Abstract
The major disadvantage of setting up a willow coppice is the low survival rate, which reduces economic efficiency and crop sustainability. The aim of this research was to test, under controlled conditions, the impact of water, gibberellic acid A3 (0.05%), and humic acid (0.2%) on the growth and development of two willow clones. Under humic acid treatment, 20 cm cuttings of the Tordis clone developed up to 15 roots, and 25 cm cuttings developed more than 23. In comparison, water stimulated more than 15 roots for both 20 and 25 cm cuttings. Gibberellins acted as an inhibitor, especially on the roots, and the cuttings dried out from the top to the middle, with weak development of shoots and callus formation. For both clones, the highest number of active buds was observed on 20 and 25 cm cuttings grown in water, with more than four for Inger and more than seven for Tordis. Root development of the Inger clone had a maximum of eight for 25 cm cuttings grown in water; it was three times lower in the same variant of Tordis and two times lower for the Tordis clone with humic acid treatment. In general, Inger cuttings of 15 and 25 cm highlighted a delayed root formation when humic acids and gibberellins were applied. In controlled condition experiments, the Tordis clone was more suitable owing to its higher development and increased growth stability.
1. Introduction
Given the current energy crisis worldwide, Romania is focusing on alternative energy sources mainly for biomass production of bioethanol or pellets. In this context, willow (Salix viminalis spp.) is intensively targeted because of its low cost and high yield; it is easily converted into alternative energy to reduce the use of conventional sources such as fossil fuel, coal, or crude oil. Therefore, this species represents a valuable green and renewable energy source [1,2,3], and the willow wood is used for combustion. Willow provides a good source of income, with commercial cultivation periods of a few years up to 15–20 years in the same field [4,5] and, on average, 25 years [6]. It provides a large amount of biomass with relatively low start-up costs, and crop success is seen immediately. In general, willow prefers humid, even regularly flooded soils, being less susceptible to waterlogging [7,8]. Fertilizers and water chemistry greatly influence its survival rate and growth performance [8,9] as a sustainable crop.
Currently, the increased interest in obtaining woody plants through short rotations is expected to have a major impact on shrub growth and increased biomass yield for the crescent energy field, without affecting food production [10,11,12,13]. These energy crops have several advantages, including 14–85 times higher levels of energy storage than fossil fuels per unit of fossil energy [14,15]. Greenhouse gas emissions are also reduced by around 9–161 times, compared to coal use [14,15]. The Glasgow COP26 climate change summit highlighted the EU’s latest plan for a green transition (available online: https://www.consilium.europa.eu/en/policies/climate-change/eu-climate-action/ (accessed on 20 April 2022)). The objectives set by the “Fit to 55” legislative package included reducing emissions by at least 55% by 2030, with measures to keep global warming below 1.5 °C. Thus, the aim is to obtain 40% of energy from renewable sources by 2030. In this context, Romania has a great potential for obtaining all forms of renewable energy, especially biomass for sustainable development [16,17]. Short rotation crops contribute to the sustainability of a specific area and offer a series of advantages in helping to reconstruct and restore the degraded land by phytoremediation [3], reducing soil erosion, recycling organic waste, and reducing the demand for wood in natural forests [18,19,20,21]. Willow fiber has also been used successfully in the cellulose and paper industry [6,22]. In addition to the above are the benefits to the environment from using energy plants as primary resources for enzymatic scarification, focusing on the release of sugars and the production of bioethanol [10]. Bioenergy-producing crops also support soil wastewater recycling [23] and decrease in heavy metals accumulation [5,24,25], which pollute the soil and thus stunt crop growth and development.
Many studies have been carried out on various willow hybrids [26,27,28,29,30,31]. In Romania, and at an international level, several problems with rooting, attachment, and the juvenile stages of willow cuttings were highlighted [32,33,34]. Up to the present day in Romania, a great diversity of willow hybrids have been studied, such as Gudrun (Salix dasyclados), Inger (Salix triandra × viminalis), Klara (Salix dasyclados, S. viminalis × schwerinii), Olof (Salix viminalis × schwerinii), Stina (S. aegyptiaca × schwerinii × viminalis × lanceolata), Sven (Salix viminalis × schwerinii), Tora (Salix schwerinii × viminalis), and Tordis (Salix schwerinii × viminalis), by setting up new crops directly in the field [35,36]. Most studies in Timiș, Mureș, and Brașov counties [37] have highlighted the biggest problem encountered in planting, namely, the very low percentage of attachment: 0% for the Gurdun and Tordis hybrid and 29% for the willow hybrid Inger [35]. For the Inger hybrid, studies of soil contaminated with heavy metals recorded 45–71.91% survival rates, with rates between 87 and 91.82% on uncontaminated soils [38]. Another study of Inger and Tordis alone indicated a lower percentage of catches for Inger of between 60.6 (contaminated soil) and 94.5% (uncontaminated soil). The values of the Tordis hybrid ranged from 83.9 to 97.1% [38]. In another study in Romania, no differences were observed between plant height and root development between Inger and Tordis. The plants’ survival rates were similar, at 59.28–94.10% for Inger and slightly higher at 59.44–96.80% for Tordis [36]. Significant differences were observed between Inger and Tordis in terms of root weight and shoot weight. Dry matter high yield was reported for Tordis at 33.1 t ha−1 year−1 and for Inger at 30.4 t ha−1 year−1, and both cultivars showed great efficiency in a short-term cropping system among other tested cultivars [6]. Growth vigor is a desirable property and represents a result of genotype and genetic interactions, together with the growth environment [39]. Most of the studies testing the effects of nitrite and nitrate nutrition on growth morphological parameters [40], and comparative tests for establishing the heavy metal tolerance range [5,25], were made in the field and only a few were in the laboratory. Salix viminalis L. cv. Inger, the so-called basket willow, had a high potential for bioremediation in the case of Cd and Zn and general intensive mineral nutrition [5,41,42]. Through the synergistic actions represented by the handling of the cuttings, soil conditions, and planting technology, the cuttings’ survival percentage was negatively influenced [37,38].
Based on all the benefits and constraints, willow represents a very interesting paradox. This plant is a valuable means of achieving sustainability, and it is notable from short-coppice to long-term cultivation. It has a good survival rate in nature, contrary to anthropic cultivation where the survival rate is very low. In this context, our study aimed to stimulate rhizogenesis and indirectly determine how to maximize the plant response of the two willow clones, Inger and Tordis. A pre-field short experiment, under controlled conditions, was necessary to develop recommendations on the growth evolution of willow clones and their behavior, in terms of root development in contact with macro- and micronutrients, humic acids, and root hormones such as gibberellins. The experiment was designed to assess the best cutting size from a total of three selected sizes. A supplementary objective was to assess the rhizogenesis development with two organic stimulators and their impact on physiological features, which will lead to a high survival rate and willow biomass formation. The following hypotheses were tested: (i) What is the right size for both Salix clones and different cuttings sizes when setting up a crop? (ii) What stimulants or nutrients have a direct impact on rhizogenesis? (iii) Are there any differences between the willow clones Inger and Tordis in the primary start-up effect in vegetation dependent on specific characteristics?
2. Materials and Methods
The experiment was set up in 2021 in the research laboratory of the Plant Physiology Department at the University of Agricultural Studies and Veterinary Medicine in Cluj-Napoca, Romania. The growth and development of two energy crop clones Salix viminalis cv. Inger and Salix viminalis cv. Tordis were assessed in a completely randomized experimental design. The two willow clones were subjected to the effects of three treatments, with three replications for each variant. All the variants were kept at room temperature 20 ± 2 °C, 40% air humidity, with a photoperiod of 15 h daylight (1000 μMol m−2 s−1) and 9 h dark. The entire set of observations was made every 5 days. The experiment was stopped in the short term, after 20 days, because most of the observed cuttings formed a complete callus at the immersed part, a phenomenon that affected their further development.
2.1. Plant Material
Plant material was represented by Inger [EU 11635], the most cultivated Swedish commercial clone in Romania [43]. This clone was created as a cross between a Russian Siberian clone, Salix triandra, and the species Salix viminalis Jorr; the reason being that it is more tolerant of pedological drought conditions. Inger is a species recommended in mixed plantations. It is the highest yielding variety for a mild warm climate with optimal water supply. The second variety, Tordis (Salix schwerinii × Salix viminalis), gives high yields and it is adapted to north-central Europe. It was obtained by crossing the species Torah and Ulv. Tordis [EU9288] is one of the best hybrids for both biomass and cuttings production. Swedish one-year short rotation energy willow stems (SalixEnergi Europa AB, Sweden) were assessed. Inger and Tordis clones were each cut to three different sizes, 15, 20, and 25 cm, with no more than a 1 cm diameter and with the same bud number. All the stem cuttings had 3 buds soaked in the solutions, and the 15 cm cuttings had 3 buds above, the 20 cm had 5 buds above, and the 25 cm cuttings had 9 buds above.
2.2. Measurements and Growth Parameters
Measurements comprised root morphological parameters (number of root insertions), bud onset (number of active buds visible), and shoot growth and development (length, number of leaves). The shoot length was measured using the caliper method (0.001 mm precision). The number of leaves on each shoot was counted. Plant images were captured with a 16 MP, 2280 × 1080 resolution camera. Shoots and roots dry weight was determined by collecting them in metallic containers at the end of the experimental trial and oven-drying them (Binder FD 115, Tuttlingen, Germany) at 105 °C for 48 h until two constant weight readings were obtained (Kern 440-21A, 0.001 g, Balingen, Germany).
2.3. Solution Compositions
The cuttings were placed in 400 mL Berzelius glasses, 7 cm loaded glass height with 250 mL solutions. This quantity was maintained throughout the experimental trial. The clone cuttings were placed in three different solutions: water, water + gibberellins (GIB), and water + humic acid (HA). The solution compositions were formed following the effect of normal doses for irrigation water, respectively, 0.125 g gibberellins (90% gibberellinic acid A3 + 10% inactive substance) (Nordic Chemicals, Cluj-Napoca, Romania) in 250 mL water (0.05%), and 0.5 mL Gekka-Bio (BIO Pyrolytic Condensate, Organic Fertilizer Solution–Pyroligeous Acid–Humic Acid) (Nordic Chemicals, Cluj-Napoca, Romania) in 250 mL water (0.2%). This solution amount was maintained throughout the experimental trial.
BIO Pyrolytic Condensate, Organic Fertilizer Solution–Pyroligeous Acid–Humic Acid is designed to help plants grow healthier. This commercial bio-product has a pH of 3.6, <5 mg/L NO2, <1.0 mg/L N Kjeldahl, <50 mg/L NO3 and different microelement contents < 2 mg/L B, <0.4 mg/L Cu, 533 Fe, <0.4 mg/L P, 0.81 mg/L Mg, 3.42 mg/L Mn, <0.2 mg/L Mo, <20 mg/L K, 0.922 mg/L Zn.
2.4. Data Analysis
The data analysis was performed in RStudio [44], version 1.4.1106, by integrating the necessary packages for each of the used tests. The distribution of all data was analyzed by histogram function, with the package “graphics” [45], prior to the extraction of basic statistics for each parameter, based on functions from the “psych” package [46]. One-way ANOVA with F test was used for the extraction of the experimental factors’ singular and combined impact; before exploring the differences through the LSD test, both were performed with the “agricolae” package [47] and extracted from RStudio with the “broom” package functions [48]. The condensed form of the data originated from leaves and shoots developed by the willow clones and were analyzed by clustering with the “ape” package [49]. The average values for root and shoot growth and development were visually expressed to detect the general reaction trend of each clone to applied treatments (Supplementary Figures S1 and S2). The synthesis of observations over the entire experiment was used as a supplementary database for the description of the physiological phenomena recorded, detailed below Supplementary Figures S1 and S2.
3. Results
ANOVA analysis of the Inger cultivar showed treatment to be the most important factor for bud development (Table 1), followed by shoot length. The overall effect of these two factors on bud development highlighted a reduction in F test values, which sustained the hypothesis of bud emergence based on a specific combination of shoot length × treatment. Five days from the experiment set-up, the highest number of bud emergences were visible for the 25 cm cuttings kept in water (IN_25_W). Significant differences were recorded between this treatment and both GIB and HA applied on cutting lengths of 15 and 25 cm.

Table 1.
Effect of length and treatment on active buds of Salix viminalis cv. Inger cuttings.
The 20 cm length cuttings displayed interesting bud emergence. Overall, GIB treatments gave the lowest number of buds, and the difference between HA and water was not significant. Application of the GIB treatment diminished the bud emergence at rates lower than 1:3 up to 1:9.
At the second assessment (M2) of Inger growth, the treatment had the highest impact on bud emergence (F test = 15.17, p < 0.001). This moment was representative of the maximum influence of cutting length, which decreased in value after this point. These trends were associated with an increase in the combined length × treatment interaction. The highest value was seen for 25 cm cuttings grown in water. Hereby, the bud numbers were significant compared with the GIB and HA treatments. The ratio between the active buds and the total number of buds registered significantly higher values for 25 and 20 cm cuttings in water of 4:9 and 4:5, followed by 20 cm in humic acid of 2:5, which was not significant in number compared with other treatments.
After 15 days, the shoot number varied significantly within treatments and shoot cutting lengths (Figure 1).


Figure 1.
Treatment effect on Salix viminalis Inger (a–c) and Salix viminalis Tordis (d–f) willow growth and development at the third assessment (M3). Treatments: (a,d) W—water, (b,e) GIB—gibberellins, (c,f) HA—humic acids. Cutting lengths: 15, 20, and 25 cm.
The lowest number of developed shoots was seen in all the treatments with GIB and was significantly lower than the other two treatments. Compared with the initial existing buds, the ratio between active and inactive buds was 2:3 for 15 cm cuttings in all treatments, 4:5 for 20 cm and 5:9 for 25 cm cuttings in water. At the last assessment, after 20 days, the number of shoots was the highest in the water treatment, around three times greater in comparison with the HA treatment for the 25 cm cutting length, and at 5% compared to HA for the 20 cm cutting length. A significant difference was registered between the treatment IN_25_W and all the other treatments except IN_20_W. The ratio between active and inactive buds respective to the number of shoots followed the same trend as the previous measurement.
The bud appearance of the Tordis cultivar was significantly influenced by treatment and cutting length factors, but not by the interaction between them (Table 2).

Table 2.
Effect of length and treatment on active buds of Salix viminalis cv. Tordis cuttings.
The overall representative treatment was TO_20_W with significantly higher bud number. At the first measurement, after 5 days, the 15 cm cuttings activated two buds out of three. Differences between treatments were significant from the cuttings of 20 and 25 cm immersed in water, compared to most of the other registered values. An interesting case was the treatment with gibberellins, which activated fewer than two buds, regardless of the cutting length. The highest ratio was 5:5 at the treatment TO_20_W, followed by 2:3 at 15 cm and 5:9 at 25 cm. After 5 and 10 days, respectively, only three treatments showed significant differences—TO_20_W, TO_25_HA, and TO_25_W. The treatments with gibberellins reduced by two times the bud number compared with humic acid and water. After 15 days the measurement indicated that humic acid and water had similar values for cuttings of 15 cm and 25 cm, respectively. In contrast, 20 cm cuttings recorded a doubled number of active buds in the case of water treatment compared to humic acid. All gibberellin treatments, regardless of cutting length, displayed two or three active buds. The last measurement, after 20 days, presented a low increase trend for water and humic acid, the differences between the variants being maintained from 5 days before. In the case of gibberellin treatments, there was a decrease in the number of active buds, under two, in the case of 15 and 20 cm cuttings. This phenomenon was due to a drying-up process that affected the bud activity and growth.
The roots of Salix viminalis cv. Inger showed significant differences within treatment factors after 10, 15, and 20 days (Table 3). Only the treatment produced significant differences, and only 10 days from the start of the experiment. In the first measurement, only the 20 cm cuttings presented roots, and among the rest of the cuttings, the 25 cm ones immersed in water.

Table 3.
Effect of length and treatment on roots of Salix viminalis cv. Inger cuttings.
The second measurement presented a high increase in the roots developed by the 20–25 cm cuttings treated with water and humic acid (only cuttings of 20 cm). The emergence of roots was visible on all cuttings, except for the treatments with gibberellins and humic acid for 15 cm cuttings. After 15 days, water treatment induced a significant development of roots in all cutting lengths, all of these values being exceeded by the application of humic acid on 20 cm cuttings. In the last measurement, only 15 cm cuttings treated with gibberellins did not develop roots (Figure 2).
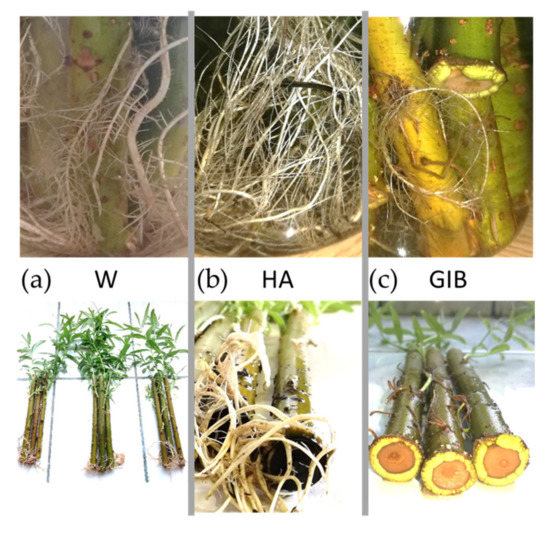
Figure 2.
General aspects reflecting the rhizogenesis ending for all three tested treatments. (a) W- water treatment; (b) HA—humic acid; (c) GIB—gibberellins. The upper part comprises all suggestive details of root morphology and the bottom is a general overview of the entire cutting from each one of three tested treatments.
The increase in root was 1 unit for the water treatment in 15 cm cuttings, almost 2.8 in the 20 cm cuttings, and more than 3 units in the 25 cm length. As for humic acid, the highest increase was for 20 cm cuttings, with more than 2.5 units. For the same treatment in the 25 cm cuttings, the roots showed a double value compared to the previous measurement, and only 0.33 units for 15 cm cuttings.
In terms of root development, the Tordis cultivar showed the most interesting impact of humic acids, compared to water and gibberellins (Table 4). Both cutting length and applied treatment had different impacts during the fourth measurement period (M4). There, we noticed that treatment provided significant values for rhizogenesis, and there was an overall impact of different cutting lengths × treatment combination.

Table 4.
Effect of length and treatment on roots of Salix viminalis cv. Tordis cuttings.
The middle of the experiment after 10 days was defined by significant impacts of both factors, but not their combination. At the end of the experiment, the last values recorded for both factors and their interaction were significant. Except for the 25 cm cuttings immersed in water, all roots recorded in the first measurement were below 1. Compared to this value, 15 and 25 cm cuttings treated with gibberellins and 20 cm cuttings treated with water showed lower values by 0.5–0.9 units, but with no significant differences.
The second measurement after 10 days showed a great difference between the experimental combinations, with clear significant differences. The two groups in terms of similar differences were separated by a range of 2.11 (25 cm HA) to 2.78 (15 cm W). The water treatment applied to 20 and 25 cm cuttings was higher than this interval. All gibberellin treatments were below the interval, regardless of the cutting length and, most importantly, in the case of 20 cm cuttings where the roots were lost.
Root number reached 13 after 15 days in the variant with 25 cm cuttings treated with water. Only two other results were close to this variant, the 20 and 25 cm cuttings treated with water. Humic acid produced similar root numbers in cuttings lengths of 15 and 20 cm, but almost three times lower than in the 25 cm ones. Gibberellins maintained the roots at reduced values for 15 and 25 cm cuttings, with 1.0 and 1.78 cm, respectively. The last measurement, after 20 days, showed a more than three times increase in the roots developed by the 20 cm size of cuttings treated with humic acid, compared to the previous measurement of the same treatment. Similar to this phenomenon, the humic acid applied on 15 and 25 cm cuttings doubled the root number. The application of water on all types of cuttings produced a gradual increase from 15 to 20 days. For this treatment, roots increased with 1.2 units for 15 cm cuttings, more than 3.5 units for 20 cm cuttings, and 5.0 units for 25 cm cuttings. Roots did not develop on the 20 cm cuttings treated with gibberellins.
The application of the graphical LSD test to explore the differences between shoots and roots biomass was useful for the establishment of the treatment hierarchy (Figure 3). For both willow cultivars, the water treatment applied on 25 cm cuttings produced the highest shoots biomass, with a slightly high value and smaller differences between replications for Tordis. For this cultivar, all humic acid treatments and water applied on 20 cm cuttings maintained the observed biomass over 0.8 g.
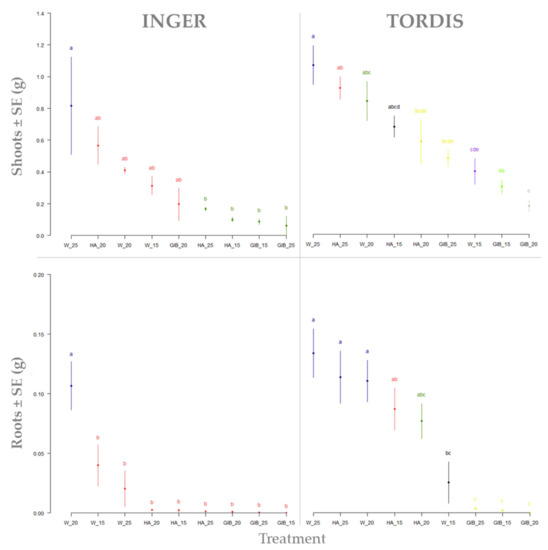
Figure 3.
Dry biomass of the shoots and roots from all treatments of both willow clones. Means ± s.e. followed by different letters present significant differences at p < 0.05. Treatments: W—water; HA—humic acid; GIB—gibberellins. Cutting lengths: 15, 20, 25 cm.
Interestingly, the gibberellin treatments altered the potential biomass of the cuttings, regardless of their initial length. Inger, in terms of shoot biomass, showed a group of variants located in the 0.2–0.6 g range (HA, W, and GIB at 20 cm, and W at 15 cm). Most of the obtained biomasses were significantly lower, only compared to water treatment and 25 cm cuttings. Root biomass showed an interesting case of biomass allocation to roots altered by the treatment application. The only treatment that produced recordable root biomass was water immersion in the case of Inger cultivars. The hierarchy for this treatment was 20—15—25 cm willow cuttings. Root biomass development status showed the opposite in the case of the Tordis cultivar. All water and humic acid treatments applied on these cultivar cuttings produced a recordable root biomass. The maximum was achieved for 25 cm cuttings immersed in water, followed by the same cutting length with humic acid. The hierarchy was continued for 20 cm in water, 15 and 20 cm in humic acid, and ended with 15 cm in water treatment. All gibberellin treatments blocked the formation of recordable root biomass and began to produce a callus after 10 days.
The first global analysis of shoot clustering in the Inger clone showed two small and reduced groups (Figure 4). The first one consisted of only the 20 cm cuttings treated with HA at the end of the experiment, a group that had less than 10 cm length for the first four shoots, but increased to 14 cm at the fifth one, and 30 cm (the maximum of the entire experiment) in the sixth shoot. The total length of developed shoots was 72.54 cm, 6 cm higher compared to the next variant. The second condensed group comprised the 25 cm cuttings treated with water, at 15 and 20 days from the start (DFS), a group that activated eight shoots with a total length of 45.68 and 66.89 cm, respectively, both variants having similar values for the first three shoots and for the seventh one.
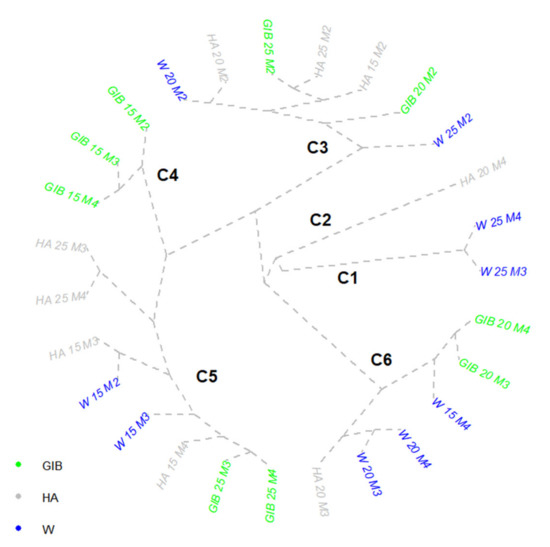
Figure 4.
Cluster representative of Inger clone shoot lengths. Treatments: W—water; HA—humic acid; GIB—gibberellins. Cutting lengths: 15, 20, 25 cm. M1–M4 are the measurement times of active buds: M1—5 days, M2—10 days, M3—15 days, M4—20 days from the beginning of the experiment.
The differences recorded between the two variants were in the range of 4–5 cm, which sustained an increase in the shoot length of 1 cm/day between the two measurements. The third cluster was a very heterogeneous one. In the middle point of the cluster, it comprised three variants at 10 DFS, HA 15, HA 25, and GIB 25, all of them activating only three shoots, with values lower than 2 cm for the first shoot, between 1 and 3 cm for the second one, and between 2 and 5 cm for the first one. Interestingly, the application of GIB stimulated the shoot elongation faster than HA for the analyzed variants. The lateral positions of this cluster were occupied by another four variants from the same 10 DFS period and the same cutting length—W 25 and GIB 20, and HA 20 and W 20. The first two, HA 20 and GIB 20, had a total of 17.27 and 18.24 cm shoot elongations, respectively, with 5/4 shoots activated, but with the length of the fourth and fifth from the first one equal to the length of the fourth from the second variant. The second sub-cluster, composed of 20 and 25 cm cuttings treated with GIB and water, had a total length of shoot elongation of 22.03–23.19 cm, with longer fourth to sixth shoots for 20 cm cuttings, and smaller shoots with the addition of another two shoots (seventh and eighth) activation for 25 cm cuttings. The fourth cluster showed a good collection of multiple similarly treated cuttings/variants, starting with the combination of all the measurements of GIB 15 cm in one cluster. These variants activated only two to three shoots, with a total length varying in the range of 10–14 cm. Shoot elongations were unbalanced, all recorded lengths being associated with shoots developed in different positions. The two HA 25 cm cuttings (15 and 20 DFS) each activated four shoots, with over 20.6 cm total length and a gradual increase from one period to another one. Their similarity was due to only a 0.4 cm/day increase from 15 to 20 DFS. Both W 15 cm (10 DFS) and HA 15 cm cutting (15 DFS) variants had similar values for all three shoots activated, with total elongation of only 11.43–11.78 cm. The last sub-cluster showed a great similarity between the water and HA treatments applied on 15 cm cuttings (15 DFS) and two GIB treated variants of 25 cm cuttings (15 and 20 DFS). All four variants activated only three shoots, with a 3–4 cm elongation of the first shoot and more than 7 cm for the last one.
Cluster analysis was suitable for the detection of similar patterns in leaf development of the Inger clone as a result of applied treatments on different length cuttings (Figure 5). The inclusion of all measurements in the analysis allowed the comparison between experimental factors for a continuous leaf development.
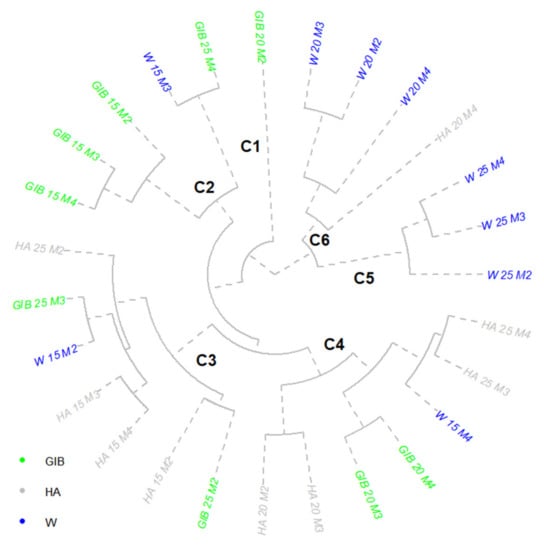
Figure 5.
Cluster representative of Inger clone leaves number. Treatments: W—water; HA—humic acid; GIB—gibberellins. Cutting lengths: 15, 20, 25 cm. M1–M4 are the measurement times of active buds: M1—5 days, M2—10 days, M3—15 days, M4—20 days from the beginning of the experiment.
One treatment was visible as a case unrelated to the other ones—the 20 cm cuttings treated with gibberellins. From the four shoots developed, three had fewer than 6 leaves and the fourth developed 20 leaves. This case presented an unbalanced allocation of resources for leaf development between shoots. A second cluster comprised five experimental combinations—the three measurements of 15 cm cuttings treated with gibberellins and a similar image for 25 cm cuttings treated with gibberellins and 15 cm cuttings treated with water, the first in the last measurement and the last one after 15 DFS. The GIB 15 cm case presented a development of leaves on the first two shoots and fewer or absent leaves on the third one. The similarity between water 15 cm at 15 days and gibberellins 25 cm at the end of the experiment was due to the development of the same number of leaves on the three shoots of 9/15/18 and 10/16/18, respectively. The pattern of similarity between W 15 cm and GIB 25 cm was visible again in the next cluster. GIB 25 at 15 DFS and W 15 at 10 DFS developed two to three fewer leaves than in the previous cluster, but the repeatability of this trend indicated a 5-day faster emergence in the case of water, or a 5-day delay caused by gibberellins. Humic acids applied on 15 cm cuttings were present in the large form of this cluster. All the data from measurements M3 and M4 were similar, with a global leaf production between 22 and 28 units. The application of gibberellins induced an allocation of 17 leaves on the third shoot, and a total leaf number similar to these treatments. The first sub-cluster (HA 20 cm M2 and M3) in the third cluster presented five shoots with numerous leaves developed. The maximum values were recorded on the last three shoots. At the opposite part of the cluster, the same treatment applied on 25 cm cuttings stimulated the appearance of leaves on only four shoots, but with a balanced allocation for each shoot. A similar trend was visible in the water treatments applied on 15 cm cuttings at the end of the experiment (M4). Although gibberellin treatment on 20 cm cuttings stimulated the emergence of leaves on six shoots (15 and 20 DFS), the maximum was recorded on shoot number four, and an unbalanced allocation of leaf emergence was recorded between the shoots within the two measurements. The most stable and cohesive treatment in terms of leaf development was water, with each of the 20 and 25 cm cuttings in the experiment showing the same development from the beginning. The 20 cm cuttings immersed in water stimulated the emergence of leaves on six shoots (10 and 15 DFS) and on the seventh shoot at the end of the experiment. In the case of 25 cm cuttings, leaf emergence was visible on eight shoots with an increased number of leaves every 5 days. The highest number of recorded leaves occurred at the end of the experiment in the variant treated with humic acid and 20 cm cuttings. A total number of six shoots presented leaves, with more than 100 leaves developed.
The number of active shoots developed by the Tordis clone, and their length, varied greatly owing to the applied treatments on different cutting lengths (Figure 6). Cluster analysis revealed that most of the treatments were grouped in the same area, a phenomenon that indicated a gradual development of these aboveground growths.
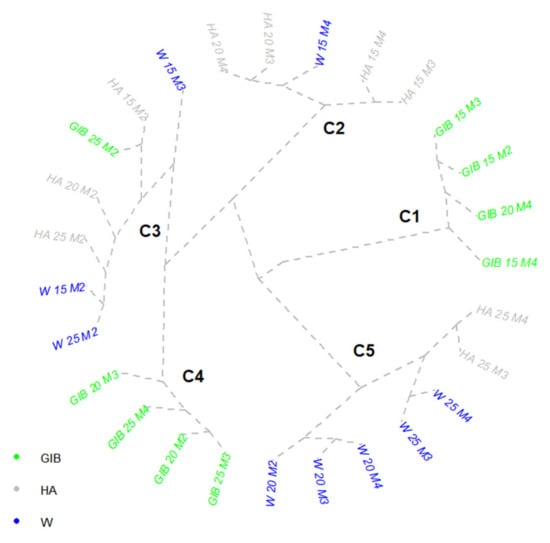
Figure 6.
Cluster representative of Tordis clone shoot length. Treatments: W—water; HA—humic acid; GIB—gibberellins. Cutting lengths: 15, 20, 25 cm. M1–M4 are the measurement times of active buds: M1—5 days, M2—10 days, M3—15 days, M4—20 days from the beginning of the experiment.
All GIB treatments applied on 15 cm cuttings, and the last measurement on 20 cm cuttings, were grouped in the first cluster for Tordis. All these variants showed an average of only two shoots activation, with a total length between 16 and 32 cm. For GIB 15 cm cuttings, the increase was almost 6 cm from 10 to 15 DFS, and 10 cm from 15 to 20 DFS. The GIB 20 M4 case was interesting because of the presence of only two active shoots, which was caused by a sudden drying-out of two shoots: one activated in 10 DFS and another one active in 15 DFS. The second cluster showed the shoot length in 15 and 20 DFS by two cuttings of 15 and 20 cm treated with humic acids. For these four variants, the 15 cm cuttings treated with water (20 DFS) were present. The HA 20 cm cuttings activated five shoots, with a 6.4 cm increase from 15 to 20 DFS. The length of these two variants increased from the bottom to the upper part. The two HA 15 cm cuttings activated six shoots each, with a total increase between 15 to 20 DFS of 13 cm. The W 15 cm variant had eight active shoots, with the first five longer and the seventh in an emergence process. The rest of the two measurements on W 15 cm cuttings were located in cluster three, each with seven shoots activated. The cluster was composed of additional variants in 10 DFS, the three cuttings treated with HA, 25 cm cuttings immersed in water, and the GIB 25 cm. The different numbers of activated shoots for these variants implied a reduction of each shoot length. The fourth cluster consisted of two cutting groups (20 and 25) in the following periods, all treated with GIB. Each of the last period variants (GIB 20 M3 and GIB 25 M4) activated a supplementary shoot. The last cluster included all 20 cm cuttings treated with water, and the last two measurements were for 25 cm cuttings treated with water and HA. The 20 cm cuttings activated 10 shoots from the beginning, with an overall 29 cm increase from 10 to 15 DFS, and 12 cm until the end of the experiment. The rest of the variants showed nine shoots activated, with increases of 15–17 cm between the two periods.
The Tordis cluster analysis of leaf development revealed the assemblage of four clusters, with a total sum of leaves developed between 23 and 139 (Figure 7). The first cluster represented a group of 20 cm cuttings treated with water, all three recorded leaf values were over 100, with a 17-leaf increase from 10 to 15 DFS, and the same value from 15 to 20 DFS and activating 10 shoots. The 25 cm cuttings from 15 and 20 DFS treated with water and HA showed similar leaf values for all the nine shoots activated, with an increase in leaf number of 13–14 from 15 to 20 DFS. The second and third clusters were dominated by GIB treatments, independent of the DFS periods.
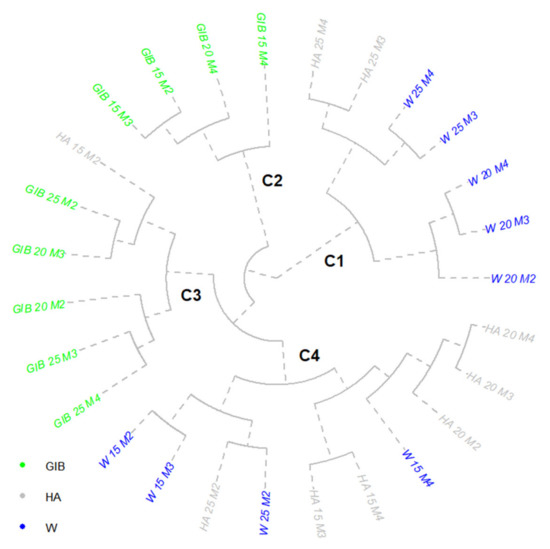
Figure 7.
Cluster representative of the Tordis clone leaf numbers. Treatments: W—water; HA—humic acid; GIB—gibberellins. Cutting lengths: 15, 20, 25 cm. M1–M4 are the measurement times of active buds: M1—5 days, M2—10 days, M3—15 days, M4—20 days from the beginning of the experiment.
These variants stimulated the appearance of leaves on only two to four shoots, with an inconsistent gradual allocation on shoot hierarchy. Only two variants activated four shoots (GIB 25 and HA 15, both at 10 DFS), and their overall leaf production was set below 50. The fourth cluster represented small groups of water and HA treatments, with two W 15 cm cuttings in 10–15 DFS with similar values. For these variants, the seven shoots activated produced 60 leaves (10 DFS) and 65 leaves, respectively, after another 5 days. An interesting case was the similarity between 25 cm cuttings treated with water and with HA, both activating eight shoots to produce 75 and 94 leaves, respectively. The leaf allocation on shoots presented similar patterns, with a maximum value of leaves on the fifth shoot. The HA treatment on 20 cm cuttings produced 52 leaves with 10 DFS, but the secondary increase in these structures was only 13 after another 5 days (15 DFS), and only 1 leaf developed after another 5 days.
4. Discussion
For willow and for perennial species in general, crop sustainability and feasibility, from an economic perspective, are determined by the survival rate and in the end by the shrubs’ yield over time [50]. A large number of studies have been conducted in the field with different willow hybrids and clones. The development of sustainable willow short rotation for biomass production lacks adapted and improved technics to succeed and promote high yield. Hereby, it is notable that a high percentage of cuttings were not activated under field conditions; therefore, the crop did not achieve a high success rate owing to the co-occurrence of multiple factors. Usually, the main reason for willow cuttings dry-out remains unknown.
Humic substances are ubiquitous in the natural soil environment in different shares and play an important role in providing easy uptake of nutrients for plants [51]. Dissolved organic matter, such as humic acid, acts like hormones for plants. Its use offers a series of advantages: it increases the root weight system, increases biomass overall, speeds up cell division, facilitates mineral absorption and nutrient transport to the plant [52,53,54,55]. Humic acids promote plant growth by altering pH, contain redox potential as an advantage for nutrient uptake, and also changes membrane properties to ensure nutrient absorption from organic matter decomposition [56]. Soil microbial activity is regulated by the quantity of dissolved organic matter [57] and represents a central point for sustainable ecosystem functioning [58]. In solutions, humic acids are soluble only if the pH is higher than 4, and those in the soil are usually insoluble and associated with mineral constituents [59].
The GIB A3 natural growth stimulator is intended for many agricultural and horticultural crops: vegetables, cereals, sunflowers, potatoes, fruit trees and shrubs, alfalfa, clover, and vines [60,61,62,63,64,65,66]. As a result, the growth stimulator, which contains gibberellic acid GA3 (gibberellin), increases yields of superior strength and quality. Additionally, the main changes that occur in plant metabolism from gibberellins are an intensification of photosynthesis, respiration, and water consumption, delaying the aging processes of plant tissue [67,68,69,70,71,72]. Although the receptor location of gibberellic acid was not proved, it was stated that the signals are perceived extracellularly in the plasma membrane [73]. Throughout the plant life cycle, plant hormones such as gibberellic acid modulate plant growth and development. Usually, growth stimulators improve plant growth by stimulating cell elongation and expansion. An inhibitory reaction to GA3 was found in carrots, where there was a reduction of root growth with a higher shoot development [74], and the roots lignified [75]. In willow clones, similar results were obtained in this study with the noting of callus formation after 10 days. This fact can be explained by the concomitant gibberellin biosynthesis [76] and GA3 presence in the solution that suppressed the development of the normal roots [77] through the development of an unorganized mass of plant xylem parenchyma cells, and the cell wall significantly thickened.
For Salix in particular, water availability produces the expansion of fine roots [78], and carbon allocation for biomass production benefits proportionally. Events with low water potential usually happen in summer periods; Salix spp. are water affinity plants, therefore, they can intercept rainfall infiltration at the root area during a rainy event, which could also lower groundwater levels [79]. Planting a Salix spp. crop in the field is a reasonable decision.
One step forward in identifying the main factors should be publishing experimental notes and observations (Supplementary Figures S1 and S2). The recorded results of the most important observations will help to preserve the essential details of a phenomenon. Fundamental studies are needed to understand the biology and survival capacity of a species relative to eco-pedo-climatic conditions. The current study attempted to highlight the most comprehensive observations with regard to both Inger and Tordis willow clones. The priming effect of general growth was established following three hypotheses. (i) The right size for the Inger clone’s best development was the medium tested value, and for the Tordis clone it was the maximum established size when the nutrients were missing, and the minimum tested size when the growth occurred in the nutrient solution. (ii) The highest success rate for both clones was supplied by growth in water, and the lowest growth and development results were seen in the treatments with gibberellins. (iii) Tordis represented the best growth pattern, a characteristic identified after the assessment of all parameters. Between replications of the same variant, the minimum, medium or higher values were always treatment-specific. This aspect, however, was not seen in the Inger clone where, compared with Tordis, low vigor was observed with thinner and smaller leaves.
Cutting length influenced the root/shoot allocation regarding bud appearance, shoot growth, and total leaf appearance. Similar studies recommended longer Inger cuttings of about 40 cm, instead of shorter cuttings of 20 cm, to ensure a higher survival rate at crop establishment [80]. The results for 20 cm Inger cuttings were contradictory; growth and development were the most efficient with highest performance. The same study emphasized that longer cuttings would fare better in the field under unfavorable conditions such as low nutrient and water supply. It was noted [80] that assessing the effect of cutting size on willow clone root system development is lacking. It can be stated that overall developmental characteristics are still insufficient and very seldom considered. Regarding the findings on Inger shoot/root development, 62.5% agree that a cutting of 25 cm, the longer cutting size tested, represents medium values of normal and efficient growth and development. One study on SRC development under nitrogen fertilization concluded that dry biomass yield is first dependent on genotype, and only afterward in small part on fertilizer [81].
A search performed on Web of Science topics “willow” × “Inger”, “willow” × “Tordis”, and “willow” × “Inger” × “Tordis” revealed a total of 42 articles. Only 10 presented information related to both clones, while 14 were associated with Inger and 18 with Tordis. Most research was focused on yield, dry biomass, management, and fertilizers in field conditions [50,82,83,84,85,86,87], and there were only a few studies on harsh conditions [31,37,38,88,89,90,91]. In both contexts, a short pre-field experiment was useful to establish and promote the rhizogenesis potential of willow clones by recreating the nutritional conditions of the planting site and recording the plant growth and development parameters. An interesting finding in the Web of Science database was the short coppice rotation system, which requires the establishment of fertilizers. These conditions can be tested, and selection of the optimal ones is easier in controlled conditions. The application of the pre-field test for contaminated soils was also useful owing to the researchers’ direct access to the changes produced by treatments. The most important information was the viability rate establishment, which can be further used for selecting a priming process before planting in the field.
5. Conclusions
The tested sizes had different effects on the buds and root appearance and the development of both clones.
The medium and high cutting sizes tested favored the Inger clone because of good bud development, with best results in water and a medium response to humic acids. Gibberellins activated an average of one to two buds on the Inger clone, independent of cutting length, but restricted the root formation of 15 and 25 cm cuttings because of callus formation.
The gibberellin application produced a callus on all the Tordis clone’s cuttings lengths tested, which blocked the rhizogenesis and the bud activation, growth, and development.
Humic acids stimulated the activation of Tordis buds with more than four buds in the 15 and 25 cm cuttings, similar to the values recorded for the water treatment. For the Tordis clone, humic acids applied on 20 cm cuttings did not favor increases in the number of activated buds, but it was the length that stimulated more than seven buds when water was applied.
A comparison of the two clones highlighted the stability and the performance of Tordis in short-term experiments, and the higher sensibility of Inger, which made it a potential test clone for reaction-type tests.
Future studies should focus on willow pre-planting studies to identify the most suitable rhizogenesis priming effect for producing resistant planting material, with higher survival chances in field transplantation.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/su14159272/s1, Supplementary Figure S1. Salix viminalis cv. Inger—trends of shoot and root development during all four assessments. Representative colors: lowest values—blue, average values—gray, highest values—red. A—Overall average of treatment (independent of cutting size). Detailed observations for each assessment listed below. Supplementary Figure S2. Salix viminalis cv. Tordis—trends of shoot and root development during all four assessments. Representative colors: lowest values—blue, average values—gray, highest values—red. A—Overall average of treatment (independent of cutting size). Detailed observations for each assessment listed below.
Author Contributions
Conceptualization, S.D.V., V.S. and V.A.S.; methodology, Ș.G. and M.Ș.; software, V.S.; validation, S.D.V., M.Ș., V.A.S. and Ș.G.; formal analysis, V.S. and R.V.; investigation, V.A.S., Ș.G. and M.Ș.; resources, S.D.V., A.H., A.V. and V.A.S.; data curation, V.S.; writing—original draft preparation, V.A.S., V.S., S.D.V., Ș.G., R.V. and M.Ș.; writing—review and editing, V.A.S., V.S., S.D.V., Ș.G., R.V. and M.Ș.; visualization, V.A.S., V.S., S.D.V., Ș.G., R.V., A.V. and M.Ș.; supervision, V.A.S. and V.S.; project administration, Ș.G., A.V. and R.V.; funding acquisition, S.D.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the University of Agricultural Sciences and Veterinary Medicine Cluj-Napoca from the National Research Development Projects to Finance Excellence (PFE)-14/2022-2024 granted by the Romanian Ministry of Research and Innovation, and from Supporting Research Grant, number 21657/01.10.2021.
Data Availability Statement
The data presented in this study are available on request from the corresponding authors.
Acknowledgments
The authors wish to acknowledge the help and support provided by Wilhelm Hollerbach.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Scordia, D.; Papazoglou, E.G.; Kotoula, D.; Sanz, M.; Ciria, C.S.; Pérez, J.; Maliarenko, O.; Prysiazhniuk, O.; von Cossel, M.; Greiner, B.E.; et al. Towards identifying industrial crop types and associated agronomies to improve biomass production from marginal lands in Europe. GCB Bioenergy 2022, 14, 710–734. [Google Scholar] [CrossRef]
- Zięty, J.J.; Olba-Zięty, E.; Stolarski, M.J.; Krzykowski, M.; Krzyżaniak, M. Legal Framework for the Sustainable Production of Short Rotation Coppice Biomass for Bioeconomy and Bioenergy. Energies 2022, 15, 1370. [Google Scholar] [CrossRef]
- Trava, I.D.; Borlea, G.F.; Hollerbach, W. Identification of the Most Productive Species from the Salix Genus and Its Use in Energetic Cultures. J. Hortic. For. Biotechnol. 2014, 18, 209–213. [Google Scholar]
- Johnston, C.R.; Walsh, L.R.; McCracken, A.R. Effect of two vs. three year harvest intervals on yields of Short Rotation Coppice (SRC) willow. Biomass Bioenergy 2022, 156, 106303. [Google Scholar] [CrossRef]
- Simon, L.; Szabó, B.; Szabó, M.; Vincze, G.; Varga, C.; Uri, Z.S.; Koncz, J. Effect of Various Soil Amendments on the Mineral Nutrition of Salix viminalis and Arundo donax Energy Crops. Eur. Chem. Bull 2013, 2, 18–21. [Google Scholar]
- Kulig, B.; Gacek, E.; Wojciechowski, R.; Oleksy, A.; Kołodziejczyk, M.; Szewczyk, W.; Klimek-Kopyra, A. Biomass Yield and Energy Efficiency of Willow Depending on Cultivar, Harvesting Frequency and Planting Density. Plant Soil Environ. 2019, 65, 377–386. [Google Scholar] [CrossRef]
- Demo, M.; Hauptvogl, M.; Prčík, M.; Húska, D. Comparison of production parameters of willow (Salix spp.) and poplar (Populus spp.) varieties in the last year of the first four-year harvest cycle. Wood Res. 2014, 59, 705–716. [Google Scholar]
- Macalpine, W.J. Identifying Drought Tolerant Short Rotation Coppice Willows. Master’s Thesis, Harper Adams University, Newport, UK, 2019. [Google Scholar]
- Liberacki, D.; Kocięcka, J.; Stachowski, P.; Rolbiecki, R.; Rolbiecki, S.; Sadan, H.A.; Figas, A.; Jagosz, B.; Wichrowska, D.; Ptach, W.; et al. Water Needs of Willow (Salix L.) in Western Poland. Energies 2022, 15, 484. [Google Scholar] [CrossRef]
- Digruber, T.; Sass, L.; Cseri, A.; Paul, K.; Nagy, A.V.; Remenyik, J.; Dudits, D. Stimulation of Energy Willow Biomass with Triacontanol and Seaweed Extract. Ind. Crops Prod. 2018, 120, 104–112. [Google Scholar] [CrossRef]
- Olba-Zięty, E.; Stolarski, M.J.; Krzyżaniak, M. Economic Evaluation of the Production of Perennial Crops for Energy Purposes—A Review. Energies 2021, 14, 7147. [Google Scholar] [CrossRef]
- Warmiński, K.; Stolarski, M.J.; Gil, Ł.; Krzyżaniak, M. Willow bark and wood as a source of bioactive compounds and bioenergy feedstock. Ind. Crops Prod. 2021, 171, 113976. [Google Scholar] [CrossRef]
- Long, A.; Bose, A.; O’Shea, R.; Monaghan, R.; Murphy, J.D. Implications of European Union recast Renewable Energy Directive sustainability criteria for renewable heat and transport: Case study of willow biomethane in Ireland. Renew. Sustain. Energy Rev. 2021, 150, 111461. [Google Scholar] [CrossRef]
- Djomo, S.N.; Kasmioui, O.E.; Ceulemans, R. Energy and Greenhouse Gas Balance of Bioenergy Production from Poplar and Willow: A Review. GCB Bioenergy 2011, 3, 181–197. [Google Scholar] [CrossRef]
- Ivan, V.; Hoefnagels, R.; Junginger, M.; van der Hilst, F. Supply potential of lignocellulosic energy crops grown on marginal land and greenhouse gas footprint of advanced biofuels—A spatially explicit assessment under the sustainability criteria of the Renewable Energy Directive Recast. GCB Bioenergy 2021, 13, 1425–1447. [Google Scholar]
- Câmpeanu, V.; Pencea, S. Renewable energy sources in Romania: From a “paradise” of investors to a possible abandon or to another boom? The impact of a new paradigm in Romanian renewable sources policy. Procedia Econ. Financ. 2014, 8, 129–137. [Google Scholar] [CrossRef][Green Version]
- Oncioiu, I.; Petrescu, A.G.; Grecu, E.; Petrescu, M. Optimizing the renewable energy potential: Myth or future trend in Romania. Energies 2017, 10, 759. [Google Scholar] [CrossRef]
- Adegbidi, H.G.; Volk, T.A.; White, E.H.; Abrahamson, L.P.; Briggs, R.D.; Bickelhaupt, D.H. Biomass and Nutrient Removal by Willow Clones in Experimental Bioenergy Plantations in New York State. Biomass Bioenergy 2001, 20, 399–411. [Google Scholar] [CrossRef]
- Labrecque, M.; Teodorescu, T.I. Influence of Plantation Site and Wastewater Sludge Fertilization on the Performance and Foliar Nutrient Status of Two Willow Species Grown under SRIC in Southern Quebec (Canada). For. Ecol. Manag. 2001, 150, 223–239. [Google Scholar] [CrossRef]
- Labrecque, M.; Teodorescu, T.I. Field Performance and Biomass Production of 12 Willow and Poplar Clones in Short-Rotation Coppice in Southern Quebec (Canada). Biomass Bioenergy 2005, 29, 1–9. [Google Scholar] [CrossRef]
- Isebrands, J.G.; Aronsson, P.; Carlson, M.; Ceulemans, R.; Coleman, M.; Dickinson, N.; Dimitriou, J.; Doty, S.; Gardiner, E.; Heinsoo, K.; et al. Environmental Applications of Poplars and Willows. In Poplars and Willows: Trees for Society and the Environment; Isebrands, J.G., Richardson, J., Eds.; FAO: Roma, Italy, 2014; pp. 258–336. [Google Scholar]
- Cerrillo, T.; Álvarez, J.; Battistella, A.; Braccini, C.; Casaubón, E.; Ceballos, D.; Cortizo, S.; Fernandez Tschieder, E.; Fernández, P.C.; Faustino, L.; et al. Salicáceas afforestation as a contribution to the sustainable development of the Paraná Delta. Disertación. XXIX Jornadas Forestales de Entre Ríos. Concordia 2015, 14, 1–14. [Google Scholar]
- Holm, B.; Heinsoo, K. Municipal wastewater application to Short Rotation Coppice of willows—Municipal wastewater application to Short Rotation Coppice of willows—Treatment efficiency and clone response in Estonian case study. Biomass Bioenergy 2013, 57, 126–135. [Google Scholar] [CrossRef]
- Mleczek, M.; Rutowski, P.; Rissman, I.; Kaczmarek, Z.; Golinski, P.; Szentner, K.; Strazynska, K.; Stachowiak, A. Biomass Productivity and Phytoremediation Potential of Salix alba and Salix viminalis. Biomass Bioenergy 2010, 34, 1410–1418. [Google Scholar] [CrossRef]
- Corneanu, M.; Hernea, C.; Butnariu, M.; Corneanu, G.; Sărac, I.; Hollerbach, W.; Petcov, A.A. Preliminary Tests for Salix Sp. Tolerance to Heavy Metals (Cd, Ni, Pb). In Proceedings of the EGU General Assembly Conference Abstracts, Vienna, Austria, 27 April–2 May 2014; p. 10620. [Google Scholar]
- Stolarski, M.J.; Niksa, D.; Krzyżaniak, M.; Tworkowski, J.; Szczukowski, S. Willow productivity from small-and large-scale experimental plantations in Poland from 2000 to 2017. Renew. Sustain. Energy Rev. 2019, 101, 461–475. [Google Scholar] [CrossRef]
- Stanton, B.J.; Bourque, A.; Coleman, M.; Eisenbies, M.; Emerson, R.M.; Espinoza, J.; Gantz, C.; Himes, A.; Rodstrom, A.; Shuren, R.; et al. The practice and economics of hybrid poplar biomass production for biofuels and bioproducts in the Pacific Northwest. BioEnergy Res. 2021, 14, 543–560. [Google Scholar] [CrossRef]
- Dillen, M.; Vanhellemont, M.; Verdonckt, P.; Maes, W.H.; Steppe, K.; Verheyen, K. Productivity, stand dynamics and the selection effect in a mixed willow clone short rotation coppice plantation. Biomass Bioenergy 2016, 87, 46–54. [Google Scholar] [CrossRef]
- Njakou Djomo, S.; Ac, A.; Zenone, T.; De Groote, T.; Bergante, S.; Facciotto, G.; Sixto, H.; Ciria Ciria, P.; Weger, J.; Ceulemans, R. Energy performances of intensive and extensive short rotation cropping systems for woody biomass production in the EU. Renew. Sustain. Energy Rev. 2015, 41, 845–854. [Google Scholar] [CrossRef]
- Weih, M.; Glynn, C.; Baum, C. Willow short-rotation coppice as model system for exploring ecological theory on biodiversity–ecosystem function. Diversity 2019, 11, 125. [Google Scholar] [CrossRef]
- Matyka, M.; Radzikowski, P. Productivity and biometric characteristics of 11 varieties of willow cultivated on marginal soil. Agriculture 2020, 10, 616. [Google Scholar] [CrossRef]
- Hernea, C.; Corneanu, M.; Sarac, I.; Petrescu, I. The behavior of willow commercial clones in the first growing season. A case study for three different sites from Banat area. Ann. Univ. Craiova-Agric. Montanology Cadastre Ser. 2017, 46, 622–627. [Google Scholar]
- Soare, M.; Panita, O.; Salceanu, C. Partial results concerning the behavior of energy willow genotypes in cultivated improper areas. Ann. Univ. Craiova-Agric. Montanology Cadastre Ser. 2015, 45, 300–305. [Google Scholar]
- Buzatu-Goanta, C.; Corneanu, M.; Babeanu, C. Salix Accessions with Potential For New Hybrids. A Case Study from Banat Area. In Scientific Papers. Series E. Land Reclamation, Earth Observation & Surveying, Environmental Engineering; University of Agronomic Sciences and Veterinary Medicine of Bucharest: Bucharest, Romania, 2021; Volume X, ISSN 2285-6064. pp. 214–220. [Google Scholar]
- Hernea, C.; Trava, I.D.; Borlea, G.F. Biomass Production of Some Swedish Willow Hybrids on the West of Romania. A Case Study. J. Hortic. For. Biotechnol. 2015, 19, 103–106. [Google Scholar]
- Soare, M.; Iancu, P.; Panita, O.; Soare, R.; Bonciu, E. Researches Concerning the Possibility of Cultivating Energetic Willow on Deposit of Ash from Thermal Power Stations. SGEM2017 Conf. Proc. 2017, 17, 519–528. [Google Scholar]
- Scriba, C.; Lunguleasa, A.; Salca, E.A.; Ciobanu, V.D. Properties of Biomass Obtained from Short-Rotation Inger Willow Clone Grown on a Contaminated and Non-Contaminated Land. Maderas. Cienc. Tecnol. 2021, 23, 1–12. [Google Scholar] [CrossRef]
- Scriba, C.; Lunguleasa, A.; Spirchez, C.; Ciobanu, V. Influence of INGER and TORDIS Energetic Willow Clones Planted on Contaminated Soil on the Survival Rates, Yields and Calorific Value. Forests 2021, 12, 826. [Google Scholar] [CrossRef]
- Orlovic, S.; Klasnja, B.; Pilipovic, A.; Radosavljevic, N. Physiological and growth characteristics of white willow (Salix alba L.). Clones 2003, 1, 223–226. [Google Scholar]
- Rewald, B.; Kunze, M.; Godbold, D.L. NH4: NO3 Nutrition Influence on Biomass Productivity and Root Respiration of Poplar and Willow Clones. GCB Bioenergy 2016, 8, 51–58. [Google Scholar] [CrossRef]
- Keller, C. Efficiency and Limitations of Phytoextraction by High Biomass Plants: The Example of Willows. In Trace Elements in the Environment; CRC Press: Boca Raton, FL, USA, 2005; pp. 629–650. [Google Scholar]
- Pulford, I.D.; Dickinson, N.M. Phytoremediation technologies using trees. In Trace Elements in the Environment. Biogeochemistry, Biotechnology, and Bioremediation; Prasad, M.N.V., Sajwan, K.S., Naidu, R., Eds.; CRC Press, Taylor and Francis Group: Boca Raton, FL, USA, 2006; pp. 383–403. [Google Scholar]
- Cornelia, H.; Hollerbach, W.; Trava, D.; Mihaela, C. The Behaviour for SRC Willow Inger in Experimental Trial Ghilad, Romania. Bull. UASVM Hortic. 2015, 72, 376–380. [Google Scholar]
- RStudio Team. RStudio: Integrated Development Environment for R. RStudio, PBC, Boston, MA, USA. 2021. Available online: http://www.rstudio.com/ (accessed on 20 April 2022).
- R Core Team. R: A language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. 2021. Available online: https://www.R-project.org/ (accessed on 20 April 2022).
- Revelle, W. Psych: Procedures for Personality and Psychological Research, Northwestern University, Evanston, Illinois, USA. 2021. Available online: https://CRAN.R-project.org/package=psych (accessed on 20 April 2022).
- De Mendiburu, F. Agricolae: Statistical Procedures for Agricultural Research. R Package Version 1.3-5. 2021. Available online: https://CRAN.R-project.org/package=agricolae (accessed on 20 April 2022).
- Robinson, D.; Hayes, A.; Couch, S. broom: Convert Statistical Objects into Tidy Tibbles. R Package Version 0.7.8. 2021. Available online: https://CRAN.R-project.org/package=broom (accessed on 20 April 2022).
- Paradis, E.; Schliep, K. Ape 5.0: An environment for modern phylogenetics and evolutionary analyses in R. Bioinformatics 2019, 35, 526–528. [Google Scholar] [CrossRef]
- Larsen, S.U.; Jørgensen, U.; Lærke, P.E. Biomass Yield, Nutrient Concentration and Nutrient Uptake by SRC Willow Cultivars Grown on Different Sites in Denmark. Biomass Bioenergy 2018, 116, 161–170. [Google Scholar] [CrossRef]
- Kałuża-Haładyn, A.; Jamroz, E.; Bekier, J. Humic substances of differently matured composts produced from municipal solid wastes and biomass of energetic plants. Soil Sci. Annu. 2019, 70, 292–297. [Google Scholar] [CrossRef]
- Rajan, R.P.; Singh, G. A review on the use of organic rooting substances for propagation of horticulture crops. Plant Arch. 2021, 21, 685–692. [Google Scholar] [CrossRef]
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural uses of plant biostimulants. Plant Soil 2014, 383, 3–41. [Google Scholar] [CrossRef]
- Nardi, S.; Schiavon, M.; Francioso, O. Chemical structure and biological activity of humic substances define their role as plant growth promoters. Molecules 2021, 26, 2256. [Google Scholar] [CrossRef]
- Makan, A. (Ed.) Humic Substances; IntechOpen: London, UK, 2021. [Google Scholar]
- Toetz, D.; Payton, M. The role of dissolved organic matter in accrual of periphytic biomass in a subalpine stream, Colorado Front Range. J. Freshw. Ecol. 2006, 21, 613–620. [Google Scholar] [CrossRef][Green Version]
- Jia, W.; Hu, C.; Xu, J.; Ming, J.; Zhao, Y.; Cai, M.; Zhao, X. Dissolved organic matter derived from rape straw pretreated with selenium in soil improves the inhibition of Sclerotinia sclerotiorum growth. J. Hazard. Mater. 2019, 369, 601–610. [Google Scholar] [CrossRef]
- Lange, M.; Roth, V.N.; Eisenhauer, N.; Roscher, C.; Dittmar, T.; Fischer-Bedtke, C.; Gleixner, G. Plant diversity enhances production and downward transport of biodegradable dissolved organic matter. J. Ecol. 2021, 109, 1284–1297. [Google Scholar] [CrossRef]
- Kaschl, A.; Chen, Y. Interactions of humic substances with trace metals and their stimulatory effects on plant growth. In Use of Humic Substances to Remediate Polluted Environments: From Theory to Practice; Springer: Dordrecht, The Netherlands, 2005; pp. 83–113. [Google Scholar]
- Ghosh, S.; Halder, S. Effect of different kinds of gibberellin on temperate fruit crops: A review. Pharma Innov. J. 2018, 7, 315–319. [Google Scholar]
- Zaman, M.; Kurepin, L.V.; Catto, W.; Pharis, R.P. Enhancing crop yield with the use of N-based fertilizers co-applied with plant hormones or growth regulators. J. Sci. Food Agric. 2015, 95, 1777–1785. [Google Scholar] [CrossRef]
- Singh, N.B.; Singh, A.; Khare, S.; Yadav, V.; Bano, C.; Yadav, R.K. Mitigating strategies of gibberellins in various environmental cues and their crosstalk with other hormonal pathways in plants: A review. Plant Mol. Biol. Report. 2021, 39, 34–49. [Google Scholar]
- Pal, S.L. Role of plant growth regulators in floriculture: An overview. J. Pharmacogn. Phytochem. 2019, 8, 789–796. [Google Scholar]
- Rademacher, W. Chemical regulators of gibberellin status and their application in plant production. Annu. Plant Rev. Online 2018, 49, 359–403. [Google Scholar]
- Urbanova, T.; Leubner-Metzger, G. Gibberellins and seed germination. Annu. Plant Rev. 2016, 49, 253–284. [Google Scholar]
- Gollagi, S.G.; Lokesha, R.; Dharmpal, S.; Sathish, B.R. Effects of growth regulators on growth, yield and quality of fruits crops: A review. J Pharm. Phytochem. 2019, 8, 979–981. [Google Scholar]
- Small, C.C.; Degenhardt, D. Plant growth regulators for enhancing revegetation success in reclamation: A review. Ecol. Eng. 2018, 118, 43–51. [Google Scholar] [CrossRef]
- Kosakivska, I.V.; Vedenicheva, N.P.; Babenko, L.M.; Voytenko, L.V.; Romanenko, K.O.; Vasyuk, V.A. Exogenous phytohormones in the regulation of growth and development of cereals under abiotic stresses. Mol. Biol. Rep. 2021, 49, 617–628. [Google Scholar] [CrossRef]
- Wu, K.; Xu, H.; Gao, X.; Fu, X. New insights into gibberellin signaling in regulating plant growth–metabolic coordination. Curr. Opin. Plant Biol. 2021, 63, 102074. [Google Scholar] [CrossRef]
- Hedden, P. The current status of research on gibberellin biosynthesis. Plant Cell Physiol. 2020, 61, 1832–1849. [Google Scholar] [CrossRef]
- Rizza, A.; Jones, A.M. The makings of a gradient: Spatiotemporal distribution of gibberellins in plant development. Curr. Opin. Plant Biol. 2019, 47, 9–15. [Google Scholar] [CrossRef]
- Wu, J.; Wang, J.; Hui, W.; Zhao, F.; Wang, P.; Su, C.; Gong, W. Physiology of Plant Responses to Water Stress and Related Genes: A Review. Forests 2022, 13, 324. [Google Scholar]
- Richards, D.E.; King, K.E.; Ait-Ali, T.; Harberd, N.P. How gibberellin regulates plant growth and development: A molecular genetic analysis of gibberellin signaling. Annu. Rev. Plant Biol. 2001, 52, 67–88. [Google Scholar] [CrossRef]
- Wang, G.L.; Que, F.; Xu, Z.S.; Wang, F.; Xiong, A.S. Exogenous gibberellin altered morphology, anatomic and transcriptional regulatory networks of hormones in carrot root and shoot. BMC Plant Biol. 2015, 15, 290. [Google Scholar] [CrossRef]
- Wang, G.L.; Que, F.; Xu, Z.S.; Wang, F.; Xiong, A.S. Exogenous gibberellin enhances secondary xylem development and lignification in carrot taproot. Protoplasma 2017, 254, 839–848. [Google Scholar] [CrossRef]
- Xiao, J.; Li, H.; Zhang, J.; Chen, R.; Zhang, Y.; Ouyang, B.; Ye, Z. Dissection of GA 20-oxidase members affecting tomato morphology by RNAi-mediated silencing. Plant Growth Regul. 2006, 50, 179–189. [Google Scholar] [CrossRef]
- Tagliavini, M.; Looney, N.E. Response of peach seedlings to root-zone temperature and root-applied growth regulators. HortScience 1991, 26, 870–872. [Google Scholar] [CrossRef]
- Rytter, R.M. The effect of limited availability of N or water on C allocation to fine roots and annual fine root turnover in Alnus incana and Salix viminalis. Tree Physiol. 2013, 33, 924–939. [Google Scholar] [CrossRef]
- Zhao, M.; Wang, W.; Wang, Z.; Chen, L.; Ma, Z.; Wang, Q. Water use of Salix in the variably unsaturated zone of a semiarid desert region based on in-situ observation. J. Hydrol. 2020, 591, 125579. [Google Scholar] [CrossRef]
- Vigl, F.; Rewald, B. Size Matters?–The Diverging Influence of Cutting Length on Growth and Allometry of Two Salicaceae Clones. Biomass Bioenergy 2014, 60, 130–136. [Google Scholar] [CrossRef]
- Styszko, L.; Dabrowski, J. Impact of the Nitrogen Fertilization on Yield, Dry Matter, Ash and Total Nitrogen Content in the Second 4-Year Rotation of Basket Willow Cultivation. Rocz. Ochr. Srodowiska 2018, 20, 1234–1251. [Google Scholar]
- Larsen, S.U.; Jørgensen, U.; Lærke, P.E. Willow yield is highly dependent on clone and site. BioEnergy Res. 2014, 7, 1280–1292. [Google Scholar] [CrossRef]
- Liu, N.; Jørgensen, U.; Lærke, P.E. Concentrations of chemical elements in willow biomass depend on clone, site and management in the field. BioEnergy Res. 2016, 9, 1216–1230. [Google Scholar] [CrossRef]
- Borzymowska, A.; Styszko, L. Influence of Planting Density on Lenght, Thikness and Number of Shoots in Willow Carp During Four-year Cultivation Cycle. Rocz. Ochr. Srodowiska 2012, 14, 481–490. [Google Scholar]
- Graß, R.; Malec, S.; Wachendorf, M. Biomass performance and competition effects in an established temperate agroforestry system of willow and grassland—Results of the 2nd rotation. Agronomy 2020, 10, 1819. [Google Scholar] [CrossRef]
- Finnan, J.M.; Donnelly, I.; Burke, B. The effect of cutting back willow after one year of growth on biomass production over two harvest cycles. Biomass Bioenergy 2016, 92, 76–80. [Google Scholar] [CrossRef]
- Sommer, J.; Hartmann, L.; Dippold, M.A.; Lamersdorf, N.P. Specific Nmin uptake patterns of two widely applied poplar and willow clones for short rotation coppices–Implications for management practices. Biomass Bioenergy 2017, 98, 236–242. [Google Scholar] [CrossRef]
- Heinsoo, K.; Dimitriou, I. Growth performance of willow clones in short rotation coppice after sewage sludge application. Balt. For. 2014, 20, 70–77. [Google Scholar]
- Castaño-Díaz, M.; Barrio-Anta, M.; Afif-Khouri, E.; Cámara-Obregón, A. Willow short rotation coppice trial in a former mining area in northern Spain: Effects of clone, fertilization and planting density on yield after five years. Forests 2018, 9, 154. [Google Scholar] [CrossRef]
- Mikó, P.; Kovács, G.P.; Alexa, L.; Balla, I.; Póti, P.; Gyuricza, C.S. Biomass production of energy willow under unfavourable field conditions. Appl. Ecol. Environ. Res. 2014, 12, 1–11. [Google Scholar] [CrossRef]
- Van Slycken, S.; Witters, N.; Meiresonne, L.; Meers, E.; Ruttens, A.; Van Peteghem, P.; Weyens, N.; Tack, F.M.; Vangronsveld, J. Field evaluation of willow under short rotation coppice for phytomanagement of metal-polluted agricultural soils. Int. J. Phytoremediation 2013, 15, 677–689. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).