Abstract
Biological processes may generate CO2, CH4, and N2O. Few studies have evaluated the impact of vermifilters (VFs) on the generation of these gases. The objective of this study was to evaluate the GHG emissions of a full-scale VF used for sewage treatment, as well as the effects of seasonality and operational condition. The study monitored the influent and effluent of a VF in a rural area. Emissions fluxes were measured using the static chamber method in fall–winter and spring–summer. The results showed that in terms of annual per capita emissions (kgCO2eq/cap·y), VFs generated less GHGs than conventional and non-conventional wastewater treatment plants (WWTPs), with CO2, CH4, and N2O emissions ranging between 0.8 and 7.5 kg/cap·y, 0.1–0.5 kgCO2eq/cap·y, and 5.7–9.5 kgCO2eq/cap·y, respectively. Regarding the effects of seasonality, CO2 increased by 139% in spring–summer compared to fall–winter, while N2O increased by 139% in fall–winter compared to spring–summer. A positive correlation between influent COD concentrations and CO2 emissions (r = 0.7) was observed, whereas the influent carbon/nitrogen ratio (C/N) and N2O emissions (r = −0.6) presented a negative correlation. These results evidenced that seasonality and sewage characteristics influenced GHG emissions in a full-scale VF.
1. Introduction
Greenhouse gas (GHG) alters the temperature of the planet, affecting climate stability, society, and ecosystems [1,2]. Among the seven gases considered by the IPCC to be the main emissions, CO2, CH4, and N2O are the most studied, since they represent 66%, 16%, and 7% of GHGs, respectively [3,4]. Regarding warming potential, CO2 has a significant greenhouse effect on the planet; however, over a 100-year time horizon, CH4 and N2O have warming potentials 23 and 296 times greater than CO2 [5]. GHGs from wastewater treatment plants (WWTPs) account for 1.5% of CO2 and 5% of non-CO2 GHG emissions and are expected to reach 42% of waste-related emissions by 2030 [6,7], meaning that the operation of these facilities has a significant effect on the climate [8]. Although these figures account for the impact of the wastewater treatment sector, most studies are based on estimates that consider conventional WWTPs such as activated sludge (AS) and anaerobic sludge (AN) facilities installed in urban areas, disregarding plants in rural areas [9].
In 2020, there was no safe treatment for 45% of the wastewater generated in the world, much of which is concentrated in rural areas with low-density, dispersed populations [10,11]. To avoid contamination in these low-population areas, decentralized technologies that treat wastewater at the source or near the source of generation are used [12]. Constructed wetlands (CWs), soil treatment units (STUs), and vermifilters (VFs) are nature-based decentralized technologies [13], with VFs being systems involving earthworms that, by interacting with microorganisms, change the physicochemical properties of wastewater [10]. Earthworms are used in vermifilters to accelerate processes that occur in soil filtration during wastewater treatment [14]. Ingestion, excavation, and excretion by earthworms reinforce this relationship and generate aerobic environments that promote the growth of microorganisms. Some studies have reported removal efficiencies of 83.4% for organic matter measured as chemical oxygen demand (COD), 54.1% for total nitrogen (TN), 96.6% for ammonium (NH4+), and 52.2% for nitrate (NO3−) [15,16,17]. However, few studies have evaluated the impact of these systems on GHG generation [18]. The role of earthworms in the generation of emissions is unclear. Some studies claim that earthworms could increase CO2 and N2O emissions from the soil by 33% and 42% [19]. While CO2 and CH4 are related to overall decomposition, N2O occurs during a particular type of decomposition (denitrification) that requires anaerobic conditions and is also associated with intermediate steps of dissimilatory nitrate reduction [20]; however, it can be produced by ammonia-oxidizing archaea through nitrification [5]. In contrast, other studies have shown that earthworms inhibit methane generation due to the burrowing behavior of earthworms that increase oxygen and stimulate the methanotrophic community (i.e., −3.3 and −9.7 mg CH4/day [21]). Regarding CO2, they can reduce emissions by increasing carbon storage through decomposition of organic matter and carbon stabilization through the formation of microaggregates and macroaggregates [22]. Ingestion and subsequent peristalsis destroy the pre-existing microstructure, but during gut transit, clay minerals and organic materials are mixed and become encrusted with mucus to create a new nucleus for microaggregate formation [23,24]. However, stabilization depends on the organic load and earthworm density of the medium [22]. Zhao et al. [25] and Huang et al. [26] showed that CO2, CH4, and N2O emissions in VFs depend on earthworm density and the C/N ratio of the influent. While decreasing C/N favors incomplete denitrification processes and generates N2O, an increase in earthworm density can increase CO2 and limit CH4 generation to 3% of CO2 [10]. Additionally, seasonal temperature variation is an important factor for the growth and metabolic activity of earthworms and microorganisms [27]. VFs operating during warm seasons (15–25 °C) have been reported to enhance the decomposition of organic matter by 15% due to increased microbial and earthworm activity [28]. In contrast, during cold seasons, low temperatures inhibit earthworm reproduction and induce hibernation, reducing overall metabolic performance [14]. These seasonal changes can lead to higher CO2 emissions in warm seasons, driven by intensified metabolic activity and elevated respiration rates within the VF [27,29]. Some studies conducted in conventional WWTPs indicate that N2O emissions increase the emissions factor by 1,8% in the cold season, possibly due to nitrification [30,31]. However, no studies have addressed these seasonal variations in vermifiltration systems (VFs). Therefore, GHG generation depends on operational conditions such as the earthworm density, temperature, C/N and the organic load of the influent (COD). Thus, the objective of this study is to evaluate the GHG emissions of a full-scale VF and determine the effects of seasonality and specific operational conditions of wastewater called sewage parameters (i.e., C/N and influent COD concentrations) on GHG emissions.
2. Materials and Methods
2.1. Design and Operating Conditions
This study was carried out in a rural WWTP at Copiulemu operated by the Municipality of Florida, Concepción, Biobío Region, Chile. The WWTP uses a sequential system that includes pretreatment for the removal of solids, followed by VF and ultraviolet radiation as a disinfection system. The VF is a vertical subsurface flow system. Wastewater enters a rotary filter that has a helical screw to sweep solids from an external container into the VF. Once treated, this wastewater is stored in an accumulator tank and transferred via two pumps to the upper part of the VF, from which it is distributed over the surface via 24 sprinklers. The VF of 276 m2 (A) operates with a flow (Q), hydraulic loading rate (HLR), and organic loading rate (OLR) of 160 m3/d, 0.6 m3/m2·d, and 0.5 kgCOD/m2·d, respectively. The filter material is stratified, with a lower layer of stones and an upper layer of wood chips measuring 0.92 ± 0.12 m. The latter layer is the active layer, which contains earthworms of the species Eisenia foetida, along with heterotrophic and autotrophic microorganisms responsible for biological reactions [32,33]. The monitored earthworm density was between 1105 and 7221 earthworms/m3.
2.2. Monitoring Strategy
The VF was installed in 2020; however, this study involved monitoring during 2022 (Period I), 2023 (Period II), and 2024 (Period III), with a sampling frequency of two to three months during the fall–winter (April to August, N = 7) and spring–summer (October to March, N = 8) of the southern hemisphere. The wood chips were replaced at the end of Period II. Figure 1 shows a schematic of the VF and the emissions monitoring strategy. GHG samples (CO2, CH4, and N2O) from between the active layer and the atmosphere were measured using the static chamber method, which involves the use of a cylinder with a lid that is 10 cm in diameter and 35 cm high to enclose a volume of atmosphere from the topsoil [34]. The cylinder was buried 5 cm deep to collect samples in vials using a 60 mL syringe attached to the chamber by a hose. The sampling frequency was every 15 min for 1 h. Solid samples of the active layer were analyzed at different times of use in Period II; and to determine the effect of wood chip replacement on the VF, samples were taken at different times in Period III (0, 2, and 8 months). For the analysis, 5 subsamples were taken at different points until 5 kg had been collected. Additionally, wastewater samples were taken from the influent (IN) at the pretreatment outlet and from the effluent (E) at the VF outlet. All samples were stored at 4 °C for less than 24 h before analysis.
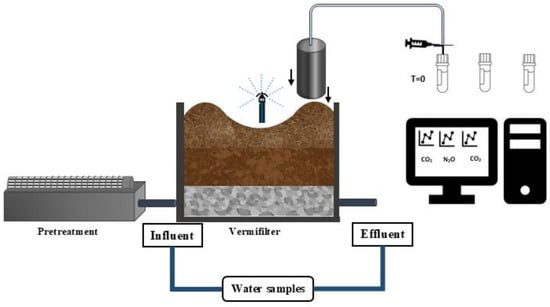
Figure 1.
Schematic representation of a VF and gas measurement.
2.3. Analysis of Gas Samples
The sample vials were collected only in 2024 in fall–winter (May and August) and spring–summer (December and March). A gas chromatographic analysis was then performed [35]. The equipment used was a gas chromatograph Shimadzu with a methanizer, a flame ionization detector, and an electron capture detector. The flux of CO2, CH4, and N2O between the soil surface and the atmosphere inside the chamber was calculated using Equation (1) [36]:
where f represents the flux of CO2, CH4, and N2O (mg/m2·h), ∆C is the change in gas mass (mg) inside the chamber due to the change in time (∆t), P is atmospheric pressure (atm) inside the chamber (assumed to be 1 atm), V is the volume of the chamber (L), R is the ideal gas constant (0.08205 atm·L/mol·°K), T is the temperature (°K) inside the chamber, and A is the area of the chamber (m2). Table 1 shows the emissions factors resulting from the static chamber measurements. These factors and the removal efficiency of COD and TN in the VF influent were considered to estimate the GHGs for Periods I, II, and III.

Table 1.
Vermifilter emissions factors in spring–summer and fall–winter (CO2, CH4, N2O).
The emissions factor (EF) is defined as the mass (kg) of CO2, CH4, or N2O produced per kilogram of COD or TN removed, and the corresponding equations are as follows (Equations (2)–(4)):
In the formula, CODinf and CODeff are inlet and outlet COD (mg/L); TNinf and TNeff are inlet and outlet total nitrogen (mg/L); MCO2, MCH4, and MN2O, respectively, are CO2, CH4, and N2O release amounts (kg/d).
To compare GHG generation in conventional WWTP, studies reporting GHG emissions normalized on per capita emissions per year were selected (kgCO2 eq/cap·y). In particular, Ranieri et al. [8] analyzed emissions from more than 180 WWTPs, while Ross et al. [37] quantified gas fluxes and normalized emissions on a per capita per year basis. Regarding non-conventional technologies, less information is available; therefore, publications from 2011 to 2024 that reported emissions normalized on a per capita per year basis (kgCO2 eq/cap·y) or provided information on system design and measured GHG fluxes were also included. For converting emissions fluxes to per capita GHG emissions (PCGHG), the following equation was used (Equation (5)):
In the formula, f represents the average flux of CO2, CH4, and N2O (mg/m2·h), A is the surface area of the VF, and PE is the population served. In this study, the system serves a population of 1000 people.
2.4. Analysis of Liquid Samples
The liquid samples, IN and E, were filtered using a Whatman membrane with a pore size of 0.7 µm and analyzed based on the protocol described in the standard method [38]. The organic matter present in the samples was identified in the form of COD (colorimetric method, 5220-D), biological oxygen demand (BOD5) (azide-modified Winkler method, 5210-B), and total organic carbon (TOC) (catalytic combustion oxidation and non-dispersive infrared detection method). The analyzed nutrients were measured in the form of NH4+-N, NO3−-N, NO2−-N (spectrophotometer UV–Vis Shimadzu UV 1800, Kyoto, Japan), and total nitrogen (TN) (Spectroquant-Nova 60, kits Merck, Darmstadt, Germany).
2.5. Analysis of Solid Samples
Solid samples of the active layer were analyzed at different times of use. Organic carbon in the wood chip active layer was measured via calcination loss at 550 °C [39]. NH4+-N and NO3−-N were measured using the KCl 2 mol/L extraction method and colorimetry in a Skalar autoanalyzer [40]. Total nitrogen was measured using the Kjeldahl method [41].
2.6. Mass Balance Analysis
The mass balances used the average of the data for 2022 (Period I), 2023 (Period II), and 2024 (Period III). Furthermore, they were calculated in terms of TOC and TN using Equation (6) [42]:
where Ci is the influent concentration of COT or TN (kg/L), Qi is the influent flow (L/d), Storage is the biomass growth and retention, Emission is the the mass of carbon or nitrogen emitted in gaseous form (specifically as C-CO2, C-CH4, or N-N2O (kg/d)), Ce is the effluent concentration of COT or TN (kg/L), and Qe is the effluent flow (L/d). Carbon and nitrogen concentrations were determined from liquid samples, as described in Section 2.4. Gaseous emissions were quantified using gas chromatography, as detailed in Section 2.3. To express emissions in terms of carbon or nitrogen content, the molecular weights of CO2 (44 g/mol), CH4 (16 g/mol), and N2O (44 g/mol) were used, converting them to C-CO2, C-CH4, and N-N2O, respectively, based on the molar mass of the element within each compound [42].
2.7. Statistical Analysis
To evaluate the effects of each period and seasonality on COD and TN storage, statistical analyses were carried out using R software 4.4.2 and RStudio version 2024.9.1.394, with a significance level of p = 0.05. The Shapiro–Wilk and Levene tests were performed to analyze normality and homogeneity of variance, respectively. Subsequently, an ANOVA test was performed, and this was followed by the Kruskal–Wallis test for data without a normal distribution [43]. Furthermore, a principal component analysis (PCA) was carried out with the first two principal components to evaluate the influence of different physicochemical parameters of the wastewater influent (i.e., C/N and influent, COD and NT concentrations) on the GHG emissions of the VF (CO2, N2O, CH4). Given a set of variables, PCA can be used to construct new variables or principal components (PCs) that are linear combinations of the initial variables. The first and second PC is the combination that maximizes the explained variance in the data set (66.9%). Moreover, a correlation matrix was performed to detect correlations between physicochemical parameters and GHG emissions. In the correlation matrix, values range from −1 (perfect negative correlation) to +1 (perfect positive correlation); values close to 0 indicate no linear relationship [44].
3. Results and Discussion
3.1. CO2, N2O, and CH4 Formation in WWTPs
Table 2 shows the mass flux of gas per capita. While conventional technologies generate emissions ranging from 67.9 to 122.6 kgCO2eq/cap·y, non-conventional technologies generate emissions that range from 1.1 to 75.5 kgCO2eq/cap·y. In WWTPs, the main source of gas emissions is biological transformations involving microorganisms [42]. Generally, in these systems, CO2 is generated by oxidation in aerobic processes and CH4 is generated by reduction in organic matter in anaerobic processes [17]. N2O is generated by nitrification and denitrification of nitrogen compounds in wastewater [8].

Table 2.
Comparison of per capita GHG emissions among different WWTPs (kgCO2eq/cap·y).
CO2 emissions in the VF were lower than in other WWTPs, with 0.6–7.3 kgCO2/cap·y. In particular, STU emissions were −2.8 kgCO2/cap·y. However, Knapper et al. [9] suggest that this is due to low organic matter removal efficiency in the biofilter. It has been reported that in some VFs with other influents (i.e., dairy and swine waste), CO2 decreases as worm density increases [21,47]. Consequently, low CO2 concentrations are linked to the role of earthworms in mineralization, carbon stabilization, and the generation of recalcitrant vermicast [22]. In the case of wetlands, although plants assimilate atmospheric CO2 through photosynthesis and transfer carbon compounds into the soil, it returns to the atmosphere during respiration [5]. This explains the higher CO2 concentrations, ranging between 1.4 and 26 kgCO2/cap·y. For conventional WWTPs such as ANS, AS, A2O, and AO plants, emissions range between 14.6 and 30.3 kgCO2/cap·y. With these technologies, organic matter is degraded through aerobic and anaerobic processes, which explains the increase in CO2 levels [37]. In aerobic processes, however, heterotrophic microbial kinetics are higher in conventional plants [48], leading to increased emissions.
CH4 emissions in the VF were lower than other WWTPs, with −0.04 and 0.5 kgCO2eq/cap·y. CH4 reduction is attributed to earthworm and microorganism activity that enhances VF aeration [49]. These systems generate aerobic environments, with ORP between 200 mV and 300 mV [13,50], which reduces CH4 [25]. On the other hand, Luth et al. [21] reported emissions of −3.3 and −9.7 mg/d CH4 with another type of influent. Negative concentrations suggest the presence of methanotrophic microorganisms [46]. Wetlands have higher CH4 than VFs, with 13.3–45.9 kgCO2eq/cap·y. Unlike in VFs [51], anaerobic microorganisms (i.e., hydrolytic, fermentative, and acetogenic) in wetlands are not inhibited and can degrade organic matter into simpler compounds (e.g., acetate, CO2, and H2). These compounds are subsequently transformed into CH4 by methanogenic bacteria [52]. Conversely, when aerobic environments are favored, methanotrophic bacteria can reduce CH4 levels by more than 50% [53]. Therefore, CH4 generation depends on the quantity, composition, and activity of methanogenic and methanotrophic microorganisms impacted by the operational conditions, i.e., temperature, UV radiation, and organic load [54]. In conventional WWTPs, such as ANS, A2O, and AO plants, emissions range between 48.2 and 64.2 kgCO2eq/cap·y. In this type of treatment, anaerobic digestion is part of the main processes and methane is generated as a by-product of methanogenesis [9]. For AS, methane emissions are much lower, at 12.5 kgCO2eq/cap·y. In these systems, the O2 is injected by pumps at a concentration of over 2 mg/L, which energetically favors aerobic degradation and generates a toxic effect on methanogenic microorganisms [41].
N2O emissions in the VF were between 5.7 and 9.5 kgCO2eq/cap·y. Such emissions are generated by nitrification and denitrification processes, depending on the operational parameters of the VF [13]. Zhao et al. [25] observed increases in denitrification and N2O generation when the influent C/N ratio dropped from 10 to 2.5. In the present study, the influent C/N ratio was between 8 and 10. This ratio, and the aerobic environment generated by the worms, inhibits the generation of N2O via a denitrification process [54]. In wetlands, N2O is between 3.7 and 5.5 kgCO2eq/cap·y. These systems have aerobic, anaerobic, and anoxic microbial sites; therefore, N2O originates in nitrification and denitrification processes [46]. Even though there is no consensus as to what the primary source is, as with VFs, there are operational conditions that cause an increase in N2O, i.e., increased nitrogen, low organic load, O2, and pH [5]. In conventional WWTPs, such as ANS, AS, A2O, and AO plants, emissions are between 2 and 36.7 kgCO2eq/cap·y. N2O can be generated under aerobic or anoxic conditions. Under aerobic conditions, oxidation of ammonium and nitrite generates N2O [37]. Under anaerobic conditions, only ammonium oxidation generates N2O. In activated sludge, 90% of emissions are generated during sludge aeration and the remaining 10% are generated in sludge storage tanks [55]. Factors that increase N2O generation are low pH levels, the presence of toxic compounds, or low oxygen concentrations [56].
3.2. Vermifilter Performance and Mass Balance
Figure 2 shows the mass balance of TOC. In the effluent, TOC is reduced to 8.3 ± 3.4 kg/d, representing 33% of the influent. Additionally, the system emits 4.9 ± 3 kg/d of C-CO2 and 7.2·10−3 ± 7.4·10−3 kg/d of C-CH4, which together account for 19% of the influent. C-CO2 and C-CH4 emissions are associated with the degradation of organic matter related to earthworm and aerobic microorganism activity [10]. Low C-CH4 emissions (7.2·10−3 kg/d) indicate prevailing aerobic conditions and methanotrophic activity [5,25]. Therefore, the remaining 48% of TOC is likely retained as worm biomass, vermicast, microbial biomass, and carbon and absorbed into the wood chips of the active layer [42].
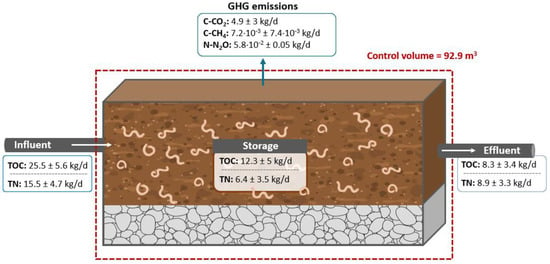
Figure 2.
Mass balance in a full-scale vermifilter. Note: TOC: total organic carbon; TN: total nitrogen; N-N2O: nitrogen from N2O; C-CO2: carbon from CO2; C-CH4: carbon from CH4.
Table 3 shows the physicochemical characteristics of the active layer analyzed in Periods II and III. The results show that during the first 8 months of Period III with the new wood chips, there was a rapid decrease in TOC of 12% (i.e., 23.074 kg to 20.482 kg). TOC decreases in the active layer have been seen in other VFs [40,57] and are related to VF acclimatization and an increase in earthworm biomass [58]. Earthworms feed on organic matter, grow, and reproduce [59], changing the microstructure and compacting the active layer with an increase in microbial enzymes in the biofilms [60] and a loss of some compounds present in the wood chips, such as lignin, cellulose, and hemicellulose [61,62].

Table 3.
Characterization of the active layer in periods of operation II and III.
The mass balance of TN can be observed in Figure 2. In the effluent, TN is reduced to 8.9 ± 3.3 kg/d, accounting for 58% of the influent. In addition, the system emits 5.8·10−2 ± 0.05 kg/d N2O. N2O emissions depend on the removal of the nitrogen that is related to nitrification and denitrification processes [59]. In the cited study, the denitrification process is inhibited by the aerobic environment of the VF [63]. However, the earthworm gut functions as a bioreactor that generates N2O through incomplete denitrification when the C/N ratio decreases [62], as evidenced in Period III (i.e., after 48 months of active layer usage; see Table 3). N2O emissions account for 0.4% of the TN in the influent. Thus, like TOC, the remaining 42% of TN is retained as worm biomass, worm vermicast, microbial biomass, and nitrogen and adsorbed into the wood chips of the active layer [42]. Table 3 shows that, unlike TOC, TN increases from 84 kg to 534 kg in the first 8 months of Period III. This is related to the adsorption of organic nitrogen, which increases from 83 kg to 514 kg, and the nitrification of NH4+-N, which increases NO3−-N from 0.1 kg to 12 kg. In Period II (48 months), the biofilm is well developed, and these processes intensify, with N-org reaching 793 kg and NO3−-N reaching 41 kg.
3.3. Effects of Seasonality
Figure 3 shows a comparison of emissions compositions between seasons, revealing that CO2 emissions were 4177 ± 1798 kg/y in fall–winter and 9992 ± 1714 kg/y in spring–summer. The significant increase (p = 0.002) of 139% in spring–summer is related to earthworm and microorganism activity. Earthworms are poikilothermic species; therefore, they are affected by temperature [14]. The optimal life range of earthworms is between 15 °C and 20 °C. In this study, the temperature range was between 11 ± 2 °C in fall–winter and 18 ± 4 °C in spring–summer. These differences affect the metabolism, growth, reproduction cycle, and activity of earthworms and microorganisms that can degrade organic matter [28]. The amount of CH4 was 17 ± 7 kg/y in fall–winter and 175 ± 30 kg/y in spring–summer. A significant increase (p = 0.001) of 90% may be related to higher organic loading due to reduced rainfall [64]. When the system receives a high input of organic matter, substrate transformations depend on the abundance of earthworms [65], and the system may experience decreased aeration. However, CO2 emissions are approximately 50 times higher than CH4. Therefore, aerobic processes represent the main mechanism of organic matter degradation in VF [63]. Although the optimal range for aerobic degradation is 25–35 °C [66], it has been observed in environments with earthworms that exothermic processes of organic compound bioconversion can increase the temperature by 1–5 °C relative to the environment [67]. This is consistent with other publications that have recorded increases in heterotrophic activity responsible for organic matter degradation in spring–summer [28]. Furthermore, temperature can also affect air diffusion in the active layer, increasing the extent of biological reactions [66].
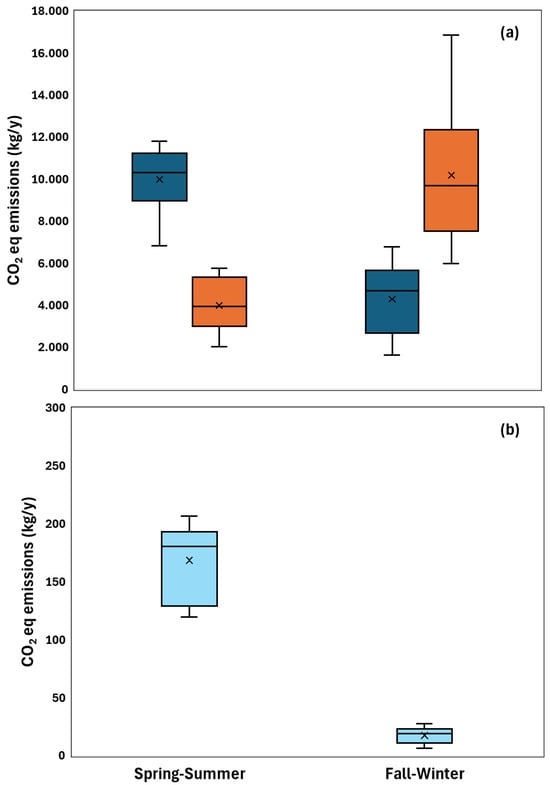
Figure 3.
Effects of seasonality on GHG in a full-scale vermifilter. (a) CO2 eq emissions due to CO2 (■) and N2O (■). (b) CO2eq emissions due to CH4. (■). Note: significant differences, p < 0.01.
Figure 3 also shows that N2O emissions were 9555 ± 3763 kgCO2eq/y in fall–winter and 3992 ± 1279 kgCO2eq/y in spring–summer. The significant increase (p < 0.001) of 139% in fall–winter may be related to variations in the C/N ratio [13]. In fall–winter, the C/N ratio was 8 ± 3 and in spring–summer it was 10 ± 2. These changes may be related to variations in TN concentrations generated by rain events that sweep along nitrogen compounds [68].
3.4. Effects of Other Parameters in GHG Emissions
Figure 4a shows a PCA, in which 66.9% of the variance is explained by component 1 (PC1) and component 2 (PC2). Component 1 is represented by N2O (0.43) and C/N (0.44) and component 2 by CO2 (0.41), COD (0.44), and TN (0.51). Figure 4b shows the correlation matrix of the physicochemical parameters measured in the influent and GHG. In addition, Table 4 presents a mass balance for different active layer usage times.
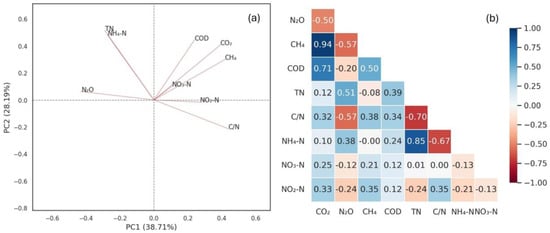
Figure 4.
Relationship between physicochemical parameters and greenhouse gas emissions: (a) principal component analysis and (b) correlation matrix. Note: TN: total nitrogen; NH4+-N: ammonium nitrogen; NO3−—N: nitrate—nitrogen; NO2−—N: nitrite—nitrogen; C/N: carbon/nitrogen ratio; N2O: nitrous oxide; CH4: methane; CO2: carbon dioxide. In the correlation matrix (Figure 4b), values range from -1 (perfect negative correlation) to +1 (perfect positive correlation); values close to 0 indicate no linear relationship. The color scale represents the strength and direction of the correlations, with blue tones indicating positive correlations and red tones indicating negative ones.

Table 4.
Mass balance in periods of operation I, II, and III of the vermifilter.
For CO2, the PCA showed a positive relationship with COD. The correlation factor between COD and CO2 was 0.71 (see Figure 4b). Generally, in all systems, CO2 is directly related to overall organic matter decomposition rates [19]. In wetlands, Gagnon et al. [69] found that high organic matter concentrations influence bacteria activity, generating an increase in CO2. In a VF, the variations in organic loads favor earthworm activity and therefore, affect the respiration of aerobic microorganisms [43,70]. Thus, the organic matter concentration in sewage affects earthworm and microorganism activity and induces a CO2 increase in a VF [13].
For N2O, the PCA showed a positive relationship with TN and a negative one with C/N. The correlation factors of N2O with TN and C/N were 0.51 and -0.57, respectively (see Figure 4b). Denitrification activity in earthworms can be 1000 times more than in the soil. This produces N2 together with N2O [24]. However, when the C/N ratio decreases, incomplete denitrification in earthworms increases, along with the release of N2O. Meanwhile, when the C/N ratio increases, complete denitrification increases, along with the release of N2 [71]. Generally, denitrification is carried out by heterotrophic bacteria that grow when organic carbon is abundant in the system [20]. The mucus secreted by earthworms has gut microbes, including heterotrophs, mixed with the active layer [10]. Zhao et al. [25] found similar results when increasing the influent TN and reducing the C/N ratio. When the C/N ratio was 10, N2O emissions were between 2.03 and 2.41 mg/m2·h. However, when the ratio was 2.5, N2O emissions increased to 12.24–12.34 mg/m2·h.
Table 4 shows a slightly significant increase in TOC storage (p = 0.06), from 40% in Period II to 61% in Period III. This is due to the replacement of the wood chips of the active layer. The C-CO2 increase from 4.0 to 4.2 kg/d was not significant (p > 0.05). Therefore, in the first year with new wood chips, the main processes may be related to wood chip adsorption and an increase in worm biomass [32,57]. TN storage is higher in Period I at 47.4%. However, the difference is not substantial relative to Period II and III. It is possible that the rainy seasons and changes in the C/N ratio could generate significant changes within the periods, thus explaining the lack of significance of the results [13].
Overall, the results highlight the relevance of VF systems as low emissions alternatives for wastewater treatment in rural areas. The observed relation between operation conditions (i.e., earthworm density, temperature, C/N and the organic load of the influent, COD) and GHG emphasizes the need for optimizing design and operation to minimize CO2 and N2O release while maintaining high pollutant removal efficiencies. These findings demonstrate that the incorporation of VF into existing wastewater treatment infrastructure could reduce the carbon footprint of the sanitation sector and provide a sustainable upgrading pathway for decentralized systems.
4. Conclusions
Considering the results of this study, the following can be concluded:
- -
- VFs generate lower GHGs than other WWTPs: CO2 7.5 kgCO2eq/cap·y, CH4 0.1 kgCO2eq/cap·y, and N2O 5.7 kgCO2eq/cap·y;
- -
- According to the mass balance, 19% of the influent TOC and 0.4% of the influent TN were converted into CO2 and N2O emissions, respectively, in the full-scale VF system;
- -
- Seasonality affects GHG emissions of the VF. CO2 emissions were 4052 ± 1722 kg/y in fall–winter and 9630 ± 1640 kg/y in spring–summer. N2O emissions were 11,423 ± 6330 kgCO2eq/y in fall–winter and 3992 ± 1279 kgCO2eq/y in spring–summer.
- -
- Influent COD concentrations and the C/N ratio were factors that determined GHG production. COD concentrations were positively correlated with emissions, with higher COD concentrations in the influent generating higher CO2 emissions. In contrast, the C/N ratio was negatively correlated with N2O emissions, with higher C/N ratios resulting in lower emissions of GHGs.
- -
- This study is the first attempt to establish GHG emissions from a full-scale VF, providing a basis for future research in this area.
Author Contributions
Conceptualization, V.G. and G.V.; methodology, V.G. and G.V.; software, V.G.; validation, G.V. and G.G.; formal analysis, V.G. and G.G.; investigation, V.G. resources, G.V.; data curation, V.G. and G.G.; writing—original draft preparation, V.G.; writing—review and editing, V.G. and G.G.; visualization, G.G.; supervision, G.V.; project administration, G.V.; funding acquisition, G.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by ANID/FONDAP/1523A0001.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author(s).
Acknowledgments
This work was supported by ANID/FONDAP/1523A0001. V. Gutiérrez thanks ANID/Scholarship Program/DOCTORADO NACIONAL/2021-21211494 for his scholarship.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Luo, Y.; Zhao, J.Y. Attentional and perceptual biases of climate change. Curr. Opin. Behav. Sci. 2021, 42, 22–26. [Google Scholar] [CrossRef]
- IPCC. Sections. In Climate Change 2023: Synthesis Report. Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2023; pp. 35–115. [Google Scholar] [CrossRef]
- World Meteorological Organization (WMO). Greenhouse Gas Bulletin: The State of Greenhouse Gases in the Atmosphere Based on Global Observations Through 2018; World Meteorological Organization (WMO): Geneva, Switzerland, 2019. [Google Scholar]
- IPCC. Climate Change 2013: The Physical Science Basis; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- Yin, X.; Jiang, C.; Xu, S.; Yu, X.; Yin, X.; Wang, J.; Maihaiti, M.; Wang, C.; Zheng, X.; Zhuang, X. Greenhouse gases emissions of constructed wetlands: Mechanisms and affecting factors. Water 2023, 15, 2871. [Google Scholar] [CrossRef]
- Bogner, J.; Pipatti, R.; Hashimoto, S.; Diaz, C.; Mareckova, K.; Diaz, L.; Kjeldsen, P.; Monni, S.; Faaij, A.; Gao, Q.; et al. Mitigation of global greenhouse gas emissions from waste: Conclusions and strategies from the Intergovernmental Panel on Climate Change (IPCC) Fourth Assessment Report. Working Group III (Mitigation). Waste Manag. Res. 2020, 26, 11–32. [Google Scholar] [CrossRef] [PubMed]
- US EPA. Global Non-CO2 GHG Emissions: 1990–2030; U.S. Environmental Protection Agency: Washington, DC, USA, 2012. [Google Scholar]
- Ranieri, E.; D’Onghia, G.; Lopopolo, L.; Gikas, P.; Ranieri, F.; Gika, E.; Spagnolo, V.; Ranieri, A.C. Evaluation of greenhouse gas emissions from aerobic and anaerobic wastewater treatment plants in Southeast of Italy. J. Environ. Manag. 2023, 337, 117767. [Google Scholar] [CrossRef]
- Knappe, J.; Somlai, C.; Laurence, L. Assessing the spatial and temporal variability of greenhouse gas emissions from different configurations of on-site wastewater treatment system using discrete and continuous gas flux measurement. Biogeosciences 2022, 19, 1067–1085. [Google Scholar] [CrossRef]
- Gutiérrez, V.; Gómez, G.; Rodríguez, D.; Vidal, G. Critical analysis of wastewater treatment using vermifilters: Operating parameters, wastewater quality, and greenhouse gas emissions. J. Environ. Chem. Eng. 2023, 11, 109683. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Technical Brief on Water, Sanitation, Hygiene and Wastewater Management to Prevent Infections and Reduce the Spread of Antimicrobial Resistance; World Health Organization (WHO): Geneva, Switzerland, 2020; 32p, Available online: https://iris.who.int/server/api/core/bitstreams/b3b1a541-91ac-474d-8a49-10660e3ef6d2/content (accessed on 10 October 2025).
- Capodaglio, A.G.; Callegari, A.; Cecconet, D.; Molognoni, M. Sustainability of decentralized wastewater treatment technologies. Water Pract. Tech. 2017, 12, 363–477. [Google Scholar] [CrossRef]
- Gutiérrez, V.; Monsalves, N.; Gómez, G.; Vidal, G. Performance of a full-scale vermifilter for sewage treatment in removing organic matter, nutrients, and antibiotic-resistant bacteria. Sustainability 2023, 15, 6842. [Google Scholar] [CrossRef]
- Arora, S.; Saraswat, S. Vermifiltration as a natural, sustainable, and green technology for environmental remediation: A new paradigm for wastewater treatment process. Curr. Res. Green Sustain. Chem. 2021, 4, 1–6. [Google Scholar] [CrossRef]
- Rajpal, A.; Arora, S.; Bhatia, A.; Kumar, T.; Bhargava, R.; Chopra, A.K.; Kazmi, A.A. Cotreatment of organic fraction of municipal solid waste (OFMSW) and sewage by vermireactor. Ecol. Eng. 2014, 73, 154–161. [Google Scholar] [CrossRef]
- Lavrnić, S.; Cristino, S.; Zapater-Pereyra, M.; Vymazal, J.; Cupido, D.; Lucchese, G.; Mancini, B.; Mancini, M.L. Effect of earthworms and plants on the efficiency of vertical flow systems treating university wastewater. Environ. Sci. Pollut. Res. 2019, 26, 10354–10362. [Google Scholar] [CrossRef]
- Kumar, A.; Khwairakpam, M. A comparative study with vermifilter and geofilter for domestic wastewater treatment and its Phyto-toxicity efficacy. J. Water Process. Eng. 2024, 60, 105245. [Google Scholar] [CrossRef]
- Lourenço, N.; Nunes, L.M. Review of dry and wet decentralized sanitation technologies for rural areas: Applicability, challenges and opportunities. Environ. Manag. 2020, 65, 642–664. [Google Scholar] [CrossRef] [PubMed]
- Lubbers, I.M.; Van Groenigen, K.J.; Fonte, S.J.; Six, J.; Brussaard, L.; Van Groenigen, J.W. Greenhouse gas emissions from soil increased by earthworms. Nat. Clim. Change. 2013, 3, 187–194. [Google Scholar] [CrossRef]
- Dey Chowdhury, S.; Bhunia, P.; Zhang, T.; Surampalli, R.Y. Nitrogen transformation dynamics in macrophyte-assisted high-rate vermifilter treating real domestic sewage. J. Water Process. Eng. 2023, 55, 104171. [Google Scholar] [CrossRef]
- Luth; Robin, P.; Germain, P.; Lecomte, M.; Landrain, B.; Li, Y.; Cluzeau, D. Earthworm effects on gaseous emissions during vermifiltration of pig fresh slurry. Bioresour. Technol. 2011, 102, 3679–3686. [Google Scholar] [CrossRef]
- Schon, N.L.; Curtin, D.; Beare, M.H.; Mackay, A.D.; Gray, R.A.; Dodd, M.B.; Van Koten, C. Earthworm induced transfer of dung-carbon into soil particle size fractions. N. Zeal. J. Agric. Res. 2020, 63, 551–558. [Google Scholar] [CrossRef]
- Six, J.; Bossuyt, H.; Degryze, S.; Denef, K.A. History of research on the link between (micro) aggregates, soil biota, and soil organic matter dynamics. Soil Tillage Res. 2004, 79, 7–31. [Google Scholar] [CrossRef]
- Singh, A.; Singh, G.S. Is earthworm a protagonist or an antagonist in greenhouse gas (GHG) emissions from the soil? Int. J. Environ. Sci. Technol. 2019, 16, 1145–1158. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, Y.; Ge, Z.; Hu, C.; Zhang, H. Effects of influent C/N ratios on wastewater nutrient removal and simultaneous greenhouse gas emission from the combinations of vertical subsurface flow constructed wetlands and earthworm ecofilters for treating synthetic wastewater. Environ. Sci. Process. Impacts 2014, 16, 567–575. [Google Scholar] [CrossRef]
- Huang, W.; Zhao, Y.; Wu, J.; Zhang, J.; Zheng, Z. Effects of different influent C/N ratios on the performance of various earthworm eco-filter systems: Nutrient removal and greenhouse gas emission. World J. Microbiol. Biotechnol. 2014, 30, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, V.; Gómez, G.; Rodríguez, D.; Vidal, G. Heterotrophic activity in the active layer of a vermifilter used for sewage treatment: Effects of worm density and seasonality. J. Environ. Chem. Eng. 2025, 13, 117639. [Google Scholar] [CrossRef]
- Arora, S.; Kazmi, A.A. The effect of seasonal temperature on pathogen removal efficacy of vermifilter for wastewater treatment. Water Res. 2015, 74, 88–99. [Google Scholar] [CrossRef]
- Sharma, P.K.; Takashi, I.; Kato, K.; Ietsugu, H.; Tomita, K.; Nagasawa, T. Effects of load fluctuations on treatment potential of a hybrid sub-surface flow constructed wetland treating milking parlor wastewater. Ecol. Eng. 2013, 57, 216–225. [Google Scholar] [CrossRef]
- Valkova, T.; Parravicini, V.; Saracevic, E.; Tauber, J.; Svardal, K.; Krampe, J. A method to estimate the direct nitrous oxide emissions of municipal wastewater treatment plants based on the degree of nitrogen removal. J. Environ. Manag. 2021, 279, 111563. [Google Scholar] [CrossRef]
- Sieranen, M.; Hilander, H.; Haimi, H.; Larsson, T.; Kuokkanen, A.; Mikola, A. Seasonality of nitrous oxide emissions at six full-scale wastewater treatment plants. Water Sci. Technol. 2024, 89, 603–612. [Google Scholar] [CrossRef]
- Chicaiza, C.; Huaraca, L.; Almeida-Naranjo, C.E.; Guerrero, V.H.; Villamar, C.A. Improvement of organic matter and nutrient removal from domestic wastewater by using intermittent hydraulic rates on earthworm–microorganism biofilters. Water Sci. Technol. 2020, 2, 281–291. [Google Scholar] [CrossRef]
- Tahar, A.; Feighan, J.; Hannon, L.; Clifford, E. Optimization of operational conditions and performances of pilot scale lumbrifiltration for real raw municipal wastewater treatment. Environ. Sci. Pollut. Res. 2022, 22, 32717–32731. [Google Scholar] [CrossRef]
- Smith, K.A.; Ball, T.; Conen, F.; Dobbie, K.E.; Massheder, J.; Rey, A. Exchange of greenhouse gases between soil and atmosphere: Interactions of soil physical factors and biological processes. Eur. J. Soil Sci. 2018, 69, 10–20. [Google Scholar] [CrossRef]
- McAuliffe, C. Gas chromatographic determination of solutes by multiple phase equilibrium. Chem. Technol. 1971, 1, 46–51. [Google Scholar]
- Were, D.; Kansiime, F.; Fetahi, T.; Hein, T. Carbon dioxide and methane fluxes from various vegetation communities of a natural tropical freshwater wetland in different seasons. Environ. Process. 2021, 8, 553–571. [Google Scholar] [CrossRef]
- Ross, B.N.; Lancellotti, B.V.; Brannon, E.Q.; Loomis, G.W.; Amador, J.A. Greenhouse gas emissions from advanced nitrogen-removal onsite wastewater treatment systems. Sci. Total Environ. 2020, 737, 140399. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2022. [Google Scholar]
- Wang, L.; Zheng, Z.; Luo, X.; Zhang, J. Performance and mechanisms of a microbial earthworm ecofilter for removing organic matter and nitrogen from synthetic domestic wastewater. J. Hazard. Mater. 2011, 195, 245–253. [Google Scholar] [CrossRef]
- Kumar, T.; Rajpal, A.; Arora, S.; Bhargava, R.; Hari Prasad, K.S.; Kazmi, A.A. A comparative study on vermifiltration using epigeic earthworm Eisenia fetida and Eudrilus eugeniae. Desalin. Water Treat. 2016, 57, 6347–6354. [Google Scholar] [CrossRef]
- Adugna, A.T.; Andrianisa, H.A.; Konate, Y.; Maiga, A.H. Fate of filter materials and microbial communities during vermifiltration process. J. Environ. Manag. 2019, 242, 98–105. [Google Scholar] [CrossRef]
- Miito, G.J.; Ndegwa, P.M.; Alege, F.P.; Coulibaly, S.S.; Harrison, J. Efficacy of a vermifilter at mitigating greenhouse gases and ammonia emissions from dairy wastewater. J. Environ. Qual. 2022, 51, 644–655. [Google Scholar] [CrossRef] [PubMed]
- Carrillo, V.; Collins, C.; Brisson, J.; Vidal, G. Evaluation of long-term phosphorus uptake by Schoenoplectus californicus and Phragmites australis plants in pilot-scale constructed wetlands. Int. J. Phytoremediat. 2021, 24, 610–621. [Google Scholar] [CrossRef]
- Leiva, A.M.; Gutierrez, E.; Arias, C.A.; Vidal, G. Influence of water quality parameters on the removal of triclosan and ibuprofen in vertical subsurface flow constructed wetlands using multivariate analysis. Environ. Technol. Innov. 2021, 24, 101846. [Google Scholar] [CrossRef]
- Pan, T.; Zhu, X.D.; Ye, Y.P. Estimate of life-cycle greenhouse gas emissions from a vertical subsurface flow constructed wetland and conventional wastewater treatment plants: A case study in China. Ecol. Eng. 2011, 37, 248–254. [Google Scholar] [CrossRef]
- Wang, B.; Li, H.; Du, X.; Cai, Y.; Peng, J.; Zhang, S.; Liu, F. Characteristics of greenhouse gas emissions from constructed wetlands vegetated with Myriophyllum aquatic: The effects of influent C/N ratio and microbial responses. Water 2024, 16, 308. [Google Scholar] [CrossRef]
- Lai, E.; Hess, M.; Mitloehner, F.M. Profiling of the microbiome associated with nitrogen removal during vermifiltration of wastewater from a commercial dairy. Front. Microbiol. 2018, 9, 1964. [Google Scholar] [CrossRef]
- Arias-Navarro, M.; Villen-Guzman, M.; Perez-Recuerda, R.; Rodriguez-Maroto, J.M. The use of respirometry as a tool for the diagnosis of wastewater treatment plants. A real case study in Southern Spain. J. Water. Process. Eng. 2019, 29, 100791. [Google Scholar] [CrossRef]
- Wang, J.; Tang, X.; Liu, Y.; Xie, B.; Li, G.; Liang, H. Self-sustained ultrafiltration coupling vermifiltration for decentralized domestic wastewater treatment: Microbial community and mechanism. Resour. Conserv. Recycl. 2022, 177, 106008. [Google Scholar] [CrossRef]
- Szögi, A.A.; Hunt, P.G.; Sadler, E.J.; Evans, D.E. Characterization of oxidation-reduction processes in constructed wetlands for swine wastewater treatment. Appl. Eng. Agric. 2004, 20, 189–200. [Google Scholar] [CrossRef]
- Wang, X.; Bai, J.; Tian, Y.; Wang, T.; Zhou, X.; Zhang, C. Synergistic effects of natural ventilation and animal disturbance on oxygen transfer, pollutants removal and microbial activity in constructed wetlands. Chemosphere 2021, 283, 131175. [Google Scholar] [CrossRef] [PubMed]
- Lopez, D.; Sepulveda-Mardones, M.; Ruiz-Tagle, N.; Sossa, K.; Uggetti, E.; Vidal, G. Potential methane production and molecular characterization of bacterial and archaeal communities in a horizontal subsurface flow constructed wetland under cold and warm seasons. Sci. Total Environ. 2019, 648, 1042–1051. [Google Scholar] [CrossRef]
- Thauer, R.K. Functionalization of methane in anaerobic microorganisms. Angew. Chem.-Int. Edit. 2010, 49, 6712–6713. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; D’Alessio, M.; Jahangeer; Meneses, Y.; Bartelt-Hunt, S.; Ray, C. Nitrogen removal in vermifiltration: Mechanisms, influencing factors, and future research needs. J. Environ. Manag. 2021, 281, 111868. [Google Scholar] [CrossRef]
- Law, Y.; Ye, L.; Pan, Y.; Yuan, Z. Nitrous oxide emissions from wastewater treatment processes. Phil. Trans. Biol. Sci. 2012, 367, 1265–1277. [Google Scholar] [CrossRef]
- Kampschreur, M.J.; Temmink, H.; Kleerebezem, R.; Jetten, M.S.M.; Van Loosdrecht, M.C.M. Nitrous oxide emission during wastewater treatment. Water Res. 2009, 43, 4093–4103. [Google Scholar] [CrossRef]
- Arora, S.; Rajpal, A.; Kazmi, A.A. Antimicrobial activity of bacterial community for removal of pathogens during vermifiltration. J. Environ. Eng. 2016, 142, 1–10. [Google Scholar] [CrossRef]
- Lourenço, N.; Nunes, L.M. Is filter packing important in a small-scale vermifiltration process of urban wastewater? Int. J. Environ. Sci. Technol. 2017, 14, 2411–2422. [Google Scholar] [CrossRef]
- Chowdhury, S.D.; Bhunia, P. Simultaneous carbon and nitrogen removal from domestic wastewater using high rate vermifilter. Indian J. Microbiol. 2021, 61, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xing, M.; Yang, J.; Lu, Y. Properties of biofilm in a vermifiltration system for domestic wastewater sludge stabilization. J. Chem. Eng. 2013, 223, 932–943. [Google Scholar] [CrossRef]
- Tejedor, J.; Cóndor, V.; Almeida-Naranjo, C.E.; Guerrero, V.H.; Villamar, C.A. Performance of wood chips/peanut shells biofilters used to remove organic matter from domestic wastewater. Sci. Total Environ. 2020, 738, 139589. [Google Scholar] [CrossRef]
- Singh, A.; Karmegam, N.; Singh, G.S.; Bhadauria, T.; Chang, S.W.; Awasthi, M.K.; Sudhakar, S.; Arunachalam, K.D.; Biruntha, M.; Ravindran, B. Earthworms and vermicompost: An eco-friendly approach for repaying nature’s debt. Environ. Geochem. Health 2020, 42, 1617–1642. [Google Scholar] [CrossRef]
- Pous, N.; Barcelona, A.; Sbardella, L.; Gili, O.; Hidalgo, M.; Colomer, J.; Serra, T.; Salvadó, V. Vermifilter and zooplankton-based reactor integration as a nature-based system for wastewater treatment and reuse. Case Stud. Chem. Environ. Eng. 2021, 4, 100153. [Google Scholar] [CrossRef]
- Vera, I.; Saez, K.; Vidal, G. Performance of 14 full-scale sewage treatment plants: Comparison between four aerobic technologies regarding effluent quality, sludge production and energy consumption. Environ. Technol. 2013, 34, 2267–2275. [Google Scholar] [CrossRef]
- Clarke, W.P.; Taylor, M.; Cossins, R. Evaluation by respirometry of the loading capacity of a high rate vermicompost bed for treating sewage sludge. Bioresour. Technol. 2007, 98, 2611–2618. [Google Scholar] [CrossRef]
- Singh, R.; Samal, K.; Dash, R.R.; Bhunia, P. Vermifiltration as a sustainable natural treatment technology for the treatment and reuse of wastewater: A review. J. Environ. Manag. 2019, 247, 140–151. [Google Scholar] [CrossRef]
- Singh, R.; Bhunia, P.; Dash, R.R. A mechanistic review on vermifiltration of wastewater: Design, operation and performance. J. Environ. Manag. 2017, 197, 656–672. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Cho, J.S.; Park, J.H.; Heo, J.S.; Ok, Y.S.; Delaune, R.D.; Seo, D.C. Long-term performance of vertical-flow and horizontal flow constructed wetlands as affected by season, N load, and operating stage for treating nitrogen from domestic sewage. Environ. Sci. Pollut. Res. 2016, 23, 1108–1119. [Google Scholar] [CrossRef] [PubMed]
- Gagnon, V.; Chazarenc, F.; Comeau, Y.; Brisson, J. Influence of macrophyte species on microbial density and activity in constructed wetlands. Water Sci. Technol. 2007, 56, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Kumar, T.; Hari Prasad, K.S.; Singh, N.K. Substrate removal kinetics and performance assessment of a vermifilter bioreactor under organic shock load conditions. Water Sci. Technol. 2016, 74, 1177–1184. [Google Scholar] [CrossRef]
- Fierer, N.; Jackson, J.A.; Vilgalys, R.; Jackson, R.B. Assessment of soil microbial community structure by use of taxon-specific quantitative PCR assays. Appl. Environ. Microbiol. 2005, 71, 4117–4120. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).