Impact of Low CO2 Footprint-Dissolution Treatment of Silica and Potassium-Rich Biomass Ashes on the Compressive Strength of Alkali-Activated Mortars
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
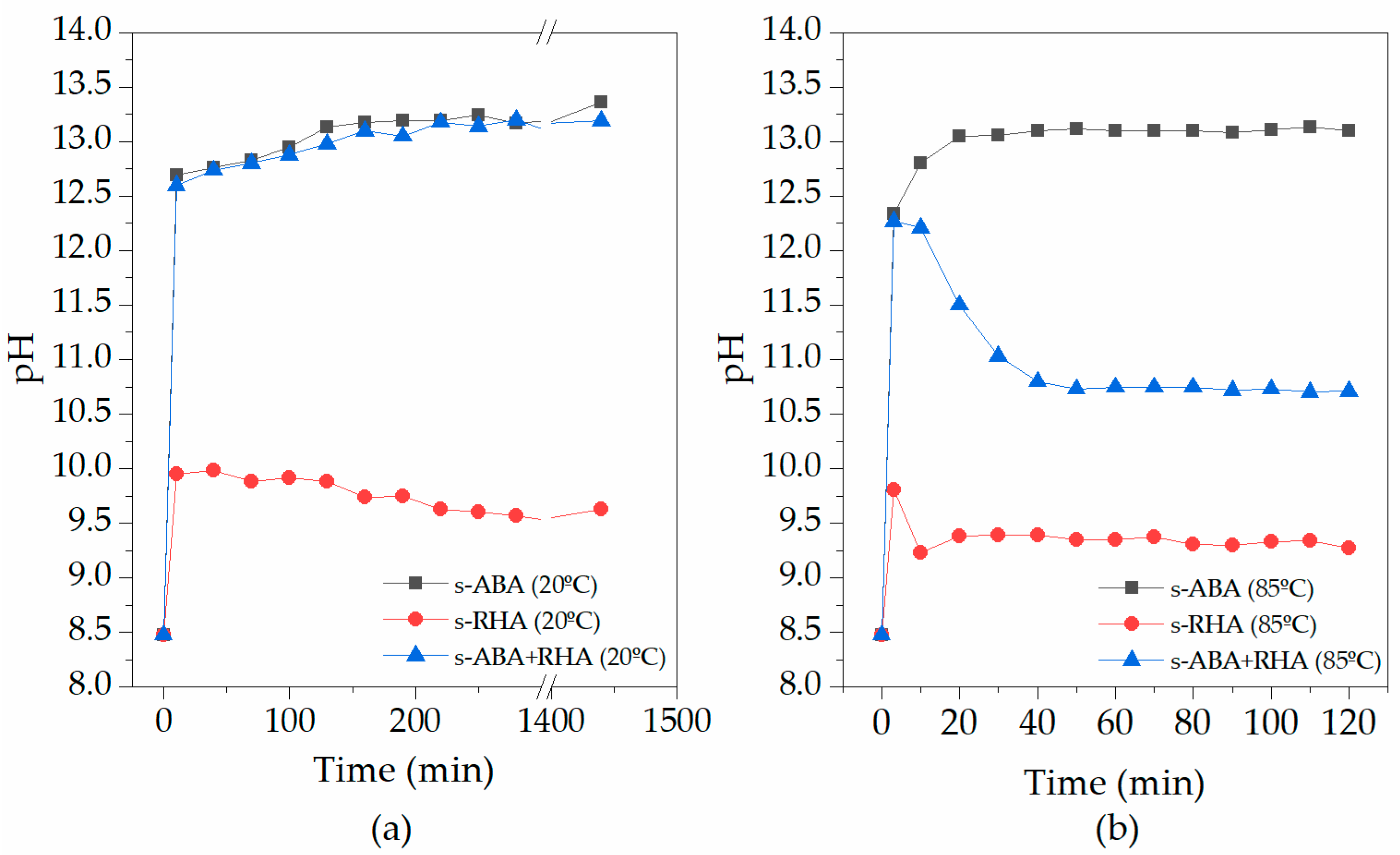
2.2.1. Analyses of the Activating Suspensions
2.2.2. Preparation of the Activating Suspensions with ABA and ABA + RHA for Mortar Production
2.2.3. Production of Binary and Ternary Alkali-Activated Mortars
2.2.4. Compressive Strength of Alkali-Activated Mortars
2.2.5. Carbon Emission Measurement of the Alkali-Activated Binders
3. Results and Discussion
3.1. Characterisation of the Activating Suspensions
3.2. Evaluation of Compressive Strength of Mortars
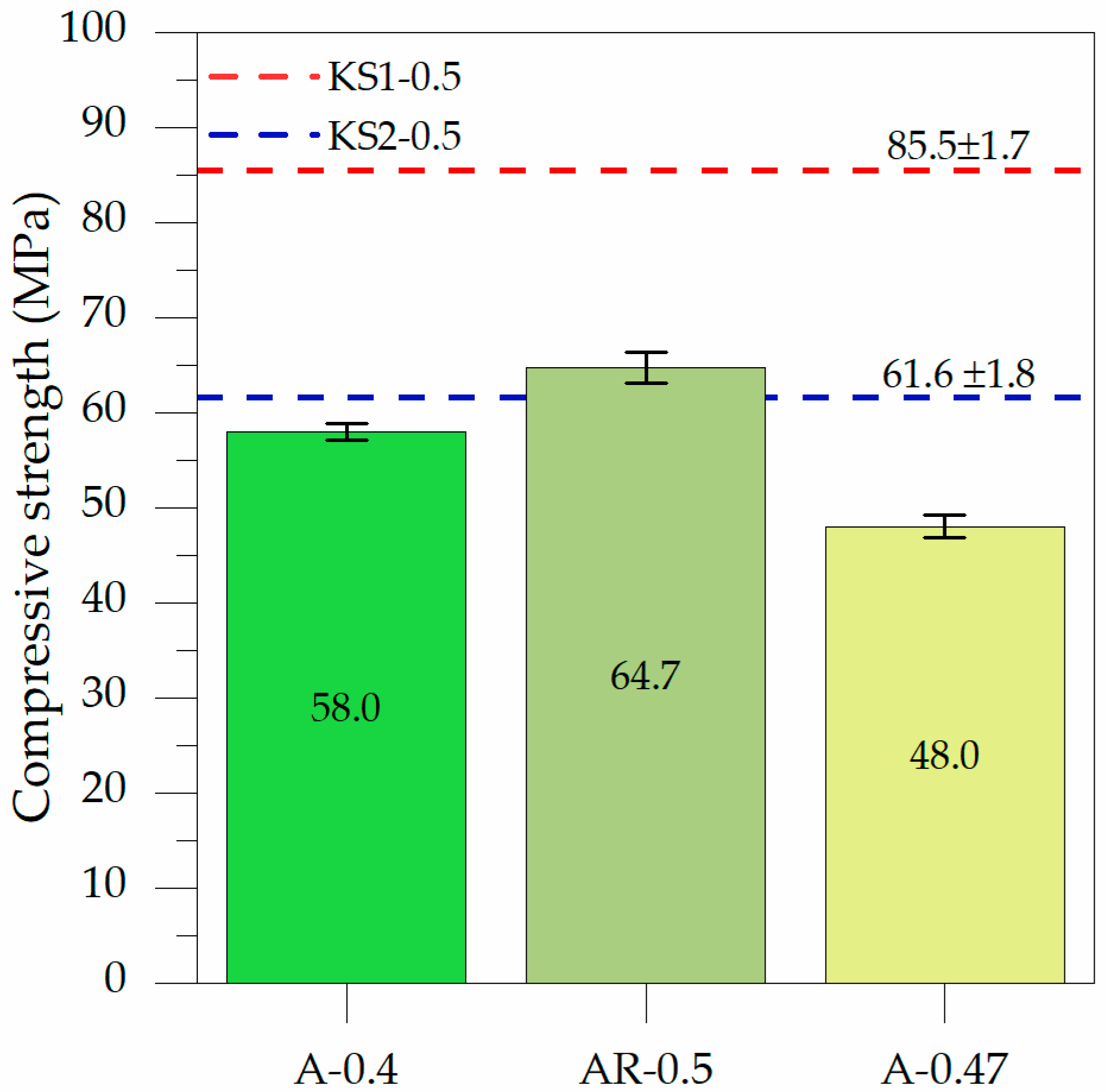
3.2.1. Influence of ABA on the Compressive Strength
3.2.2. Effect of the Preactivation of RHA Combined with ABA
3.2.3. Evaluation of the Compressive Strength Development at Room Temperature of Binary and Ternary Alkali-Activated Mortars
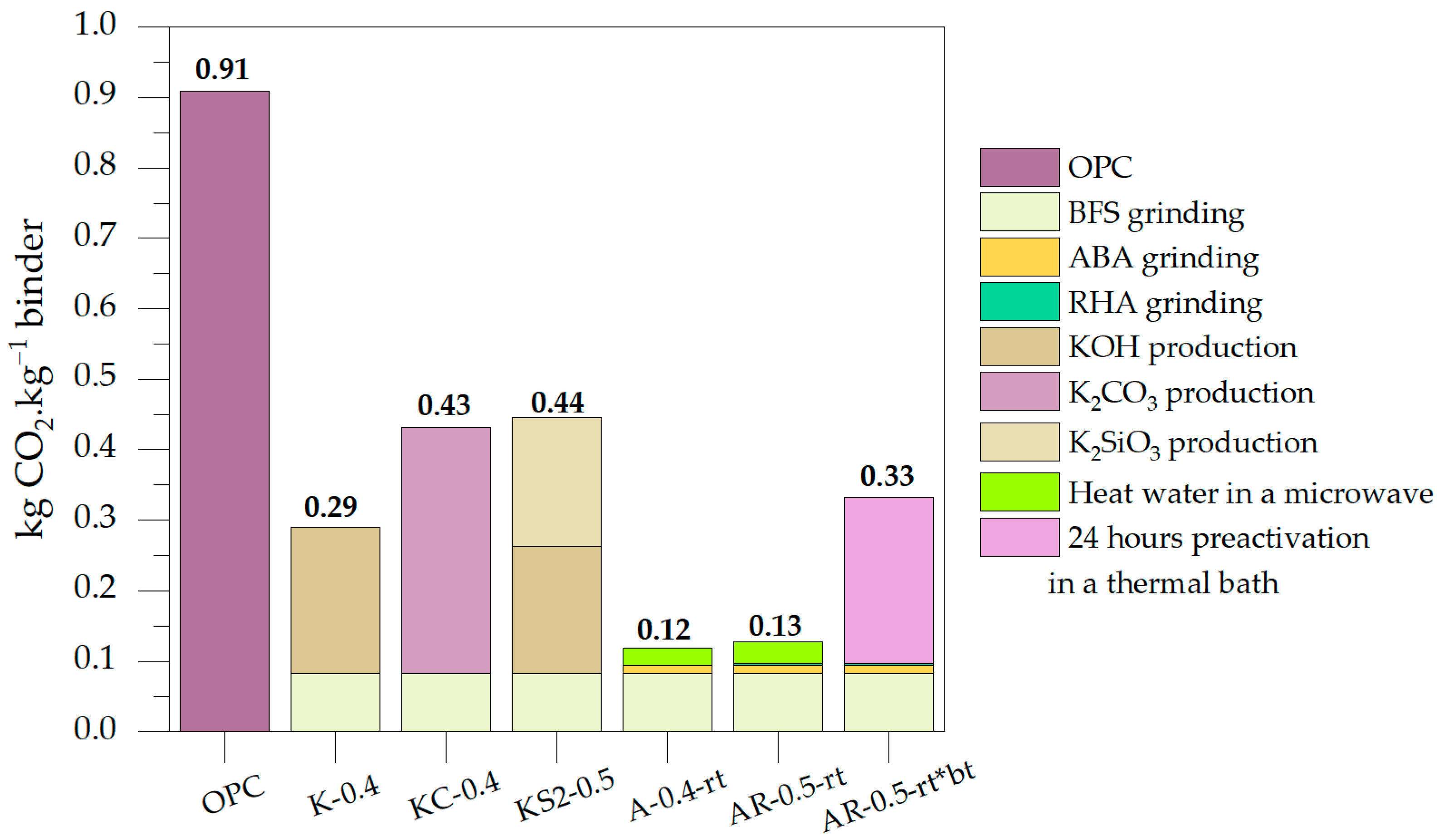
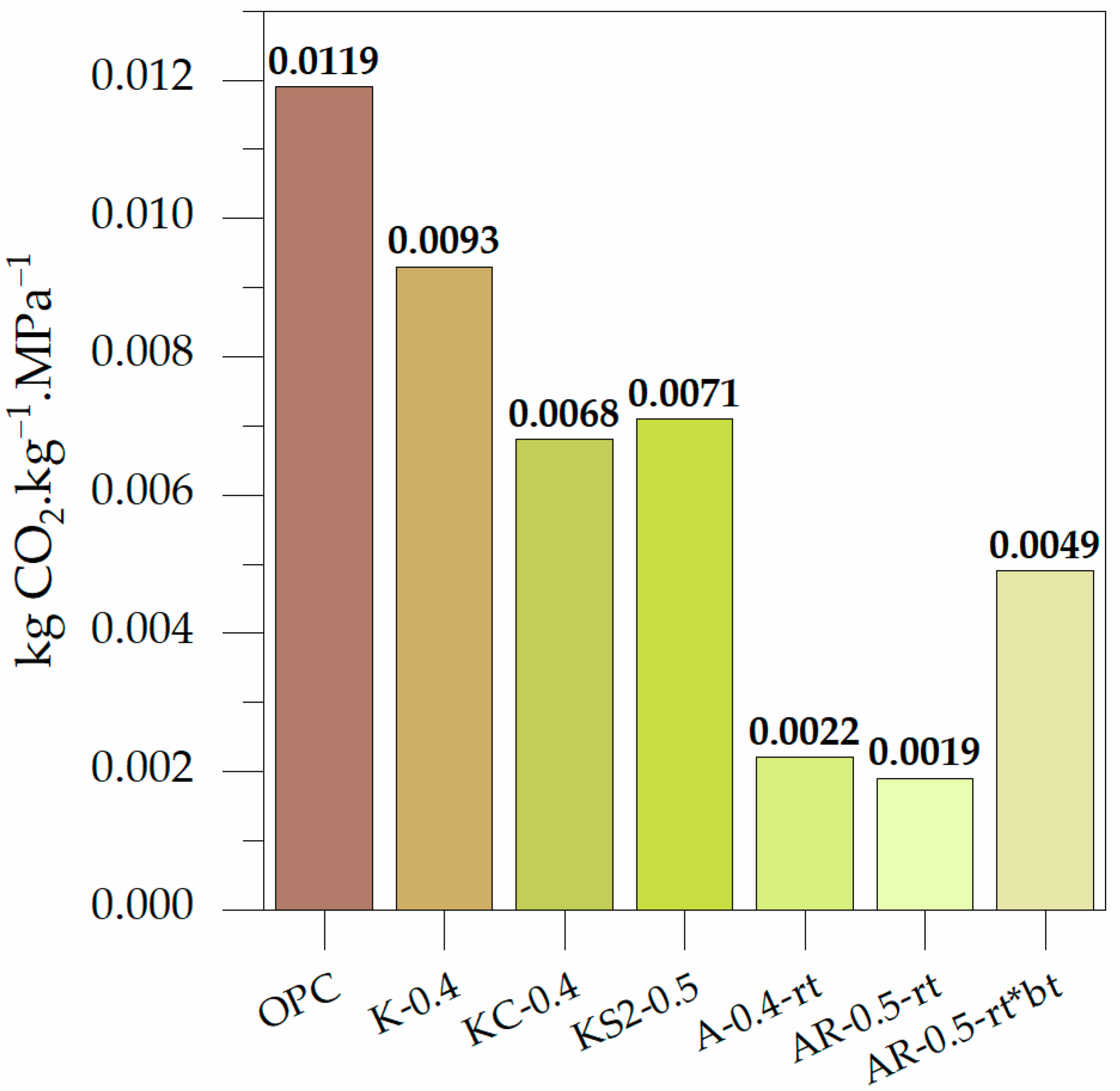
3.2.4. Carbon Footprint Calculations
4. Conclusions
- Based on the compressive strength of binary and ternary alkali-activated mortars, one-hour treatment was the optimal duration for preparing the activating solutions in a TIB filled with hot water (85 °C). For larger preactivation times, the viscosity of the activating suspensions increased due to a process of jellification, accompanied by water absorption by undissolved particles.
- The dissolution of biomass ashes (ABA and RHA) employing the TIB with hot water technique enhances the compressive strength performance of binary and ternary alkali-activated mortars. Notably, when RHA was treated jointly with high-potassium biomass ash, the compressive strength performance of ternary alkali-activated mortars exceeded that of binary alkali-activated mortars by 34.8%.
- The compressive strength development of binary and ternary alkali-activated mortars, when cured at room temperature (20 °C), exhibited a slower rate compared to those cured in a thermal bath at 65 °C. After 135 curing days at room temperature, the binary and ternary alkali-activated mortars reached 61.4 MPa and 71.9 MPa.
- The preactivation of RHA with ABA played a crucial role in enabling RHA to exhibit reactive behaviour in the ternary alkali-activated mortar, which was cured at room temperature. The significant impact of RHA on the strength was particularly evident in the substantial increase in compressive strength, amounting to 61.4%, observed between the 7th and 28th curing days.
- The BFS-alkali activated mortars made with ABA and ABA plus RHA reduce CO2 emissions by 86.8% and 85.7% compared to OPC-based mortars, respectively.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Geissdoerfer, M.; Savaget, P.; Bocken, N.M.P.; Hultink, E.J. The circular economy—A new sustainability paradigm? J. Clean. Prod. 2017, 143, 757–768. [Google Scholar] [CrossRef]
- Khalife, E.; Sabouri, M.; Kaveh, M.; Szymanek, M. Recent advances in the application of agricultural waste in construction. Appl. Sci. 2024, 14, 2355. [Google Scholar] [CrossRef]
- Navaratnam, S.; Tushar, Q.; Jahan, I.; Zhang, G. Environmental sustainability of industrial waste-based cementitious materials: A review, experimental investigation and life-cycle assessment. Sustainability 2023, 15, 1873. [Google Scholar] [CrossRef]
- Awoyera, P.O.; Adesina, A.; Sivakrishna, A.; Gobinath, R.; Kumar, K.R.; Srinivas, A. Alkali activated binders: Challenges and opportunities. Mater. Today Proc. 2019, 27, 40–43. [Google Scholar] [CrossRef]
- Shehata, N.; Sayed, E.T.; Abdelkareem, M.A. Recent progress in environmentally friendly geopolymers: A review. Sci. Total Environ. 2021, 762, 143166. [Google Scholar] [CrossRef]
- Ślosarczyk, A.; Fořt, J.; Klapiszewska, I.; Thomas, M.; Klapiszewski, Ł.; Černý, R. A literature review of the latest trends and perspectives regarding alkali-activated materials in terms of sustainable development. J. Mater. Res. Technol. 2023, 25, 5394–5425. [Google Scholar] [CrossRef]
- Provis, J.L. Alkali-Activated Materials. Cem. Concr. Res. 2018, 114, 40–48. [Google Scholar] [CrossRef]
- Xiao, R.; Jiang, X.; Zhang, M.; Polaczyk, P.; Huang, B. Analytical Investigation of Phase Assemblages of Alkali-Activated Materials in CaO-SiO2-Al2O3 Systems: The Management of Reaction Products and Designing of Precursors. Mater. Des. 2020, 194, 108975. [Google Scholar] [CrossRef]
- Fennell, P.S.; Davis, S.J.; Mohammed, A. Decarbonizing cement production. Joule 2021, 5, 1305–1311. [Google Scholar] [CrossRef]
- Griffiths, S.; Sovacool, B.K.; Furszyfer Del Rio, D.D.; Foley, A.M.; Bazilian, M.D.; Kim, J.; Uratani, J.M. Decarbonizing the cement and concrete industry: A systematic review of socio-technical systems, technological innovations, and policy options. Renew. Sustain. Energy Rev. 2023, 180, 113291. [Google Scholar] [CrossRef]
- Mangat, P.; Lambert, P. Sustainability of alkali-activated cementitious materials and geopolymers. In Sustainability of Construction Materials; Elsevier: Amsterdam, The Netherlands, 2016; pp. 459–476. ISBN 9780081003701. [Google Scholar]
- Palomo, A.; Maltseva, O.; Garcia-Lodeiro, I.; Fernández-Jiménez, A. Portland versus alkaline cement: Continuity or clean break: “A key decision for global sustainability”. Front. Chem. 2021, 9, 705475. [Google Scholar] [CrossRef]
- Habert, G.; Ouellet-Plamondon, C. Recent update on the environmental impact of geopolymers. RILEM Tech. Lett. 2016, 1, 17–23. [Google Scholar] [CrossRef]
- Mendes, B.C.; Pedroti, L.G.; Vieira, C.M.F.; Marvila, M.; Azevedo, A.R.G.; Franco de Carvalho, J.M.; Ribeiro, J.C.L. Application of eco-friendly alternative activators in alkali-activated materials: A review. J. Build. Eng. 2021, 35, 102010. [Google Scholar] [CrossRef]
- Kumar, S.S.; Rithuparna, R.; Senthilkumar, R.; Bahurudeen, A. Cleaner Production of Waste-Derived Alkali Activators from Industrial and Agricultural by-Products for Sustainable Alkali Activated Binders. Constr. Build. Mater. 2023, 391, 131824. [Google Scholar] [CrossRef]
- Jamieson, E.; van Riessen, A.; McLellan, B.; Penna, B.; Kealley, C.; Nikraz, H. Introducing Bayer liquor–derived geopolymers. In Handbook of Low Carbon Concrete; Elsevier: Amsterdam, The Netherlands, 2017; pp. 159–193. [Google Scholar] [CrossRef]
- van Riessen, A.; Jamieson, E.; Kealley, C.S.; Hart, R.D.; Williams, R.P. Bayer-geopolymers: An exploration of synergy between the alumina and geopolymer industries. Cem. Concr. Compos. 2013, 41, 29–33. [Google Scholar] [CrossRef]
- Choo, H.; Lim, S.; Lee, W.; Lee, C. Compressive strength of one-part alkali activated fly ash using red mud as alkali supplier. Constr. Build. Mater. 2016, 125, 21–28. [Google Scholar] [CrossRef]
- Phetchuay, C.; Horpibulsuk, S.; Suksiripattanapong, C.; Chinkulkijniwat, A.; Arulrajah, A.; Disfani, M.M. Calcium carbide residue: Alkaline activator for clay–fly ash geopolymer. Constr. Build. Mater. 2014, 69, 285–294. [Google Scholar] [CrossRef]
- Pourabbas Bilondi, M.; Toufigh, M.M.; Toufigh, V. Using calcium carbide residue as an alkaline activator for glass powder–clay geopolymer. Constr. Build. Mater. 2018, 183, 417–428. [Google Scholar] [CrossRef]
- Fernández-Jiménez, A.; Cristelo, N.; Miranda, T.; Palomo, Á. Sustainable alkali activated materials: Precursor and activator derived from industrial wastes. J. Clean. Prod. 2017, 162, 1200–1209. [Google Scholar] [CrossRef]
- Ban, C.S. The high volume reuse of hybrid biomass ash as a primary binder in cementless mortar block. Am. J. Appl. Sci. 2014, 11, 1369–1378. [Google Scholar] [CrossRef]
- Peys, A.; Rahier, H.; Pontikes, Y. Potassium-rich biomass ashes as activators in metakaolin-based inorganic polymers. Appl. Clay Sci. 2016, 119, 401–409. [Google Scholar] [CrossRef]
- Lima, F.S.; Gomes, T.C.F.; Moraes, J.C.B. Effect of coffee husk ash as alkaline activator in one-part alkali-activated binder. Constr. Build. Mater. 2023, 362, 129799. [Google Scholar] [CrossRef]
- Alonso, M.M.; Gascó, C.; Morales, M.M.; Suárez-Navarro, J.A.; Zamorano, M.; Puertas, F. Olive biomass ash as an alternative activator in geopolymer formation: A study of strength, radiology and leaching behaviour. Cem. Concr. Compos. 2019, 104, 103384. [Google Scholar] [CrossRef]
- Moraes Pinheiro, S.M.; Font, A.; Soriano, L.; Tashima, M.M.; Monzó, J.; Borrachero, M.V.; Payá, J. Olive-stone biomass ash (OBA): An alternative alkaline source for the blast furnace slag activation. Constr. Build. Mater. 2018, 178, 327–338. [Google Scholar] [CrossRef]
- Font, A.; Soriano, L.; de Moraes Pinheiro, S.M.; Tashima, M.M.; Monzó, J.; Borrachero, M.V.; Payá, J. Design and properties of 100% waste-based ternary alkali-activated mortars: Blast furnace slag, olive-stone biomass ash and rice husk ash. J. Clean. Prod. 2020, 243, 118568. [Google Scholar] [CrossRef]
- Soriano, L.; Font, A.; Tashima, M.M.; Monzó, J.; Borrachero, M.V.; Bonifácio, T.; Payá, J. Almond-shell biomass ash (ABA): A greener alternative to the use of commercial alkaline reagents in alkali-activated cement. Constr. Build. Mater. 2021, 290, 123251. [Google Scholar] [CrossRef]
- Bouzón, N.; Payá, J.; Borrachero, M.V.; Soriano, L.; Tashima, M.M.; Monzó, J. Refluxed rice husk ash/NaOH suspension for preparing alkali activated binders. Mater. Lett. 2014, 115, 72–74. [Google Scholar] [CrossRef]
- Moraes, J.C.B.; Font, A.; Soriano, L.; Akasaki, J.L.; Tashima, M.M.; Monzó, J.; Borrachero, M.V.; Payá, J. New Use of Sugar Cane Straw Ash in Alkali-Activated Materials: A Silica Source for the Preparation of the Alkaline Activator. Constr. Build. Mater. 2018, 171, 611–621. [Google Scholar] [CrossRef]
- UNE-EN 196-1; Methods of Testing Cement—Part 1: Determination of Strength. Spanish Association for Standardisation: Madrid, Spain, 2018.
- Leemann, A.; Góra, M.; Lothenbach, B.; Heuberger, M. Alkali–silica reaction in concrete: Revealing the expansion mechanism by surface force measurements. Cem. Concr. Res. 2024, 176, 107392. [Google Scholar] [CrossRef]
- Lide, D.R. (Ed.) CRC Handbook of Chemistry and Physics, 90th ed.; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- He, J.; Yu, S.; Sang, G.; He, J.; Wang, J.; Chen, Z. Properties of alkali-activated slag cement activated by weakly alkaline activator. Materials 2023, 16, 3871. [Google Scholar] [CrossRef]
- Zhou, S.; Tan, C.; Gao, Y.; Li, Y.; Guo, S. One-part alkali activated slag using Ca(OH)2 and Na2CO3 instead of NaOH as activator: More excellent compressive strength and microstructure. Mater. Res. Express 2021, 8, 085501. [Google Scholar] [CrossRef]
- Kovtun, M.; Kearsley, E.P.; Shekhovtsova, J. Chemical acceleration of a neutral granulated blast-furnace slag activated by sodium carbonate. Cem. Concr. Res. 2015, 72, 1–9. [Google Scholar] [CrossRef]
- Bernal, S.A.; Provis, J.L.; Myers, R.J.; San Nicolas, R.; van Deventer, J.S.J. Role of carbonates in the chemical evolution of sodium carbonate-activated slag binders. Mater. Struct. 2015, 48, 517–529. [Google Scholar] [CrossRef]
- Yuan, B.; Yu, Q.L.; Brouwers, H.J.H. Time-dependent characterization of Na2CO3-activated slag. Cem. Concr. Compos. 2017, 84, 188–197. [Google Scholar] [CrossRef]
- Siddika, A.; Hajimohammadi, A.; Sahajwalla, V. Alkali-activated foam: Understanding the relationship between rheology, activator–precursor interaction, and pore characteristics. Constr. Build. Mater. 2023, 409, 134111. [Google Scholar] [CrossRef]
- Saceda, J.J.F.; De Leon, R.L.; Rintramee, K.; Prayoonpokarach, S.; Wittayakun, J. Properties of silica from rice husk and rice husk ash and their utilization for zeolite Y synthesis. Química Nova 2011, 34, 1394–1397. [Google Scholar] [CrossRef]
- Lin, J.; Zhang, Y.; Yang, Z. A Review of Recent Advances in Alkali-Activated Materials from Silica-Rich Wastes Derived Sodium Silicate Activators. J. Adv. Concr. Technol. 2023, 21, 189–203. [Google Scholar] [CrossRef]
- Medina-Serna, T.; Arredondo-Rea, S.; Gómez-Soberón, J.; Rosas-Casarez, C.; Corral-Higuera, R. Effect of curing temperature in the alkali-activated blast-furnace slag paste and their structural influence of porosity. Adv. Sci. Technol. Res. J. 2016, 10, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Olagunju, B.D.; Olanrewaju, O.A. Life cycle assessment of ordinary Portland cement (OPC) using both problem oriented (midpoint) approach and damage oriented approach (endpoint). In Product Life Cycle—Opportunities for Digital and Sustainable Transformation; IntechOpen: London, UK, 2021. [Google Scholar]
- CarbonCloud. Climate Footprint Report: Potassium Hydroxide (KOH)—E525. 2025. Available online: https://apps.carboncloud.com/climatehub/product-reports/id/1394351136979 (accessed on 31 May 2025).
- CarbonCloud. Climate Footprint Report: Potassium Carbonate—E501(i). 2025. Available online: https://apps.carboncloud.com/climatehub/product-reports/id/3285120312383 (accessed on 31 May 2025).
- Torres-Carrasco, M.; Rodríguez-Puertas, C.; Alonso, M.d.M.; Puertas, F. Alkali-activated slag cements using waste glass as alternative activators: Rheological behaviour. Boletín De La Soc. Española De Cerámica Y Vidr. 2015, 54, 45–57. [Google Scholar] [CrossRef]
- Mellado, A.; Catalán, C.; Bouzón, N.; Borrachero, M.V.; Monzó, J.M.; Payá, J. Carbon footprint of geopolymeric mortar: Study of the contribution of the alkaline activating solution and assessment of an alternative route. RSC Adv. 2014, 4, 23846–23852. [Google Scholar] [CrossRef]
- Instituto para la Diversificación y Ahorro de la Energía (IDAE). Factores De Conversión Para El Cálculo De Consumos Y Emisiones De CO2; IDEA: Madrid, Spain, 2010. [Google Scholar]
- Faridmehr, I.; Nehdi, M.L.; Nikoo, M.; Huseien, G.F.; Ozbakkaloglu, T. Life-cycle assessment of alkali-activated materials incorporating industrial byproducts. Materials 2021, 14, 2401. [Google Scholar] [CrossRef]
- Alhassan, M.; Alkhawaldeh, A.; Betoush, N.; Alkhawaldeh, M.; Huseien, G.F.; Amaireh, L.; Elrefae, A. Life cycle assessment of the sustainability of alkali-activated binders. Biomimetics 2023, 8, 58. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Pignatta, G.; Sepasgozar, S.M.E. Life-cycle assessment of fly ash and cenosphere-based geopolymer material. Sustainability 2021, 13, 11167. [Google Scholar] [CrossRef]
- Krajnović, I.; Komkova, A.; Barragán, B.; Tardy, G.; Bos, L.; Matthys, S. Explorative study into alkali-activated repair mortars using blast furnace slag and glass waste. Sustainability 2024, 16, 764. [Google Scholar] [CrossRef]
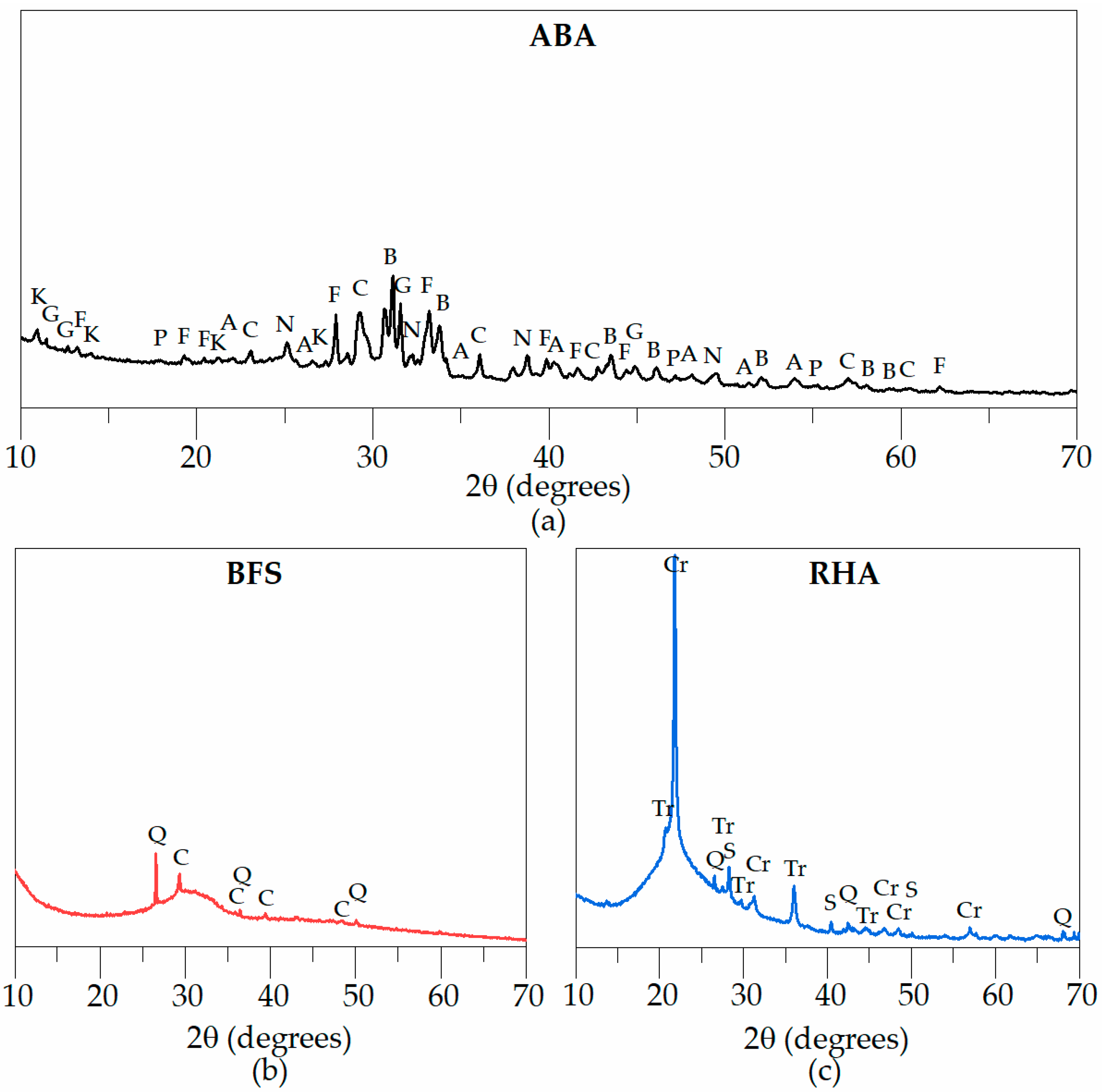





| Oxides | BFS | ABA | RHA |
|---|---|---|---|
| % (by Mass) | |||
| SiO2 | 30.53 | 1.46 | 85.58 |
| CaO | 40.15 | 26.69 | 1.83 |
| Al2O3 | 10.55 | 0.45 | 0.25 |
| Fe2O3 | 1.29 | 0.48 | 0.21 |
| Na2O | 0.87 | 0.24 | *,1 |
| MgO | 7.43 | 2.91 | 0.50 |
| K2O | 0.57 | 49.73 | 3.39 |
| P2O5 | 0.26 | 4.40 | 0.67 |
| SO3 | 1.93 | 0.66 | 0.26 |
| Others | 0.89 | 0.48 | 0.32 |
| LOI *,2 | 5.53 | 12.50 | 6.99 |
| Granulometric Parameters | BFS | ABA | RHA |
|---|---|---|---|
| (in µm) | |||
| Mean particle size | 30.4 | 21.5 | 20.3 |
| d(0.1) | 1.8 | 4.6 | 2.5 |
| d(0.5) | 20.9 | 14.5 | 10.5 |
| d(0.9) | 73.0 | 42.1 | 41.3 |
| Analysis | Type of Suspension | Raw Materials | Thermal Treatments | ||
|---|---|---|---|---|---|
| Water | ABA | RHA | |||
| (in g) | |||||
| pH | s-RHA | 50 | - | 10.0 |
|
| s-ABA | 25.0 | - | |||
| s-ABA + RHA | 10.0 | ||||
| Temperature increment | s-ABA | 50 | 25 | - |
|
| Viscosity | s-ABA + RHA | 50 | 25 | 10.0 |
|
| s-ABA + 2RHA | 20.0 | ||||
| FESEM-EDS | s-ABA | 50 | 25 | - |
|
| s-ABA + RHA | 10 | ||||
| Mortar’s Identification | BFS | B:s | w/B | Activating Suspensions/Solutions | Curing Condition | ||||
|---|---|---|---|---|---|---|---|---|---|
| in % with Respect to BFS | |||||||||
| (in %) | ABA | RHA | KOH | K2CO3 | K2SiO3 | ||||
| KC-0.4 | 100 | 1:3 | 0.4 | - | - | - | 14.6 | - | 7 days at 65 °C |
| K-0.4 | 0.4 | - | - | 14.0 | - | - | |||
| KS1-0.5 | 0.5 | - | 10 | 7.8 | - | 41.4 | |||
| KS2-0.5 | 0.5 | - | - | 12.2 | - | 15.2 | |||
| A-0.4 | 0.4 | 25 | - | - | - | ||||
| A-0.47 | 0.47 | 25 | - | - | - | ||||
| AR-0.5 | 0.5 | 25 | 10 | - | - | ||||
| A-0.4-rt | 0.4 | 25 | - | - | - | 7, 28, and 135 days at 20 °C | |||
| AR-0.5-rt | 0.5 | 25 | 10 | - | - | ||||
| * R-A0.5-rt | 0.5 | 25 | 10 | - | - | ||||
| Suspension Type | Retained Solids | Residual Solids |
|---|---|---|
| % Regarding the Initial Total Mass of Raw Materials | ||
| s-ABA (85 °C—1 h) | 76.3% | 25.7% |
| s-ABA + RHA (85 °C—1 h) | 91.2% | 21.2% |
| Sample | Oxides | ABA | Retained Solids | Residual Solids |
|---|---|---|---|---|
| (%) | ||||
| s-ABA (85 °C—1 h) | K2O | 61.8 ± 1.4 | 24.0 ± 4.4 | 96.0 ± 1.0 |
| CaO | 25.9 ± 1.6 | 50.6 ± 2.4 | 0.0 ± 0.0 | |
| SiO2 | 2.1 ± 0.4 | 5.9 ± 0.7 | 4.0 ± 1.0 | |
| Others | 10.2 ± 1.8 | 19.6 ± 1.7 | 0.0 ± 0.0 | |
| Total | 100.0 | 100.0 | 100.0 | |
| Sample | Oxides | ABA + RHA | Retained Solids | Residual Solids |
|---|---|---|---|---|
| (%) | ||||
| s-ABA + RHA (85 °C—1 h) | SiO2 | 40.8 ± 6.7 | 42.8 ± 7.0 | 5.7 ± 2.5 |
| K2O | 36.5 ± 5.6 | 21.9 ± 2.6 | 91.4 ± 2.6 | |
| CaO | 15.5 ± 1.2 | 25.8 ± 4.9 | 0.0 ± 0.0 | |
| Others | 7.2 ± 1.0 | 9.4 ± 1.2 | 2.8 ± 0.7 | |
| Total | 100.0 | 100.0 | 100.0 | |
| Curing Time | A-0.4-rt | R-A0.5-rt | AR-0.5-rt |
|---|---|---|---|
| (in MPa) | |||
| 7 days | 43.3 ± 0.7 | 41.1 ± 1.3 | 41.5 ± 1.9 |
| 28 days | 54.7 ± 1.3 | 47.7 ± 2.6 | 67.0 ± 3.7 |
| 135 days | 61.4 ± 1.4 | 56.1 ± 1.8 | 71.9 ± 4.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Istuque, D.B.; Soriano, L.; Monzó, J.; Borrachero, M.V.; Tashima, M.M.; Payá, J. Impact of Low CO2 Footprint-Dissolution Treatment of Silica and Potassium-Rich Biomass Ashes on the Compressive Strength of Alkali-Activated Mortars. Sustainability 2025, 17, 10359. https://doi.org/10.3390/su172210359
Istuque DB, Soriano L, Monzó J, Borrachero MV, Tashima MM, Payá J. Impact of Low CO2 Footprint-Dissolution Treatment of Silica and Potassium-Rich Biomass Ashes on the Compressive Strength of Alkali-Activated Mortars. Sustainability. 2025; 17(22):10359. https://doi.org/10.3390/su172210359
Chicago/Turabian StyleIstuque, Danilo Bordan, Lourdes Soriano, José Monzó, Maria Victoria Borrachero, Mauro Mitsuuchi Tashima, and Jordi Payá. 2025. "Impact of Low CO2 Footprint-Dissolution Treatment of Silica and Potassium-Rich Biomass Ashes on the Compressive Strength of Alkali-Activated Mortars" Sustainability 17, no. 22: 10359. https://doi.org/10.3390/su172210359
APA StyleIstuque, D. B., Soriano, L., Monzó, J., Borrachero, M. V., Tashima, M. M., & Payá, J. (2025). Impact of Low CO2 Footprint-Dissolution Treatment of Silica and Potassium-Rich Biomass Ashes on the Compressive Strength of Alkali-Activated Mortars. Sustainability, 17(22), 10359. https://doi.org/10.3390/su172210359









