2017, 125, 57–71. [Google Scholar] [CrossRef]
- Yuan, W.Y.; Xu, W.T.; Wu, Z.B.; Zhang, Z.W.; Wang, L.C.; Bai, J.F.; Wang, X.Y.; Zhang, Q.W.; Zhu, X.F.; Zhang, C.L.; et al. Mechanochemical treatment of Cr(VI) contaminated soil using a sodium sulfide coupled solidification/stabilization process. Chemosphere 2018, 212, 540–547. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.J.; Guo, Y.P.; Yang, H.Y.; Wang, S.; Ding, H.; Qi, Y. Synthesis of a water-soluble thiourea-formaldehyde (WTF) resin and its application to immobilize the heavy metal in MSWI fly ash. J. Environ. Manag. 2016, 182, 328–334. [Google Scholar] [CrossRef]
- Ma, W.C.; Chen, D.M.; Pan, M.H.; Gu, T.B.; Zhong, L.; Chen, G.Y.; Yan, B.B.; Cheng, Z.J. Performance of chemical chelating agent stabilization and cement solidification on heavy metals in MSWI fly ash: A comparative study. J. Environ. Manag. 2019, 247, 169–177. [Google Scholar] [CrossRef]
- Zhang, M.L.; Guo, M.R.; Zhang, B.R.; Li, F.T.; Wang, H.T.; Zhang, H.B. Stabilization of heavy metals in MSWI fly ash with a novel dithiocarboxylate-functionalized polyaminoamide dendrimer. Waste Manag. 2020, 105, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Li, S.D.; Jiang, X.G.; Zhao, Y.M.; Yan, J.H. Disposal technology and new progress for dioxins and heavy metals in fly ash from municipal solid waste incineration: A critical review. Environ. Pollut. 2022, 311, 119878. [Google Scholar] [CrossRef]
- Zhu, J.M.; Hao, Q.J.; Chen, J.J.; Hu, M.L.; Tu, T.T.; Jiang, C.S. Distribution characteristics and comparison of chemical stabilization ways of heavy metals from MSW incineration fly ashes. Waste Manag. 2020, 113, 488–496. [Google Scholar] [CrossRef]
- Wang, L.; Cho, D.W.; Tsang, D.C.W.; Cao, X.; Hou, D.; Shen, Z.; Alessi, D.S.; Ok, Y.S.; Poon, C.S. Green remediation of As and Pb contaminated soil using cement-free clay-based stabilization/solidification. Environ. Int. 2019, 126, 336–345. [Google Scholar] [CrossRef]
- HJ 77.3-2008; Solid Waste Determination of Polychlorinated Dibenzo-P-Dioxins (PCDDs) and Polychlorinated Dibenzofurans (PCDFs) Isotope Dilution HRGC-HRMS. China Environmental Science Press: Beijing, China, 2008.
- HJ/T 300-2007; Solid Waste-Extraction Procedure for Leaching Toxicity-Acetic Acid Buffer Solution Method. China Environmental Science Press: Beijing, China, 2007.
- Raclavská, H.; Corsaro, A.; Hartmann-Koval, S.; Juchelková, D. Enrichment and distribution of 24 elements within the sub-sieve particle size distribution ranges of fly ash from wastes incinerator plants. J. Environ. Manag. 2017, 203, 1169–1177. [Google Scholar] [CrossRef]
- Sarkar, A.; Rano, R.; Mishra, K.K.; Sinha, I.N. Particle size distribution profile of some Indian fly ash—A comparative study to assess their possible uses. Fuel Process. Technol. 2005, 86, 1221–1238. [Google Scholar] [CrossRef]
- Sun, X.L.; Ou, Z.X.; Xu, Q.; Qin, X.; Guo, Y.C.; Lin, J.X.; Yuan, J.S. Feasibility analysis of resource application of waste incineration fly ash in asphalt pavement materials. Environ. Sci. Pollut. Res. 2023, 30, 5242–5257. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.N.; Luo, J.J.; Sun, S.Q. Characteristics of MSWI fly ash with acid leaching treatment. J. Fuel Chem. Technol. 2021, 49, 1208–1218. [Google Scholar] [CrossRef]
- Li, Z.G.; Kondoa, R.; Ikeda, K. Development of Foamed Geopolymer with Addition of Municipal Solid Waste Incineration Fly Ash. J. Adv. Concr. Technol. 2021, 19, 830–846. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Z.D.; Xie, G.M.; Li, Z.L.; Fan, X.; Zhang, W.Z.; Xing, F.; Tang, L.P.; Ren, J. Resource utilization of municipal solid waste incineration fly ash—Cement and alkali-activated cementitious materials: A review. Sci. Total Environ. 2022, 852, 158254. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Jiang, J.H.; Zheng, R.D.; Yu, C.M.; Zhou, Z.H.; Hantoko, D. Experimental study on the washing characteristics of fly ash from municipal solid waste incineration. Waste Manag. Res. 2021, 40, 1212–1219. [Google Scholar] [CrossRef]
- Nedkvitne, E.N.; Borgan, Ø.; Eriksen, D.Ø.; Rui, H. Variation in chemical composition of MSWI fly ash and dry scrubber residues. Waste Manage. 2021, 126, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Marieta, C.; Guerrero, A.; Leon, I. Municipal solid waste incineration fly ash to produce eco-friendly binders for sustainable building construction. Waste Manag. 2021, 120, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.D.; Zhang, Y.H.; Heberlein, S.; Lisak, G.; Yi, Y.L. Characterization and comparison of gasification and incineration fly ashes generated from municipal solid waste in Singapore. Waste Manag. 2022, 146, 44–52. [Google Scholar] [CrossRef]
- GB16889-2024; Standard for Pollution Control on the Landfill Site of Municipal Solid Waste. China Environmental Science Press: Beijing, China, 2024.
- Wei, X.K.; Xie, F.; Dong, C.L.; Wang, P.J.; Xu, J.Y.; Yan, F.; Zhang, Z.T. Safe disposal of hazardous waste incineration fly ash: Stabilization/solidification of heavy metals and removal of soluble salts. J. Environ. Manag. 2022, 324, 116246. [Google Scholar] [CrossRef]
- Zhao, X.Y.; Yang, J.Y.; Ning, N.; Yang, Z.S. Chemical stabilization of heavy metals in municipal solid waste incineration fly ash: A review. Environ. Sci. Pollut. Res. 2022, 29, 40384–40402. [Google Scholar] [CrossRef]
- Ren, Z.S.; Wang, L.; Wang, H.; Liu, S.H.; Liu, M. Solidification/stabilization of lead-contaminated soils by phosphogypsum slag-based cementitious materials. Sci. Total Environ. 2023, 857, 159552. [Google Scholar] [CrossRef]
- Tang, Q.; Liu, Y.; Gu, F.; Zhou, T. Solidification/Stabilization of Fly Ash from a Municipal Solid Waste Incineration Facility Using Portland Cement. Adv. Mater. Sci. Eng. 2016, 2016, 7101243. [Google Scholar] [CrossRef]
- Xu, D.D.; Huang, Y.; Jin, X.; Sun, T. Synergistic treatment of heavy metals in municipal solid waste incineration fly ash with geopolymer and chemical stabilizers. Process Saf. Environ. 2022, 160, 763–774. [Google Scholar] [CrossRef]
- Crannell, B.S.; Eighmy, T.T.; Krzanowski, J.E.; Eusden, J.D.; Shaw, E.L.; Francis, C.A. Heavy metal stabilization in municipal solid waste combustion bottom ash using soluble phosphate. Waste Manag. 2000, 20, 135–148. [Google Scholar] [CrossRef]
- Xin, M.X.; Sun, Y.J.; Wu, Y.K.; Li, W.H.; Yin, J.Q.; Long, Y.Y.; Wang, X.B.; Wang, Y.N.; Huang, Y.M.; Wang, H.W. Stabilized MSW incineration fly ash co-landfilled with organic waste: Leaching pattern of heavy metals and related influencing factors. Process Saf. Environ. 2022, 165, 445–452. [Google Scholar] [CrossRef]
- Liu, W.G.; Duan, H.; Wei, D.Z.; Cui, B.Y.; Wang, X.Y. Stability of diethyl dithiocarbamate chelates with Cu(II), Zn(II) and Mn(II). J. Mol. Struct. 2019, 1184, 375–381. [Google Scholar] [CrossRef]
- Xiao, H.Z.; Wang, G.F.; Liang, G.C.; Zhu, J.L.; Qiu, J.; Ding, C.H.; Komarneni, S. Stabilization of heavy metals from lead-zinc ore tailings with sodium diethyl dithiocarbamate functionalized montmorillonite (DDTC-Mt): Leaching characteristics and remediating mechanism. Miner. Eng. 2022, 183, 107608. [Google Scholar] [CrossRef]
- Tian, Y.X.; Themelis, N.J.; Zhao, D.D.; Thanos Bourtsalas, A.C.; Kawashima, S. Stabilization of Waste-to-Energy (WTE) fly ash for disposal in landfills or use as cement substitute. Waste Manag. 2022, 150, 227–243. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Deng, Z.; Wang, W.; Fang, H.; Zhou, H.; Deng, F.; Huang, L.; Li, H. Degradation characteristics of dioxin in the fly ash by washing and ball-milling treatment. J. Hazard. Mater. 2017, 339, 191–199. [Google Scholar] [CrossRef]
- Pan, Y.; Yang, L.B.; Zhou, J.Z.; Liu, J.Y.; Qian, G.R.; Ohtsuka, N.; Motegi, M.; Oh, K.; Hosono, S. Characteristics of dioxins content in fly ash from municipal solid waste incinerators in China. Chemosphere 2013, 92, 765–771. [Google Scholar] [CrossRef]
- Wei, G.X.; Liu, H.Q.; Liu, F.; Zang, D.D.; Liu, G.S.; Zhu, Y.W. Effect of flotation on the dioxin distribution in size-fractioned fly ash of hospital solid waste incineration. Sep. Sci. Technol. 2017, 52, 2622–2631. [Google Scholar] [CrossRef]
- Wu, S.M.; Zhou, J.Z.; Pan, Y.; Zhang, J.; Zhang, L.G.; Ohtsuka, N.; Motegi, M.; Yonemochi, S.; Oh, K.; Hosono, S.; et al. Dioxin distribution characteristics and health risk assessment in different size particles of fly ash from MSWIs in China. Waste Manag. 2016, 50, 113–120. [Google Scholar] [CrossRef]
- Zhang, D.; Yang, Z.J.; Kang, D.; Fang, C.Y.; Jiao, Y.; Mi, S.Z. Experimental Study on Subgrade Material of Calcium Silicate Slag. Materials 2022, 15, 2304. [Google Scholar] [CrossRef]
- Zhang, J.J.; Sun, G.W.; Wang, C.H.; Zhang, Y.; Wang, P.S.; Yan, N. Activation effects and micro quantitative characterization of high-volume ground granulated blast furnace slag in cement-based composites. Cem. Concr. Compos. 2020, 109, 103556. [Google Scholar] [CrossRef]
- Zhu, Z.H.; Guo, Y.Y.; Zhao, Y.C.; Zhou, T. A novel waste-recycled chelating agent for the stabilization of lead in municipal solid waste incineration fly ash: Preparation, feasibility, and mechanism analysis. J. Hazard. Mater. 2022, 427, 127914. [Google Scholar] [CrossRef]
- Jiang, J.G.; Xu, X.; Wang, J.; Yang, S.J.; Zhang, Y. Investigation of basic properties of fly ash from urban waste incinerators in China. J. Environ. Sci. 2007, 19, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Xiang, J.; Hu, S.; Sun, L.S.; Su, S.; Li, P.S.; Sun, X.X. Characterization of solid residues from municipal solid waste incinerator. Fuel 2004, 83, 1397–1405. [Google Scholar] [CrossRef]
- Tian, X.; Rao, F.; León-Patiño, C.A.; Song, S. Effects of aluminum on the expansion and microstructure of alkali-activated MSWI fly ash-based pastes. Chemosphere 2020, 240, 124986. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.C.; Min, X.B.; Ke, Y.; Liu, D.G.; Tang, C.J. Preparation of red mud-based geopolymer materials from MSWI fly ash and red mud by mechanical activation. Waste Manag. 2019, 83, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhao, Q.X.; Guo, W.C.; Xue, C.H.; Bai, Y.Y.; Pan, H.M.; Qiu, Y.X. Preparation and characterization of mortar specimens based on municipal solid waste incineration fly ash-activated slag. J. Build. Eng. 2023, 69, 106254. [Google Scholar] [CrossRef]
- Moghal, A.A.B. State-of-the-Art Review on the Role of Fly Ashes in Geotechnical and Geoenvironmental Applications. J. Mater. Civ. Eng. 2017, 29, 04017072. [Google Scholar] [CrossRef]
- Yuan, Q.X.; Yang, G.; Zhang, Y.S.; Wang, T.; Wang, J.W.; Romero, C.E. Supercritical CO2 coupled with mechanical force to enhance carbonation of fly ash and heavy metal solidification. Fuel 2022, 315, 123154–123156. [Google Scholar] [CrossRef]
- Tian, Z.P.; Zhang, B.R.; He, C.J.; Tang, R.Z.; Zhao, H.P.; Li, F.T. The physiochemical properties and heavy metal pollution of fly ash from municipal solid waste incineration. Process Saf. Environ. 2015, 98, 333–341. [Google Scholar]
- Huang, T.; Zhou, L.L.; Chen, L.; Liu, W.H.; Zhang, S.W.; Liu, L.F. Mechanism exploration on the aluminum supplementation coupling the electrokinetics-activating geopolymerization that reinforces the solidification of the municipal solid waste incineration fly ashes. Waste Manag. 2020, 103, 361–369. [Google Scholar] [CrossRef] [PubMed]
| Composition | wt./% | Composition | wt./% |
|---|---|---|---|
| CaO | 32.55 | Na2O | 3.94 |
| Cl | 15.76 | ZnO | 3.54 |
| SO3 | 9.97 | Al2O3 | 2.13 |
| SiO2 | 9.04 | MgO | 1.32 |
| K2O | 6.63 | PbO | 0.07 |
| Fe2O3 | 6.12 | CuO | 0.11 |
| P2O5 | 5.64 | Others | 3.18 |
| No. 1 | Stabilizing Agent | Heavy Metal Leaching Concentration (mg/L) | RVE | |||||
|---|---|---|---|---|---|---|---|---|
| Pb | Zn | Cd | Ni | Cu | Cr | |||
| 01 | - | 0.78 | 244.75 | 0.25 | 0.55 | 8.78 | 0.18 | 1 |
| 1 | 1% Cement | 0.71 ± 0.048 | 236.51 ± 5.754 | 0.23 ± 0.030 | 0.53 ± 0.059 | 5.84 ± 0.555 | 0.17 ± 0.019 | 1.13 ± 0.017 |
| 2 | 5% Cement | 0.56 ± 0.069 | 200.84 ± 7.530 | 0.20 ± 0.004 | 0.51 ± 0.071 | 5.05 ± 0.559 | 0.15 ± 0.018 | 1.17 ± 0.038 |
| 3 | 10% Cement | 0.34 ± 0.044 | 190.64 ± 7.425 | 0.16 ± 0.055 | 0.47 ± 0.029 | 4.29 ± 0.104 | 0.13 ± 0.015 | 1.26 ± 0.029 |
| 4 | 50% Cement | 0.05 ± 0.006 | 146.62 ± 5.870 | 0.05 ± 0.011 | 0.36 ± 0.066 | 1.32 ± 0.241 | 0.14 ± 0.032 | 1.69 ± 0.040 |
| 5 | 100% Cement | n.d. 3 | 107.42 ± 3.443 | 0.01 ± 0.001 | 0.31 ± 0.017 | 0.24 ± 0.037 | 0.13 ± 0.027 | 2.14 ± 0.035 |
| 6 | 150% Cement | n.d. | 68.72 ± 4.048 | 0.02 ± 0.004 | 0.30 ± 0.029 | 0.14 ± 0.014 | 0.12 ± 0.010 | 2.7 ± 0.158 |
| 7 | 200% Cement | n.d. | 39.16 ± 3.038 | 0.01 ± 0.002 | 0.32 ± 0.074 | 0.11 ± 0.004 | 0.10 ± 0.009 | 3.17 ± 0.125 |
| 8 | 5% NaH2PO4 | 0.27 ± 0.017 | 122.8 ± 7.075 | 0.09 ± 0.014 | 0.39 ± 0.038 | 3.79 ± 0.597 | 0.11 ± 0.008 | 1.16 ± 0.019 |
| 9 | 10% NaH2PO4 | 0.03 ± 0.004 | 111.2 ± 7.262 | 0.09 ± 0.011 | 0.36 ± 0.054 | 2.75 ± 0.214 | 0.08 ± 0.012 | 1.23 ± 0.006 |
| 10 | 15% NaH2PO4 | 0.01 ± 0.002 | 98.47 ± 7.510 | 0.05 ± 0.011 | 0.32 ± 0.041 | 2.20 ± 0.213 | 0.08 ± 0.016 | 1.28 ± 0.029 |
| 11 | 20% NaH2PO4 | n.d. | 80.13 ± 7.669 | 0.05 ± 0.010 | 0.31 ± 0.051 | 1.67 ± 0.224 | 0.11 ± 0.017 | 1.28 ± 0.009 |
| 12 | 25% NaH2PO4 | n.d. | 78.90 ± 5.060 | 0.07 ± 0.013 | 0.27 ± 0.019 | 1.33 ± 0.200 | 0.11 ± 0.003 | 1.28 ± 0.022 |
| 13 | 5% SDD | 0.52 ± 0.031 | 89.33 ± 0.565 | 0.21 ± 0.011 | 0.45 ± 0.010 | 1.35 ± 0.208 | 0.12 ± 0.012 | 1.19 ± 0.010 |
| 14 | 10% SDD | 0.35 ± 0.061 | 47.35 ± 4.952 | 0.17 ± 0.006 | 0.37 ± 0.069 | 0.8 ± 0.173 | 0.11 ± 0.018 | 1.26 ± 0.007 |
| 15 | 15% SDD | 0.21 ± 0.022 | 17.60 ± 1.385 | 0.12 ± 0.012 | 0.30 ± 0.012 | 0.45 ± 0.102 | 0.10 ± 0.016 | 1.31 ± 0.012 |
| 16 | 20% SDD | 0.15 ± 0.020 | 14.15 ± 2.249 | 0.07 ± 0.007 | 0.25 ± 0.038 | 0.2 ± 0.022 | 0.13 ± 0.022 | 1.31 ± 0.010 |
| 17 | 25% SDD | n.d. | 11.25 ± 1.249 | 0.05 ± 0.008 | 0.15 ± 0.035 | 0.2 ± 0.026 | 0.1 ± 0.026 | 1.33 ± 0.017 |
| Limit 2 | 0.25 | 100 | 0.15 | 0.5 | 40 | 4.5 | ||
| No. 1 | Stabilizing Agent | Heavy Metal Leaching Amount (mg/L) | RVE | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cement | SDD | NaH2PO4 | Pb | Zn | Cd | Ni | Cu | Cr | ||
| 01 | - | - | - | 0.78 | 244.75 | 0.25 | 0.55 | 8.78 | 0.18 | 1 |
| 1 | 5% | 5% | 5% | 0.27 ± 0.007 | 59.09 ± 2.440 | 0.12 ± 0.020 | 0.30 ± 0.035 | 0.25 ± 0.020 | 0.1 ± 0.009 | 1.30 ± 0.019 |
| 2 | 5% | 5% | 10% | 0.05 ± 0.007 | 43.25 ± 1.967 | 0.10 ± 0.004 | 0.15 ± 0.014 | 0.11 ± 0.009 | 0.12 ± 0.023 | 1.32 ± 0.012 |
| 3 | 5% | 5% | 15% | n.d. 3 | 39.35 ± 0.756 | 0.15 ± 0.036 | 0.09 ± 0.010 | 0.20 ± 0.015 | 0.1 ± 0.019 | 1.33 ± 0.013 |
| 4 | 5% | 5% | 20% | n.d. | 36.15 ± 1.135 | 0.11 ± 0.021 | 0.10 ± 0.007 | 0.05 ± 0.011 | 0.1 ± 0.016 | 1.35 ± 0.008 |
| 5 | 5% | 10% | 5% | 0.15 ± 0.005 | 22.45 ± 0.644 | 0.16 ± 0.037 | 0.25 ± 0.048 | 0.20 ± 0.002 | 0.1 ± 0.007 | 1.31 ± 0.010 |
| 6 | 5% | 10% | 10% | 0.08 ± 0.004 | 23.35 ± 0.996 | 0.14 ± 0.036 | 0.20 ± 0.030 | 0.15 ± 0.026 | 0.12 ± 0.010 | 1.32 ± 0.014 |
| 7 | 5% | 10% | 15% | 0.03 ± 0.005 | 9.90 ± 0.216 | 0.15 ± 0.009 | 0.15 ± 0.009 | 0.13 ± 0.029 | 0.13 ± 0.004 | 1.34 ± 0.012 |
| 8 | 5% | 10% | 20% | n.d. | 13.81 ± 0.196 | 0.05 ± 0.004 | 0.11 ± 0.009 | 0.10 ± 0.007 | 0.13 ± 0.008 | 1.36 ± 0.014 |
| 9 | 5% | 15% | 5% | 0.05 ± 0.004 | 15.05 ± 0.541 | 0.05 ± 0.006 | 0.22 ± 0.019 | 0.25 ± 0.024 | 0.05 ± 0.004 | 1.33 ± 0.006 |
| 10 | 5% | 15% | 10% | 0.02 ± 0.002 | 13.51 ± 0.705 | 0.07 ± 0.011 | 0.10 ± 0.021 | 0.15 ± 0.026 | 0.02 ± 0.002 | 1.31 ± 0.013 |
| 11 | 5% | 15% | 15% | n.d. | 14.60 ± 0.615 | 0.04 ± 0.003 | 0.11 ± 0.016 | 0.15 ± 0.006 | 0.05 ± 0.006 | 1.32 ± 0.009 |
| 12 | 5% | 15% | 20% | n.d. | 14.65 ± 0.060 | 0.05 ± 0.004 | 0.10 ± 0.009 | n.d. | n.d. | 1.35 ± 0.015 |
| 13 | 5% | 20% | 5% | 0.03 ± 0.004 | 10.30 ± 0.558 | 0.05 ± 0.004 | 0.15 ± 0.005 | 0.15 ± 0.008 | 0.1 ± 0.012 | 1.31 ± 0.018 |
| 14 | 5% | 20% | 10% | 0.01 ± 0.002 | 12.45 ± 1.051 | 0.07 ± 0.003 | 0.12 ± 0.019 | 0.15 ± 0.023 | 0.15 ± 0.014 | 1.32 ± 0.010 |
| 15 | 5% | 20% | 15% | n.d. | 16.58 ± 0.575 | 0.04 ± 0.002 | 0.10 ± 0.017 | 0.15 ± 0.002 | 0.15 ± 0.010 | 1.34 ± 0.016 |
| 16 | 5% | 20% | 20% | n.d. | 14.70 ± 1.246 | 0.05 ± 0.07 | 0.13 ± 0.019 | 0.10 ± 0.012 | 0.12 ± 0.012 | 1.38 ± 0.013 |
| Limit 2 | 0.25 | 100 | 0.15 | 0.5 | 40 | 4.5 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Z.; Huang, X.; Wang, L. Stabilization Effect of Combined Stabilizing Agent on Heavy Metals in Hazardous Waste Incineration Fly Ash and Effect on Solidification Volume. Sustainability 2025, 17, 9926. https://doi.org/10.3390/su17229926
Zhao Z, Huang X, Wang L. Stabilization Effect of Combined Stabilizing Agent on Heavy Metals in Hazardous Waste Incineration Fly Ash and Effect on Solidification Volume. Sustainability. 2025; 17(22):9926. https://doi.org/10.3390/su17229926
Chicago/Turabian StyleZhao, Zhen, Xiaofan Huang, and Lei Wang. 2025. "Stabilization Effect of Combined Stabilizing Agent on Heavy Metals in Hazardous Waste Incineration Fly Ash and Effect on Solidification Volume" Sustainability 17, no. 22: 9926. https://doi.org/10.3390/su17229926
APA StyleZhao, Z., Huang, X., & Wang, L. (2025). Stabilization Effect of Combined Stabilizing Agent on Heavy Metals in Hazardous Waste Incineration Fly Ash and Effect on Solidification Volume. Sustainability, 17(22), 9926. https://doi.org/10.3390/su17229926





