Abstract
Coastal marine ecosystems provide essential benefits and services to humanity, but many are rapidly degrading. Human activities are leading to significant land take along coastlines and to major changes in ecosystems. Ecological engineering tools capable of promoting large-scale restoration of coastal ecosystems are needed today in the face of intensifying climatic stress and human activities. Concrete is one of the materials most commonly used in the construction of coastal and marine infrastructure. Immersed in seawater, concretes are rapidly colonized by microorganisms and macroorganisms. Surface colonization and subsequent biofilm and biofouling formation provide numerous advantages to these organisms and support critical ecological and biogeochemical functions in the changing marine environment. The new challenge of the 21st century is to develop innovative concretes that, in addition to their usual properties, provide improved bioreceptivity in order to enhance marine biodiversity. The aim of this study is to master and clarify the intrinsic parameters that influence the bioreceptivity (biocolonization) of cementitious materials in the marine environment. By coupling biofilm (culture-based methods) and biofouling (image-analysis-based method and wet-/dry-weight biomass measurement) quantification techniques, this study showed that the application of a curing compound to the concrete surface reduced the biocolonization of cementitious materials in seawater, whereas green formwork oil had the opposite effect. This study also found that certain surface conditions (faceted and patterned surface, rough surface) promote the bacterial and macroorganism colonization of cementitious materials. Among the parameters examined, surface roughness proved to be the factor that promotes biocolonization most effectively. These results could be taken up in future recommendations to enable engineers to eco-design more eco-friendly marine infrastructure and develop green-engineering projects.
1. Introduction
The management and conservation of the world’s oceans require synthesis of spatial data on the distribution and intensity of human activities and the overlap of their impacts on marine ecosystems [1]. Human activities are leading to significant land take along coastlines [1] and to major changes in ecosystems [2]. “Sprawl” in natural marine areas is leading to a loss of spatial connectivity [3] and erosion of biodiversity [4] in this Anthropocene epoch [5]. The recent global assessment report by the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services (IPBES) on biodiversity and ecosystem services (May 2019) [6] clearly identifies five direct drivers of change in nature, first among them being changes in land and sea use due to urbanization, industrial complexes, roads and mining. The challenge of the 21st century must be to find new solutions in the construction field. One such solution is to combine engineering techniques and ecological understanding to provide cost-effective ways to maintain or enhance biodiversity [7,8,9].
In the marine environment, urban, coastal and offshore structures provide an important protective function, but can also have unintended ecological consequences, such as the loss or modification of habitat and the alteration of hydrological and ecological flows [3,10,11,12,13]. The challenge for managers now is to design marine infrastructure in a way that minimizes its ecological impact and increases its bioreceptivity (the ability to be colonized by living organisms) in order to enhance marine biodiversity [9,14,15]. The results of this paper are intended for technical applications in the area of concrete protection for breakwaters.
In the last decade, concrete has been one of the materials most widely used for the construction of marine infrastructure such as ports and coastal defenses [16,17]. It has proved to be a reliable structural material with very good durability when properly used [18]. However, the durability of a concrete structure is influenced by the exposure environment [19]. In seawater, chemical, physical and biological actions affect the aging and durability of a concrete structure [20,21,22,23]. Influences of a physical and chemical nature are generally well studied, and are subject to standards and recommendations [24,25]. Several scientific publications state that actions of a chemical nature such as chloride and magnesium sulfate attack are the main cause of concrete structure deterioration in the marine environment [18,22,26,27]. However, the actions of a biological nature (e.g., colonization of concrete by marine organisms) have been less studied, and less is known about them [24]. The effect of these actions on the durability of concrete in the marine environment remains unclear, but most scientists agree that marine organisms adhered to the concrete surface have a protective effect (bioprotection) against chemical attack in seawater [7,23,28,29,30,31,32,33,34]; they form a physical barrier that reduces surface permeability. The decrease in surface permeability leads to less-efficient diffusion of aggressive ions (Cl−, Mg2+ and OH−), which can increase the durability of a concrete structure in the marine environment [23,29,35,36]. Understanding and mastering the biological actions affecting concrete in this environment is therefore an essential step toward meeting the new challenge of designing sustainable maritime structures that enhance the regeneration of marine biodiversity [36].
Actions of a biological nature result from the interaction between the material and the marine organisms that colonize the surface, leading to biofouling. Biofouling in the marine environment is described as the colonization of any solid surface, living or dead, natural or artificial, by micro- and macroorganisms. Biofouling formation is hence a phenomenon common to the majority of materials submerged in seawater [37,38,39]. It is generally divided into two main stages: microfouling and macrofouling (Figure 1). The submerged material is quickly colonized by marine bacteria, which form a bacterial biofilm on the surface (microfouling). This bacterial biofilm facilitates colonization by other micro- and macroorganisms such as cyanobacteria, fungi, diatoms, barnacles, algae and protozoa (macrofouling) [40,41,42,43,44,45].
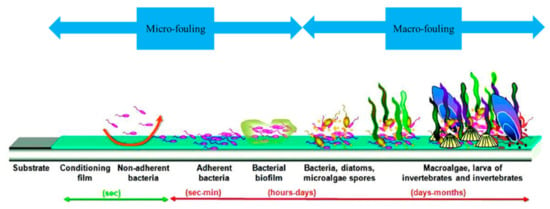
Figure 1.
Schematic representation of marine biofouling formation [45].
The stages of cementitious material biocolonization in the marine environment are illustrated in Figure 1. After immersion, organic and mineral molecules are quickly adsorbed at the surface. This adsorption, known as “conditioning film formation” or “surface conditioning,” modifies the physicochemical properties of the surface and makes it favorable to the stable adhesion of bacteria [45,46]. One or more pioneer bacterial species then adhere to the surface and form a bacterial biofilm (microfouling). Mature bacterial biofilms have complex three-dimensional structures, composed of one or more bacterial species adhered to the surface and enclosed in a matrix of extracellular polymeric substances (EPS) consisting of proteins, glycoproteins, glycolipids, extracellular DNA and polysaccharides [45,47,48,49]. A few days to a few months later, other micro- and macroorganisms such as diatoms, algae and larva adhere to the surface, leading to the formation of macrofouling [50,51]. Biofouling in seawater is hence the process whereby fouling organisms collect and grow on a surface, and their morphology is characterized by their thickness, bioadhesive strength and weight [52].
Generally speaking, the factors that influence the bioreceptivity (biofouling formation) of cementitious materials are the nature and the physicochemical properties of the surface [53,54]: chemical composition [55,56,57,58], roughness [59,60], porosity [60,61,62], hydrophobicity [56,63,64,65] and pH [60,66]. However, these factors are less well known in the marine environment [9,67,68]. We showed in a previous study that the bacterial colonization of cementitious materials in seawater is influenced by the pH and the type of cement [69,70]. In this present paper, the effect of several parameters in the two main stages (microfouling and macrofouling) of cementitious material biocolonization in the marine environment is tested. The main factors studied are:
- The surface conditions: smooth surface (SS); faceted and patterned surface (FPS), which describes an irregular and patterned surface mimicking natural rocks at the surface of the ECOPODE™ unit; and rough surface (RS), which allows a biomimetic rocky surface to be obtained.
- The use of two products during concrete production: a curing agent and a “green” formwork oil.
- The type of cement: ordinary Portland cement (OPC) CEM I and slag cement CEM III.
The aim of this study is to master and clarify the intrinsic parameters that influence the bioreceptivity of cementitious materials in the marine environment, in the context of coastal infrastructure construction. It is also to identify the parameters that influence the biocolonization of marine structures such as breakwaters most effectively, in order to help engineers in their new challenge of designing marine infrastructures using green concrete. The results of this study will be used directly by Artelia (an international engineering company specializing in the field of marine infrastructure construction) to enhance biocolonization of its armor-facing blocks.
2. Materials and Methods
The choice of materials and the techniques used to prepare the surfaces of the cementitious materials used in this study were inspired by the existing artificial concrete units developed by Artelia for use in breakwater armor layers. Several types of concrete armor units have been developed by Artelia since 1953, among them there are two single-layer systems called ACCROPODE™ (smooth surface) and ECOPODE™ (faceted and patterned surface).
This study was carried out using two types of specimen—mortar and concrete—dedicated to the quantification of microfouling (bacterial biofilm) and macrofouling (algae, etc.), respectively. These specimens were subsequently immersed in a seawater basin for 5 months, during which time micro- and macrofouling were measured periodically.
2.1. Preparation of Mortar Specimens
OPC CEM I cement 52.5 N CE CP2 NF “SB” (provided by Ciments Calcia) and blast-furnace slag cement CEM III (composed of 60% ground granulated blast-furnace slag NF EN 15167-1, provided by Ecocem, CAS no.: 65996-69-2) were used in this study to produce six types of mortar specimens (Table 1). The mortar had a water/cement ratio (w/c) of 0.5 and was composed of 450 g cement and 1350 g sand (sand 0/4 EN 196-1).

Table 1.
Types and compositions of mortar specimens investigated in this study.
After mixing, the mortars were cast in cylindrical molds measuring 2.7 cm in diameter and 2.9 cm high. After 24 h of hardening, the mortar specimens were removed from the molds and placed in a laboratory room at 20 °C for 30 days (Figure 2).
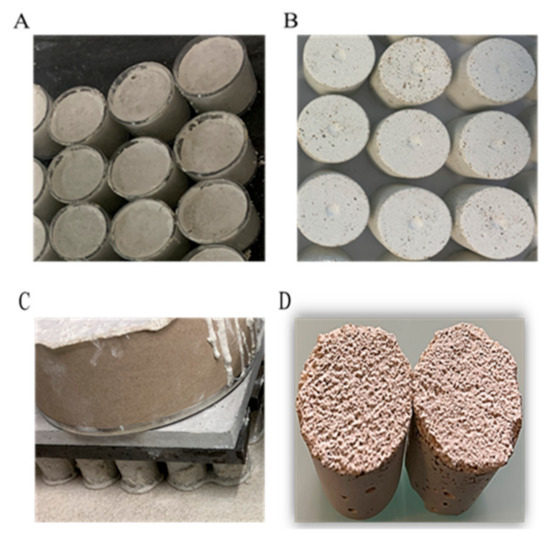
Figure 2.
Preparation of mortar specimens. (A) Preparation of SS mortar specimens (mortars in molds); (B) SS mortar specimens obtained after stripping; (C) preparation of RS mortar specimens on rough silicone skin; (D) RS mortar specimens obtained after stripping.
Formwork oil (vegetable oil, BIODEM SI 3) was spread on the molds, using absorbent paper to avoid any surplus, before the mortar was poured. Curing compound (SikaCem® Cure) was added to the top of the specimens in accordance with the supplier’s instructions 1 h after stripping. The rough mortars were prepared with a rough silicone skin; no release agent was needed. The cylindrical molds filled with mortar were poured on the silicone skin and held in place with a weight (Figure 2).
2.2. Preparation of Concrete Specimens
The same types of cement used for the mortar specimens were used to produce seven types of concrete. Table 2 presents an overview of the specimens investigated, their size, their surface condition, the various components used and their compressive strength.

Table 2.
Size, surface condition, compressive strength and components used to prepare the concrete specimens.
The concrete specimens were prepared with a water-to-cement ratio of 0.45, using cement (CEM I, CEM III), sand (0/4), a natural limestone gravel (2/4 and 4/6) and a superplasticizer with very good water-reducing properties (CHRYSOȐFluid Optima 220). The size of these specimens varied slightly depending on the type of mold used during preparation (Table 2).
All the concrete specimens were incubated in their molds for 7 days (hardening phase). After hardening, the concrete specimens were removed from the molds and placed in a laboratory room at 20 °C for 30 days. As with the mortar specimens, formwork oil was added to the molds before the concrete was poured, and the curing agent was added as soon as possible after stripping.
Smooth concrete specimens (control, CC and OC) were cast after mixing in circular cardboard molds fixed with silicone on a glass table. ECOPODE™ concrete specimens (EC1 and EC3) were cast after mixing in plastic cylindrical molds (12 cm in diameter and 2.2 cm high) representing an ECOPODE™ surface (Figure 3).

Figure 3.
Photo of an ECOPODE™ concrete block [71].
In addition, biomimetic concrete specimens (BC1 and BC3) were cast in circular cardboard molds fixed on a rough silicone skin (Figure 4).

Figure 4.
Preparation of concrete specimens. (A) Preparation of SS concrete specimens; (B) preparation of RS concrete specimens; (C) preparation of FPS concrete specimens.
2.3. Immersion Conditions
An immersion test in seawater was carried out using flat-bottomed basins (polyester, 6 m long, 0.6 m high and 2 m wide) located at the IFREMER station (biology of exploited marine organisms research unit in Palavas, France). The basins featured a seawater inlet and outlet allowing for an open seawater circuit (Figure 5). To ensure that the experiment would be completed smoothly and avoid contamination of any type, the basins were cleaned before the cementitious material specimens were placed in them.
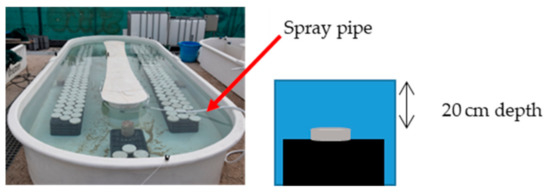
Figure 5.
Basins used during this study.
2.4. Quantification of Bacterial Biofilm
Quantification of the bacterial biofilm adhered to the surface of the mortar specimens was performed using “culture-based methods” (Figure 6) [72,73,74]. This quantification was carried out after 0, 1, 2, 6, 8, 10, 15, 24, 26 and 28 days of immersion in seawater. This method involves three main steps: (i) recovering the bacterial biofilm from the surface, (ii) cultivating the solution obtained on a bacterial culture medium and (iii) counting the bacterial colonies after sufficient incubation time for bacterial growth. With this method, the culture medium has a major impact on bacterial growth. In this study, we used marine agar (MA) medium. MA is widely used for the cultivation of marine bacteria [75,76,77].

Figure 6.
Culture-based method used in the quantification of bacterial biofilm formed on the surface of mortar specimens.
After each incubation period, three specimens of each mortar type were placed in three sterile tubes containing 10 mL of sterile seawater. Bacteria adhering to the mortar surface were detached by immersing the tubes in an ultrasonic bath (Bandelin SONOREX™) for 10 min at 20 °C. The solution obtained was diluted using sterile seawater, then 100 µL of diluted solution was spread on plates containing MA (Dutscher, 490614). The plates were then incubated at 20 °C, and a colony count was performed at least 72 h after incubation. The results are expressed as colony-forming units per cm3 of mortar (CFU/cm3).
2.5. Evaluation and Quantification of Marine Fouler Organisms (Macro-Fouling)
Various techniques were used in this study to quantify the marine fouler organisms (adhered to the surfaces of the concrete specimens) and assess their visual appearance: an image-analysis-based method [61,64,78,79], and wet-/dry-weight biomass measurement [80,81,82] (Figure 7).

Figure 7.
Techniques used to quantify macrofouling.
For the image-analysis-based method, photographs of the concrete specimens were taken using a Nikon D5300 camera. The photographs were taken under constant lighting using a photography lab. The images were subsequently processed using the ImageJ 1.38x software [83]. To distinguish the biofouling from the noncolonized surface of the concrete, a threshold color was used in the CIE Lab color space. Afterward, the images were processed with a binary system (Figure 8) to obtain white pixels (noncolonized area) and black pixels (colonized area, biofouling) from the initial photograph. These data were compiled and used to calculate the biofouling coverage for each concrete type as a percentage of surface area.
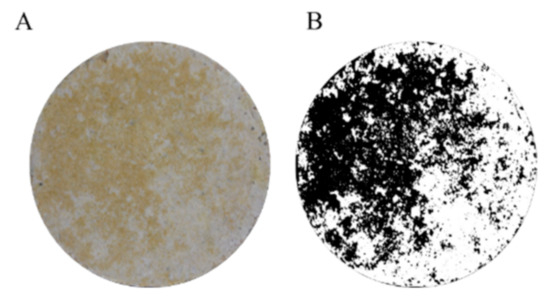
Figure 8.
Example of the analyses performed on a concrete surface image. (A) Original picture obtained with the Nikon D5300 camera. Image processed using ImageJ; (B) the black and white pixels indicate the fouled and unfouled surfaces, respectively.
This method was applied at 0, 8, 15, 22, 29, 43, 57, 71, 78, 85, 99, 106 and 113 days of immersion using 15 replicates of each concrete type.
For the dry-weight biomass measurement technique, the first step was to recover the marine fouler organisms from the concrete surface. The recovered cells were placed in tubes and dried at 60 °C for 24 h [80]. The weight of dry biomass was then measured using scales (accuracy 0.01 g). This quantification method was applied at 71, 78, 85, 99, 106 and 113 days of immersion using 3 replicates of each concrete type per time; the data are reported as grams of biomass (g) per cm2 of concrete surface (g/cm2).
The principle of the wet-weight biomass measurement technique was the same as that of the dry biomass quantification technique, i.e., quantifying the weight of the marine fouler organisms adhered to the concrete specimens. To apply this technique, the weight of 15 concrete specimens was measured before they were immersed using scales (Sodipro, 300619, accuracy 1 g). Since concrete is a porous material, the immersed specimens absorb water and increase in weight. Then, the weight of these specimens after 15 days of immersion was taken as W0 (concrete + absorbed water weight), and the weight of biomass was subsequently calculated by subtracting the weight obtained after X days of immersion (Wx) from W0. Before the evaluation of W0 and Wx, the concrete specimens were drained for 15 min. This quantification method was applied at 0, 8, 15, 22, 29, 43, 57, 71, 78, 85, 99, 106 and 113 days of immersion using 15 replicates of each concrete type; the data are reported as grams of biomass (g) per cm2 of concrete surface (g/cm2).
2.6. pH Measurement
The alkalinity of the medium and the surface of a submerged cementitious material can affect biocolonization [70,84,85]. Therefore, the pH of the seawater and the surface of the mortar specimens were measured in triplicate throughout the immersion period using a pH electrode (Hanna instrument, HI1230, accuracy 0.1 pH unit) and pH indicator paper (Whatman, 0.0 to 14.0, accuracy 1 pH unit), respectively, according to the protocol described by Hayek et al. [69]. The pH of the mortar specimens was verified using phenolphthalein indicators (which go from colorless to pink at pH ≥9). However, the use of phenolphthalein and pH indicator paper gives a fairly good-quality estimate of the pH of the surface, but does not accurately quantify the pH of the material [86].
2.7. Hydrophobicity Evaluation
The hydrophobicity of the mortar surface during bacterial colonization was evaluated using a drop-shape analyzer (KRÜSS, DSA 30), which measures the angles and diameters of contact between a drop of water and a surface [70,87]. After each period of incubation in seawater, 3 mortar specimens were drained for 2 h. Then, a drop (3 μL) of water was placed on mortar surface. Absorption of the drop was monitored using a camera (8 images per second). The contact angle between the drop and the material was measured using the Advance machine software, and a minimum of three tests were carried out at different points.
2.8. Statistical Analyses
In order to evaluate the significance of the various results obtained, statistical analysis was performed using GraphPad Prism 5 (GraphPad Software, San Diego, CA, USA) and t-test, one-way and two-way ANOVA tests. Statistical significance was accepted by p value <0.05 obtained using Bonferroni or Tukey’s multiple comparison post hoc tests.
3. Results and Discussion
3.1. Preliminary Result: Seawater pH and Temperature Measurement
Marine environmental conditions such as seawater temperature and alkalinity influence bacterial growth and biofilm formation [88,89,90]. In order to ensure that environmental conditions were favorable to the growth of marine bacteria during this study, the temperature and the alkalinity of seawater used in this experiment were measured throughout the immersion period (Figure 9).
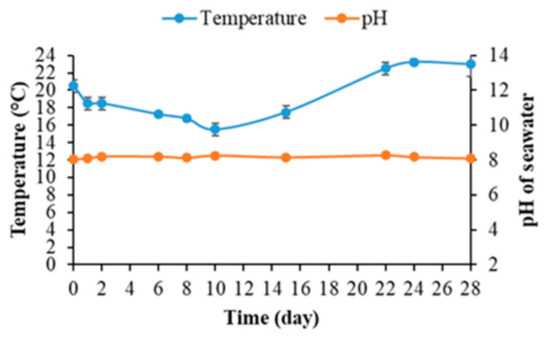
Figure 9.
The pH and temperature of seawater during the quantification of bacterial biofilm on mortar specimens.
Figure 9 shows that the seawater temperature remained between 16 and 23 °C depending on variations in the weather at IFREMER’s outdoor experimental platform in Palavas-les-Flots over the course of the experiment. During the subsequent process of bacterial-biofilm quantification, the seawater temperature was optimal for the growth of most marine bacteria [75,91,92,93].
Moreover, the seawater pH remained constant at an average of 8.16 throughout the experiment (Figure 9). This value was consistent with the literature; the pH of seawater varied between 7.5 and 9.0, with an average of around 8.2 [69,94,95]. Seawater alkalinity was hence unaffected by the immersion of cementitious material specimens (the release of Ca(OH)2 and KOH resulting from the leaching reaction) and remained optimal for the growth of marine bacteria [96,97,98].
3.2. Examination of the Mortar Specimens
When immersed in seawater, mortar and concrete surfaces are rapidly colonized by marine bacteria. These bacteria form on the surface a highly complex dynamic and 3D biofilm structures [47,48,99,100].
To study the influence of intrinsic parameters on the bacterial colonization of cementitious materials in the marine environment, six types of mortar specimen (Table 1) were immersed in seawater under natural light. The bacterial biofilm formed on the mortar surfaces was quantified after 0, 1, 2, 6, 8, 10, 15, 22, 24 and 28 days. At the same time, the pH of the seawater and mortar surfaces were determined.
3.2.1. pH of Mortar Surfaces
Cementitious materials have a very high surface alkalinity (pH~13) [101,102]. Due to this very basic pH, their constituent materials have been cited as inhibitors of the recruitment of marine biota [36]. In addition, a decrease in pH from 13 to around 9 has been reported as necessary for bacterial colonization of cementitious materials submerged in seawater [69,103]. Therefore, the pH of the mortar surfaces was evaluated throughout the experiment in order to discern whether the pH was indeed responsible for inhibiting bacterial colonization when obtained.
Figure 10 shows that the pH of the CEM I mortar specimens (Ref, CM, OM1 and BM1) at T0 was around 11.7. The pH was also verified using phenolphthalein indicators, which gave a pink coloration after contact with the surface of the specimens, indicating a pH higher than 9. Therefore, the surface pH measured was lower than expected (pH~13) [101,102]. This might be due to the 30 days of contact between air and mortar specimens before the start of the immersion test. In such conditions, mortar specimens undergo a carbonation reaction due to the presence of carbon dioxide. Carbon dioxide from air reacts with calcium hydroxide and calcium silicate hydrate in the mortar to form calcium carbonate and water. This carbonation reaction decreases the pH of mortar specimens [104,105].
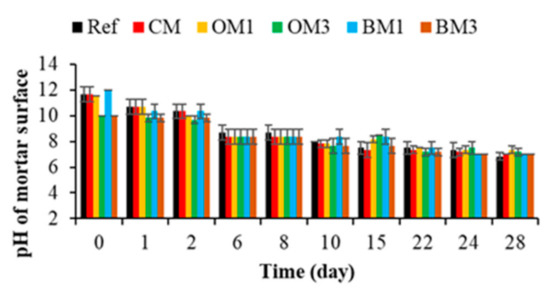
Figure 10.
Evaluation of the pH of mortar surfaces. Each evaluation was performed in triplicate, and the error bars present the standard deviation from the values obtained. Ref = control mortar; CM = cured mortar; OM1 = oiled mortar prepared with CEM I; OM3 = oiled mortar prepared with CEM III; BM1 = biomimetic mortar prepared with CEM I; BM3 = biomimetic mortar prepared with CEM III.
Moreover, when CEM I was replaced by CEM III, the pH of the CEM III mortar specimens (OM3 and BM3) at T0 was around 10 (due to the carbonation reaction). This difference in pH between the CEM I and CEM III specimens was probably one reason why the cementitious materials prepared with CEM III were more bioreceptive than those formulated using CEM I, according to [69,70,106].
After immersion, the pH of the CEM I and CEM III mortar specimens decreased quickly and reached a value of 8.3 at T6. Then, the pH decreased gradually and reached a value of 7.3 at T28. This drop in pH can be explained by the action of the leaching reaction in seawater. In contact with water, alkali metals such as potassium, calcium and hydroxide ions will leach out of the mortar specimens and thus reduce the pH of the surface [107,108].
Therefore, the pH values measured were lower than 9 from 6 days of immersion for both the CEM I and the CEM III mortar specimens. This pH was favorable for the adhesion and growth of marine bacteria [98,109].
3.2.2. Quantification of Bacterial-Biofilm Formation
The bacterial colonization of cementitious materials and biofilm formation are complex processes that are affected by many factors, including the environmental conditions, the bacterial properties and the physical/chemical characteristics of the material surface [109,110,111]. In this study, the mortar specimens were incubated under the same environmental conditions (pH and temperature) in the presence of the same marine bacteria. Therefore, only the physicochemical properties of the mortar surfaces could generate a different rate of bacterial colonization. The results concerning bacterial colonization of the six types of mortar specimen used in this study are presented in Figure 11.
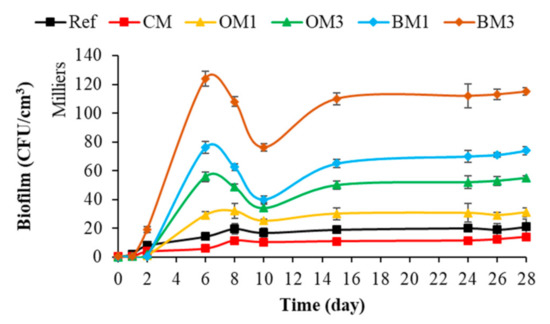
Figure 11.
Quantification of bacterial-biofilm formation on mortar specimens. Each quantification was performed in triplicate using the culture-based method, and the error bars present the standard deviation from the values obtained. Ref = control mortar; CM = cured mortar; OM1 = oiled mortar prepared with CEM I; OM3 = oiled mortar prepared with CEM III; BM1 = biomimetic mortar prepared with CEM I; BM3 = biomimetic mortar prepared with CEM III.
Figure 11 shows that the bacterial colonization of mortar surfaces (all types of mortar) started with a latency phase, followed by a phase of cell growth and accumulation on the surfaces and ending with a plateau phase. These colonization kinetics were also observed in most of the studies quantifying bacterial-biofilm formation on cementitious-material surfaces or on other surface types [60,61,69,112,113,114]. Moreover, a decrease in the bacterial colonization of mortar surfaces was observed between 6 and 10 days. This decrease could be justified by the drop in water temperature (from 19 to 16 °C) observed during the immersion period [115,116,117].
Therefore, based on the rate of bacterial colonization, the mortar types can be classified from less to more bioreceptive in the following order: CM < Ref < OM1 < OM3 < BM1 < BM3. Then, surface chemical characteristics, chemical composition (type of cement) and surface roughness influenced the bacterial colonization of cementitious materials submerged in seawater. Among these parameters, surface roughness seemed to be the parameter that promoted the bacterial colonization of cementitious materials most effectively in the marine environment.
3.2.3. Effect of Surface Roughness on Bacterial Colonization
Surface roughness is one of the main physical factors influencing the bioreceptivity of materials. The effect of roughness has been determined for different types of material and in several types of environment [118,119,120,121,122]. Figure 12 shows that, in the marine environment, a rough mortar surface significantly increased bacterial colonization in comparison with a smooth surface (Ref). This influence of surface roughness also was identified in several studies concerning the bacterial colonization of cementitious materials [123,124].
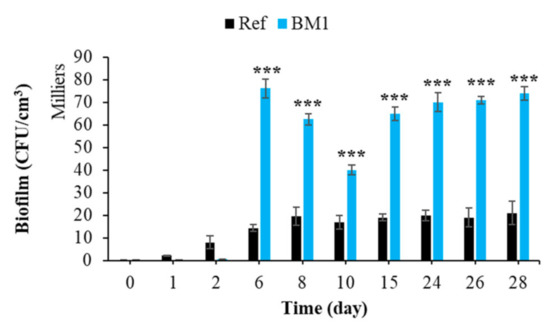
Figure 12.
Quantification of bacterial biofilm on smooth and rough mortars. Each experiment was performed in triplicate, and the error bars present the standard deviation of the values obtained. Ref = control mortar; BM1 = biomimetic mortar prepared with CEM I. The experiments highlighted with asterisks differed significantly from the control (Bonferroni; ***: p < 0.001) at the indicated time.
Moreover, nanoscale bumps on the cementitious surface can act as anchoring sites and micro-refuges for the installation of microorganisms [125]. These microrefuges protect the microorganisms from the hydrodynamic forces that tend to detach them during the adhesion phase [125,126]. Therefore, surface roughness increased the bacterial colonization (bioreceptivity) of cementitious materials in the marine environment.
3.2.4. Effect of Cement Type on Bacterial Colonization
Chemical composition has a significant influence on the biocolonization of cementitious materials [36,54]. Figure 13 shows that the type of cement influenced the bacterial colonization of cementitious materials in the marine environment. The formation of bacterial biofilm was much greater in the case of CEM III mortars, regardless of the surface topography or whether formwork oil was applied. These results were in keeping with the literature, in which a similar effect of cement type on the bacterial colonization of cementitious materials has been reported [63,69,127]. Therefore, the use of CEM III binder increased the bioreceptivity of cementitious materials in the marine environment.
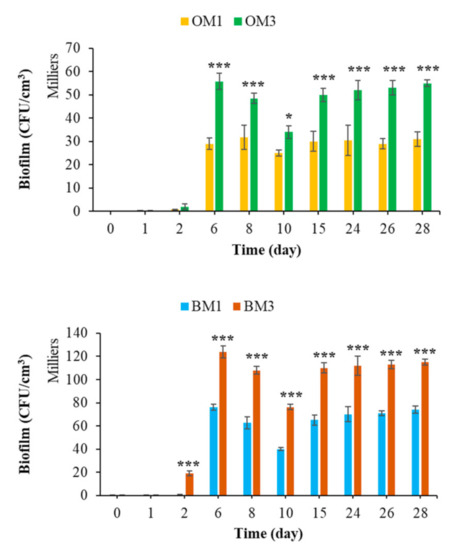
Figure 13.
Quantification of bacterial biofilm on CEM1 and CEM3 mortar specimens. Each experiment was performed in triplicate, and the error bars present the standard deviation of the values obtained. Ref = control mortar; OM1 = oiled mortar prepared with CEM I; OM3 = oiled mortar prepared with CEM III; BM1 = biomimetic mortar prepared with CEM I; BM3 = biomimetic mortar prepared with CEM III. The experiments highlighted with asterisks differed significantly from the control (Bonferroni; *: p < 0.05, ***: p < 0.001) at the indicated time.
3.2.5. Effect of Curing Compound on Bacterial Colonization
The curing agent is a liquid applied to the concrete or mortar surface to protect the material from water evaporation and give it greater aesthetic and mechanical durability, preventing early-age surface cracking [128,129,130].
To the best of our knowledge, the effect of a curing product on the biocolonization of cementitious materials in seawater has not been studied. Figure 14 shows that the curing compound significantly inhibited the bacterial colonization of mortar submerged in seawater. Cells accumulated and grew faster and more extensively on an untreated surface.
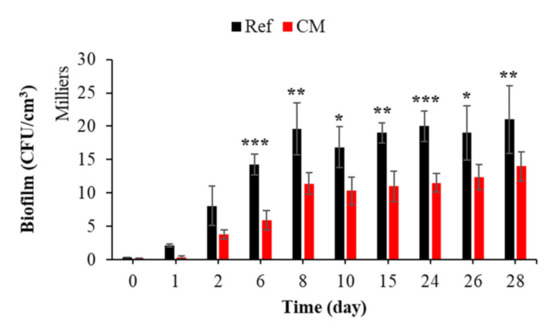
Figure 14.
Quantification of bacterial biofilm on treated and untreated mortars with curing compound. Each experiment was performed in triplicate, and the error bars present the standard deviation of the values obtained. The experiments highlighted with asterisks differed significantly from the control (Bonferroni; *: p < 0.05, **: p < 0.01, ***: p < 0.001) at the indicated time.
In the marine environment, one of the anti-biofouling strategies used to inhibit bacterial adhesion and the adhesion of other organisms is the treatment of the material by a hydrophobic surface coating [99,131,132,133,134,135]. To verify the effect of the curing film on the hydrophobicity of the mortar surface, the contact angle with a drop of water was measured using a drop-shape analyzer (Figure 15). From their preparation until the start of the immersion test, the mortars treated with the curing compound presented a contact angle greater than 110°, indicating a hydrophobic surface [136,137]. After immersion and under the action of seawater, the contact angle gradually decreased with time and became equal to that of the control mortar at 28 days of immersion (data not shown).
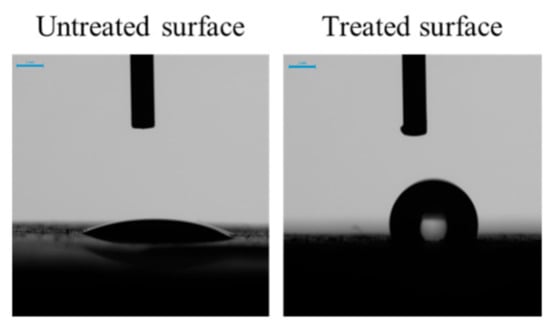
Figure 15.
Contact angle of a water drop on the surface of untreated mortar and mortar treated with a curing compound. Photos were taken two seconds after contact between the water and the surface.
Moreover, the curing compound used in this study contained a mixture of alkyl (C14-C18) bis (2-hydroxyethyl) amine, 5-chloro-2-methyl-2H-isothiazol-3-one and 2-methyl-2H-isothiazol-3-one (FDS Sikacem cure). These elements or their derivatives have been cited as anti-biofouling molecules that inhibited the formation of bacterial biofilm and the biofouling process [138,139,140].
Therefore, we propose that the curing compound inhibited the formation of bacterial biofilm on mortar surfaces in seawater because of its chemical composition (anti-biofouling) and its effect on surface hydrophobicity. Therefore, surface treatment with this type of curing compound reduced the bacterial colonization of cementitious materials in the marine environment.
3.2.6. Effect of Formwork Oil on Bacterial Colonization
Formwork oil is a mold-release agent that is applied to a wall of a mold to ensure easy separation of the hardened concrete from the mold by reducing the adhesion forces at the concrete/mold interface [141,142]. Two types of formwork oil with different chemical compositions are mentioned in the literature: mineral and green (plant-based) [143]. Mineral formwork oils have been reported to cause environmental pollution and health problems for construction workers [144]. For this reason, plant-based formwork oil (which is nontoxic and biodegradable) is the release agent used most widely today to prevent damage to the hydraulic concrete surface during mold-stripping operations [143,144,145]. This oil forms a well-adsorbed and stable “lubricant monolayer” on the surface of cementitious materials, leading to improved release performance [144]; the free fatty acids from the oil react in a basic medium with calcium hydroxides and produce a layer of calcium carboxylates (soaps) and glycerol [144,146]. Van der Waals cohesion forces stabilize the glycerol and make the layer more resistant to stress [144].
To the best of our knowledge, the effect of formwork oil on the biocolonization of cementitious materials in seawater has not been studied to date. Figure 16 shows that the colonization process was significantly increased if the surface was treated with formwork oil. The composition of the green formwork oil used is not given in this study. Detailed information is given neither in the technical product information nor in the bibliography. Therefore, given that green formwork oil is biodegradable, we propose that this oil applied on the surface of the mortar specimens was used as a carbon source by marine bacteria, according to the study and the results of [147,148,149,150]. Therefore, the presence of a biodegradable oil as an additional source of nutrients increased the biofilm formation on oiled mortars; Dusane et al. showed through a laboratory test that the carbon sources differentially affected the formation of biofilms. Lactic acid, erythritol, glycerol, glucose and edible oils increase this process [151]. Therefore, surface treatment with this type of formwork oil increased the bacterial colonization of cementitious materials in the marine environment.
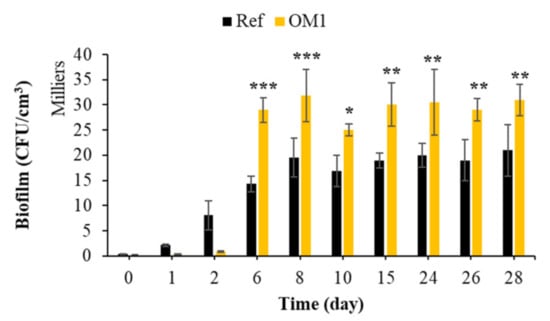
Figure 16.
Quantification of bacterial biofilm on untreated mortars and mortars treated with formwork oil. Each experiment was performed in triplicate, and the error bars present the standard deviation of the values obtained. The experiments highlighted with asterisks differed significantly from the control (Bonferroni; *: p < 0.05, **: p < 0.01, ***: p < 0.001) at the indicated time.
3.3. Quantification of Marine Fouler Organisms on the Concrete Specimens
After the colonization of immersed materials by marine bacteria, biocolonization continues through the adhesion and growth of macroorganisms such as algae and larvae. This adhesion is known as macrofouling, and is the second main stage of biofouling in the marine environment [40,41,43,44,45]. The first part of this study showed that intrinsic parameters such as surface treatment with curing compound or formwork oil, chemical composition and surface condition (SS, RS) influence the bacterial colonization of cementitious materials in the marine environment. To test the effect of these parameters on the macrofouling, seven types of concrete specimens were immersed in seawater under the same conditions (the same incubation period) as those used for the mortar specimens. The marine fouler organisms adhered on the concrete surfaces were quantified after 0, 8, 15, 22, 29, 43, 57, 71, 78, 85, 99, 106 and 113 days.
3.3.1. Quantification of Marine Fouler Organisms Using an Image-Analysis-Based Method
Image analysis is a quantification technique used widely in performing biofouling tests on cementitious materials [61,64,78,79].
Figure 17 shows that the colonization of concrete surfaces (all types of concrete) by marine fouler organisms started with a latency phase, followed by a phase during which cells accumulated and grew on the surfaces (from 43 days to 113 days). These kinetics of the colonization process were also observed in most of the other studies quantifying biofouling on cementitious-material surfaces [61,79,106,152].
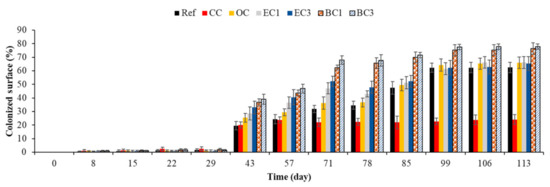
Figure 17.
Quantification of marine fouler organisms using an image-analysis-based method. Ref = control concrete; CC = cured concrete; OC = oiled concrete; EC1 = ECOPODE™ concrete prepared with CEM I; EC3 = ECOPODE™ concrete prepared with CEM III; BC1 = biomimetic concrete prepared with CEM I; BC3 = biomimetic concrete prepared with CEM III. Each experiment was performed on 15 replicates, and the error bars present the standard deviation of the values obtained.
As was the case with bacterial colonization, curing compound inhibited the adhesion of marine fouler organisms to the surface of concrete (Table 3). This inhibition may have been due to a toxic chemical composition and the hydrophobicity of the material surface treated with the curing agent [131,132,133,134,135,138,140].

Table 3.
Statistical comparison (GraphPad Prism 5) of the macrofouling quantification results obtained using an image-analysis-based method. Ref = control concrete; CC = cured concrete; OC = oiled concrete; EC1 = ECOPODE™ concrete prepared with CEM I; EC3 = ECOPODE™ concrete prepared with CEM III; BC1 = biomimetic concrete prepared with CEM I; BC3 = biomimetic concrete prepared with CEM III. (Bonferroni; ns: not significant, p > 0.05, *: p < 0.05, **: p < 0.01, ***: p < 0.001).
To assess the effect of other parameters on macrofouling, a statistical analysis of the results was performed using GraphPad Prism 5 software (Table 3). This analysis showed that:
- Treating the surface with formwork oil only supported greater biocolonization during the first days of macrofouling (Ref vs. OC); after 71 days, the quantity of marine fouler organisms on the oiled surface was equal to that on the control surface.
- Surface topography (FPS surface) and the use of CEM III cement did not affect biocolonization after 78 days of immersion (OC vs. EC1, OC vs. EC3 and EC1 vs. EC3).
- Surface roughness supported greater biocolonization from the start of macrofouling (43 days) until the end of our study at 133 days of immersion (Ref vs. BC1).
- The effect of surface roughness on macrofouling dominated the effect of chemical composition (use of CEM III cement). The biocolonization was significantly different when the control concrete and the rough concrete were compared (Ref vs. BC1, Ref vs. BC3), whereas no significant differences were observed when biomimetic concretes prepared using CEM I or CEM III were compared (BC1 vs. BC3).
Therefore, surface-chemical characteristics, chemical composition (type of cement) and surface roughness influenced the macrofouling of cementitious materials submerged in seawater.
Based on the persistence of their significant effect, the intrinsic parameters that supported a greater biocolonization of cementitious materials in the marine environment can be classified from less to more effective in the following order: surface treatment with formwork oil < surface topography (FPS surface) < chemical composition (type of cement) < surface roughness (biomimetic surface). Therefore, as was the case with bacterial colonization, surface roughness was the parameter that promoted the macrofouling of cementitious materials in the marine environment most effectively. We suggest that surface roughness was the most important factor in the design of bioreceptive concrete in the marine environment.
These results and conclusions are in keeping with those from the study performed by Hsiung et al., which showed that the decrease in pH did not affect the long-term bioreceptivity of cementitious materials in the marine environment. At the end of their study, Hsiung et al. suggested that manipulation of the physical structure (surface roughness) of the concrete was a more effective eco-engineering approach to enhancing ecological value and species diversity in the marine environment [102].
3.3.2. Quantification of Marine Fouler Organisms Using Wet-Weight Biomass Measurement
The second method used to quantify macrofouling on concrete surfaces is wet-weight biomass measurement. This technique is quick and easy to apply. It allows a rapid assessment of biocolonization in the marine environment. To the best of our knowledge, this is the first study in which this technique has been applied in this way (see the Materials and Methods section) to quantify macrofouling on concrete surfaces in a marine environment.
The results obtained with this method (Figure 18) were in keeping with those obtained using the image-analysis-based method. The statistical analysis of the results (data not shown) was similar and the conclusions were the same—surface roughness was the most important factor in the design of bioreceptive concrete in the marine environment.
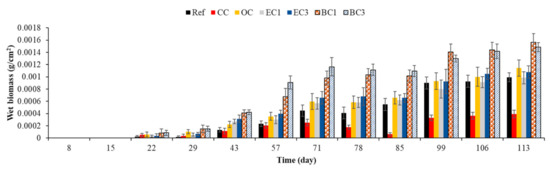
Figure 18.
Quantification of marine fouler organisms using wet-weight biomass measurement. Ref = control concrete. CC = cured concrete; OC = oiled concrete; EC1 = ECOPODE™ concrete prepared with CEM I; EC3 = ECOPODE™ concrete prepared with CEM III; BC1 = biomimetic concrete prepared with CEM I; BC3 = biomimetic concrete prepared with CEM III. Each experiment was performed on 15 replicates, and the error bars present the standard deviation of the values obtained.
3.3.3. Quantification of Marine Fouler Organisms Using Dry-Weight Biomass Measurement
To be sure that the lack of significance in concrete surface biocolonization after 85 days of immersion was not due to a detection limit inherent to the method applied, we used dry-weight biomass measurement to quantify the marine fouler organisms on the surfaces of control, cured, oiled and ECOPODE™ concrete [80,81,82]. Cured concrete was used as a control during this experiment.
Statistical analysis of obtained results (data not shown) and Figure 19 show that no significant difference was observed after 85 days of incubation, which confirmed the results obtained using wet-weight biomass measurement and image analysis. Surface treatment with formwork oil and surface topography only influenced the initial macrofouling of concrete in the marine environment.
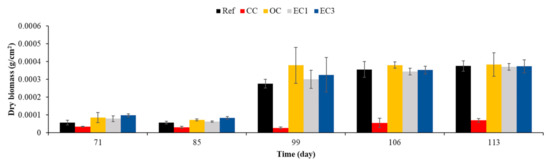
Figure 19.
Quantification of marine fouler organisms using dry-weight biomass measurement. Ref = control concrete; CC = cured concrete; OC = oiled concrete; EC1 = ECOPODE™ concrete prepared with CEM I; EC3 = ECOPODE™ concrete prepared with CEM III. Each experiment was performed in triplicate, and the error bars present the standard deviation of the values obtained.
4. Conclusions
In summary, this study indicates that the intrinsic parameters of cementitious materials influence the biocolonization and the biofouling formation in the marine environment. Regarding the parameters tested in the preset work, we can summarize that: (1) the surface design (smooth surface, faceted and patterned surface, rough surface) affect the bacterial and macroorganism colonization of cementitious materials in seawater; (2) the chemical composition such as the type of binder (ordinary Portland cement CEM I, slag cement CEM III) significantly influences the biocolonization of cementitious materials; (3) work practices such as the use of a curing agent and/or formwork oil have an impact on biocolonization.
Using mortar specimens and culture-based methods, we have shown that in the marine environment: (1) surface roughness seems to be the factor enhancing the bacterial colonization of cementitious materials most effectively; (2) the use of slag cement CEM III increases the bioreceptivity of cementitious materials; (3) the application of a curing compound to the surface reduces the bacterial colonization of cementitious materials, whereas green formwork oil has the opposite effect.
Using concrete specimens, an image-analysis-based method and wet-/dry-weight biomass measurements, we have shown that the surface design, the chemical composition (type of cement) and the chemical characteristics of the surface (curing compound or formwork oil) have a similar effect to that observed with mortar on the macrofouling of cementitious materials submerged in seawater.
Based on the persistence of their significant effect, the intrinsic parameters that support greater biocolonization are classified from more to less effective in the following order: surface design (roughness of biomimetic specimens) > chemical composition (slag cement CEM III) > faceted and patterned surface > chemical characteristics (formwork oil).
Therefore, at the material scale, surface roughness is the most effective factor in designing bioreceptive concrete that enhances marine biodiversity [9,14,15]. Nevertheless, faceted and patterned surfaces seem to improve the initial colonization stages, maybe because they induce a local hydrological change in environmental conditions. This characteristic should be investigated in detail, especially in real conditions, at the macroscopic scale.
In regard to improving construction techniques used in marine ecosystems, this conclusion will be used directly by an engineering and project-management company specializing in the field of marine-infrastructure construction to design a new innovative concrete block that promotes marine-infrastructure colonization more effectively. The use of CEM III cement has a positive effect in terms of both durability and biocolonization of the concrete by marine structures. This research must be pursued further at a 1:1 scale, on real works, to develop new concrete-block designs and study the macroscopic aspects of the structure of concrete armor used to protect breakwaters.
Author Contributions
Writing—review and editing, M.H., M.S., J.-C.S., E.C., C.G. and O.P. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Artelia, an international, multidisciplinary consultancy, engineering and project-management company specializing in the field of marine-infrastructure construction.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors thank Emmanuel Rezzouk (IFREMER, Biology Research Unit for exploited marine organisms) for receiving them at the Palavas-les-Flots oceanographic station and thank LIB industry for the supply of the silicone matrix and their assistance. The authors also thank IMT Mines Ales and University of Montpellier 3 for their participation and support. The authors wish to acknowledge the help provided by Florian Stratta, Habiba Lharti, Léa Capitan-Fernandez, Thomas Davignon, Oumaïma Saâdoune, Claire Desoubry, Emma Guillot and Mathilde Metges.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Halpern, B.S.; Walbridge, S.; Selkoe, K.A.; Kappel, C.V.; Micheli, F.; D’Agrosa, C.; Bruno, J.F.; Casey, K.S.; Ebert, C.; Fox, H.E.; et al. A Global Map of Human Impact on Marine Ecosystems. Science 2008, 319, 948–952. [Google Scholar] [CrossRef]
- Bulleri, F.; Chapman, M.G. The Introduction of Coastal Infrastructure as a Driver of Change in Marine Environments. J. Appl. Ecol. 2010, 47, 26–35. [Google Scholar] [CrossRef]
- Bishop, M.J.; Mayer-Pinto, M.; Airoldi, L.; Firth, L.B.; Morris, R.L.; Loke, L.H.; Hawkins, S.J.; Naylor, L.A.; Coleman, R.A.; Chee, S.Y.; et al. Effects of Ocean Sprawl on Ecological Connectivity: Impacts and Solutions. J. Exp. Mar. Biol. Ecol. 2017, 492, 7–30. [Google Scholar] [CrossRef]
- Barnosky, A.D.; Hadly, E.A.; Bascompte, J.; Berlow, E.L.; Brown, J.H.; Fortelius, M.; Getz, W.M.; Harte, J.; Hastings, A.; Marquet, P.A.; et al. Approaching a State Shift in Earth’s Biosphere. Nat. Cell Biol. 2012, 486, 52–58. [Google Scholar] [CrossRef]
- Corlett, R.T. The Anthropocene Concept in Ecology and Conservation. Trends Ecol. Evol. 2015, 30, 36–41. [Google Scholar] [CrossRef]
- Bai, X.; Geschke, A.; Molnár, Z.; Jaureguiberry, P.; Hendry, A.; Liu, J.; Martin, A.; Midgley, G.F.; Scheffran, J.; Seppelt, R.; et al. IPBES Global Assessment on Biodiversity and Ecosystem Services Chapter 1. Assessing a Planet in Transformation: Rationale and Approach of the IPBES Global Assessment on Biodiversity and Ecosystem Services. In IPBES Global Assessment on Biodiversity and Ecosystem Services; IPBES Secretariat: Bonn, Germany, 2019; p. 69. [Google Scholar]
- Pioch, S.; Relini, G.; Souche, J.; Stive, M.; De Monbrison, D.; Nassif, S.; Simard, F.; Allemand, D.; Saussol, P.; Spieler, R.; et al. Enhancing Eco-Engineering of Coastal Infrastructure with Eco-Design: Moving from Mitigation to Integration. Ecol. Eng. 2018, 120, 574–584. [Google Scholar] [CrossRef]
- Martins, G.M.; Thompson, R.C.; Neto, A.I.; Hawkins, S.J.; Jenkins, S.R. Enhancing Stocks of the Exploited Limpet Patella Candei D’Orbigny via Modifications in Coastal Engineering. Biol. Conserv. 2010, 143, 203–211. [Google Scholar] [CrossRef]
- Souche, J.-C.; Pioch, S.; Salgues, M.; De Weerdt, K.; Agostini, A.; Hayek, M. De la Conception à L’éCo-conception Des Ouvrages Maritimes: Intégrer la Nature AU Projet D’Aménagement Maritime. Rev. Paralia 2019, 12, n01.1–n01.26. [Google Scholar] [CrossRef]
- Heery, E.C.; Bishop, M.J.; Critchley, L.P.; Bugnot, A.B.; Airoldi, L.; Mayer-Pinto, M.; Sheehan, E.V.; Coleman, R.A.; Loke, L.H.; Johnston, E.L.; et al. Identifying the Consequences of Ocean Sprawl for Sedimentary Habitats. J. Exp. Mar. Biol. Ecol. 2017, 492, 31–48. [Google Scholar] [CrossRef]
- Dugan, J.E.; Airoldi, L.; Chapman, M.G.; Walker, S.J.; Schlacher, T. 8.02—Estuarine and Coastal Structures: Environmental Effects, A Focus on Shore and Nearshore Structures. In Treatise on Estuarine and Coastal Science; Wolanski, E., McLusky, D., Eds.; Academic Press: Waltham, MA, USA, 2011; pp. 17–41. ISBN 978-0-08-087885-0. [Google Scholar]
- Powell, E.J.; Tyrrell, M.C.; Milliken, A.; Tirpak, J.M.; Staudinger, M.D. A Review of Coastal Management Approaches to Support the Integration of Ecological and Human Community Planning for Climate Change. J. Coast. Conserv. 2019, 23, 1–18. [Google Scholar] [CrossRef]
- Waltham, N.J.; Dafforn, K.A. Ecological Engineering in the Coastal Seascape. Ecol. Eng. 2018, 120, 554–559. [Google Scholar] [CrossRef]
- Dafforn, A.K.; Glasby, T.M.; Airoldi, L.; Rivero, N.K.; Mayer-Pinto, M.; Johnston, E.L. Marine Urbanization: An Ecological Framework for Designing Multifunctional Artificial Structures. Front. Ecol. Environ. 2015, 13, 82–90. [Google Scholar] [CrossRef]
- Browne, M.A.; Chapman, M.G. Ecologically Informed Engineering Reduces Loss of Intertidal Biodiversity on Artificial Shorelines. Environ. Sci. Technol. 2011, 45, 8204–8207. [Google Scholar] [CrossRef] [PubMed]
- Moradllo, M.K.; Shekarchi, M.; Hoseini, M. Time-Dependent Performance of Concrete Surface Coatings in Tidal Zone of Marine Environment. Constr. Build. Mater. 2012, 30, 198–205. [Google Scholar] [CrossRef]
- Oleson, J.P.; Brandon, C.; Cramer, S.M.; Cucitore, R.; Gotti, E.; Hohlfelder, R.L. The ROMACONS Project: A Contribution to the Historical and Engineering Analysis of Hydraulic Concrete in Roman Maritime Structures. Int. J. Naut. Archaeol. 2004, 33, 199–229. [Google Scholar] [CrossRef]
- Costa, A.; Appleton, J. Case Studies of Concrete Deterioration in a Marine Environment in Portugal. Cem. Concr. Compos. 2002, 24, 169–179. [Google Scholar] [CrossRef]
- Pandey, A.; Kumar, B. Investigation on the Effects of Acidic Environment and Accelerated Carbonation on Concrete Admixed with Rice Straw Ash and Microsilica. J. Build. Eng. 2020, 29, 101125. [Google Scholar] [CrossRef]
- Song, H.-W.; Lee, C.-H.; Ann, K.Y. Factors Influencing Chloride Transport in Concrete Structures Exposed to Marine Environments. Cem. Concr. Compos. 2008, 30, 113–121. [Google Scholar] [CrossRef]
- Collepardi, M. Concrete Durability in a Marine Environment. In Proceedings of the Canmet/Aci International Conference on Advances in Concrete Technology in the Middle East, Dubai, United Arab Emirates, 15 October 2008; pp. 19–20. [Google Scholar]
- Yi, Y.; Zhu, D.; Guo, S.; Zhang, Z.; Shi, C. A Review on the Deterioration and Approaches to Enhance the Durability of Concrete in the Marine Environment. Cem. Concr. Compos. 2020, 113, 103695. [Google Scholar] [CrossRef]
- Chlayon, T.; Iwanami, M.; Chijiwa, N. Combined Protective Action of Barnacles and Biofilm on Concrete Surface in Intertidal Areas. Constr. Build. Mater. 2018, 179, 477–487. [Google Scholar] [CrossRef]
- Jacob, C.; Buffard, A.; Pioch, S.; Thorin, S. Marine Ecosystem Restoration and Biodiversity Offset. Ecol. Eng. 2018, 120, 585–594. [Google Scholar] [CrossRef]
- Ting, M.Z.Y.; Wong, K.S.; Rahman, M.E.; Meheron, S.J. Deterioration of Marine Concrete Exposed to Wetting-Drying Action. J. Clean. Prod. 2021, 278, 123383. [Google Scholar] [CrossRef]
- Song, H.-W.; Shim, H.-B.; Petcherdchoo, A.; Park, S.-K. Service Life Prediction of Repaired Concrete Structures under Chloride Environment Using Finite Difference Method. Cem. Concr. Compos. 2009, 31, 120–127. [Google Scholar] [CrossRef]
- De Rincón, O.T.; Sánchez, M.; Millano, V.; Fernández, R.; De Partidas, E.; Andrade, C.; Martinez, I.; Castellote, M.; Barboza, M.; Irassar, F.; et al. Effect of the Marine Environment on Reinforced Concrete Durability in Iberoamerican Countries: DURACON project/CYTED. Corros. Sci. 2007, 49, 2832–2843. [Google Scholar] [CrossRef]
- Lv, J.; Mao, J.; Ba, H. Influence Ofcrassostrea Gigason the Permeability and Microstructure of the Surface Layer of Concrete Exposed to the Tidal Zone of the Yellow Sea. Biofouling 2015, 31, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Coombes, M.A.; Viles, H.A.; Naylor, L.A.; La Marca, E.C. Cool Barnacles: Do Common Biogenic Structures Enhance or Retard Rates of Deterioration of Intertidal Rocks and Concrete? Sci. Total Environ. 2017, 580, 1034–1045. [Google Scholar] [CrossRef]
- Coombes, M.A.; La Marca, E.C.; Naylor, L.A.; Thompson, R.C. Getting into the Groove: Opportunities to Enhance the Ecological Value of Hard Coastal Infrastructure Using Fine-Scale Surface Textures. Ecol. Eng. 2015, 77, 314–323. [Google Scholar] [CrossRef]
- Gowell, M.R.; Coombes, M.A.; Viles, H.A. Rock-Protecting Seaweed? Experimental Evidence of Bioprotection in the Intertidal Zone. Earth Surf. Process. Landf. 2015, 40, 1364–1370. [Google Scholar] [CrossRef]
- Coombes, M.A.; Naylor, L.A.; Viles, H.A.; Thompson, R.C. Bioprotection and Disturbance: Seaweed, Microclimatic Stability and Conditions for Mechanical Weathering in the Intertidal Zone. Geomorphology 2013, 202, 4–14. [Google Scholar] [CrossRef]
- Coombes, M.A.; Naylor, L.; Thompson, R.; Roast, S.D.; Gómez-Pujol, L.; Fairhurst, R.J. Colonization and Weathering of Engineering Materials by Marine Microorganisms: An SEM Study. Earth Surf. Process. Landforms 2010, 36, 582–593. [Google Scholar] [CrossRef]
- Naylor, L.A.; Coombes, M.A.; Viles, H.A. Reconceptualising the Role of Organisms in the Erosion of Rock Coasts: A New Model. Geomorphology 2012, 157, 17–30. [Google Scholar] [CrossRef]
- Soleimani, S.; Ormeci, B.; Isgor, O.B. Growth and Characterization of Escherichia Coli DH5α Biofilm on Concrete Surfaces as a Protective Layer against Microbiologically Influenced Concrete Deterioration (MICD). Appl. Microbiol. Biotechnol. 2013, 97, 1093–1102. [Google Scholar] [CrossRef] [PubMed]
- Perkol-Finkel, S.; Sella, I. Ecologically Active Concrete for Coastal and Marine Infrastructure: Innovative Matrices and Designs. In From Sea to Shore? Meeting the Challenges of the Sea; ICE Publishing: London, UK, 2014; pp. 1139–1149. [Google Scholar]
- Flemming, H.-C. Biofouling in Water Systems—Cases, Causes and Countermeasures. Appl. Microbiol. Biotechnol. 2002, 59, 629–640. [Google Scholar] [CrossRef] [PubMed]
- Dobretsov, S.; Abed, R.M.; Teplitski, M. Mini-Review: Inhibition of Biofouling by Marine Microorganisms. Biofouling 2013, 29, 423–441. [Google Scholar] [CrossRef]
- Hayek, M.; Salgues, M.; Habouzit, F.; Bayle, S.; Souche, J.-C.; De Weerdt, K.; Pioch, S. La Bioréceptivité de Matériaux Cimentaires Dans L’Eau de Mer: Mécanismes, Facteurs Agissants ET Conséquences. Rev. Paralia 2020, 13, n03.1–n03.22. [Google Scholar] [CrossRef]
- Chambers, L.D.; Stokes, K.R.; Walsh, F.C.; Wood, R.J.K. Modern Approaches to Marine Antifouling Coatings. Surf. Coatings Technol. 2006, 201, 3642–3652. [Google Scholar] [CrossRef]
- Kanematsu, H.; Barry, D.M. A Sequence Between Microfouling and Macrofouling in Marine Biofouling. Monit. Artif. Mater. Microbes Marine Ecosyst. Interact. Assess. Methods 2020, 2, 67–80. [Google Scholar] [CrossRef]
- Dobretsov, S.V.; Railkin, A.I. Correlative Relationship between Marine Microfouling and Macrofouling; Biologiya Morya: Vladivostok, Russia, 1994; pp. 115–119. [Google Scholar]
- Dobretsov, S.; Rittschof, D. Love at First Taste: Induction of Larval Settlement by Marine Microbes. Int. J. Mol. Sci. 2020, 21, 731. [Google Scholar] [CrossRef]
- Mieszkin, S.; Callow, M.E.; Callow, J.A. Interactions between Microbial Biofilms and Marine Fouling Algae: A Mini Review. Biofouling 2013, 29, 1097–1113. [Google Scholar] [CrossRef] [PubMed]
- Grzegorczyk, M.; Pogorzelski, S.J.; Pospiech, A.; Boniewicz-Szmyt, K. Monitoring of Marine Biofilm Formation Dynamics at Submerged Solid Surfaces with Multitechnique Sensors. Front. Mar. Sci. 2018, 5, 5. [Google Scholar] [CrossRef]
- Siboni, N.; Lidor, M.; Kramarsky-Winter, E.; Kushmaro, A. Conditioning Film and Initial Biofilm Formation on Ceramics Tiles in the Marine Environment. FEMS Microbiol. Lett. 2007, 274, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Salta, M.; Wharton, J.A.; Blache, Y.; Stokes, K.R.; Briand, J.-F. Marine Biofilms on Artificial Surfaces: Structure and Dynamics. Environ. Microbiol. 2013, 15, 2879–2893. [Google Scholar] [CrossRef] [PubMed]
- Davey, M.E.; O’Toole, G.A. Microbial Biofilms: From Ecology to Molecular Genetics. Microbiol. Mol. Biol. Rev. 2000, 64, 847–867. [Google Scholar] [CrossRef] [PubMed]
- Dang, H.; Lovell, C.R. Bacterial Primary Colonization and Early Succession on Surfaces in Marine Waters as Determined by Amplified rRNA Gene Restriction Analysis and Sequence Analysis of 16S rRNA Genes. Appl. Environ. Microbiol. 2000, 66, 467–475. [Google Scholar] [CrossRef]
- Qian, P.-Y.; Lau, S.C.K.; Dahms, H.-U.; Dobretsov, S.V.; Harder, T. Marine Biofilms as Mediators of Colonization by Marine Macroorganisms: Implications for Antifouling and Aquaculture. Mar. Biotechnol. 2007, 9, 399–410. [Google Scholar] [CrossRef]
- Gittens, J.E.; Smith, T.J.; Suleiman, R.; Akid, R. Current and Emerging Environmentally-Friendly Systems for Fouling Control in the Marine Environment. Biotechnol. Adv. 2013, 31, 1738–1753. [Google Scholar] [CrossRef]
- Bixler, G.D.; Theiss, A.; Bhushan, B.; Lee, S.C. Anti-Fouling Properties of Microstructured Surfaces Bio-Inspired by Rice Leaves and Butterfly Wings. J. Colloid Interface Sci. 2014, 419, 114–133. [Google Scholar] [CrossRef]
- Pande, S.; Shitut, S.; Freund, L.; Westermann, M.; Bertels, F.; Colesie, C.; Bischofs, I.B.; Kost, C. Metabolic Cross-Feeding via Intercellular Nanotubes among Bacteria. Nat. Commun. 2015, 6, 6238. [Google Scholar] [CrossRef]
- Lors, C.; Feugeas, F.; Tribollet, B. Interactions Materials—Microorganisms: Concrete and Metals More Resistant to Biodeterioration; EDP Sciences: Les Ulis, France, 2019; ISBN 978-2-7598-2317-8. [Google Scholar]
- Dubosc, A.; Escadeillas, G.; Blanc, P.J. Characterization of Biological Stains on External Concrete Walls and Influence of Concrete as Underlying Material. Cem. Concr. Res. 2001, 31, 1613–1617. [Google Scholar] [CrossRef]
- Dalod, E. Influence de la Composition Chimique de Mortiers Sur Leur Biodétérioration Par Les Algues. Ph.D. Thesis, Ecole Nationale Supérieure des Mines de Saint-Etienne, Saint-Etienne, France, 4 May 2015. [Google Scholar]
- Dalod, E.; Govin, A.; Guyonnet, R.; Grosseau, P.; Lors, C.; Damidot, D. Influence of the Chemical Composition of Mortars on Algal Biofouling. In Calcium Aluminates: Proceedings of the International Conference 2014; Fentiman, K.L., Mangabhai, C.H., Scrivener, R.J., Eds.; IHS BRE Press: Avignon, France, 2014; pp. 523–534. [Google Scholar]
- Manso, S.; De Muynck, W.; Segura, I.; Aguado, A.; Steppe, K.; Boon, N.; De Belie, N. Bioreceptivity Evaluation of Cementitious Materials Designed to Stimulate Biological Growth. Sci. Total Environ. 2014, 481, 232–241. [Google Scholar] [CrossRef]
- Souche, J.-C.; Le Saout, G.; Salgues, M.; Pioch, S. Effets de Bétons Bio-Actifs Sur La Colonisation Marine En Environnement Méditerranéen. Matér. Tech. 2016, 104, 504. [Google Scholar] [CrossRef]
- Tran, T.H.; Govin, A.; Guyonnet, R.; Grosseau, P.; Lors, C.; Damidot, D.; Deves, O.; Ruot, B. Influence of the Intrinsic Characteristics of Mortars on Their Biofouling by Pigmented Organisms: Comparison between Laboratory and Field-Scale Experiments. Int. Biodeterior. Biodegrad. 2014, 86, 334–342. [Google Scholar] [CrossRef]
- Azevedo, J.; Antunes, J.T.; Machado, A.M.; Vasconcelos, V.; Leão, P.N.; Froufe, E. Monitoring of Biofouling Communities in a Portuguese Port Using a Combined Morphological and Metabarcoding Approach. Sci. Rep. 2020, 10, 13461. [Google Scholar] [CrossRef]
- Govin, A.; Tran, T.H.; Guyonnet, R.; Grosseau, P.; Lors, C.; Damidot, D.; Devès, O.; Ruot, B. Ability in Biofouling by Klebsormidium Flaccidum of Mortars: Influence of the Intrinsic Characteristics. First Int. Conf. Concr. Sustain. ICCS 2013, 914–919. [Google Scholar]
- Guillitte, O. Bioreceptivity: A New Concept for Building Ecology Studies. Sci. Total Environ. 1995, 167, 215–220. [Google Scholar] [CrossRef]
- Giannantonio, D.J.; Kurth, J.C.; Kurtis, K.E.; Sobecky, P.A. Effects of Concrete Properties and Nutrients on Fungal Colonization and Fouling. Int. Biodeterior. Biodegrad. 2009, 63, 252–259. [Google Scholar] [CrossRef]
- Miller, A.; Sanmartín, P.; Pereira-Pardo, L.; Dionísio, A.; Saiz-Jimenez, C.; Macedo, M.; Prieto, B. Bioreceptivity of Building Stones: A Review. Sci. Total Environ. 2012, 426, 1–12. [Google Scholar] [CrossRef]
- Manso, S.; Mestres, G.; Ginebra, M.P.; De Belie, N.; Segura, I.; Aguado, A. Development of a Low PH Cementitious Material to Enlarge Bioreceptivity. Constr. Build. Mater. 2014, 54, 485–495. [Google Scholar] [CrossRef]
- McManus, R.S.; Archibald, N.; Comber, S.; Knights, A.M.; Thompson, R.C.; Firth, L.B. Partial Replacement of Cement for Waste Aggregates in Concrete Coastal and Marine Infrastructure: A Foundation for Ecological Enhancement? Ecol. Eng. 2018, 120, 655–667. [Google Scholar] [CrossRef]
- Chalee, W.; Ausapanit, P.; Jaturapitakkul, C. Utilization of Fly Ash Concrete in Marine Environment for Long Term Design Life Analysis. Mater. Des. 2010, 31, 1242–1249. [Google Scholar] [CrossRef]
- Hayek, M.; Salgues, M.; Habouzit, F.; Bayle, S.; Souche, J.-C.; De Weerdt, K.; Pioch, S. In Vitro and in Situ Tests to Evaluate the Bacterial Colonization of Cementitious Materials in the Marine Environment. Cem. Concr. Compos. 2020, 113, 103748. [Google Scholar] [CrossRef]
- Hayek, M.; Salgues, M.; Habouzit, F.; Bayle, S.; Souche, J.-C.; De Weerdt, K.; Pioch, S. L’Influence de la Carbonatation Sur la Biocolonisation de Matériaux Cimentaires Dans Le Milieu Marin. Matériaux Techniques 2020, 108, 202. [Google Scholar] [CrossRef]
- Denechere, M.; Sallaberry, A.; Giraudel, C. Le Bloc ECOPODETM: Une Alternative Aux Enrochements Naturels. 2009. Available online: https://www.semanticscholar.org/paper/Le-bloc-ECOPODE%E2%84%A2-%3A-une-alternative-aux-enrochements-Denechere-Sallaberry/e957a879b75dc13fb21e0acdb307a8fe21dcf17b (accessed on 1 January 2021).
- De Muynck, W.; Debrouwer, D.; De Belie, N.; Verstraete, W. Bacterial Carbonate Precipitation Improves the Durability of Cementitious Materials. Cem. Concr. Res. 2008, 38, 1005–1014. [Google Scholar] [CrossRef]
- Verdier, T.; Coutand, M.; Bertron, A.; Roques, C. A Review of Indoor Microbial Growth across Building Materials and Sampling and Analysis Methods. Build. Environ. 2014, 80, 136–149. [Google Scholar] [CrossRef]
- Lv, J.; Mao, J.; Ba, H. Influence of Marine Microorganisms on the Permeability and Microstructure of Mortar. Constr. Build. Mater. 2015, 77, 33–40. [Google Scholar] [CrossRef]
- Hayek, M.; Baraquet, C.; Lami, R.; Blache, Y.; Molmeret, M. The Marine Bacterium Shewanella woodyi Produces C8-HSL to Regulate Bioluminescence. Microb. Ecol. 2019, 79, 865–881. [Google Scholar] [CrossRef]
- Hansen, G.H.; Sørheim, R. Improved Method for Phenotypical Characterization of Marine Bacteria. J. Microbiol. Methods 1991, 13, 231–241. [Google Scholar] [CrossRef]
- Díaz-García, A.; Borrero-Santiago, A.R.; Riba, I. Implications in Studies of Environmental Risk Assessments: Does Culture Medium Influence the Results of Toxicity Tests of Marine Bacteria? Chemosphere 2018, 205, 24–30. [Google Scholar] [CrossRef]
- Stender, Y.; Foley, M.; Rodgers, K.; Jokiel, P.; Singh, A. Evaluating the Feasibility and Advantage of a Multi-Purpose Submerged Breakwater for Harbor Protection and Benthic Habitat Enhancement at Kahului Commercial Harbor, Hawai’i: Case Study. SN Appl. Sci. 2021, 3, 167. [Google Scholar] [CrossRef]
- De Muynck, W.; Ramirez, A.M.; De Belie, N.; Verstraete, W. Evaluation of Strategies to Prevent Algal Fouling on White Architectural and Cellular Concrete. Int. Biodeterior. Biodegrad. 2009, 63, 679–689. [Google Scholar] [CrossRef]
- Escadeillas, G.; Bertron, A.; Blanc, P.; Dubosc, A. Accelerated Testing of Biological Stain Growth on External Concrete Walls. Part 1: Development of the Growth Tests. Mater. Struct. 2006, 40, 1061–1071. [Google Scholar] [CrossRef]
- Woods, C.M.C.; Floerl, O.; Hayden, B.J. Biofouling on Greenshell™ Mussel (Perna canaliculus) Farms: A Preliminary Assessment and Potential Implications for Sustainable Aquaculture Practices. Aquac. Int. 2011, 20, 537–557. [Google Scholar] [CrossRef]
- Allan, V.J.M.; Callow, M.E.; Macaskie, L.E.; Paterson-Beedle, M. Effect of Nutrient Limitation on Biofilm Formation and Phosphatase Activity of a Citrobacter sp. Microbiology 2002, 148, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to Image J: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Vishwakarmaa, V.; George, R.; Ramachandran, D.; Anandkumar, B.; Mudali, U.K.; Vishwakarmaa, V. Studies of Detailed Biofilm Characterization on Fly Ash Concrete in Comparison with Normal and Superplasticizer Concrete in Seawater Environments. Environ. Technol. 2013, 35, 42–51. [Google Scholar] [CrossRef]
- Olivia, M.; Moheimani, N.; Javaherdashti, R.; Nikraz, H.R.; Borowitzka, M.A. The Influence of Micro Algae on Corrosion of Steel in Fly Ash Geopolymer Concrete: A Preliminary Study. Adv. Mater. Res. 2012, 626, 861–866. [Google Scholar]
- Manso, S.; Aguado, A. A Review of Sample Preparation and Its Influence on pH Determination in Concrete Samples. Mater. Construcción 2017, 67, 108. [Google Scholar] [CrossRef]
- Munzer, C.; Belhaj, E.; Meylheuc, T.; LeComte, A.; Feugeas, F. Effets D’Un Bioadjuvant Sur Les Caractéristiques de Surface de Pâtes Cimentaires. Matériaux Tech. 2015, 103, 208. [Google Scholar] [CrossRef]
- Hoštacká, A.; Čižnár, I.; Štefkovičová, M. Temperature and pH Affect the Production of Bacterial Biofilm. Folia Microbiol. 2010, 55, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Ling, J.; Blancheton, J.-P. Nitrification Kinetics of Biofilm as Affected by Water Quality Factors. Aquac. Eng. 2006, 34, 179–197. [Google Scholar] [CrossRef]
- Arp, G.; Hofmann, J.; Reitner, J. Microbial Fabric Formation in Spring Mounds (“Microbialites”) of Alkaline Salt Lakes in the Badain Jaran Sand Sea, PR China. Palaios 1998, 13, 581. [Google Scholar] [CrossRef]
- Stanley, S.O.; Morita, R.Y. Salinity Effect on the Maximal Growth Temperature of Some Bacteria Isolated from Marine Envi-ronments. J. Bacteriol. 1968, 95, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Zhang, Z.; Wang, Y.; Zhuo, L.; Yin, L.; Du, Z. Screening, Identification and Properties Study of Marine Bacteria Producing Alkaline Cellulase. Mar. Sci. 2009, 7. [Google Scholar] [CrossRef]
- Delille, D.; Perret, E. Influence of Temperature on the Growth Potential of Southern Polar Marine Bacteria. Microb. Ecol. 1989, 18, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Halevy, I.; Bachan, A. The Geologic History of Seawater pH. Science 2017, 355, 1069–1071. [Google Scholar] [CrossRef]
- Odier, M.; Plichon, V. Le Cuivre en Solution Dans L’Eau de Mer: Forme Chimique et Dosage. Anal. Chim. Acta 1971, 55, 209–220. [Google Scholar] [CrossRef]
- Reichelt, J.L.; Baumann, P. Effect of Sodium Chloride on Growth of Heterotrophic Marine Bacteria. Arch. Microbiol. 1974, 97, 329–345. [Google Scholar] [CrossRef]
- Mountfort, D.O.; Pybus, V. Effect of pH, Temperature and Salinity on the Production of Gamma Aminobutyric Acid (GABA) from Amines by Marine Bacteria. FEMS Microbiol. Lett. 1992, 101, 237–244. [Google Scholar] [CrossRef]
- Loh, W.L.C.; Huang, K.-C.; Ng, H.S.; Lan, J.C.-W. Exploring the Fermentation Characteristics of a Newly Isolated Marine Bacteria Strain, Gordonia Terrae TWRH01 for Carotenoids Production. J. Biosci. Bioeng. 2020, 130, 187–194. [Google Scholar] [CrossRef]
- Huggett, M.J.; Nedved, B.T.; Hadfield, M.G. Effects of Initial Surface Wettability on Biofilm Formation and Subsequent Settlement of Hydroides Elegans. Biofouling 2009, 25, 387–399. [Google Scholar] [CrossRef]
- Molino, P.J.; Childs, S.; Hubbard, M.R.E.; Carey, J.M.; Burgman, M.A.; Wetherbee, R. Development of the Primary Bacterial Microfouling Layer on Antifouling and Fouling Release Coatings in Temperate and Tropical Environments in Eastern Australia. Biofouling 2009, 25, 149–162. [Google Scholar] [CrossRef]
- De Weerdt, K.; Ben Haha, M.; Le Saout, G.; Kjellsen, K.; Justnes, H.; Lothenbach, B. Hydration Mechanisms of Ternary Portland Cements Containing Limestone Powder and Fly Ash. Cem. Concr. Res. 2011, 41, 279–291. [Google Scholar] [CrossRef]
- Hsiung, A.R.; Tan, W.T.; Loke, L.H.; Firth, L.B.; Heery, E.C.; Ducker, J.; Clark, V.; Pek, Y.S.; Birch, W.R.; Ang, A.C. Little Evidence That Lowering the pH of Concrete Supports Greater Biodiversity on Tropical and Temperate Sea-Walls. Mar. Ecol. Prog. Ser. 2020, 656, 193–205. [Google Scholar] [CrossRef]
- Huang, X.; Wang, Z.; Liu, Y.; Hu, W.; Ni, W. On the Use of Blast Furnace Slag and Steel Slag in the Preparation of Green Artificial Reef Concrete. Constr. Build. Mater. 2016, 112, 241–246. [Google Scholar] [CrossRef]
- Bertos, M.F.; Li, X.; Simons, S.J.R.; Hills, C.D.; Carey, P.J. Investigation of Accelerated Carbonation for the Stabilisation of MSW Incinerator Ashes and the Sequestration of CO2. Green Chem. 2004, 6, 428–436. [Google Scholar] [CrossRef]
- Liu, X.; Niu, D.; Li, X.; Lv, Y. Effects of Ca(OH)2—CaCO3 Concentration Distribution on the pH and Pore Structure in Natural Carbonated Cover Concrete: A Case Study. Constr. Build. Mater. 2018, 186, 1276–1285. [Google Scholar] [CrossRef]
- Grosseau, P.; Dalod, E.; Govin, A.; Lors, C.; Guyonnet, R.; Damidot, D. Effect of the Chemical Composition of Building Materials on Algal Biofouling. In Proceedings of the 27th Biennial National Conference of the Concrete Institute of Australia in conjunction with the 69th RILEM Week, Melbourne, Australia, 30 August–2 September 2015; pp. 735–744. [Google Scholar]
- Tang, Y.-J.; Zuo, X.-B.; Yin, G.-J.; Davoudi, H.; Li, X.-N. Influence of Calcium Leaching on Chloride Diffusivity in Cement-Based Materials. Constr. Build. Mater. 2018, 174, 310–319. [Google Scholar] [CrossRef]
- Jacques, D.; Wang, L.; Martens, E.; Mallants, D. Modelling Chemical Degradation of Concrete during Leaching with Rain and Soil Water Types. Cem. Concr. Res. 2010, 40, 1306–1313. [Google Scholar] [CrossRef]
- Habimana, O.; Semião, A.; Casey, E. The Role of Cell-Surface Interactions in Bacterial Initial Adhesion and Consequent Biofilm Formation on Nanofiltration/Reverse Osmosis Membranes. J. Membr. Sci. 2014, 454, 82–96. [Google Scholar] [CrossRef]
- Rao, D.; Webb, J.S.; Kjelleberg, S. Competitive Interactions in Mixed-Species Biofilms Containing the Marine Bacterium Pseudoalteromonas tunicata. Appl. Environ. Microbiol. 2005, 71, 1729–1736. [Google Scholar] [CrossRef]
- Grasland, B.; Mitalane, J.; Briandet, R.; Quemener, E.; Meylheuc, T.; Linossier, I.; Vallee-Rehel, K.; Haras, D. Bacterial Biofilm in Seawater: Cell Surface Properties of Early-attached Marine Bacteria. Biofouling 2003, 19, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Crouzet, M.; Le Senechal, C.; Brözel, V.S.; Costaglioli, P.; Barthe, C.; Bonneu, M.; Garbay, B.; Vilain, S. Exploring Early Steps in Biofilm Formation: Set-Up of an Experimental System for Molecular Studies. BMC Microbiol. 2014, 14, 1–12. [Google Scholar] [CrossRef]
- Shih, P.-C. Effects of Quorum-Sensing Deficiency on Pseudomonas aeruginosa Biofilm Formation and Antibiotic Resistance. J. Antimicrob. Chemother. 2002, 49, 309–314. [Google Scholar] [CrossRef]
- Scheidweiler, D.; Peter, H.; Pramateftaki, P.; De Anna, P.; Battin, T.J. Unraveling the Biophysical Underpinnings to the Success of Multispecies Biofilms in Porous Environments. ISME J. 2019, 13, 1700–1710. [Google Scholar] [CrossRef]
- Rica, H.C.; Boutouil, M.; Boudart, B.; Claquin, P.; Leroy, F. Colonisation et Détérioration Des Bétons Incor-porant Des Coquilles Pour Récifs Artificiels. Matér. Tech. 2016, 104, 503. [Google Scholar] [CrossRef]
- Gallego-Cartagena, E.; Morillas, H.; Maguregui, M.; Patiño-Camelo, K.; Marcaida, I.; Morgado-Gamero, W.; Silva, L.F.; Madariaga, J.M. A Comprehensive Study of Biofilms Growing on the Built Heritage of a Caribbean Industrial City in Correlation with Construction Materials. Int. Biodeterior. Biodegrad. 2020, 147, 104874. [Google Scholar] [CrossRef]
- Nijland, T.G.; Adan, O.C.; Van Hees, R.P.; van Etten, B.D. Evaluation of the Effects of Expected Climate Change on the Durability of Building Materials with Suggestions for Adaptation. Heron 2009, 54, 37–48. [Google Scholar]
- Kerr, A.; Beveridge, C.M.; Cowling, M.J.; Hodgkiess, T.; Parr, A.C.S.; Smith, M.J. Some Physical Factors Affecting the Accumulation of Biofouling. J. Mar. Biol. Assoc. UK 1999, 79, 357–359. [Google Scholar] [CrossRef]
- Teughels, W.; Van Assche, N.; Sliepen, I.; Quirynen, M. Effect of Material Characteristics and/or Surface Topography on Biofilm Development. Clin. Oral Implant. Res. 2006, 17, 68–81. [Google Scholar] [CrossRef]
- Rodriguez, A.; Autio, W.R.; McLandsborough, L.A. Effect of Surface Roughness and Stainless Steel Finish on Listeria monocytogenes Attachment and Biofilm Formation. J. Food Prot. 2008, 71, 170–175. [Google Scholar] [CrossRef]
- Rashid, H. The Effect of Surface Roughness on Ceramics Used in Dentistry: A Review of Literature. Eur. J. Dent. 2014, 8, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Truong, V.K.; Lapovok, R.; Estrin, Y.S.; Rundell, S.; Wang, J.Y.; Fluke, C.J.; Crawford, R.J.; Ivanova, E.P. The Influence of Nano-Scale Surface Roughness on Bacterial Adhesion to Ultrafine-Grained Titanium. Biomaterials 2010, 31, 3674–3683. [Google Scholar] [CrossRef] [PubMed]
- Voegel, C.; Durban, N.; Bertron, A.; Landon, Y.; Erable, B. Evaluation of Microbial Proliferation on Cementitious Materials Exposed to Biogas Systems. Environ. Technol. 2019, 41, 2439–2449. [Google Scholar] [CrossRef] [PubMed]
- Kurth, J.C. Mitigating Biofilm Growth through the Modification of Concrete Design and Practice. Ph.D. Thesis, Georgia Institute of Technology, Atlanta, GA, USA, 1 April 2008. [Google Scholar]
- Ammar, Y.; Swailes, D.; Bridgens, B.; Chen, J. Influence of Surface Roughness on the Initial Formation of Biofilm. Surf. Coat. Technol. 2015, 284, 410–416. [Google Scholar] [CrossRef]
- Bellon-Fontaine, M.-N.; Benezech, T.; Boutroux, K.; Hermon, C. Conception Hygiénique de Matériel et Nettoy-Age-Désinfection; Sciences et Techniques Agroalimentaires: Lavoisier, Paris, 2016; p. 12. [Google Scholar]
- Ahmed, K.B. Etude de L’Encrassement Biologique de Matériaux Cimentaires en Eau de Rivière: Analyse de L’Influence Des Paramètres de Surface Des Pâtes Cimentaires. Ph.D. Thesis, Université de Strasbourg, Strasbourg, France, 22 June 2016. [Google Scholar]
- Dang, Y.; Qian, J.; Qu, Y.; Zhang, L.; Wang, Z.; Qiao, D.; Jia, X. Curing Cement Concrete by Using Shrinkage Reducing Admixture and Curing Compound. Constr. Build. Mater. 2013, 48, 992–997. [Google Scholar] [CrossRef]
- Joshaghani, A.; Bhardwaj, R.; Zollinger, D.G.; Mukhopadhyay, A.K. Investigating the Effects of Curing Quality on Key Concrete Pavement Surface Properties. Transp. Res. Rec. J. Transp. Res. Board 2019, 2673, 71–80. [Google Scholar] [CrossRef]
- Nahata, Y.; Kholia, N.; Tank, T. Effect of Curing Methods on Efficiency of Curing of Cement Mortar. APCBEE Procedia 2014, 9, 222–229. [Google Scholar] [CrossRef]
- Blainey, B.L.; Marshall, K.C. The Use of Block Copolymers to Inhibit Bacterial Adhesion and Biofilm Formation on Hydrophobic Surfaces in Marine Habitats. Biofouling 1991, 4, 309–318. [Google Scholar] [CrossRef]
- Sun, K.; Yang, H.; Xue, W.; He, A.; Zhu, D.; Liu, W.; Adeyemi, K.; Cao, Y. Anti-biofouling Superhydrophobic Surface Fabricated by Picosecond Laser Texturing of Stainless Steel. Appl. Surf. Sci. 2018, 436, 263–267. [Google Scholar] [CrossRef]
- Hartshorne, R.S.; Tustin, G.J. Systems and Methods for Marine Anti-Fouling. Available online: https://patents.google.com/patent/AU2010329600B2/en (accessed on 1 January 2021).
- Razavi, S.M.R.; Oh, J.; Haasch, R.T.; Kim, K.; Masoomi, M.; Bagheri, R.; Slauch, J.M.; Miljkovic, N. Environment-Friendly Antibiofouling Superhydrophobic Coatings. ACS Sustain. Chem. Eng. 2019, 7, 14509–14520. [Google Scholar] [CrossRef]
- Zhang, B.; Xu, W.; Zhu, Q.; Yuan, S.; Li, Y. Lotus-Inspired Multiscale Superhydrophobic AA5083 Resisting Surface Contamination and Marine Corrosion Attack. Materials 2019, 12, 1592. [Google Scholar] [CrossRef] [PubMed]
- She, W.; Yang, J.; Hong, J.; Sun, D.; Mu, S.; Miao, C. Superhydrophobic Concrete with Enhanced Mechanical Robustness: Nanohybrid Composites, Strengthen Mechanism and du-Rability Evaluation. Constr. Build. Mater. 2020, 247, 118563. [Google Scholar] [CrossRef]
- Bixler, G.D.; Bhushan, B. Biofouling: Lessons from Nature. Philos. Trans. R. Soc. Math. Phys. Eng. Sci. 2012, 370, 2381–2417. [Google Scholar] [CrossRef]
- Andjouh, S.; Blache, Y. Screening of Bromotyramine Analogues as Antifouling Compounds against Marine Bacteria. Biofouling 2016, 32, 871–881. [Google Scholar] [CrossRef]
- Schoenfeld, R.C.; Conova, S.; Rittschof, D.; Ganem, B. Cytotoxic, Antifouling Bromotyramines: A Synthetic Study on Simple Marine Natural Products and Their Analogues. Bioorgan. Med. Chem. Lett. 2002, 12, 823–825. [Google Scholar] [CrossRef]
- Andjouh, S.; Blache, Y. Click-Based Synthesis of Bromotyrosine Alkaloid Analogs as Potential Anti-biofilm Leads for SAR Studies. Bioorgan. Med. Chem. Lett. 2015, 25, 5762–5766. [Google Scholar] [CrossRef]
- Spitz, N.; Coniglio, N.; El Mansori, M.; Montagne, A.; Mezghani, S. Quantitative and Representative Adherence Assessment of Coated and Uncoated Concrete-Formwork. Surf. Coatings Technol. 2018, 352, 247–256. [Google Scholar] [CrossRef]
- Djelal, C.; Vanhove, Y.; De Caro, P.; Magnin, A. Role of Demoulding Agents during Self-Compacting Concrete Casting in Formwork. Mater. Struct. 2002, 35, 470–476. [Google Scholar] [CrossRef]
- Djelal, C.; De Caro, P.; Libessart, L.; Dubois, I.; Pébère, N. Comprehension of Demoulding Mechanisms at the Formwork/Oil/Concrete Interface. Mater. Struct. 2007, 41, 571–581. [Google Scholar] [CrossRef]
- León-Martínez, F.M.; Abad-Zarate, E.F.; Lagunez-Rivera, L.; Cano-Barrita, P.F.D.J. Laboratory and Field Performance of Biodegradable Release Agents for Hydraulic Concrete. Mater. Struct. 2015, 49, 2731–2748. [Google Scholar] [CrossRef]
- Leahy, K.C. Biodegradable Concrete Form Release Agent. Available online: https://patents.google.com/patent/US5194584A/en (accessed on 1 January 2021).
- Bouharoun, S.; De Caro, P.; Dubois, I.; Djelal, C.; Vanhove, Y. Effect of a Superplasticizer on the Properties of the Concrete/Oil/Formwork Interface. Constr. Build. Mater. 2013, 47, 1137–1144. [Google Scholar] [CrossRef]
- Mehdi, H.; Giti, E. Investigation of Alkane Biodegradation Using the Microtiter Plate Method and Correlation between Biofilm Formation, Biosur-Factant Production and Crude Oil Biodegradation. Int. Biodeterior. Biodegrad. 2008, 62, 170–178. [Google Scholar] [CrossRef]
- Saadatullah; Malook, I.; Jan, M.; Waheedullah; Muhammmad, N.; ur Rehman, Z. Isolation, Identification and Characterization of a Lipase Producing Pseudomonas. J. Biomater. 2018, 2, 51. [Google Scholar] [CrossRef]
- Li, Z.; Wrenn, B.A.; Venosa, A.D. Anaerobic Biodegradation of Vegetable Oil and Its Metabolic Intermediates in Oil-Enriched Freshwater Sediments. Biogeochemics 2005, 16, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Al-Darbi, M.M.; Saeed, N.O.; Islam, M.R.; Lee, K. Biodegradation of Natural Oils in Seawater. Energy Sources 2005, 27, 19–34. [Google Scholar] [CrossRef]
- Dusane, D.H.; Nancharaiah, Y.V.; Venugopalan, V.P.; Kumar, A.R.; Zinjarde, S.S. Biofilm Formation by a Biotechnologically Important Tropical Marine Yeast Isolate, Yarrowia lipolytica NCIM 3589. Water Sci. Technol. 2008, 58, 1221–1229. [Google Scholar] [CrossRef]
- Quagliarini, E.; Gianangeli, A.; D’Orazio, M.; Gregorini, B.; Osimani, A.; Aquilanti, L.; Clementi, F. Effect of Temperature and Relative Humidity on Algae Biofouling on Different Fired Brick Surfaces. Constr. Build. Mater. 2019, 199, 396–405. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).