An Initial Survey on Occurrence, Fate, and Environmental Risk Assessment of Organophosphate Flame Retardants in Romanian Waterways
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Sampling Design
2.3. Sample Collection
2.4. Sample Preparation
2.5. Instrumental Analysis
2.6. Quality Assurance and Quality Control
2.7. Results Evaluation
2.8. Statistical Analysis
3. Results and Discussion
3.1. Occurrence of OPFRs in WWTPs
3.1.1. Occurrence and Concentration of OPFRs in Wastewater
3.1.2. Removal Efficiencies of OPFRs in the Five WWTPs
3.1.3. Daily Mass Loading and Mass Emission
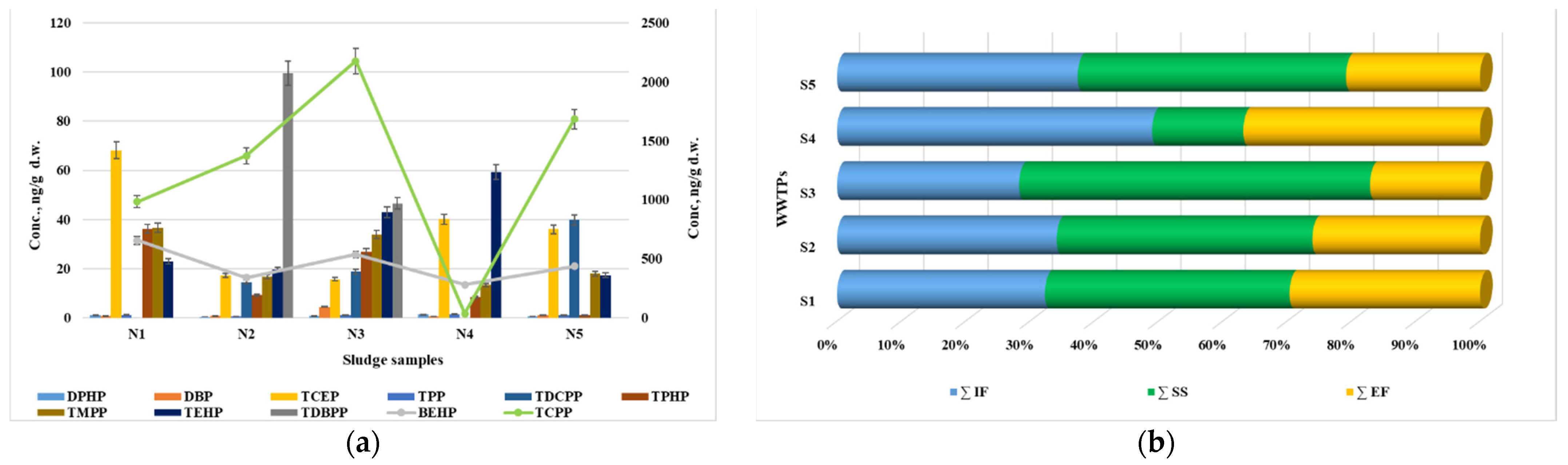
3.1.4. Occurrence and Concentration of OPFRs in Sewage Sludge
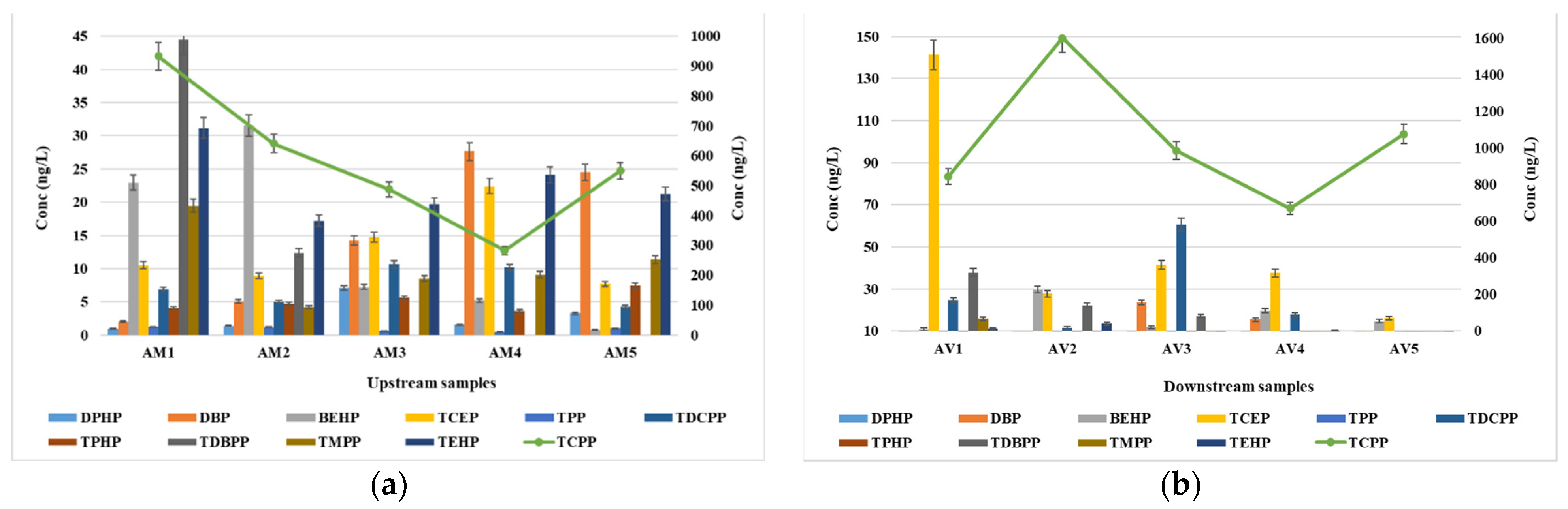
3.2. Occurrence of OPFRs in Surface Waters
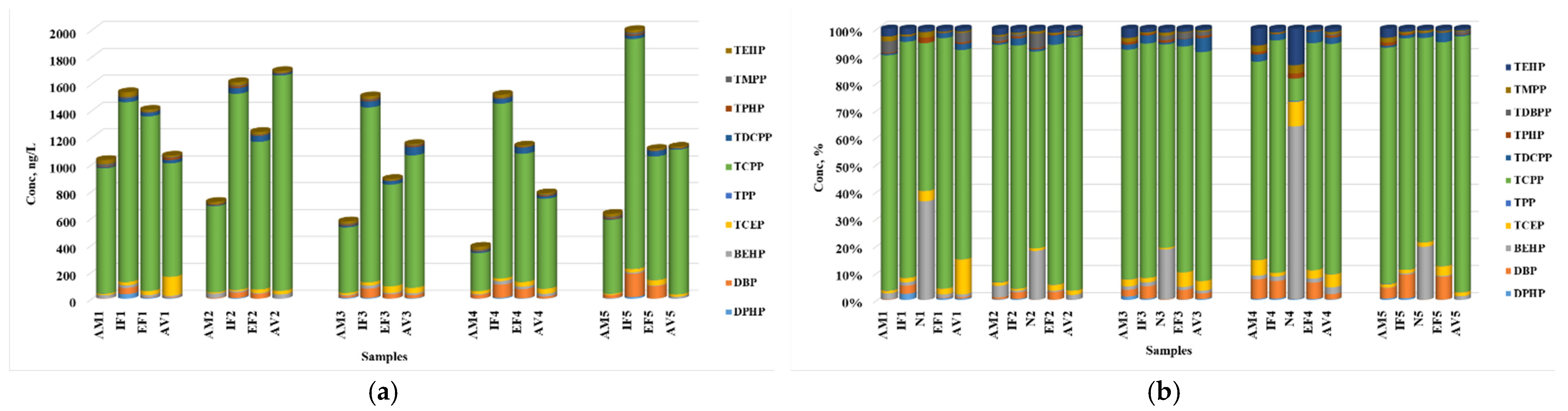
3.3. Transfer of OPFRs from WWTPs to the Aquatic Environment
3.4. Environmental Risk Assessment
4. Possible Mitigation or Control Strategies of OPFR Contamination
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, B.; Lin, H.; Bartlett, S.L.; Houghton, E.M.; Robertson, D.M.; Guo, L.D. Partitioning and transformation of organic and inorganic phosphorus among dissolved, colloidal and particulate phases in a hypereutrophic freshwater estuary. Water Res. 2021, 196, 117025. [Google Scholar] [CrossRef] [PubMed]
- Withers, P.J.A.; van Dijk, K.C.; Neset, T.S.S.; Nesme, T.; Oenema, O.; Rubaek, G.H.; Schoumans, O.F.; Smit, B.; Pellerin, S. Stewardship to tackle global phosphorus inefficiency: The case of Europe. Ambio 2015, 44, S193–S206. [Google Scholar] [CrossRef] [PubMed]
- Hao, C.; Helm, P.A.; Morse, D.; Reiner, E.J. Liquid chromatography-tandem mass spectrometry direct injection analysis of organophosphorus flame retardants in Ontario surface water and wastewater effluent. Chemosphere 2018, 191, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.B.; Shen, Z.Z.; Gao, J.X.; Qiu, Y.Q.; Li, J.; Wang, Z.Y.; Lyu, J. Adsorption-regeneration process for removing dimethoate and recovering phosphorus with three-dimensional hierarchically porous carbon. J. Environ. Chem. Eng. 2022, 10, 107716. [Google Scholar] [CrossRef]
- Kim, K.; Mun, H.; Shin, H.; Park, S.; Yu, C.; Lee, J.; Yoon, Y.; Chung, H.; Yun, H.; Lee, K.; et al. Nitrogen Stimulates Microcystis-Dominated Blooms More than Phosphorus in River Conditions That Favor Non-Nitrogen-Fixing Genera. Environ. Sci. Technol. 2020, 54, 7185–7193. [Google Scholar] [CrossRef] [PubMed]
- Flame Retardant Chemicals: Technologies and Global Markets. Available online: https://www.bccresearch.com/market-research/chemicals/flame-retardant-chemicals-markets-report.html (accessed on 2 October 2023).
- Van der Veen, I.; de Boer, J. Phosphorus flame retardants: Properties, production, environmental occurrence, toxicity and analysis. Chemosphere 2012, 88, 1119–1153. [Google Scholar] [CrossRef] [PubMed]
- Egloff, C.; Crump, D.; Porter, E.; Williams, K.L.; Letcher, R.J.; Gauthier, L.T.; Kennedy, S.W. Tris(2-butoxyethyl) phosphate and triethyl phosphate alter embryonic development, hepatic mRNA expression, thyroid hormone levels, and circulating bile acid concentrations in chicken embryos. Toxicol. Appl. Pharmacol. 2014, 279, 303–310. [Google Scholar] [CrossRef]
- Möller, A.; Sturm, R.; Xie, Z.; Cai, M.; He, J.; Ebinghaus, R. Organophosphorus Flame Retardants and Plasticizers in Airborne Particles over the Northern Pacific and Indian Ocean toward the Polar Regions: Evidence for Global Occurrence. Environ. Sci. Technol. 2012, 46, 3127–3134. [Google Scholar] [CrossRef]
- European Commission. Commission Directive 2014/79/EU. 2014. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32014L0079&from=DE (accessed on 2 October 2023).
- Lassen, C.; Brinch, A.; Corden, C.; de Carlos, A.; Esparrago, J.; Kjølholt, J. Chlorinated Phosphorous-Based Flame Retardants in Children’s Articles Containing Foam; The Danish Environmental Protection Agency: Odense, Denmark, 2016. [Google Scholar]
- European Commission. Commission Directive 2014/79/EU of 20 June 2014 Amending Appendix C of Annex II to Directive 2009/48/EC of the European Parliament and of the Council on the Safety of Toys, as Regards TCEP, TCPP and TDCP. Off. J. Eur. Union 2014, 182, 49–51. Available online: http://data.europa.eu/eli/dir/2014/79/oj (accessed on 23 May 2023).
- US EPA. Petition to Order Testing of the Chlorinated Phosphate Ester Cluster Flame Retardants (TCEP, TCPP AND TDCPP) under Section 4(a) of the Toxic Substances Control Act; U.S. Environmental Protection Agency: Washington, DC, USA, 2017.
- Li, Y.; Yao, C.; Zheng, Q.; Yang, W.; Niu, X.; Zhang, Y.; Lu, G. Occurrence and ecological implications of organophosphate triesters and diester degradation products in wastewater, river water, and tap water. Environ. Pollut. 2020, 259, 113810. [Google Scholar] [CrossRef]
- Wolschke, H.; Sühring, R.; Xie, Z.; Ebinghaus, R. Organophosphorus flame retardants and plasticizers in the aquatic environment: A case study of the Elbe River, Germany. Environ. Pollut. 2015, 206, 488–493. [Google Scholar] [CrossRef] [PubMed]
- Plantak, L.; Sirocic, A.P.; Grcic, I.; Biondic, R. Detection of Organophosphorus Esters (OPEs) in Groundwater. Environ. Sci. Proc. 2023, 25, 47. [Google Scholar] [CrossRef]
- Dou, W.; Zhang, Z.; Huang, W.; Wang, X.; Zhang, R.; Wu, Y.; Sun, A.; Shi, X.; Chen, J. Contaminant occurrence, spatiotemporal variation, and ecological risk of organophosphorus flame retardants (OPFRs) in Hangzhou Bay and east China sea ecosystem. Chemosphere 2022, 303, 135032. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Xiong, S.; Wang, P.; Yang, R.; Pei, Z.; Li, Y.; Zhang, Q.; Jiang, G. Novel brominated and organophosphate flame retardants in the atmosphere of Fildes Peninsula, West Antarctica: Continuous observations from 2011 to 2020. J. Hazard. Mat. 2022, 440, 129776. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xiong, S.; Hao, Y.; Yang, R.; Zhang, Q.; Wania, F.; Jiang, G. Organophosphate esters in Arctic air from 2011 to 2019: Concentrations, temporal trends, and potential sources. J. Hazard. Mat. 2022, 434, 128872. [Google Scholar] [CrossRef]
- Castro, G.; Sørmo, E.; Yu, G.; Sait, S.T.L.; González, S.V.; Arp, H.P.H.; Asimakopoulos, A.G. Analysis, occurrence and removal efficiencies of organophosphate flame retardants (OPFRs) in sludge undergoing anaerobic digestion followed by diverse thermal treatments. Sci. Total Environ. 2023, 870, 161856. [Google Scholar] [CrossRef]
- Mekni, S.; Barhoumi, B.; Touil, S.; Driss, M.R.; Eljarrat, E. Occurrence of Halogenated and Organophosphate Flame Retardants in Sediments and Eels (Anguilla anguilla) From Bizerte Lagoon, Tunisia. Front. Environ. Sci. 2020, 8, 67. [Google Scholar] [CrossRef]
- Tian, Y.X.; Chen, H.Y.; Ma, J.; Liu, Q.Y.; Qu, Y.J.; Zhao, W.H. A critical review on sources and environmental behavior of organophosphorus flame retardants in the soil: Current knowledge and future perspectives. J. Hazard. Mat. 2023, 452, 131161. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, J.; Lin, S.; Ying, Z.; Hu, S.; Wang, Y.; Mo, X. Organophosphate flame retardants in Hang-zhou tap water system: Occurrence, distribution, and exposure risk assessment. Sci. Total Environ. 2022, 849, 157644. [Google Scholar] [CrossRef]
- Lu, Q.O.; Jung, C.C.; Chao, H.R.; Chen, P.S.; Lee, C.W.; Tran, Q.T.P.; Ciou, J.Y.; Chang, W.H. Investigating the associations between organophosphate flame retardants (OPFRs) and fine particles in paired indoor and outdoor air: A probabilistic prediction model for deriving OPFRs in indoor environments. Environ. Int. 2023, 174, 107871. [Google Scholar] [CrossRef]
- Li, P.; Jin, J.; Wang, Y.; Hu, J.; Xu, M.; Sun, Y.; Ma, Y. Concentrations of organophosphorus, polybromobenzene, and polybrominated diphenyl ether flame retardants in human serum, and relationships between concentrations and donor ages. Chemosphere 2017, 171, 654–660. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Isobe, T.; Muto, M.; Tue, N.M.; Katsura, K.; Malarvannan, G.; Sudaryanto, A.; Chang, K.H.; Prudente, M.; Viet, P.H.; et al. Organophosphorus flame retardants (PFRs) in human breast milk from several Asian countries. Chemosphere 2014, 116, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zheng, N.; Sun, S.; Ji, Y.; An, Q.; Li, X.; Li, Z.; Zhang, W. Bioaccumulation of organophosphorus flame retardants in marine organisms in Liaodong Bay and their potential ecological risks based on species sensitivity distribution. Environ. Pollut. 2023, 317, 120812. [Google Scholar] [CrossRef] [PubMed]
- Chigusa, K.; Kanda, K.; Iwata, H. Developmental toxicity in early chicken embryos on exposure to an organophosphorus flame retardant, tris(2-chloroisopropyl) phosphate. Ecotox Environ. Safe 2023, 264, 115445. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Hong, X.; Yan, S.; Zha, J. Three organophosphate flame retardants (OPFRs) reduce sperm quality in Chinese rare minnows (Gobiocypris rarus). Environ. Pollut. 2020, 263 Part A, 114525. [Google Scholar] [CrossRef]
- Rosenmai, A.K.; Winge, S.B.; Möller, M.; Lundqvist, J.; Wedebye, E.B.; Nikolov, N.G.; Johansson, H.K.L.; Vinggaard, A.M. Organophosphate ester flame retardants have antiandrogenic potential and affect other endocrine related endpoints in vitro and in silico. Chemosphere 2021, 263, 127703. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, Y.J.; Zhang, Y.; Li, R.; Chen, W.; Yan, S.C.; Qi, Z.; Chen, Z.F.; Cai, Z. Determination of HFRs and OPFRs in PM2.5 by ultrasonic-assisted extraction combined with multi-segment column purification and GC-MS/MS. Talanta 2019, 194, 320–328. [Google Scholar] [CrossRef]
- Santín, G.; Eljarrat, E.; Barcelo, D. Simultaneous determination of 16 organophosphorus flame retardants and plasticizers in fish by liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2016, 1441, 34–43. [Google Scholar] [CrossRef]
- Kosarac, I.; Kubwabo, C.; Foster, W.G. Quantitative determination of nine urinary metabolites of organophosphate flame retardants using solid phase extraction and ultra performance liquid chromatography coupled to tandem mass spectrometry (UPLC-MS/MS). J. Chromatogr. B 2016, 1041, 24–30. [Google Scholar] [CrossRef]
- Bergh, C.; Torgrip, R.; Ostman, C. Simultaneous selective detection of organophosphate and phthalate esters using gas chromatography with positive ion chemical ionization tandem mass spectrometry and its application to indoor air and dust. Rapid Commun. Mass Spectrom. 2010, 24, 2859–2867. [Google Scholar] [CrossRef]
- Ma, Y.; Hites, R.A. Electron impact, electron capture negative ionization and positive chemical ionization mass spectra of organophosphorus flame retardants and plasticizers. J. Mass Spectrom. 2013, 48, 931–936. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, K.; Garantziotis, S.; Birnbaum, L.S.; Stapleton, H.M. Monitoring indoor exposure to organophosphate flame retardants: Hand wipes and house dust. Environ. Health Perspect. 2015, 123, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Hiltscher, M.; Püttmann, W. Occurrence and human exposure assessment of organophosphate flame retardants in indoor dust from various microenvironments of the Rhine/Main region, Germany. Indoor Air 2017, 27, 1113–1127. [Google Scholar] [CrossRef]
- Yon, T.; Saramito, G.; Lecorgne, A.; Goyat, Q.; Glorennec, P.; Le Bot, B.; Mercier, F. Simultaneous determination of organophosphorus and brominated flame retardants and polychlorinated biphenyls in hair by pressurized liquid extraction and gas chromatography/tandem mass spectrometry. ChemRxiv 2022. [Google Scholar] [CrossRef]
- Han, J.; Tian, J.; Feng, J.; Guo, W.; Dong, S.; Yan, X.; Su, X.; Sun, J. Spatiotemporal distribution and mass loading of organophosphate flame retardants (OPFRs) in the Yellow River of China (Henan segment). Environ. Pollut. 2021, 290, 118000. [Google Scholar] [CrossRef]
- Shi, Y.; Gao, L.; Li, W.; Wang, Y.; Liu, J.; Cai, Y. Occurrence, distribution and seasonal variation of organophosphate flame retardants and plasticizers in urban surface water in Beijing, China. Environ. Pollut. 2016, 209, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Technical Guidance Document on Risk Assessment. Part II. 2003. Available online: https://echa.europa.eu/documents/10162/987906/tgdpart2_2ed_en.pdf/138b7b71-a069-428e-9036-62f4300b752f (accessed on 16 August 2023).
- Schreder, E.D.; La Guardia, M.J. Flame retardant transfers from U.S. households (dust and laundry wastewater) to the aquatic environment. Environ. Sci. Technol. 2014, 48, 11575–11583. [Google Scholar] [CrossRef]
- Marklund, A.; Andersson, B.; Haglund, P. Organophosphorus Flame Retardants and Plasticizers in Swedish Sewage Treatment Plants. Environ. Sci. Technol. 2005, 39, 7423–7429. [Google Scholar] [CrossRef]
- Deng, M.; Kuo, D.T.F.; Wu, Q.; Zhang, Y.; Liu, X.; Liu, S.; Hu, X.; Mai, B.; Liu, Z.; Zhang, H. Organophosphorus flame retardants and heavy metals in municipal landfill leachate treatment system in Guangzhou, China. Environ. Pollut. 2018, 236, 137–145. [Google Scholar] [CrossRef]
- O’Brien, J.W.; Thai, P.K.; Brandsma, S.H.; Leonards, P.E.G.; Ort, C.; Mueller, J.F. Wastewater analysis of census day samples to investigate per capita input of organophosphorus flame retardants and plasticizers into wastewater. Chemosphere 2015, 138, 328–334. [Google Scholar] [CrossRef]
- García-López, M.; Rodríguez, I.; Cela, R. Mixed-mode solid-phase extraction followed by liquid chromatography-tandem mass spectrometry for the determination of tri- and di-substituted organophosphorus species in water samples. J. Chromatogr. A 2010, 1217, 1476–1484. [Google Scholar] [CrossRef] [PubMed]
- Woudneh, M.B.; Benskin, J.P.; Wang, G.; Grace, R.; Hamilton, M.C.; Cosgrove, J.R. Quantitative determination of 13 organophosphorous flame retardants and plasticizers in a wastewater treatment system by high performance liquid chromatography tandem mass spectrometry. J. Chromatogr. A 2015, 1400, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Quintana, J.B.; Reemtsma, T. Potential of membrane-assisted solvent extraction for the determination of phosphoric acid triesters in wastewater samples by liquid chromatography–tandem mass spectrometry. J. Chromatogr. A 2006, 1124, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Carballo, E.; González-Barreiro, C.; Sitka, A.; Scharf, S.; Gans, O. Determination of selected organophosphate esters in the aquatic environment of Austria. Sci. Total Environ. 2007, 388, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Meyera, J.; Bester, K. Organophosphate flame retardants and plasticisers in wastewater treatment plants. J. Environ. Monit. 2004, 6, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Kim, U.J.; Oh, J.K.; Kannan, K. Occurrence, Removal and Environmental Emission of Organophosphate Flame Retardants/Plasticizers in a Wastewater Treatment Plant in New York State, USA. Environ. Sci. Technol. 2017, 51, 7872–7880. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Shi, Y.; Li, W.; Liu, J.; Cai, Y. Occurrence and distribution of organophosphate triesters and diesters in sludge from sewage treatment plants of Beijing, China. Sci. Total Environ. 2016, 544, 143–149. [Google Scholar] [CrossRef]
- Loos, R.; Tavazzi, S.; Mariani, G.; Suurkuusk, G.; Paracchini, B.; Umlauf, G. Analysis of emerging organic contaminants in water, fish and suspended particulate matter (SPM) in the Joint Danube Survey using solid-phase extraction followed by UHPLC-MS-MS and GC–MS analysis. Sci. Total Environ. 2017, 607–608, 1201–1212. [Google Scholar] [CrossRef]
- Teo, T.L.L.; Mcdonald, J.A.; Coleman, H.M.; Khan, S.J. Analysis of organophosphate flame retardants and plasticisers in water by isotope dilution gas chromatography electron ionisation tandem mass spectrometry. Talanta 2015, 143, 114–120. [Google Scholar] [CrossRef]
- Gao, L.; Shi, Y.; Li, W.; Ren, W.; Liu, J.; Cai, Y. Determination of organophosphate esters in water samples by mixed-mode liquid chromatography and tandem mass spectrometry. J. Sep. Sci. 2015, 38, 2193–2200. [Google Scholar] [CrossRef]
- Cristale, J.; García Vázquez, A.; Barata, C.; Lacorte, S. Priority and emerging flame retardants in rivers: Occurrence in water and sediment, Daphnia magna toxicity and risk assessment. Environ. Int. 2013, 59, 232–243. [Google Scholar] [CrossRef] [PubMed]
- Hou, R.; Xu, Y.; Wang, Z. Review of OPFRs in animals and humans: Absorption, bioaccumulation, metabolism, and internal exposure research. Chemosphere 2016, 153, 78–90. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Cho, H.; Choi, W.; Moon, H. Organophosphate flame retardants (OPFRs) in water and sediment: Occurrence, distribution, and hotspots of contamination of Lake Shihwa. Korea Mar. Pollut. Bull. 2018, 130, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Bollmann, U.E.; Möller, A.; Xie, Z.; Ebinghaus, R.; Einax, J.W. Occurrence and fate of organophosphorus flame retardants and plasticizers in coastal and marine surface waters. Water Res. 2012, 46, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Cristale, J.; Katsoyiannis, A.; Sweetman, A.; Jones, K.; Lacorte, S. Occurrence and risk assessment of organophosphorus and brominated flame retardants in the river Aire (UK). Environ. Pollut. 2013, 179, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, N.; Castro-Jimenez, J.; Fauvelle, V.; Ourgaud, M.; Sempere, R. Occurrence of organic plastic additives in surface waters of the Rhone River (France). Environ. Pollut. 2020, 257, 113637. [Google Scholar] [CrossRef] [PubMed]
- Bacaloni, A.; Cavaliere, C.; Foglia, P.; Nazzari, M.; Samperi, R.; Laganà, A. Liquid chromatography/tandem mass spectrometry determination of organophosphorus flame retardants and plasticizers in drinking and surface waters. Rapid Commun. Mass Spectrom. 2007, 21, 1123–1130. [Google Scholar] [CrossRef]
- Gustavsson, J.; Wiberg, K.; Ribeli, E.; Nguyen, M.A.; Josefsson, S.; Ahrens, L. Screening of organic flame retardants in Swedish river water. Sci. Total Environ. 2018, 625, 1046–1055. [Google Scholar] [CrossRef]
- Chokwe, T.B.; Mporetji, S.M. Organophosphorus flame retardants in surface and effluent water samples from the Vaal River catchment, South Africa: Levels and risk to aquatic life. Water SA 2019, 45, 3. [Google Scholar] [CrossRef]
- Lian, M.S.; Lin, C.Y.; Wu, T.T.; Xin, M.; Gu, X.; Lu, S.; Cao, Y.X.; Wang, B.D.; Quyang, W.; Lium, X.T. Occurrence, spatiotemporal distribution, and ecological risks of organophosphate esters in the water of the Yellow River to the Laizhou Bay Bohai Sea. Sci. Total Environ. 2021, 78, 147528. [Google Scholar] [CrossRef]
- Liu, Y.E.; Luo, X.J.; Ding, H.C.; Qi, L.; Tang, B.; Mai, B.X.; Poma, G.; Covaci, A. Organophosphate diesters (DAPs) and hydroxylated organophosphate flame retardants (HO-OPFRs) as biomarkers of OPFR contamination in a typical freshwater food chain. Chemosphere 2023, 339, 139649. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.K.; Wang, G.W.; Gao, S.X.; Wang, Z.Y. Aryl organophosphate flame retardants induced cardiotoxicity during zebrafish embryogenesis: By disturbing expression of the transcriptional regulators. Aquat. Toxicol. 2015, 161, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Verbruggen, E.M.J.; Rila, J.P.; Traas, T.P.; Posthuma-Doodeman, C.J.A.M.; Posthumus, R. Environmental risk limits for several phosphate esters, with possible application as flame retardant. ChemRxiv 2005. Available online: http://refhub.elsevier.com/S0160-4120(13)00129-3/rf0285 (accessed on 2 October 2023).
- European Commission. European Union Risk Assessment Report: Tris (2-Chloroethyl) Phosphate, TCEP. 2009. Available online: https://echa.europa.eu/documents/10162/2663989d-1795-44a1-8f50-153a81133258 (accessed on 2 October 2023).
- NICNAS (National Industrial Chemicals Notification and Assessment Scheme). Priority Existing Chemical Assessment Report No. 27. 2005. Available online: https://www.nicnas.gov.au/__data/assets/word_doc/0020/34832/PEC27-TBBP.docx (accessed on 2 October 2023).

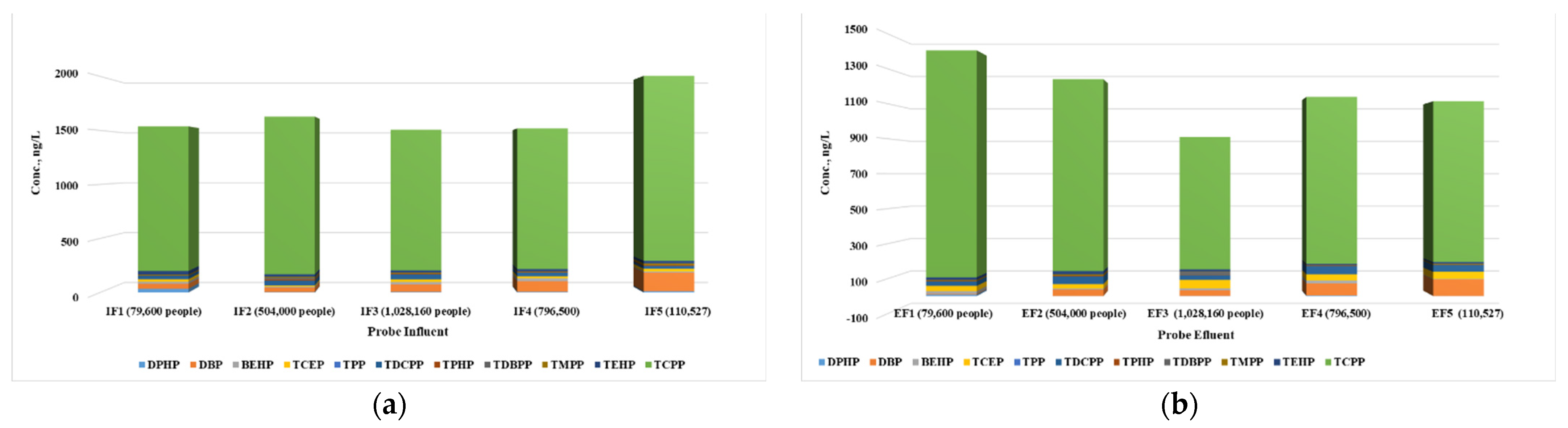

| OPFRs | Influent | Effluent | ||||||
|---|---|---|---|---|---|---|---|---|
| Min | Max | Average | Freq. | Min | Max | Average | Freq. | |
| DPHP | 5.9 | 34 | 13 | 100 | <LOQ | 8.2 | 2.8 | 100 |
| DBP | 39 | 173 | 85.0 | 100 | 3.7 | 94 | 47 | 100 |
| BEHP | 10 | 26 | 17.6 | 100 | 3.7 | 17 | 10 | 100 |
| TCEP | 11 | 26 | 21 | 100 | 26.8 | 49 | 36 | 100 |
| TPP | <LOQ | 1.5 | 0.9 | 80 | <LOQ | 0.9 | 0.6 | 100 |
| TCPP | 1301 | 1711 | 1422 | 100 | 757 | 1299 | 1006 | 100 |
| TDCPP | 23 | 46 | 35.3 | 100 | 25 | 47 | 36 | 100 |
| TPHP | 2.7 | 11 | 6.7 | 100 | 1.8 | 3.6 | 2.6 | 100 |
| TDBPP | ND | 15 | 3.0 | 20 | ND | 19 | 3.7 | 20 |
| TMPP | 6.0 | 15 | 9.0 | 100 | 1.7 | 6.8 | 4.2 | 100 |
| TEHP | 21 | 35 | 25.1 | 100 | 8.6 | 19 | 13 | 100 |
| Daily Mass Loading (mg/zi/1000 People) | |||||||||||
| Samples | DPHP | DBP | BEHP | TCEP | TPP | TCPP | TDCPP | TPHP | TDBPP | TMPP | TEHP |
| IF1 | 20.4 | 27.8 | 11.7 | 14.5 | 0.53 | 800 | 17.6 | 1.64 | - | 4.71 | 20.6 |
| IF2 | 2.62 | 17.4 | 4.52 | 5.14 | 0.65 | 650 | 19.1 | 4.88 | 6.83 | 3.46 | 11.3 |
| IF3 | 3.84 | 138 | 12.5 | 13.7 | 0.44 | 731 | 25.8 | 4.57 | - | 4.68 | 11.6 |
| IF4 | 7.31 | 95.9 | 25.6 | 19.2 | 0.41 | 1275 | 34.2 | 3.33 | - | 5.92 | 21.3 |
| IF5 | 9.65 | 138 | 8.23 | 20.4 | 1.26 | 1364 | 18.7 | 6.62 | - | 12.3 | 19.1 |
| Daily Mass Emission (mg/zi/1000 People) | |||||||||||
| Samples | DPHP | DBP | BEHP | TCEP | TPP | TCPP | TDCPP | TPHP | TDBPP | TMPP | TEHP |
| EF1 | 4.94 | 2.17 | 9.53 | 17.9 | 0.45 | 777 | 15.6 | 1.46 | - | 2.98 | 9.31 |
| EF2 | 0.25 | 16.2 | 2.36 | 12.4 | 0.38 | 489 | 19.6 | 1.62 | - | 3.11 | 8.64 |
| EF3 | 0.53 | 68.3 | 5.48 | 27.5 | 0.22 | 425 | 14.2 | 1.12 | 10.5 | 1.74 | 6.35 |
| EF4 | 3.34 | 67.2 | 16.9 | 34.4 | 0.31 | 935 | 46.2 | 3.25 | - | 1.62 | 8.37 |
| EF5 | 0.65 | 74.8 | 3.49 | 31.8 | 0.74 | 734 | 31.3 | 1.54 | - | 3.53 | 8.28 |
| OPFRs | Sewage Sludge | |||
|---|---|---|---|---|
| Min | Max | Average | Freq. | |
| DPHP | 0.42 | 1.34 | 0.88 | 100 |
| DBP | 0.51 | 4.46 | 1.53 | 100 |
| BEHP | 279 | 655 | 449 | 100 |
| TCEP | 16.3 | 68.5 | 35.7 | 100 |
| TPP | 0.55 | 1.48 | 1.19 | 100 |
| TCPP | 36.1 | 2178 | 1251 | 100 |
| TDCPP | ND | 40.3 | 15.3 | 60 |
| TPHP | 1.09 | 36.7 | 16.7 | 100 |
| TDBPP | ND | 100 | 29.2 | 40 |
| TMPP | 13.3 | 37.2 | 24.8 | 100 |
| TEHP | 17.1 | 59.8 | 32.4 | 100 |
| WWTPs | ∑OPFRs | ||
|---|---|---|---|
| IF (ng/L) | SS (ng/g d.w.) | EF (ng/L) | |
| S1 | 1537 ± 77 | 1807 ± 85 | 1406 ± 59 |
| S2 | 1627 ± 81 | 1893 ± 89 | 1241 ± 52 |
| S3 | 1507 ± 75 | 2905 ± 137 | 909 ± 38 |
| S4 | 1518 ± 76 | 439 ± 21 | 1139 ± 48 |
| S5 | 2004 ± 100 | 2235 ± 105 | 1115 ± 47 |
| OPFRs | Upstream | Downstream | ||||||
|---|---|---|---|---|---|---|---|---|
| Min | Max | Average | Freq. | Min | Max | Average | Freq. | |
| DPHP | 1.02 | 7.14 | 2.98 | 100 | 0.78 | 5.53 | 2.64 | 100 |
| DBP | 2.08 | 28.3 | 15.3 | 100 | <LOQ | 24.8 | 9.18 | 100 |
| BEHP | 0.81 | 32.6 | 14.7 | 100 | 11.5 | 30.6 | 17.3 | 100 |
| TCEP | 7.73 | 22.1 | 13.9 | 100 | 16.2 | 141 | 52.8 | 100 |
| TPP | 0.54 | 1.2.5 | 0.92 | 100 | 0.53 | 1.04 | 0.73 | 100 |
| TCPP | 283 | 933 | 579 | 100 | 670 | 1601 | 1036 | 100 |
| TDCPP | 4.33 | 11.8 | 7.47 | 100 | 6.14 | 61.3 | 24.5 | 100 |
| TPHP | 3.76 | 7.43 | 5.14 | 100 | 3.67 | 9.44 | 6.06 | 100 |
| TDBPP | ND | 45.4 | 11.2 | 40 | ND | 37.8 | 15.4 | 60 |
| TMPP | 4.22 | 19.8 | 11.8 | 100 | 2.98 | 16.1 | 7.11 | 100 |
| TEHP | 17.1 | 31.5 | 23.4 | 100 | 8.13 | 13.4 | 10.1 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paun, I.; Pirvu, F.; Iancu, V.I.; Niculescu, M.; Pascu, L.F.; Chiriac, F.L. An Initial Survey on Occurrence, Fate, and Environmental Risk Assessment of Organophosphate Flame Retardants in Romanian Waterways. J. Xenobiot. 2024, 14, 31-50. https://doi.org/10.3390/jox14010003
Paun I, Pirvu F, Iancu VI, Niculescu M, Pascu LF, Chiriac FL. An Initial Survey on Occurrence, Fate, and Environmental Risk Assessment of Organophosphate Flame Retardants in Romanian Waterways. Journal of Xenobiotics. 2024; 14(1):31-50. https://doi.org/10.3390/jox14010003
Chicago/Turabian StylePaun, Iuliana, Florinela Pirvu, Vasile Ion Iancu, Marcela Niculescu, Luoana Florentina Pascu, and Florentina Laura Chiriac. 2024. "An Initial Survey on Occurrence, Fate, and Environmental Risk Assessment of Organophosphate Flame Retardants in Romanian Waterways" Journal of Xenobiotics 14, no. 1: 31-50. https://doi.org/10.3390/jox14010003
APA StylePaun, I., Pirvu, F., Iancu, V. I., Niculescu, M., Pascu, L. F., & Chiriac, F. L. (2024). An Initial Survey on Occurrence, Fate, and Environmental Risk Assessment of Organophosphate Flame Retardants in Romanian Waterways. Journal of Xenobiotics, 14(1), 31-50. https://doi.org/10.3390/jox14010003







