Mechanisms of VOR Suppression in Brainstem Pathology: Insights from the Absence of Anti-Compensatory Saccades Despite Normal VOR Gain
Abstract
1. Introduction
2. Methods
2.1. Participants
2.2. Assessment
2.3. Statistical Analysis
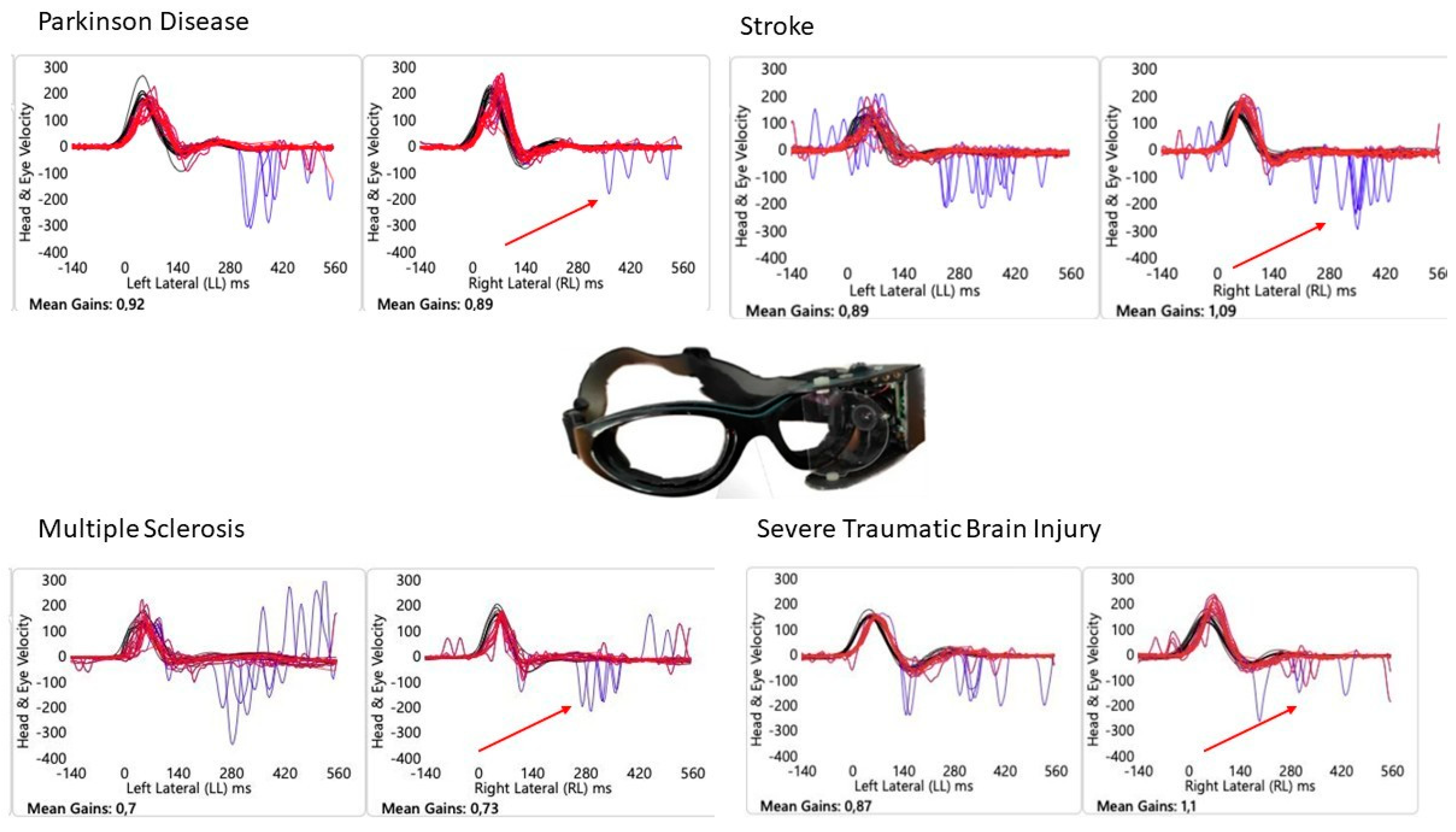
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Waele, C.; Shen, Q.; Magnani, C.; Curthoys, I.S. A Novel Saccadic Strategy Revealed by Suppression Head Impulse Testing of Patients with Bilateral Vestibular Loss. Front. Neurol. 2017, 8, 419. [Google Scholar] [CrossRef] [PubMed]
- Welgampola, M.S.; Taylor, R.L.; Halmagyi, G.M. Video Head Impulse Testing. Adv. Oto-Rhino-Laryngol. 2019, 82, 56–66. [Google Scholar] [CrossRef]
- MacDougall, H.G.; McGarvie, L.A.; Halmagyi, G.M.; Curthoys, I.S.; Weber, K.P. Application of the video head impulse test to detect vertical semicircular canal dysfunction. Otol. Neurotol. 2013, 34, 974–979. [Google Scholar] [CrossRef]
- Shen, Q.; Magnani, C.; Sterkers, O.; Lamas, G.; Vidal, P.-P.; Sadoun, J.; Curthoys, I.S.; de Waele, C. Saccadic Velocity in the New Suppression Head Impulse Test: A New Indicator of Horizontal Vestibular Canal Paresis and of Vestibular Compensation. Front. Neurol. 2016, 7, 160. [Google Scholar] [CrossRef]
- Tramontano, M.; Ferri, N.; Turolla, A.; Orejel Bustos, A.S.; Casagrande Conti, L.; Sorge, C.; Pillastrini, P.; Manzari, L. Video head impulse test in subacute and chronic stroke survivors: New perspectives for implementation of assessment in rehabilitation. Eur. Arch. Oto-Rhino-Laryngol. 2024, 281, 5129–5134. [Google Scholar] [CrossRef]
- Pavlović, I.; Ruška, B.; Pavičić, T.; Skorić, M.K.; Crnošija, L.; Adamec, I.; Habek, M. Video head impulse test can detect brainstem dysfunction in multiple sclerosis. Mult. Scler. Relat. Disord. 2017, 14, 68–71. [Google Scholar] [CrossRef]
- Ferri, N.; Whitney, S.L.; Verrecchia, L.; Casagrande Conti, L.; Turolla, A.; Lelli, T.; Formisano, R.; Buzzi, M.G.; Pillastrini, P.; Manzari, L.; et al. Video Head Impulse Test in Survivors From Severe Traumatic Brain Injury. J. Head. Trauma. Rehabil. 2025, in press.
- Berkiten, G.; Tutar, B.; Atar, S.; Kumral, T.L.; Saltürk, Z.; Akan, O.; Sari, H.; Onaran, Ö.; Biltekin Tuna, Ö.; Uyar, Y. Assessment of the Clinical Use of Vestibular Evoked Myogenic Potentials and the Video Head Impulse Test in the Diagnosis of Early-Stage Parkinson’s Disease. Ann. Otol. Rhinol. Laryngol. 2023, 132, 41–49. [Google Scholar] [CrossRef]
- Manzari, L.; Princi, A.A.; De Angelis, S.; Tramontano, M. Clinical value of the video head impulse test in patients with vestibular neuritis: A systematic review. Eur. Arch. Oto-Rhino-Laryngol. 2021, 278, 4155–4167. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Chen, Z.; Zhang, Y.; Wei, X.; Zhao, H.; Hu, J.; Cheng, Y.; Ren, X.; Zhang, Q. Association Analysis of HIMP and SHIMP Quantitative Parameters in Patients With Vestibular Neuritis and Healthy Participants. Front. Neurol. 2021, 12, 748990. [Google Scholar] [CrossRef]
- Wagner, A.R.; Grove, C.R.; Loyd, B.J.; Dibble, L.E.; Schubert, M.C. Compensatory saccades differ between those with vestibular hypofunction and multiple sclerosis pointing to unique roles for peripheral and central vestibular inputs. J. Neurophysiol. 2022, 128, 934–945. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, K.E.; Rey-Martinez, J.; Chiarovano, E.; Paul, S.S.; Valldeperes, A.; MacDougall, H.G.; Curthoys, I.S. Suppression head impulse test paradigm (SHIMP) characteristics in people with Parkinson’s disease compared to healthy controls. Exp. Brain Res. 2021, 239, 1853–1862. [Google Scholar] [CrossRef]
- Nham, B.; Wang, C.; Reid, N.; Calic, Z.; Kwok, B.Y.C.; Black, D.A.; Bradshaw, A.; Halmagyi, G.; Welgampola, M.S. Modern vestibular tests can accurately separate stroke and vestibular neuritis. J. Neurol. 2023, 270, 2031–2041. [Google Scholar] [CrossRef]
- Hawkins, K.E.; Chiarovano, E.; Paul, S.S.; Burgess, A.M.; MacDougall, H.G.; Curthoys, I.S. Vestibular semicircular canal function as detected by video Head Impulse Test (vHIT) is essentially unchanged in people with Parkinson’s disease compared to healthy controls. J. Vestib. Res. Equilib. Orientat. 2022, 32, 261–269. [Google Scholar] [CrossRef]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef]
- Polman, C.H.; Reingold, S.C.; Banwell, B.; Clanet, M.; Cohen, J.A.; Filippi, M.; Fujihara, K.; Havrdova, E.; Hutchinson, M.; Kappos, L.; et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann. Neurol. 2011, 69, 292–302. [Google Scholar] [CrossRef]
- Kurtzke, J.F. Rating neurologic impairment in multiple sclerosis: An expanded disability status scale (EDSS). Neurology 1983, 33, 1444–1452. [Google Scholar] [CrossRef]
- Gouvier, W.D.; Blanton, P.D.; LaPorte, K.K.; Nepomuceno, C. Reliability and validity of the Disability Rating Scale and the Levels of Cognitive Functioning Scale in monitoring recovery from severe head injury. Arch. Phys. Med. Rehabil. 1987, 68, 94–97. [Google Scholar] [CrossRef] [PubMed]
- Viosca, E.; Martínez, J.L.; Almagro, P.L.; Gracia, A.; González, C. Proposal and validation of a new functional ambulation classification scale for clinical use. Arch. Phys. Med. Rehabil. 2005, 86, 1234–1238. [Google Scholar] [CrossRef]
- Manzari, L.; Tramontano, M. Suppression Head Impulse Paradigm (SHIMP) in evaluating the vestibulo-saccadic interaction in patients with vestibular neuritis. Eur. Arch. Oto-Rhino-Laryngol. 2020, 277, 3205–3212. [Google Scholar] [CrossRef] [PubMed]
- Lal, V.; Truong, D. Eye movement abnormalities in movement disorders. Clin. Park. Relat. Disord. 2019, 1, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Serra, A.; Chisari, C.G.; Matta, M. Eye Movement Abnormalities in Multiple Sclerosis: Pathogenesis, Modeling, and Treatment. Front. Neurol. 2018, 9, 31. [Google Scholar] [CrossRef]
- Heitger, M.H.; Anderson, T.J.; Jones, R.D.; Dalrymple-Alford, J.C.; Frampton, C.M.; Ardagh, M.W. Eye movement and visuomotor arm movement deficits following mild closed head injury. Brain J. Neurol. 2004, 127, 575–590. [Google Scholar] [CrossRef] [PubMed]
- Rowland, M.J.; Garry, P.; Westbrook, J.; Corkill, R.; Antoniades, C.A.; Pattinson, K.T.S. Acute impairment of saccadic eye movements is associated with delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage. J. Neurosurg. 2017, 127, 754–760. [Google Scholar] [CrossRef] [PubMed]
| Multiple Sclerosis | Severe Traumatic Brain Injury | Stroke | Parkinson’s Disease | |
|---|---|---|---|---|
| Sample Size (n) | 27 | 22 | 36 | 35 |
| Age (years, mean ± SD) | 47.93 ± 8.51 | 42 ± 15.02 | 55.11 ± 15.09 | 69.9 ± 8.4 |
| Sex (Female, %) | 66.67% | 14.3% | 30.5% | 31.4% |
| HIMP aVOR Gain (mean ± SD) | ||||
| Left Anterior | 0.78 ± 0.22 | 0.81 ± 0.15 | 0.81 ± 0.20 | 0.79 ± 0.19 |
| Right Anterior | 0.86 ± 0.14 | 0.71 ± 0.18 | 0.83 ± 0.26 | 0.85 ± 0.25 |
| Horizontal Left | 0.92 ± 0.19 | 0.87 ± 0.15 | 0.9 ± 0.12 | 0.94 ± 0.16 |
| Horizontal Right | 0.98 ± 0.24 | 0.97 ± 0.21 | 0.98 ± 0.14 | 0.99 ± 0.20 |
| Left Posterior | 0.82 ± 0.12 | 0.86 ± 0.15 | 0.91 ± 0.16 | 0.85 ± 0.22 |
| Right Posterior | 0.76 ± 0.17 | 0.91 ± 0.25 | 0.79 ± 0.12 | 0.79 ± 0.19 |
| SHIMP aVOR Gain (mean ± SD) | ||||
| Horizontal Left | 0.78 ± 0.21 | 0.81 ± 0.26 | 0.88 ± 0.20 | 0.85 ± 0.19 |
| Horizontal Right | 0.87 ± 0.23 | 0.74 ± 0.23 | 0.77 ± 0.19 | 0.85 ± 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tramontano, M.; Casagrande Conti, L.; Ferri, N.; Manzari, L. Mechanisms of VOR Suppression in Brainstem Pathology: Insights from the Absence of Anti-Compensatory Saccades Despite Normal VOR Gain. Audiol. Res. 2025, 15, 154. https://doi.org/10.3390/audiolres15060154
Tramontano M, Casagrande Conti L, Ferri N, Manzari L. Mechanisms of VOR Suppression in Brainstem Pathology: Insights from the Absence of Anti-Compensatory Saccades Despite Normal VOR Gain. Audiology Research. 2025; 15(6):154. https://doi.org/10.3390/audiolres15060154
Chicago/Turabian StyleTramontano, Marco, Laura Casagrande Conti, Nicola Ferri, and Leonardo Manzari. 2025. "Mechanisms of VOR Suppression in Brainstem Pathology: Insights from the Absence of Anti-Compensatory Saccades Despite Normal VOR Gain" Audiology Research 15, no. 6: 154. https://doi.org/10.3390/audiolres15060154
APA StyleTramontano, M., Casagrande Conti, L., Ferri, N., & Manzari, L. (2025). Mechanisms of VOR Suppression in Brainstem Pathology: Insights from the Absence of Anti-Compensatory Saccades Despite Normal VOR Gain. Audiology Research, 15(6), 154. https://doi.org/10.3390/audiolres15060154








