Two-Step Air/Water Oxidation Process for the Long-Lasting Photoluminescence and Biological Viability (MTT Assay) of Porous Silicon Particles
Abstract
1. Introduction
2. Materials and Methods
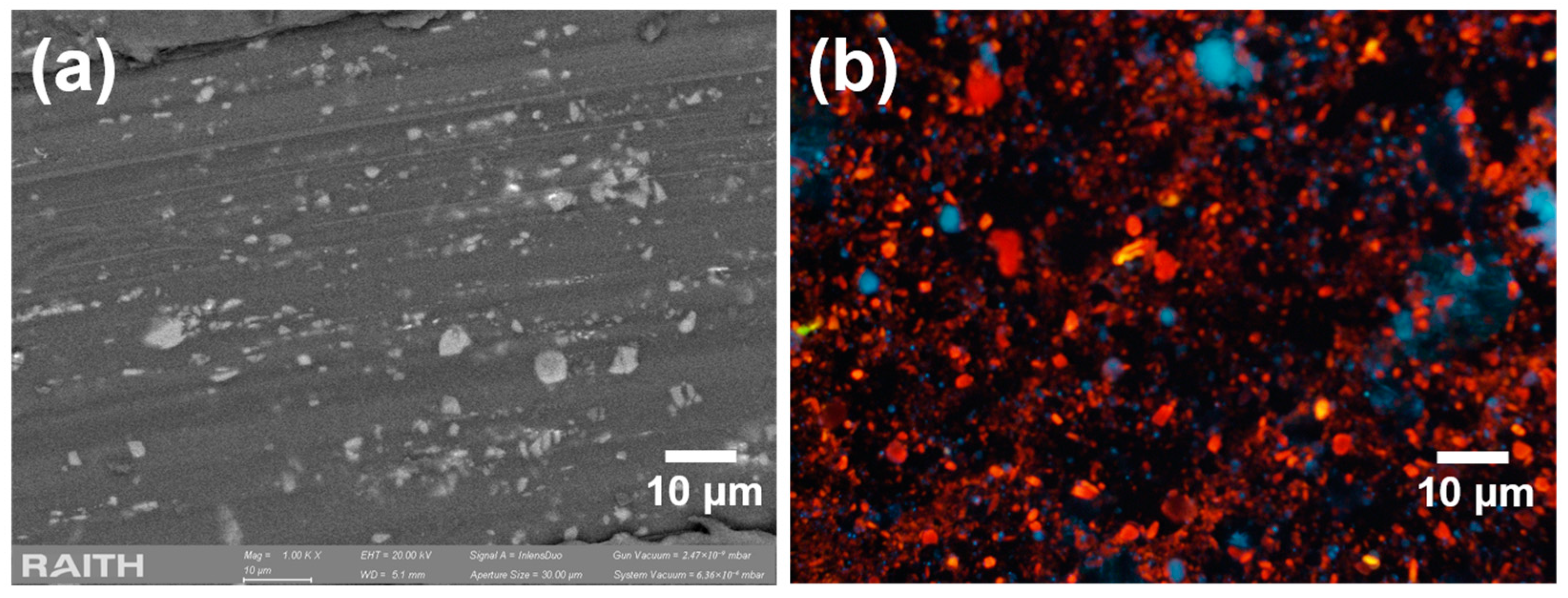
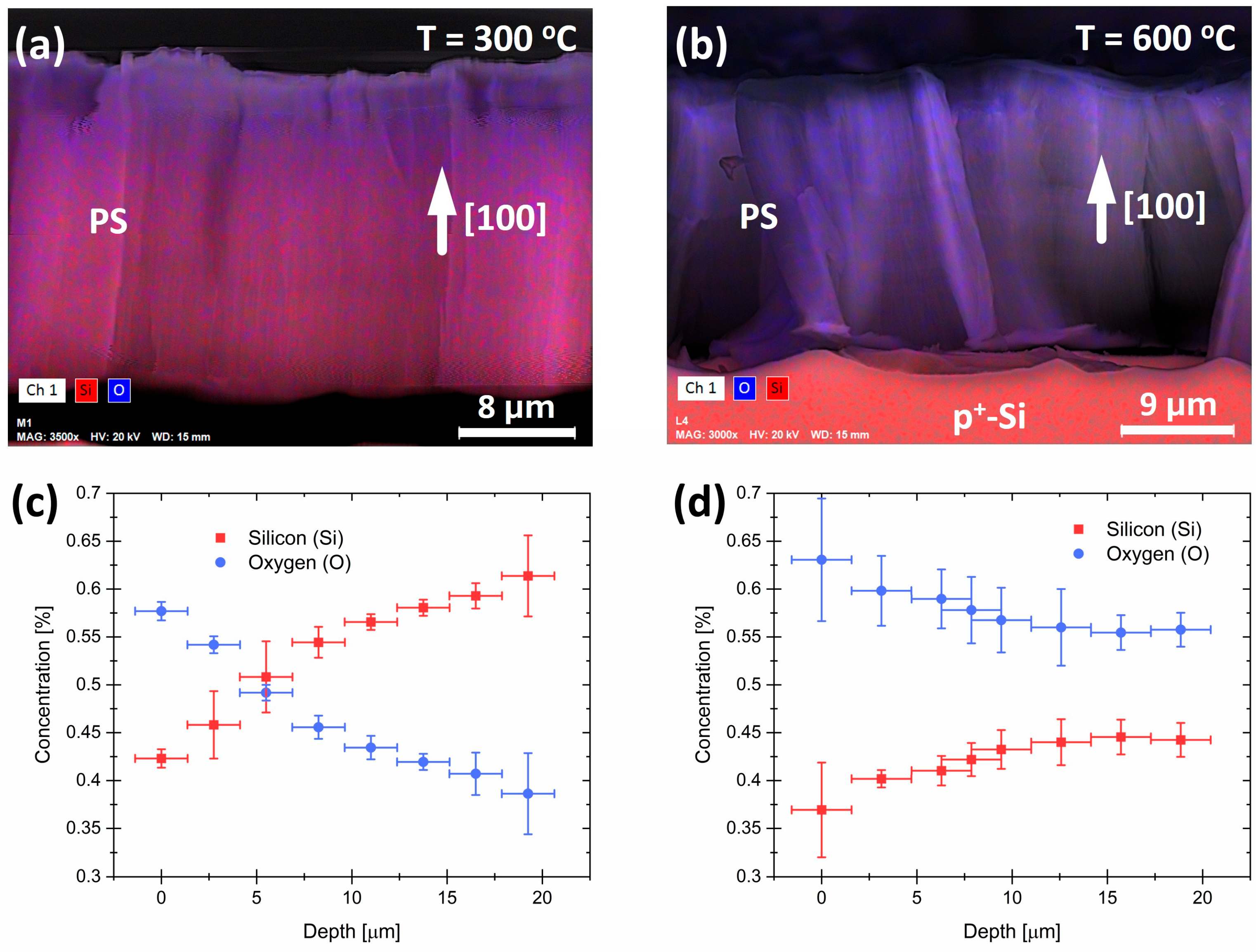
2.1. Synthesis and Characterization of Porous Silicon Particles (PSps)
2.2. Cell Viability Assay in Presence of Porous Silicon Particles (PSps)
3. Results and Discussion
3.1. Photoluminescence Evolution of Porous Silicon Particles (PSps)
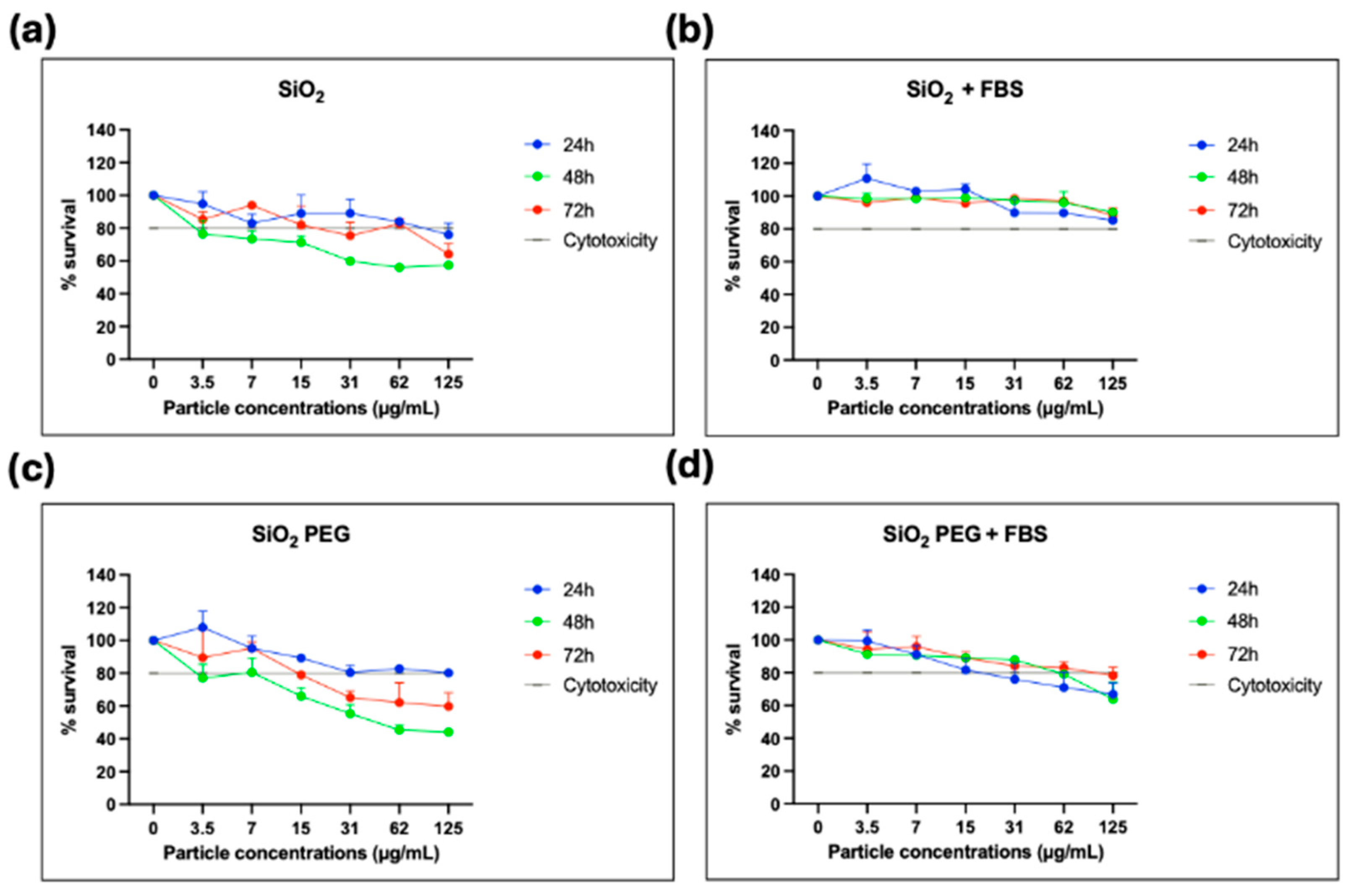
3.2. Cell Viability Assay in Presence of Porous Silicon Particles (PSps)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| EDX | Energy-Dispersive X-Ray Spectroscopy |
| FBS | Fetal Bovine Serum |
| PEG | Polyethylene Glycol |
| PL | Photoluminescence |
| PS | Porous Silicon |
| PSps | Porous Silicon Particles |
| QDs | Quantum Dots |
| SEM | Scanning Electron Microscopy |
| UV | Ultraviolet |
References
- Cullis, A.; Canham, L. Visible light emission due to quantum size effects in highly porous crystalline silicon. Nature 1991, 353, 335–338. [Google Scholar] [CrossRef]
- Anglin, E.J.; Cheng, L.; Freeman, W.R.; Sailor, M.J. Porous silicon in drug delivery devices and materials. Adv. Drug Deliv. Rev. 2008, 60, 1266–1277. [Google Scholar] [CrossRef]
- Park, J.-H.; Gu, L.; von Maltzahn, G.; Ruoslahti, E.; Bhatia, S.N.; Sailor, M.J. Biodegradable luminescent porous silicon nanoparticles for in vivo applications. Nat. Mater. 2009, 8, 331–336. [Google Scholar] [CrossRef]
- Lockwood, D.J.; Wang, A.G. Quantum confinement induced photoluminescence in porous silicon. Solid State Commun. 1995, 94, 905–909. [Google Scholar] [CrossRef]
- Jung, Y.; Kang, S.; An, J.; Jung, J.; Kim, D. Porous silicon-based fluorescent nanoprobe for the detection of anthrax biomarker and its practical sensing applications. Dyes Pigments 2020, 182, 108700. [Google Scholar] [CrossRef]
- Delerue, C.; Allan, G.; Lannoo, M. Theoretical aspects of the luminescence of porous silicon. Phys. Rev. B 1993, 48, 11024–11036. [Google Scholar] [CrossRef] [PubMed]
- Pérez, M.; Ramos, E.; de la Mora, M.B.; Santana, G.; Dutt, A. Absorption and emission of porous silicon based on quantum dots models by TD-DFT: Experimental and theoretical approach. Mater. Lett. 2021, 302, 130411. [Google Scholar] [CrossRef]
- Gole, J.L.; Dudel, F.P.; Grantier, D.; Dixon, D.A. Origin of Porous Silicon Photoluminescence: Evidence for a Surface Bound Oxyhydride-Like Emitter. Phys. Rev. B 1997, 56, 2137–2153. [Google Scholar] [CrossRef]
- Gole, J.L.; Dixon, D.A. Potential Role of Silanone and Silylenes in the photoluminescence-excitation, visible-photoluminescence-emission, and infrared spectra of porous silicon. Phys. Rev. B 1998, 57, 12002–12016. [Google Scholar] [CrossRef]
- Sailor, M.J. Chemical Reactivity and Surface Chemistry of Porous Silicon. In Handbook of Porous Silicon; Canham, L., Ed.; Springer International Publishing: Cham, Switzerland, 2014; pp. 355–380. [Google Scholar]
- Nakamura, T.; Ogawa, T.; Hosoya, N.; Adachi, S. Effects of thermal oxidation on the photoluminescence properties of porous silicon. J. Lumin. 2010, 130, 682–687. [Google Scholar] [CrossRef]
- Björkqvist, M.; Salonen, J.; Laine, E.; Niinistö, L. Comparison of stabilizing treatments on porous silicon for sensor applications. Phys. Status Solidi (A) Appl. Mater. Sci. 2023, 197, 374–377. [Google Scholar] [CrossRef]
- Boukherroub, R.; Wayner, D.D.M.; Sproule, G.I.; Lockwood, D.J.; Canham, L.T. Stability enhancement of partially-oxidized porous silicon nanostructures modified with ethyl undecylenate. Nano Lett. 2001, 1, 713–717. [Google Scholar] [CrossRef]
- Petrova-Koch, V.; Muschik, T.; Kux, A.; Meyer, B.K.; Koch, F.; Lehmann, V. Rapid-thermal-oxidized porous Si-The superior photoluminescent Si. Appl. Phys. Lett. 1992, 61, 943–945. [Google Scholar] [CrossRef]
- Duan, J.; Yu, Y.; Li, Y.; Yu, Y.; Li, Y.; Zhou, X.; Huang, P.; Sun, Z. Toxic effect of silica nanoparticles on endothelial cells through DNA damage response via Chk1-dependent G2/M checkpoint. PLoS ONE 2013, 8, e62087. [Google Scholar] [CrossRef] [PubMed]
- Napierska, D.; Thomassen, L.C.J.; Rabolli, V.; Lison, D.; Gonzalez, L.; Kirsch-Volders, M.; Martens, J.A.; Hoet, P.H. Size-dependent cytotoxicity of monodisperse silica nanoparticles in human endothelial cells. Small 2009, 5, 846–853. [Google Scholar] [CrossRef]
- Bauer, A.T.; Strozyk, E.A.; Gorzelanny, C.; Westerhausen, C.; Desch, A.; Schneider, M.F.; Schneider, S.W. Cytotoxicity of silica nanoparticles through exocytosis of von Willebrand factor and necrotic cell death in primary human endothelial cells. Biomaterials 2011, 32, 8385–8393. [Google Scholar] [CrossRef]
- Corbalan, J.J.; Medina, C.; Jacoby, A.; Malinski, T.; Radomski, M.W. Amorphous silica nanoparticles trigger nitric oxide/peroxynitrite imbalance in human endothelial cells: Inflammatory and cytotoxic effects. Int. J. Nanomed. 2011, 6, 2821–2835. [Google Scholar] [CrossRef]
- Jacobsohn, L.G.; Cooke, D.W.; Bennett, B.L.; Muenchausen, R.E.; Nastasi, M. Effects of thermal annealing and ageing on porous silicon photoluminescence. Philos. Mag. 2005, 85, 2611–2620. [Google Scholar] [CrossRef]
- Shin, H.J.; Lee, M.K.; Hwang, C.C.; Kim, K.; Kang, T.H.; Kim, B.; Kim, G.B.; Hong, C.K.; Lee, K.-W.W.; Kim, Y.Y. Photoluminescence degradation in porous silicon upon annealing at high temperature in vacuum. J. Korean Phys. Soc. 2003, 42, 808–813. [Google Scholar]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Rodríguez, J.A.; Vásquez-Agustín, M.A.; Morales-Sánchez, A.; Aceves-Mijares, M. Emission Mechanisms of Si Nanocrystals and Defects in SiO2 Materials. J. Nanomater. 2014, 409482. [Google Scholar] [CrossRef]
- Morita, M.; Ohmi, T.; Hasegawa, E.; Kawakami, M.; Ohwada, M.J. Growth of native oxide on a silicon surface. Appl. Phys. 1990, 68, 1272–1281. [Google Scholar] [CrossRef]
- Shimasaki, M.; Show, Y.; Iwase, M.; Izumi, T.; Ichinohe, T.; Nozaki, S.; Morisaki, H. Correlation between light emission and dangling bonds in porous silicon. Appl. Surf. Sci. 1996, 92, 617–620. [Google Scholar] [CrossRef]
- Ookubo, N.; Ono, H.; Ochiai, Y.; Mochizuki, Y.; Matsui, S. Effects of thermal annealing on porous silicon photoluminescence dynamics. Appl. Phys. Lett. 1992, 61, 940–942. [Google Scholar] [CrossRef]
- Docter, D.; Bantz, C.; Westmeier, D.; Galla, H.J.; Wang, Q.; Kirkpatrick, J.C.; Nielsen, P.; Maskos, M.; Stauber, R.H. The protein corona protects against size- and dose-dependent toxicity of amorphous silica nanoparticles. Beilstein J. Nanotechnol. 2014, 5, 1380–1392. [Google Scholar] [CrossRef]
- Mahmoud, N.; Abu-Dahab, R.; Abdallah, M.; Al-Dabash, S.; Abuarqoub, D.; Albasha, A.; Khalil, E.A. Interaction of gold nanorods with cell culture media: Colloidal stability, cytotoxicity and cellular death modality. J. Drug Deliv. Sci. Technol. 2020, 60, 101965. [Google Scholar] [CrossRef]
- Liang, S.; Chen, Y.; Zhang, S.; Cao, Y.; Duan, J.; Wang, Y.; Sun, Z. RhB-encapsulating silica nanoparticles modified with PEG impact the vascular endothelial function in endothelial cells and zebrafish model. Sci. Total Environ. 2020, 711, 134493. [Google Scholar] [CrossRef]
- Baghirov, H.; Karaman, D.; Viitala, T.; Duchanoy, A.; Lou, Y.-R.; Mamaeva, V.; Pryazhnikov, E.; Khiroug, L.; de Lange Davies, C.; Sahlgren, C.; et al. Feasibility study of the permeability and uptake of mesoporous silica nanoparticles across the blood-brain barrier. PLoS ONE 2016, 11, e0160705. [Google Scholar] [CrossRef] [PubMed]
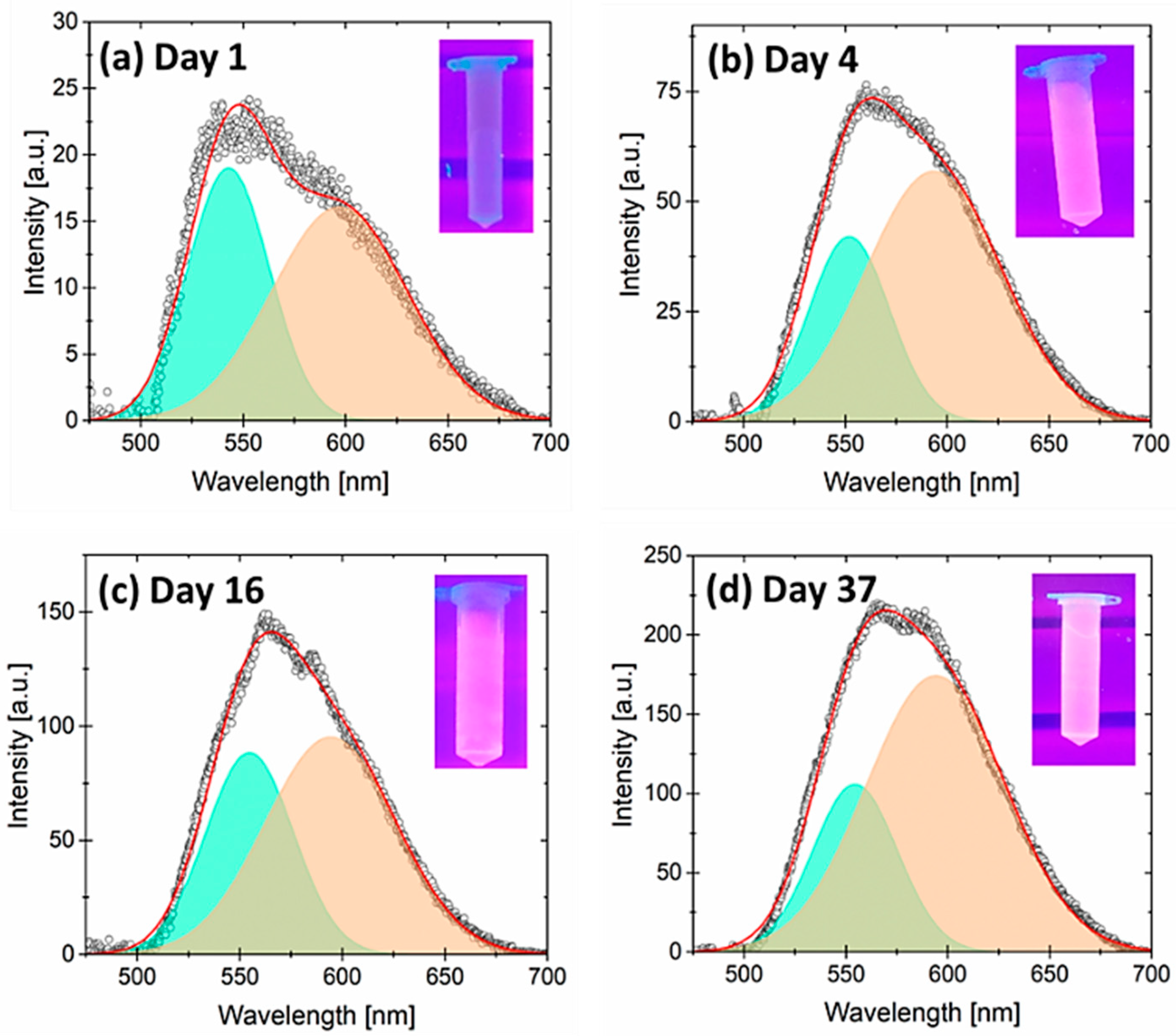
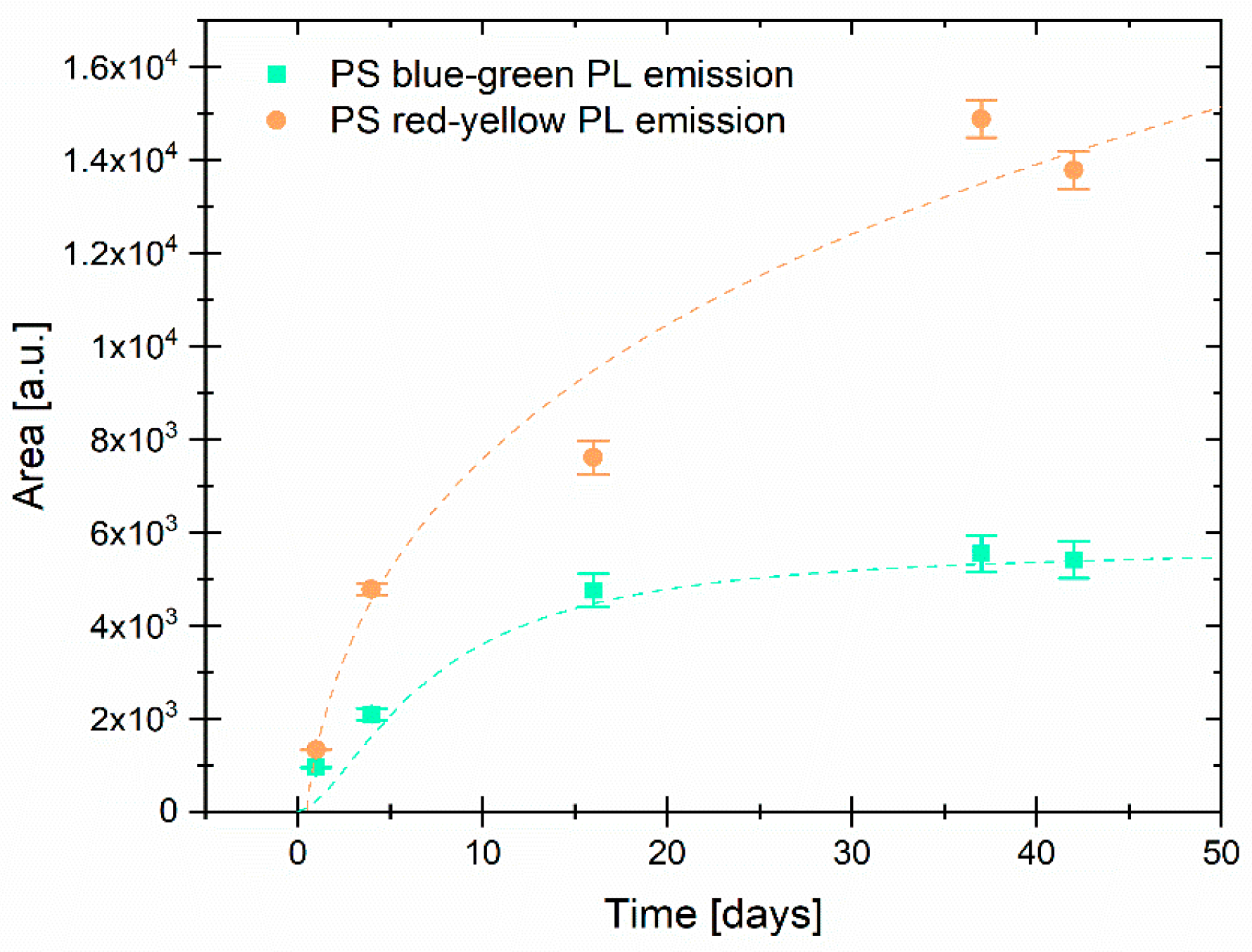
| Yellow–Green Emission | Yellow-Red–Emission | |||||
|---|---|---|---|---|---|---|
| Day | Peak Position [nm] | Area [a.u.] | FWHM [nm] | Peak Position [nm] | Area [a.u.] | FWHM [nm] |
| 1 | 542.8 ± 0.2 | 957 ± 8 | 47.3 ± 0.4 | 597 ± 1 | 1330 ± 10 | 78.0 ± 0.7 |
| 4 | 551.8 ± 0.3 | 2100 ± 100 | 46.8 ± 0.8 | 593 ± 1 | 4800 ± 100 | 79 ± 1 |
| 16 | 554.7 ± 0.4 | 4800 ± 400 | 50.5 ± 0.9 | 594 ± 2 | 7600 ± 400 | 75 ± 2 |
| 37 | 554.3 ± 0.3 | 5500 ± 400 | 49.2 ± 1.0 | 594 ± 1 | 14,900 ± 400 | 80 ± 1 |
| 42 | 555.9 ± 0.3 | 5400 ± 400 | 48.3 ± 1.0 | 595 ±1 | 13,800 ± 400 | 79 ± 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castillo Calvente, C.; Gilsanz-Muñoz, M.F.; Pérez-Piñeiro, J.; Cerpa-Naranjo, A.; Blasco, R.; Bragado-García, E.; Fernández-Alfonso, M.S.; Gallach-Pérez, D. Two-Step Air/Water Oxidation Process for the Long-Lasting Photoluminescence and Biological Viability (MTT Assay) of Porous Silicon Particles. J. Xenobiot. 2025, 15, 168. https://doi.org/10.3390/jox15050168
Castillo Calvente C, Gilsanz-Muñoz MF, Pérez-Piñeiro J, Cerpa-Naranjo A, Blasco R, Bragado-García E, Fernández-Alfonso MS, Gallach-Pérez D. Two-Step Air/Water Oxidation Process for the Long-Lasting Photoluminescence and Biological Viability (MTT Assay) of Porous Silicon Particles. Journal of Xenobiotics. 2025; 15(5):168. https://doi.org/10.3390/jox15050168
Chicago/Turabian StyleCastillo Calvente, Claudia, María F. Gilsanz-Muñoz, Javier Pérez-Piñeiro, Arisbel Cerpa-Naranjo, Rodrigo Blasco, Elvira Bragado-García, María S. Fernández-Alfonso, and Darío Gallach-Pérez. 2025. "Two-Step Air/Water Oxidation Process for the Long-Lasting Photoluminescence and Biological Viability (MTT Assay) of Porous Silicon Particles" Journal of Xenobiotics 15, no. 5: 168. https://doi.org/10.3390/jox15050168
APA StyleCastillo Calvente, C., Gilsanz-Muñoz, M. F., Pérez-Piñeiro, J., Cerpa-Naranjo, A., Blasco, R., Bragado-García, E., Fernández-Alfonso, M. S., & Gallach-Pérez, D. (2025). Two-Step Air/Water Oxidation Process for the Long-Lasting Photoluminescence and Biological Viability (MTT Assay) of Porous Silicon Particles. Journal of Xenobiotics, 15(5), 168. https://doi.org/10.3390/jox15050168








