Heavy Metals Affect the Antioxidant Defences in the Soil Ciliate Rigidohymena tetracirrata
Abstract
1. Introduction
2. Materials and Methods
2.1. Origin and Culture Conditions of the Soil Ciliate R. tetracirrata
2.2. Experimental Setup: Metal Salts (Chemicals), Preliminary Range-Finding, and Determination of Final Exposure Concentrations
2.3. Toxicity Tests with Single Metals (Cu, Zn and Cd)
2.4. Cytotoxicity Bioassays with Bimetallic Mixtures (Cd + Zn, Cu + Zn, and Cd + Cu)
2.5. Total Phenolic Content and Antioxidant Activity Assays
2.6. Estimation of Total Phenolic Content (TPC)
2.7. DPPH Scavenging Assay
2.8. Hydroxyl Radical Scavenging Assay (HRSA)
2.9. Statistical Analysis
3. Results
3.1. Cytotoxicity of Single Metals (Cu, Zn and Cd)
3.2. Cytotoxicity of Bimetallic Mixtures (Cd + Zn, Cu + Zn and Cd + Cu)
3.3. Antioxidant Properties of R. tetracirrata Extracts Treated with Cu, Zn, Cd, and Cd +Zn
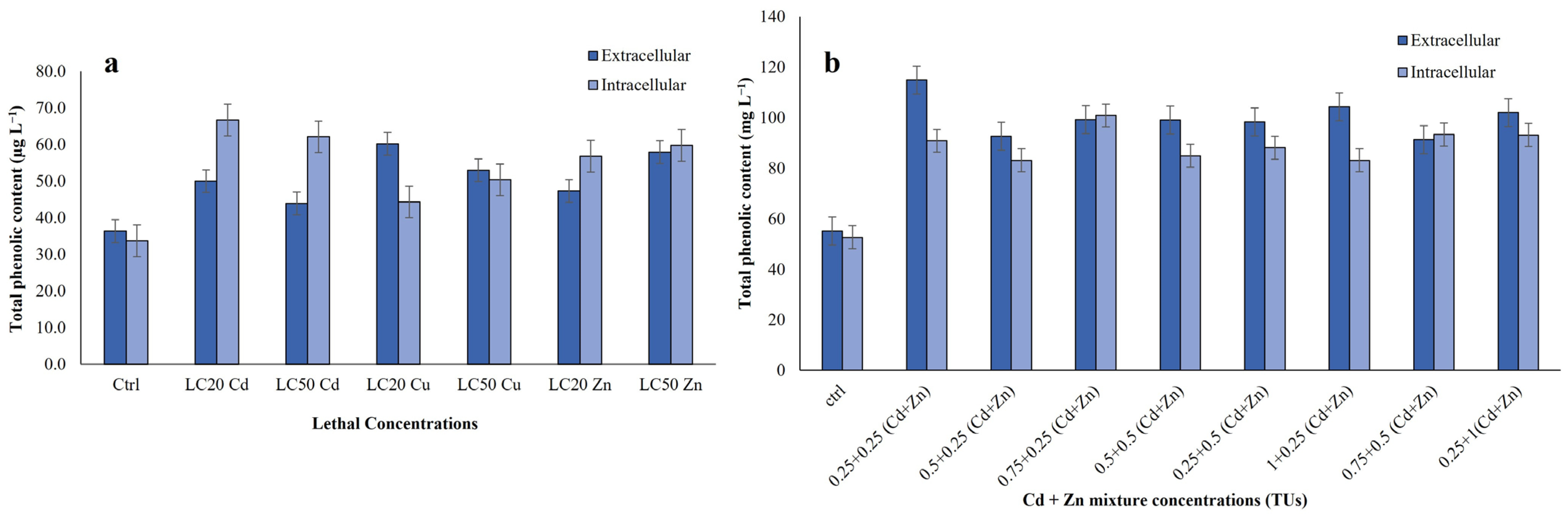
3.3.1. Total Phenolic Content (TPC) from Extracts of R. tetracirrata
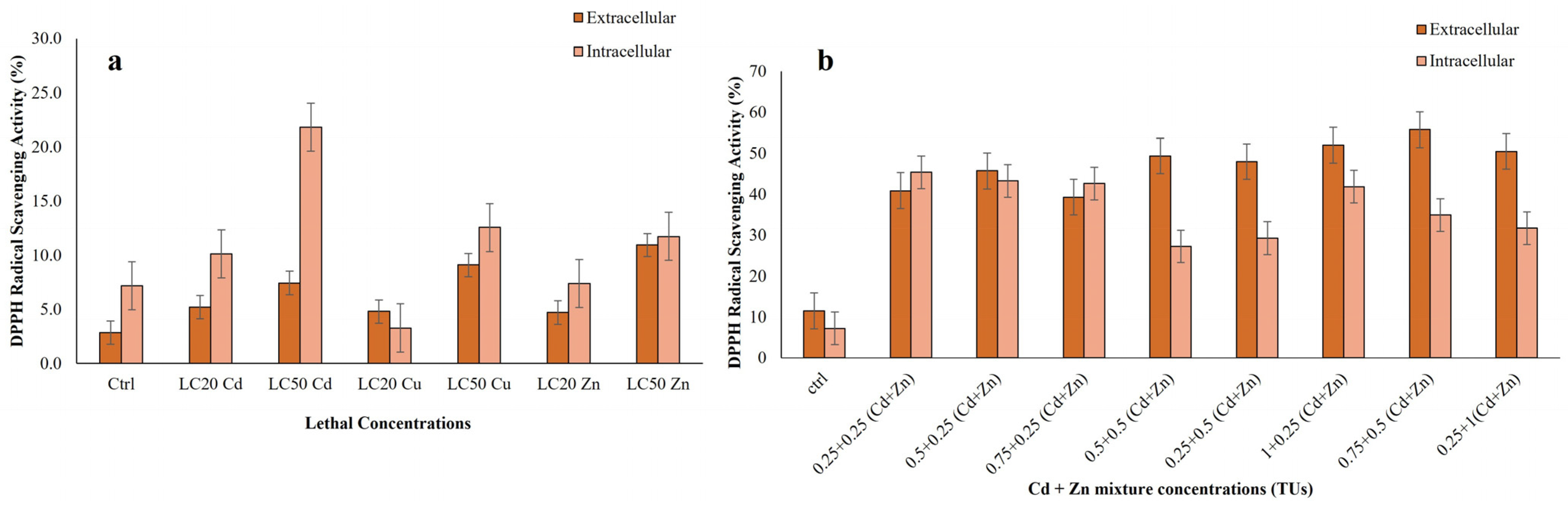
3.3.2. DPPH Scavenging Activity from Extracts of R. tetracirrata
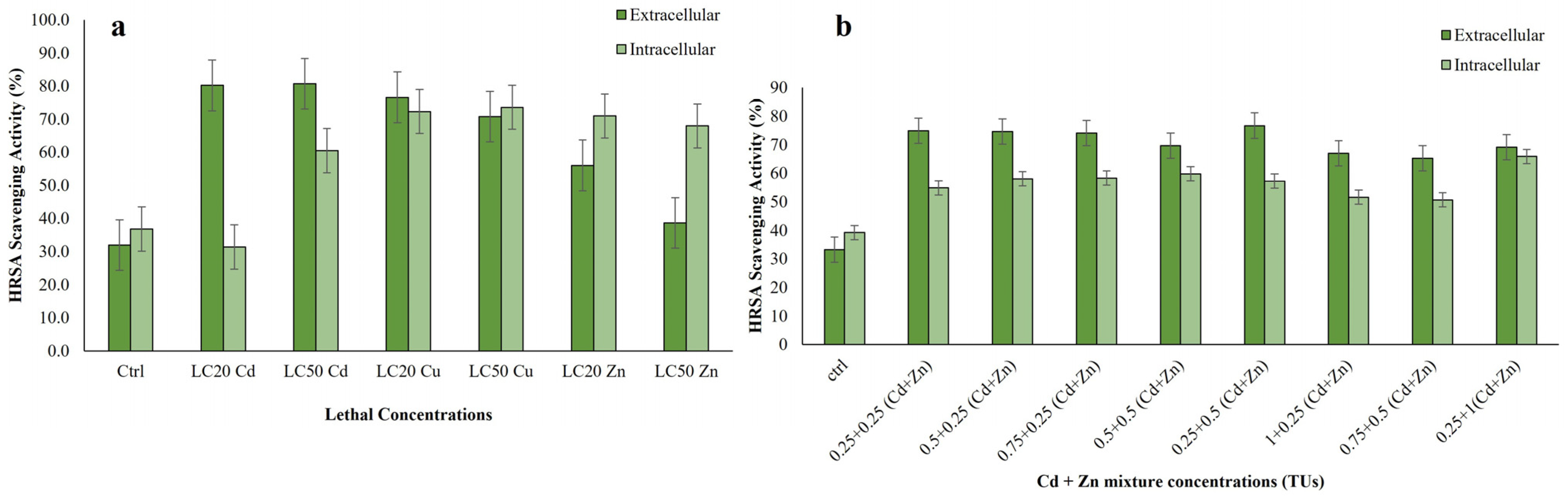
3.3.3. Hydroxyl Radical Scavenging Activity of Extracts of R. tetracirrata
3.4. A Brief Note on the Genus Rigidohymena and Its Potential as a Bioindicator of Soil Health
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Calisi, A.; Cappello, T.; Angelelli, M.; Maisano, M.; Rotondo, D.; Gualandris, D.; Semeraro, T.; Dondero, F. Non-destructive biomarkers in non-target species earthworm Lumbricus terrestris for assessment of different agrochemicals. Environments 2024, 11, 276. [Google Scholar] [CrossRef]
- Sanchez-Hernandez, J.C. Bioremediation of Agricultural Soils; CRC Press: Boca Raton, FL, USA, 2019; Volume 244. [Google Scholar]
- Järup, L. Hazards of heavy metal contamination. Br. Med. Bull. 2003, 68, 167–182. [Google Scholar] [CrossRef]
- Wuana, R.A.; Okieimen, F.E. Heavy metals in contaminated soils: A review of sources, chemistry, risks and best available strategies for remediation. Int. Sch. Res. Not. 2011, 2011, 402647. [Google Scholar] [CrossRef]
- Calisi, A.; Semeraro, T.; Giordano, M.E.; Dondero, F.; Lionetto, M.G. Earthworms multi-biomarker approach for ecotoxicological assessment of soils irrigated with reused treated wastewater. Appl. Soil Ecol. 2025, 206, 105866. [Google Scholar] [CrossRef]
- Friedlova, M. The influence of heavy metals on soil biological and chemical properties. Soil Water Res. 2010, 5, 21–27. [Google Scholar] [CrossRef]
- Bååth, E. Effects of heavy metals in soil on microbial processes and populations (a review). Water Air Soil Pollut. 1989, 47, 335–379. [Google Scholar] [CrossRef]
- Giller, K.E.; Witter, E.; Mcgrath, S.P. Toxicity of heavy metals to microorganisms and microbial processes in agricultural soils: A review. Soil Biol. Biochem. 1998, 30, 1389–1414. [Google Scholar] [CrossRef]
- Chang, L.W. Toxicology of Metals, Volume I; CRC press: Boca Raton, FL, USA, 2023. [Google Scholar]
- Xin, Z.; Zang, W.; Yan, Z.; Hong, Y.; Liu, Z.; Yi, X.; Wang, X.; Liu, T.; Zhou, L. Species sensitivity analysis of heavy metals to freshwater organisms. Ecotoxicology 2015, 24, 1621–1631. [Google Scholar] [CrossRef] [PubMed]
- Schuler, M.S.; Relyea, R.A. A review of the combined threats of road salts and heavy metals to freshwater systems. BioScience 2018, 68, 327–335. [Google Scholar] [CrossRef]
- Vilas–Boas, J.A.; Cardoso, S.J.; Senra, M.V.X.; Rico, A.; Dias, R.J.P. Ciliates as model organisms for the ecotoxicological risk assessment of heavy metals: A meta–analysis. Ecotoxicol. Environ. Saf. 2020, 199, 110669. [Google Scholar] [CrossRef]
- Fountain, M.T.; Hopkin, S.P. Folsomia candida (Collembola): A “standard” soil arthropod. Annu. Rev. Entomol. 2005, 50, 201–222. [Google Scholar] [CrossRef]
- Filser, J.; Wiegmann, S.; Schröder, B. Collembola in ecotoxicology—Any news or just boring routine? Appl. Soil Ecol. 2014, 83, 193–199. [Google Scholar] [CrossRef]
- Triebskorn, R.; Weeks, J. Biomarkers in terrestrial invertebrates for ecotoxicological soil risk assessment. Rev. Environ. Contam. Toxicol. 2000, 164, 93–147. [Google Scholar]
- Dallinger, R.; Berger, B.; Triebskorn-Köhler, R.; Köhler, H. Soil Biology and Ecotoxicology. In The Biology of Terrestrial Molluscs; CABI Wallingford: Oxfordshire, UK, 2001; pp. 489–525. [Google Scholar]
- Bharti, D.; Kumar, S.; La Terza, A. Rigidosticha italiensis n. gen., n. sp.(Ciliophora, Spirotricha), a novel large hypotrich ciliate from the soil of Lombardia, Italy. Eur. J. Protistol. 2016, 56, 112–118. [Google Scholar] [CrossRef]
- Kumar, S.; Kamra, K.; Bharti, D.; La Terza, A.; Sehgal, N.; Warren, A.; Sapra, G.R. Morphology, morphogenesis, and molecular phylogeny of Sterkiella tetracirrata n. sp. (Ciliophora, Oxytrichidae), from the Silent Valley National Park, India. Eur. J. Protistol. 2015, 51, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Warren, A.; Patterson, D.J.; Dunthorn, M.; Clamp, J.C.; Achilles-Day, U.E.M.; Aescht, E.; Al-Farraj, S.A.; Al-Quraishy, S.; Al-Rasheid, K.; Carr, M.; et al. Beyond the “Code”: A guide to the description and documentation of biodiversity in ciliated protists (Alveolata, Ciliophora). J. Eukaryot. Microbiol. 2017, 64, 539–554. [Google Scholar] [CrossRef]
- Fenchel, T. Ecology of Protozoa: The Biology of Free-Living Phagotropic Protists; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Jiang, J.-G.; Wu, S.-G.; Shen, Y.-F. Effects of seasonal succession and water pollution on the protozoan community structure in an eutrophic lake. Chemosphere 2007, 66, 523–532. [Google Scholar] [CrossRef]
- Weisse, T. Functional diversity of aquatic ciliates. Eur. J. Protistol. 2017, 61, 331–358. [Google Scholar] [CrossRef] [PubMed]
- Geisen, S.; Mitchell, E.A.; Wilkinson, D.M.; Adl, S.; Bonkowski, M.; Brown, M.W.; Fiore-Donno, A.M.; Heger, T.J.; Jassey, V.E.; Krashevska, V.; et al. Soil protistology rebooted: 30 fundamental questions to start with. Soil Biol. Biochem. 2017, 111, 94–103. [Google Scholar] [CrossRef]
- Bonkowski, M.; Clarholm, M. Stimulation of plant growth through interactions of bacteria and protozoa: Testing the auxiliary microbial loop hypothesis. Acta Protozool. 2012, 51, 237–247. [Google Scholar]
- Gualandris, D.; Rotondo, D.; Lorusso, C.; La Terza, A.; Calisi, A.; Dondero, F. The Metallothionein System in Tetrahymena thermophila Is Iron-Inducible. Toxics 2024, 12, 725. [Google Scholar] [CrossRef]
- Fulgentini, L.; Passini, V.; Colombetti, G.; Miceli, C.; La Terza, A.; Marangoni, R. UV radiation and visible light induce hsp70 gene expression in the antarctic psychrophilic ciliate euplotes focardii. Microb. Ecol. 2015, 70, 372–379. [Google Scholar] [CrossRef]
- La Terza, A.; Barchetta, S.; Buonanno, F.; Ballarini, P.; Miceli, C. The protozoan ciliate Tetrahymena thermophila as biosensor of sublethal levels of toxicants in the soil. Fresenius Environ. Bull. 2008, 17, 1144–1150. [Google Scholar]
- Bharti, D.; Kumar, S.; Basuri, C.K.; La Terza, A. Ciliated Protist Communities in Soil: Contrasting Patterns in Natural Sites and Arable Lands across Italy. Soil Syst. 2024, 8, 64. [Google Scholar] [CrossRef]
- Delmonte Corrado, M.; Trielli, F.; Amaroli, A.; Ognibene, M.; Falugi, C. Protists as tools for environmental biomonitoring: Importance of cholinesterase enzyme activities. Water Pollut. New Res. 2005, 1, 181–200. [Google Scholar]
- Gilron, G.L.; Lynn, D.H. Ciliated Protozoa as Test Organisms in Toxicity Assessments. In Microscale Testing in Aquatic Toxicology; CRC Press: Boca Raton, FL, USA, 2018; pp. 323–336. [Google Scholar]
- Halliwell, B.; Gutteridge, J.M. Oxygen free radicals and iron in relation to biology and medicine: Some problems and concepts. Arch. Biochem. Biophys. 1986, 246, 501–514. [Google Scholar] [CrossRef]
- Liu, C.-B.; Qu, G.-B.; Cao, M.-X.; Liang, Y.; Hu, L.-G.; Shi, J.-B.; Cai, Y.; Jiang, G.-B. Distinct toxicological characteristics and mechanisms of Hg2+ and MeHg in Tetrahymena under low concentration exposure. Aquat. Toxicol. 2017, 193, 152–159. [Google Scholar] [CrossRef]
- Varatharajan, G.R.; Calisi, A.; Kumar, S.; Bharti, D.; Dondero, F.; La Terza, A. Cytotoxicity and Antioxidant Defences in Euplotes aediculatus Exposed to Single and Binary Mixtures of Heavy Metals and Nanoparticles. Appl. Sci. 2024, 14, 5058. [Google Scholar] [CrossRef]
- Bharti, D.; Kumar, S.; La Terza, A. Description and molecular phylogeny of a novel hypotrich ciliate from the soil of Marche Region, Italy; including notes on the MOSYSS project. J. Eukaryot. Microbiol. 2017, 64, 678–690. [Google Scholar] [CrossRef] [PubMed]
- Chapman-Andresen, C. Pinocytosis of inorganic salts by Amoeba proteus (Chaos diffluens). Comptes rendus des travaux du Laboratoire Carlsberg. Ser. Chim. 1958, 31, 77–92. [Google Scholar]
- Dangeard, P. Notice sur la vie et les Travaux de Camille Sauvageau (1861–1936). Bull. La Société Bot. Fr. 1937, 84, 13–18. [Google Scholar] [CrossRef][Green Version]
- Strober, W. Trypan blue exclusion test of cell viability. Curr. Protoc. Immunol. 2015, 111, A3. B. 1–A3. B. 3. [Google Scholar] [CrossRef]
- Gallego, A.; Martín-González, A.; Ortega, R.; Gutiérrez, J.C. Flow cytometry assessment of cytotoxicity and reactive oxygen species generation by single and binary mixtures of cadmium, zinc and copper on populations of the ciliated protozoan Tetrahymena thermophila. Chemosphere 2007, 68, 647–661. [Google Scholar] [CrossRef]
- Sprague, J.B. Measurement of pollutant toxicity to fish. II. Utilizing and applying bioassay results. Water Res. 1970, 4, 3–32. [Google Scholar] [CrossRef]
- Ravindran, C.; Varatharajan, G.R.; Rajasabapathy, R.; Vijayakanth, S.; Kumar, A.H.; Meena, R.M. A role for antioxidants in acclimation of marine derived pathogenic fungus (NIOCCá1) to salt stress. Microb. Pathog. 2012, 53, 168–179. [Google Scholar] [CrossRef] [PubMed]
- Vattem, D.A.; Shetty, K. Solid-state production of phenolic antioxidants from cranberry pomace by Rhizopus oligosporus. Food Biotechnol. 2002, 16, 189–210. [Google Scholar] [CrossRef]
- Yıldırım, A.; Mavi, A.; Kara, A.A. Determination of antioxidant and antimicrobial activities of Rumex crispus L. extracts. J. Agric. Food Chem. 2001, 49, 4083–4089. [Google Scholar] [CrossRef] [PubMed]
- Kunchandy, E.; Rao, M. Oxygen radical scavenging activity of curcumin. Int. J. Pharm. 1990, 58, 237–240. [Google Scholar] [CrossRef]
- Berger, H. Monograph of the Gonostomatidae and Kahliellidae (Ciliophora, Hypotricha); Springer Science & Business Media: Berlin/Heidelberg, Germany, 2011; Volume 90. [Google Scholar]
- Gellért, J. Életegyüttes a fakéreg zöldporos bevonatában (Lebensgemeinschaft in dem grünpulverigen Überzug der Baumrinde). In Acta Scientiarum Mathematicarum et Naturalium; Universitas Francisco-Josephina (Nagy J. és Fia Könyvnyomdája): Bogotá, Colombia, 1942; Volume 8. [Google Scholar]
- Dragesco, J.; Njiné, T. Compléments à la connaissance des ciliés libres du Cameroun. Ann. Fac. Sci. Cameroun 1971, 7, 79–140. [Google Scholar]
- Kahl, A. Urtiere oder protozoa. I. Wimpertiere oder ciliata (Infusoria). 3. Spirotricha. Tierwelt Angrenzenden Meeresteile 1932, 25, 399–650. [Google Scholar]
- Berger, H. Monograph of the Oxytrichidae (Ciliophora, Hypotrichia); Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012; Volume 78. [Google Scholar]
- Kumar, S.; Foissner, W. Biogeographic specializations of two large hypotrich ciliates: Australocirrus shii and A. australis and proposed synonymy of Australocirrus and Cyrtohymenides. Eur. J. Protistol. 2015, 51, 210–228. [Google Scholar] [CrossRef]
- Berger, H.; Foissner, W. Cladistic relationships and generic characterization of oxytrichid hypotrichs (Protozoa, Ciliophora). Arch. Für Protistenkd. 1997, 148, 125–155. [Google Scholar] [CrossRef]
- Abraham, J.S.; Sripoorna, S.; Choudhary, A.; Toteja, R.; Gupta, R.; Makhija, S.; Warren, A. Assessment of heavy metal toxicity in four species of freshwater ciliates (Spirotrichea: Ciliophora) from Delhi, India. Curr. Sci. 2017, 113, 2141–2150. [Google Scholar] [CrossRef]
- Somasundaram, S.; Abraham, J.S.; Maurya, S.; Sood, U.; Lal, R.; Makhija, S.; Toteja, R. Computational Insights into Antioxidant Enzymes and Metalloproteins of Tetmemena sp. SeJ-2015 (Spirotrichea; Ciliophora) in Combating Heavy Metal Stress. Indian J. Microbiol. 2025, 65, 1263–1277. [Google Scholar] [CrossRef]
- Foissner, W. Morphologie und Infraciliatur einiger neuer und wenig bekannter terrestrischer und limnischer Ciliaten (Protozoa, Ciliophora). Sber. Akad. Wiss. Wien 1989, 196, 173–247. [Google Scholar]
- Song, W. Morphogenesis of Cyrtohymena tetracirrata (Ciliophora, Hypotrichia, Oxytrichidae) during binary fission. Eur. J. Protistol. 2004, 40, 245–254. [Google Scholar] [CrossRef]
- Lorusso, C.; Calisi, A.; Sanchez-Hernandez, J.C.; Varodi, C.; Pogăcean, F.; Pruneanu, S.; Dondero, F. Carbon nanomaterial functionalization with pesticide-detoxifying carboxylesterase. Chemosphere 2022, 309, 136594. [Google Scholar] [CrossRef] [PubMed]
- Kong, R.; Sun, Q.; Cheng, S.; Fu, J.; Liu, W.; Letcher, R.J.; Liu, C. Uptake, excretion and toxicity of titanate nanotubes in three stains of free-living ciliates of the genus Tetrahymena. Aquat. Toxicol. 2021, 233, 105790. [Google Scholar] [CrossRef] [PubMed]
- Díaz, S.; Martín-González, A.; Gutiérrez, J.C. Evaluation of heavy metal acute toxicity and bioaccumulation in soil ciliated protozoa. Environ. Int. 2006, 32, 711–717. [Google Scholar] [CrossRef]
- Luu, H.T.; Esteban, G.F.; Butt, A.A.; Green, I.D. Effects of copper and the insecticide cypermethrin on a soil ciliate (protozoa: Ciliophora) community. Protist 2022, 173, 125855. [Google Scholar] [CrossRef] [PubMed]
- Madoni, P.; Romeo, M.G. Acute toxicity of heavy metals towards freshwater ciliated protists. Environ. Pollut. 2006, 141, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Martín-González, A.; Díaz, S.; Borniquel, S.; Gallego, A.; Gutiérrez, J.C. Cytotoxicity and bioaccumulation of heavy metals by ciliated protozoa isolated from urban wastewater treatment plants. Res. Microbiol. 2006, 157, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Echavez, F.L.C.; Leal, J.C.M. Ecotoxicological effect of heavy metals in free-living ciliate protozoa of Lake Maracaibo, Venezuela. J. Water Land Dev. 2021, 51, 102–116. [Google Scholar] [CrossRef]
- Marín-Leal, J.C.; Rincón-Miquilena, N.J.; Díaz-Borrego, L.C.; Pire-Sierra, M.C. Acute toxicity of potentially toxic elements on ciliated protozoa from Lake Maracaibo (Venezuela). Acta Limnol. Bras. 2022, 34, e21. [Google Scholar] [CrossRef]
- Fargašová, A. Winter third-to fourth-instar larvae of Chironomus plumosus as bioassay tools for assessment of acute toxicity of metals and their binary combinations. Ecotoxicol. Environ. Saf. 2001, 48, 1–5. [Google Scholar] [CrossRef]
- Ince, N.; Dirilgen, N.; Apikyan, I.; Tezcanli, G.; Üstün, B. Assessment of toxic interactions of heavy metals in binary mixtures: A statistical approach. Arch. Environ. Contam. Toxicol. 1999, 36, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Negilski, D.; Ahsanullah, M.; Mobley, M. Toxicity of zinc, cadmium and copper to the shrimp Callianassa australiensis. II. Effects of paired and triad combinations of metals. Mar. Biol. 1981, 64, 305–309. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, M.; Xu, J.; Wang, Y. Reciprocal effect of Cu, Cd, Zn on a kind of marine alga. Water Res. 1995, 29, 209–214. [Google Scholar] [CrossRef]
- Otitoloju, A.A. Evaluation of the joint-action toxicity of binary mixtures of heavy metals against the mangrove periwinkle Tympanotonus fuscatus var radula (L.). Ecotoxicol. Environ. Saf. 2002, 53, 404–415. [Google Scholar] [CrossRef]
- Mehta, R.; Templeton, D.M.; O’Brien, P.J. Mitochondrial involvement in genetically determined transition metal toxicity: II. Copper toxicity. Chem.-Biol. Interact. 2006, 163, 77–85. [Google Scholar] [CrossRef]
- Norwood, W.; Borgmann, U.; Dixon, D.; Wallace, A. Effects of metal mixtures on aquatic biota: A review of observations and methods. Hum. Ecol. Risk Assess. 2003, 9, 795–811. [Google Scholar] [CrossRef]
- Hajdú, Z.; Hohmann, J.; Forgo, P.; Martinek, T.; Dervarics, M.; Zupkó, I.; Falkay, G.; Cossuta, D.; Máthé, I. Diterpenoids and flavonoids from the fruits of Vitex agnus-castus and antioxidant activity of the fruit extracts and their constituents. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2007, 21, 391–394. [Google Scholar] [CrossRef]
- Yıldırım, A.; Mavi, A.; Oktay, M.; Kara, A.A.; Algur, Ö.F.; Bilaloǧlu, V. Comparison of antioxidant and antimicrobial activities of Tilia (Tilia argentea Desf ex DC), sage (Salvia triloba L.), and Black tea (Camellia sinensis) extracts. J. Agric. Food Chem. 2000, 48, 5030–5034. [Google Scholar] [CrossRef]
- Uddin, G.; Rauf, A.; Arfan, M.; Ali, M.; Qaisar, M.; Saadiq, M.; Atif, M. Preliminary phytochemical screening and antioxidant activity of Bergenia caliata. Middle-East J. Sci. Res. 2012, 11, 1140–1142. [Google Scholar]
- Horta, A.; Pinteus, S.; Alves, C.; Fino, N.; Silva, J.; Fernandez, S.; Rodrigues, A.; Pedrosa, R. Antioxidant and antimicrobial potential of the Bifurcaria bifurcata epiphytic bacteria. Mar. Drugs 2014, 12, 1676–1689. [Google Scholar] [CrossRef]
- Halliwell, B. Reactive oxygen species in living systems: Source, biochemistry, and role in human disease. Am. J. Med. 1991, 91, S14–S22. [Google Scholar] [CrossRef]
- Sharma, P.; Ravikumar, G.; Kalaiselvi, M.; Gomathi, D.; Uma, C. In vitro antibacterial and free radical scavenging activity of green hull of Juglans regia. J. Pharm. Anal. 2013, 3, 298–302. [Google Scholar] [CrossRef]
- Boldrin, F.; Santovito, G.; Gaertig, J.; Wloga, D.; Cassidy-Hanley, D.; Clark, T.G.; Piccinni, E. Metallothionein gene from Tetrahymena thermophila with a copper-inducible-repressible promoter. Eukaryot. Cell 2006, 5, 422–425. [Google Scholar] [CrossRef]
- Santovito, G.; Formigari, A.; Boldrin, F.; Piccinni, E. Molecular and functional evolution of Tetrahymena metallothioneins: New insights into the gene family of Tetrahymena thermophila. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2007, 144, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Ferro, D.; Bakiu, R.; De Pittà, C.; Boldrin, F.; Cattalini, F.; Pucciarelli, S.; Miceli, C.; Santovito, G. Cu, Zn superoxide dismutases from Tetrahymena thermophila: Molecular evolution and gene expression of the first line of antioxidant defenses. Protist 2015, 166, 131–145. [Google Scholar] [CrossRef] [PubMed]
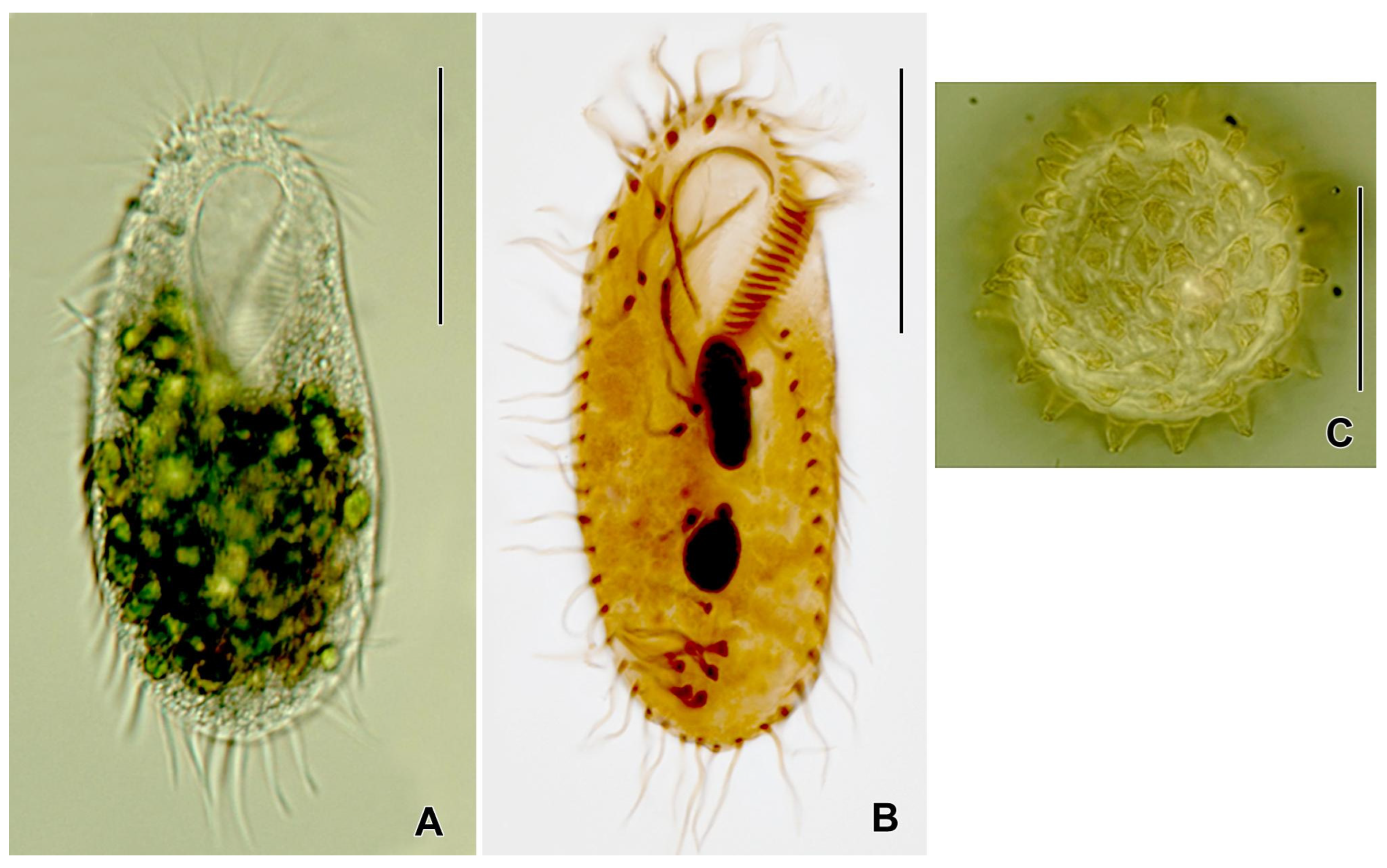

| S. No: | HMs | Parameter | Estimate (±SE) | 95% CI | R2 |
|---|---|---|---|---|---|
| 1 | Cu | LC20 | 0.16 ± 0.03 | 0.11–0.23 | 0.974 |
| LC50 | 0.25 ± 0.04 | 0.18–0.38 | |||
| 2 | Zn | LC20 | 19.86 ± 3.25 | 14.32–27.54 | 0.961 |
| LC50 | 44.12 ± 6.18 | 32.18–58.66 | |||
| 3 | Cd | LC20 | 0.68 ± 0.09 | 0.52–0.89 | 0.985 |
| LC50 | 1.12 ± 0.12 | 0.85–1.48 |
| Cd + Zn Total TU a | Concentrations (TU) a for Each Metal | Obtained Cytotoxicity b | Expected Cytotoxicity b | Interaction Type | |
|---|---|---|---|---|---|
| Cd | Zn | ||||
| 0.5 | 0.5 | 0 | 12.77 ± 2.54 | 18 ± 0.04 | Not significant different |
| 0.25 | 0.25 | 6.07 ± 2.54 | 13 ± 0.59 | Antagonism | |
| 0 | 0.5 | 3.87 ± 0.98 | 4 ± 1.17 | Not significant different | |
| 0.75 | 0.75 | 0 | 29.4 ± 3.48 | 30 ± 0.06 | Not significant different |
| 0.5 | 0.25 | 4.97 ± 1.65 | 20 ± 0.59 | Antagonism | |
| 0.25 | 0.5 | 18.83 ± 2.54 | 15 ± 1.17 | Not significant different | |
| 0 | 0.75 | 16.63 ± 1.65 | 18 ± 1.76 | Not significant different | |
| 1 | 1 | 0 | 49.97 ± 1.65 | 50 ± 0.08 | Not significant different |
| 0.75 | 0.25 | 22.2 ± 2.55 | 32 ± 0.59 | Antagonism | |
| 0.5 | 0.5 | 3.87 ± 0.98 | 22 ± 0.59 | Antagonism | |
| 0.25 | 0.75 | 8.87 ± 0.98 | 29 ± 1.76 | Antagonism | |
| 0 | 1 | 49.43 ± 0.98 | 50 ± 2.34 | Not significant different | |
| 1.25 | 1.25 | 0 | 61.07 ± 2.54 | 68 ± 0.1 | Not significant different |
| 1 | 0.25 | 33.3 ± 1.7 | 52 ± 0.59 | Antagonism | |
| 0.75 | 0.5 | 45.53 ± 3.87 | 34 ± 1.17 | Synergism | |
| 0.5 | 0.75 | 32.77 ± 2.54 | 36 ± 1.76 | Not significant different | |
| 0.25 | 1 | 14.97 ± 1.65 | 61 ± 2.34 | Antagonism | |
| 0 | 1.25 | 77.77 ± 2.54 | 82 ± 2.93 | Not significant different | |
| Cu + Zn Total TU a | Concentrations (TU) a for Each Metal | Obtained Cytotoxicity b | Expected Cytotoxicity b | Interaction Type | |
|---|---|---|---|---|---|
| Cu | Zn | ||||
| 0.5 | 0.5 | 0 | 12.17 ± 1.19 | 11 ± 0.04 | Not significant different |
| 0.25 | 0.25 | 4.43 ± 2.54 | 4 ± 0.59 | Antagonism | |
| 0 | 0.5 | 6.07 ± 0.98 | 4 ± 1.17 | Not significant different | |
| 0.75 | 0.75 | 0 | 28.30 ± 3.44 | 30 ± 0.04 | Not significant different |
| 0.5 | 0.25 | 6.07 ± 7.86 | 13 ± 0.59 | Antagonism | |
| 0.25 | 0.5 | 9.97 ± 0.92 | 6 ± 1.17 | Synergism | |
| 0 | 0.75 | 22.20 ± 2.54 | 18 ± 1.76 | Not significant different | |
| 1 | 1 | 0 | 48.83 ± 3.48 | 50 ± 0.05 | Not significant different |
| 0.75 | 0.25 | 34.93 ± 1.65 | 32 ± 0.59 | Not significant different | |
| 0.5 | 0.5 | 11.63 ± 2.54 | 15 ± 0.59 | Not significant different | |
| 0.25 | 0.75 | 23.87 ± 2.55 | 20 ± 1.76 | Synergism | |
| 0 | 1 | 52.20 ± 2.54 | 50 ± 2.34 | Not significant different | |
| 1.25 | 1.25 | 0 | 69.40 ± 2.55 | 71 ± 0.06 | Not significant different |
| 1 | 0.25 | 31.63 ± 3.48 | 52 ± 0.59 | Antagonism | |
| 0.75 | 0.5 | 28.87 ± 3.81 | 34 ± 1.17 | Not significant different | |
| 0.5 | 0.75 | 32.77 ± 2.54 | 29 ± 1.76 | Not significant different | |
| 0.25 | 1 | 72.77 ± 1.65 | 52 ± 2.34 | Synergism | |
| 0 | 1.25 | 80.53 ± 1.96 | 82 ± 2.93 | Not significant different | |
| Cd + Cu Total TU a | Concentrations (TU) a for Each Metal | Obtained Cytotoxicity b | Expected Cytotoxicity b | Interaction Type | |
|---|---|---|---|---|---|
| Cd | Cu | ||||
| 0.5 | 0.5 | 0 | 16.30 ± 1.91 | 18 ± 0.04 | Not significant different |
| 0.25 | 0.25 | 10.53 ± 2.54 | 13 ± 0.02 | Not significant different | |
| 0 | 0.5 | 13.87 ± 0.98 | 11 ± 0.03 | Not significant different | |
| 0.75 | 0.75 | 0 | 28.87 ± 3.44 | 30 ± 0.06 | Not significant different |
| 0.5 | 0.25 | 13.87 ± 7.86 | 20 ± 0.04 | Antagonism | |
| 0.25 | 0.5 | 20.53 ± 0.92 | 22 ± 0.03 | Not significant different | |
| 0 | 0.75 | 32.77 ± 2.54 | 30 ± 0.04 | Not significant different | |
| 1 | 1 | 0 | 49.40 ± 3.48 | 50 ± 0.08 | Not significant different |
| 0.75 | 0.25 | 34.97 ± 1.65 | 32 ± 0.06 | Synergism | |
| 0.5 | 0.5 | 38.83 ± 2.54 | 29 ± 0.05 | Synergism | |
| 0.25 | 0.75 | 44.40 ± 2.55 | 41 ± 0.04 | Synergism | |
| 0 | 1 | 48.83 ± 2.54 | 50 ± 0.05 | Not significant different | |
| 1.25 | 1.25 | 0 | 72.70 ± 2.55 | 68 ± 0.10 | Not significant different |
| 1 | 0.25 | 55.50 ± 3.54 | 52 ± 0.08 | Not significant different | |
| 0.75 | 0.5 | 34.40 ± 3.81 | 41 ± 0.07 | Antagonism | |
| 0.5 | 0.75 | 45.53 ± 2.54 | 48 ± 0.05 | Not significant different | |
| 0.25 | 1 | 84.97 ± 1.65 | 61 ± 0.05 | Synergism | |
| 0 | 1.25 | 73.87 ± 1.96 | 71 ± 0.06 | Not significant different | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varatharajan, G.R.; Calisi, A.; Kumar, S.; Bharti, D.; Ghosh, A.; Singh, S.; Kharkwal, A.C.; Coletta, M.; Dondero, F.; La Terza, A. Heavy Metals Affect the Antioxidant Defences in the Soil Ciliate Rigidohymena tetracirrata. J. Xenobiot. 2025, 15, 169. https://doi.org/10.3390/jox15050169
Varatharajan GR, Calisi A, Kumar S, Bharti D, Ghosh A, Singh S, Kharkwal AC, Coletta M, Dondero F, La Terza A. Heavy Metals Affect the Antioxidant Defences in the Soil Ciliate Rigidohymena tetracirrata. Journal of Xenobiotics. 2025; 15(5):169. https://doi.org/10.3390/jox15050169
Chicago/Turabian StyleVaratharajan, Govindhasamay R., Antonio Calisi, Santosh Kumar, Daizy Bharti, Arnab Ghosh, Shikha Singh, Amit C. Kharkwal, Martina Coletta, Francesco Dondero, and Antonietta La Terza. 2025. "Heavy Metals Affect the Antioxidant Defences in the Soil Ciliate Rigidohymena tetracirrata" Journal of Xenobiotics 15, no. 5: 169. https://doi.org/10.3390/jox15050169
APA StyleVaratharajan, G. R., Calisi, A., Kumar, S., Bharti, D., Ghosh, A., Singh, S., Kharkwal, A. C., Coletta, M., Dondero, F., & La Terza, A. (2025). Heavy Metals Affect the Antioxidant Defences in the Soil Ciliate Rigidohymena tetracirrata. Journal of Xenobiotics, 15(5), 169. https://doi.org/10.3390/jox15050169











