Management of Facial Paralysis Following Skull Base Surgery: A Comprehensive Narrative Review
Abstract
1. Introduction
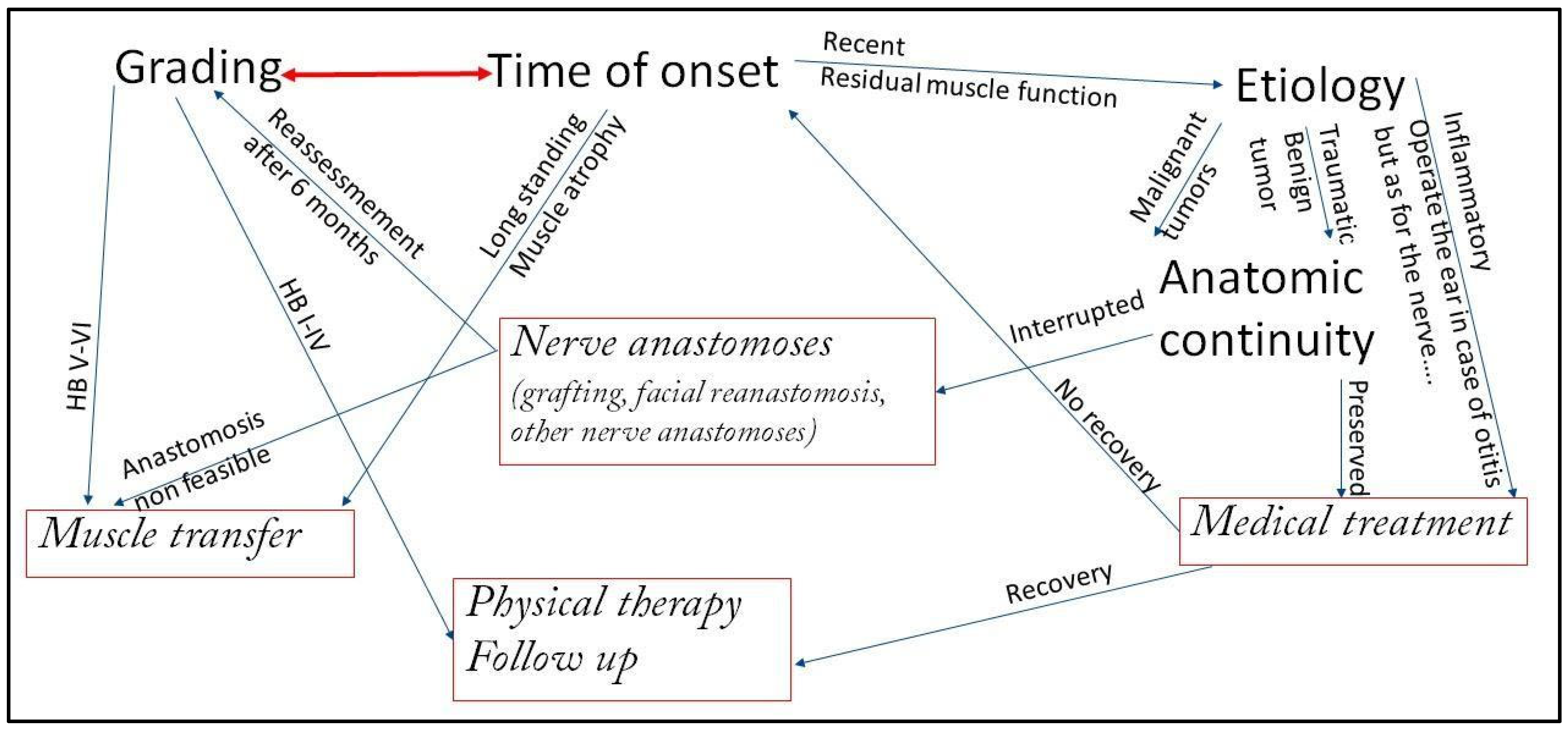
Criteria for Determining the Management Approach
- Grading of the Deficit: The House–Brackmann scale [5] is the most widely used system [1,3]. However, the Sunnybrook Facial Grading System and the eFACE clinician-graded scale provide more granular detail on resting symmetry, dynamic function, and synkinesis, which is crucial for pre-operative planning and outcome assessment [2]. In general, incomplete facial paralysis with residual function does not determine atrophy of the facial musculature and gives more time for non-surgical strategies and monitoring, while in complete facial paralysis.
- Duration of Paralysis becomes the most critical factor. In fact, the presence of viable facial musculature determines the options. Immediate to <18 months management: the facial muscles are still viable and may be reinnervated via nerve-based procedures (grafting or transfers). >18–24 months management: Chronic denervation results in irreversible muscle atrophy and fibrosis. Dynamic restoration requires muscle transfer (regional or free);
- Etiology of the paralysis: The nature of the nerve injury (neoplastic infiltration, transection, stretch, compression) and the availability of proximal and distal nerve stumps guide repair strategies;
- Prognosis of the Underlying Pathology and Patient’s General Condition: The patient’s life expectancy, oncological prognosis, and fitness for prolonged microsurgical procedures are paramount. A patient with a poor prognosis may benefit more from simpler static procedures, while a healthy, young patient is an ideal candidate for complex free tissue transfer.
2. Surgical Options
2.1. Nerve Grafting
2.2. Anastomosis with Other Motor Nerves
- Termino-terminal: The entire hypoglossal nerve is divided and connected to the facial nerve with excellent resting tone symmetry and good voluntary movements. The long term morbidity (speech, chewing, swallowing impairment) from hemilingual atrophy can be minimal, provided that tongue rehabilitation is initiated immediately;
- Side-to-end with graft interposition (“jump graft” hypoglossal to facial nerve transfer): A jump graft is connected end-to-side to the hypoglossal nerve (without transecting it) and end-to-end to the facial nerve. It preserves most tongue function while providing good facial tone and movement [3];
- “Side-to-end” without grafting: the main trunk of the facial nerve is sectioned in its intratemporal portion, just inferiorly to the second genu, in order to increase the length of the distal stump of facial nerve. That allows the direct connection end-to-side of the hypoglossal nerve with the facial nerve, avoiding any graft interposition. The advantage, in this case, is to perform a single nerve suture, increasing the number of fibers growing from the donor nerve into the distal stump of facial nerve [11];
- Descending Hypoglossal Branch (Ansa hypoglossi): The descendens hypoglossi branch (to the ansa cervicalis) is used instead of the main trunk, drastically reducing tongue morbidity while providing promising results [3]. There are no comparative studies of the results of this technique with other hypoglossal nerve techniques.
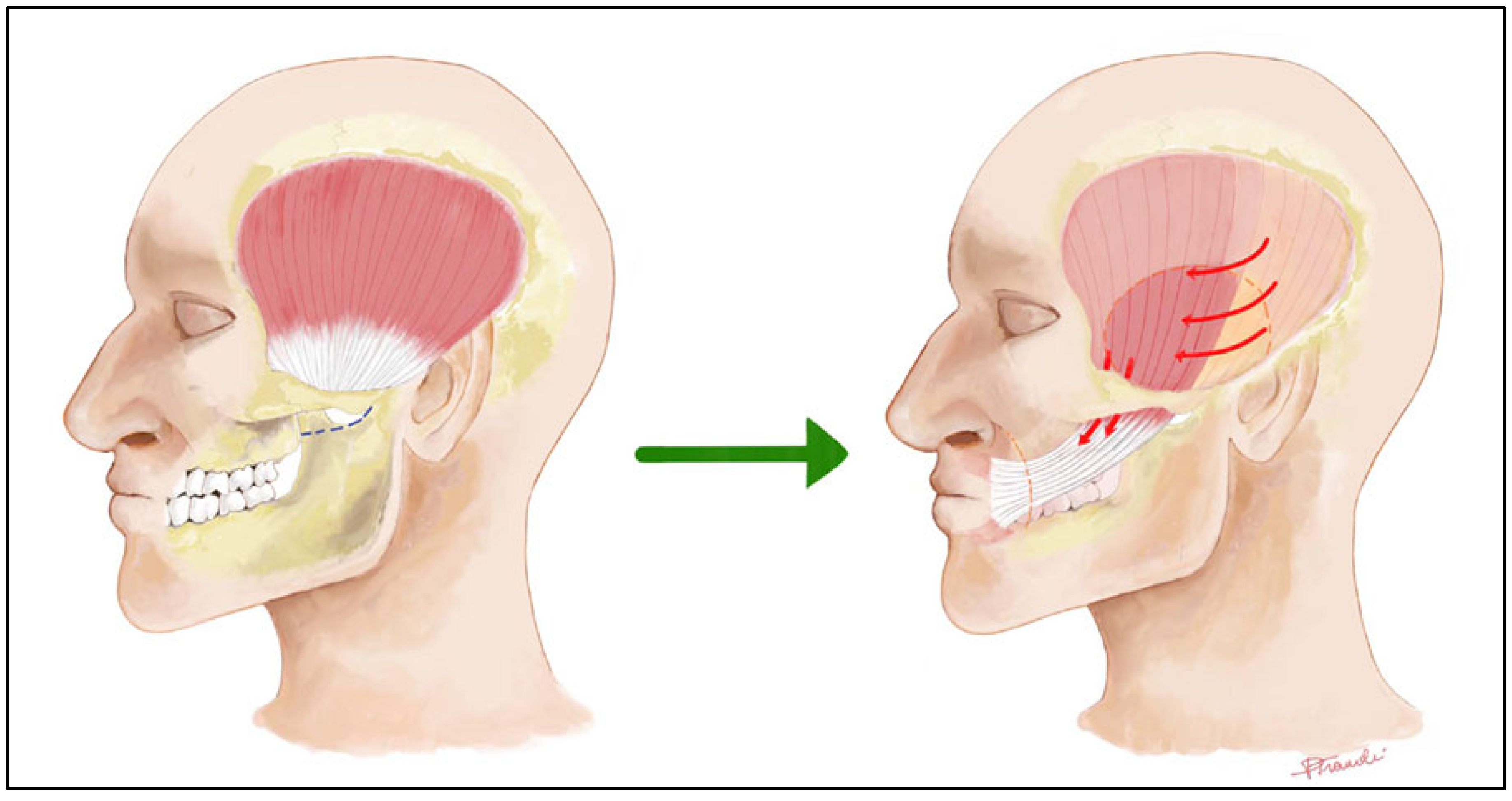
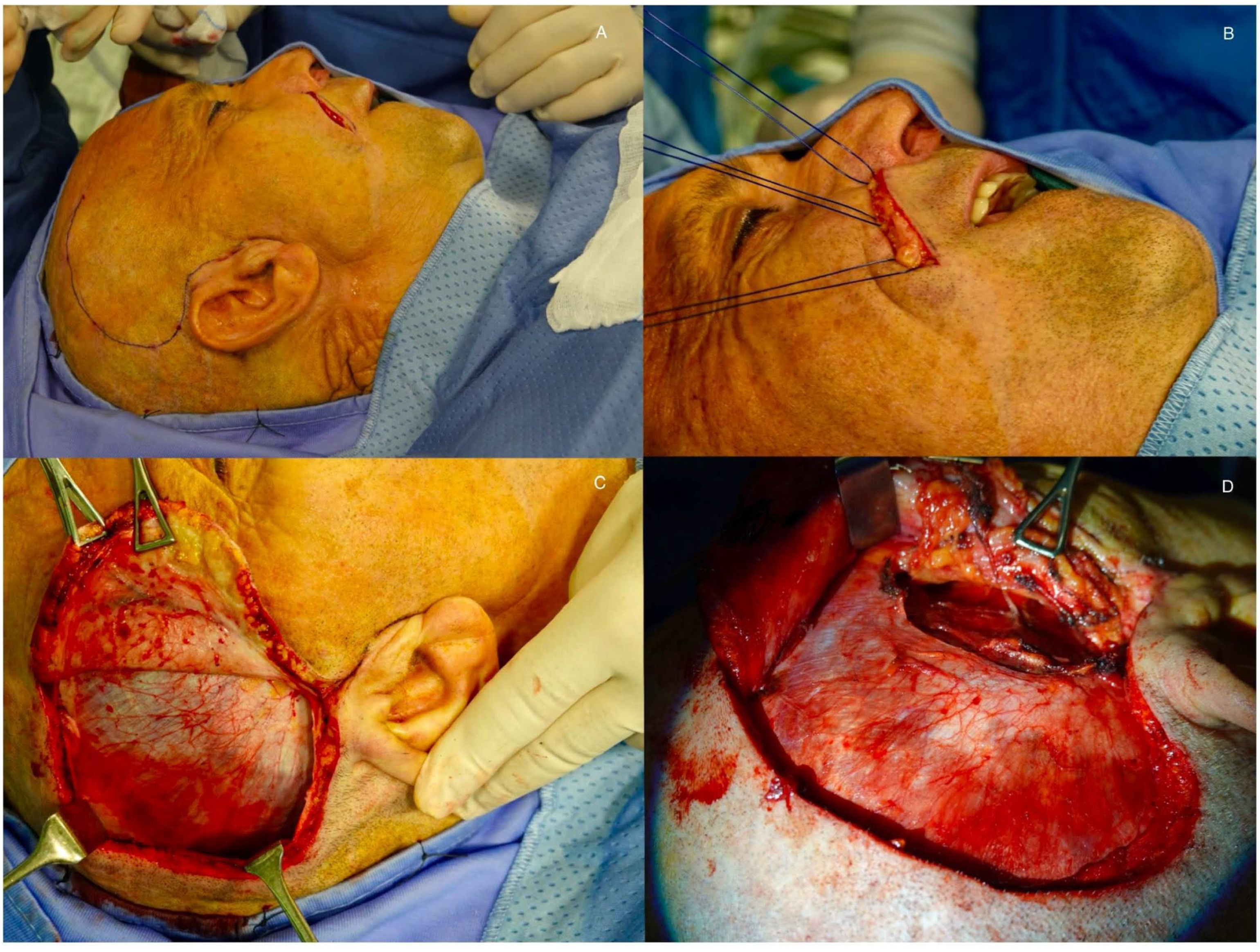
2.3. Muscle Transfer
- Latissimus Dorsi: Offers a large volume of tissue for more extensive paralysis but is more technically challenging [2];
- Pectoralis Minor: Can be used but is less popular than gracilis or latissimus;
2.4. Static Procedures
- Brow lift: Corrects ptosis;
- Upper eyelid gold/platinum weight placement: Enables eyelid closure with gravity;
- Lower eyelid tightening (canthoplasty): Corrects lid laxity;
- Static slings (fascia lata, allograft): Suspends the corner of the mouth and nasolabial fold.
2.5. Non-Surgical Options
3. Discussion
A Reasoned Approach to the Management of Facial Paralysis Following Skull Base Surgery
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| HB | House-Brackmann scale |
| eFACE | electronic Facial Clinician-graded scale |
| CPA | Cerebellopontine Angle |
| CSF | Cerebrospinal Fluid |
| CFNG | Cross-Face Nerve Grafting |
| FFMT | Free Functioning Muscle Transfer |
References
- Lassaletta, L.; Morales-Puebla, J.M.; González-Otero, T.; Moraleda, S.; Roda, J.M.; Gavilán, J. The Experience of a Facial Nerve Unit in the Treatment of Patients with Facial Paralysis Following Skull Base Surgery. Otol. Neurotol. 2020, 41, e1340–e1349. [Google Scholar] [CrossRef]
- Pinkiewicz, M.; Dorobisz, K.; Zatoński, T. A Comprehensive Approach to Facial Reanimation: A Systematic Review. J. Clin. Med. 2022, 11, 2890. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.H.; Kim, Y.C.; Oh, T.S. Facial Palsy Reconstruction. Arch. Craniofac. Surg. 2024, 25, 1. [Google Scholar] [CrossRef] [PubMed]
- Hadlock, T.; Cheney, M.L. Facial Reanimation: An Invited Review and Commentary. Arch. Facial Plast. Surg. 2008, 10, 413–417. [Google Scholar] [CrossRef]
- House, J.W.; Brackmann, D.E. Facial Nerve Grading System. Otolaryngol. Head Neck Surg. 1985, 93, 146–147. [Google Scholar] [CrossRef]
- Prasad, S.C.; Balasubramanian, K.; Piccirillo, E.; Taibah, A.; Russo, A.; He, J.; Sanna, M. Surgical Technique and Results of Cable Graft Interpositioning of the Facial Nerve in Lateral Skull Base Surgeries: Experience with 213 Consecutive Cases. J. Neurosurg. 2018, 128, 631–638. [Google Scholar] [CrossRef]
- Leong, S.C.; Lesser, T.H. Long-Term Outcomes of Facial Nerve Function in Irradiated and Nonirradiated Nerve Grafts. Ann. Otol. Rhinol. Laryngol. 2013, 122, 695–700. [Google Scholar] [CrossRef]
- Ramos, D.S.; Bonnard, D.; Franco-Vidal, V.; Liguoro, D.; Darrouzet, V. Stitchless Fibrin Glue-Aided Facial Nerve Grafting after Cerebellopontine Angle Schwannoma Removal: Technique and Results in 15 Cases. Otol. Neurotol. 2015, 36, 498–502. [Google Scholar] [CrossRef]
- Molinari, G.; Serafini, E.; Barbazza, A.; Marchioni, D.; Presutti, L.; Nizzoli, F.; Reggiani, E.; Guidotti, M.; Borghi, A.; Fernandez, I.J. Endoscopic Approach to Geniculate Ganglion: A Multicentric Experience. Eur. Arch. Otorhinolaryngol. 2024, 281, 1761–1771. [Google Scholar] [CrossRef]
- Sergesketter, A.R.; Shammas, R.L.; Massa, L.A.; Phillips, B.T.; Marcus, J.R. Early Simultaneous Cross Facial Nerve Graft and Masseteric Nerve Transfer for Facial Paralysis after Tumor Resection. Plast. Reconstr. Surg. Glob. Open 2023, 11, e4869. [Google Scholar] [CrossRef]
- Lassaletta, L.; Morales-Puebla, J.M.; Molnar, D.; González-Otero, T.; Gavilán, J. Side-to-End Intratemporal Hypoglossal-to-Facial Transfer. Atlas Oral Maxillofac. Surg. Clin. N. Am. 2023, 31, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Jeong, S.S.; Oh, T.S. Masseter Nerve-Based Facial Palsy Reconstruction. Arch. Craniofac. Surg. 2020, 21, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Liu, Y.; Zhang, S.; Yang, W.; Zuo, M.; Liu, X. Multiple Model Evaluation of the Masseteric-to-Facial Nerve Transfer for Reanimation of the Paralyzed Face and Quick Prognostic Prediction. Front. Surg. 2022, 9, 735231. [Google Scholar] [CrossRef] [PubMed]
- Labbè, D.; Bussu, F.; Iodice, A. A Comprehensive Approach to Long-Standing Facial Paralysis Based on Lengthening Temporalis Myoplasty. Acta Otorhinolaryngol. Ital. 2012, 32, 145–153. [Google Scholar]
- Bussu, F.; Tramaloni, P.; Tropiano, P.; Bonomo, M.; Crescio, C.; Sotgiu, N.; Perla, M.; Rizzo, D.; Galli, J. The Role of Lengthening Temporalis Myoplasty in the Management of Facial Paralysis: Evaluating Patient-Reported Quality of Life Improvements. Otol. Neurotol. 2025, 46, e526–e532. [Google Scholar] [CrossRef]
- Labbé, D.; Huault, M. Lengthening Temporalis Myoplasty and Lip Reanimation. Plast. Reconstr. Surg. 2000, 105, 1289–1297, discussion 1298. [Google Scholar] [CrossRef]
- Balaji, S.M. A Modified Temporalis Transfer in Facial Reanimation. Int. J. Oral Maxillofac. Surg. 2002, 31, 584–591. [Google Scholar] [CrossRef]
- Janik, S.; Marijic, B.; Faisal, M.; Grasl, S.; Tzou, C.-H.J.; Rodriquez-Lorenzo, A.; Seemann, R.; Leonhard, M.; Erovic, B.M. Using the Serratus Anterior Free Flap for Dynamic Facial Reanimation: Systematic Review. Head Neck 2023, 45, 266–274. [Google Scholar] [CrossRef]
- Alagöz, M.S.; Alagöz, A.N.; Comert, A. Neuroanatomy of Extensor Digitorum Brevis Muscle for Reanimation of Facial Paralysis. J. Craniofac. Surg. 2011, 22, 2308–2311. [Google Scholar] [CrossRef]
- Chi, J.J. Management of the Eye in Facial Paralysis. Facial Plast. Surg. Clin. N. Am. 2016, 24, 21–28. [Google Scholar] [CrossRef]
- Diels, H.J.; Combs, D. Neuromuscular Retraining for Facial Paralysis. Otolaryngol. Clin. N. Am. 1997, 30, 727–743. [Google Scholar] [CrossRef]
- Neville, C.; Beurskens, C.; Diels, J.; MacDowell, S.; Rankin, S. Consensus among International Facial Therapy Experts for the Management of Adults with Unilateral Facial Palsy: A Two-Stage Nominal Group and Delphi Study. Facial Plast. Surg. Aesthet. Med. 2024, 26, 405–417. [Google Scholar] [CrossRef] [PubMed]
- Molinari, G.; Calvaruso, F.; Barbazza, A.; Vanelli, E.; Nizzoli, F.; Reggiani, E.; Guidotti, M.; Borghi, A.; Marchioni, D.; Presutti, L.; et al. Patterns and Timing of Recovery from Facial Nerve Palsy after Nerve-Sparing Parotid Surgery: The Role of Neuromuscular Retraining. Eur. Arch. Otorhinolaryngol. 2024, 281, 5465–5472. [Google Scholar] [CrossRef] [PubMed]
- Guntinas-Lichius, O.; Prengel, J.; Cohen, O.; Mäkitie, A.A.; Vander Poorten, V.; Ronen, O.; Shaha, A.; Ferlito, A. Pathogenesis, Diagnosis and Therapy of Facial Synkinesis: A Systematic Review and Clinical Practice Recommendations by the International Head and Neck Scientific Group. Front. Neurol. 2022, 13, 1019554. [Google Scholar] [CrossRef]
- Marco, B.; Federico, C.; Tozzi, A.; Cinzia, D.G.; Federica, N.; Elena, R.; Alfredo, L.M.; Martina, S.; Daniele, M.; Giuseppe, F.; et al. Rehabilitation of Facial Nerve Palsy Combining Neuromuscular Retraining and Botulinum Toxin A Injection: A Tertiary Referral Centre Experience and a New Working Protocol Proposal. Eur. Arch. Otorhinolaryngol. 2025, 282, 3757–3769. [Google Scholar] [CrossRef]
- Mattioli, F.; Galloni, C.; Alberti, C.; Bonali, M.; Lo Manto, A.; Baraldi, S.; Tonelli, R.; Nizzoli, F.; Reggiani, E.; Barbazza, A.; et al. Facial Nerve Graft in Malignant Tumors: The Role of Facial Rehabilitation. J. Clin. Med. 2025, 14, 968. [Google Scholar] [CrossRef]
- Ezzibdeh, R.; Meyer, M.R.; Stranberg, S.; Pepper, J.-P. Functional Outcomes of Dual Nerve Transfer in Patients with Facial Paralysis. J. Plast. Reconstr. Aesthet. Surg. 2025, 103, 89–94. [Google Scholar] [CrossRef]
- Harb, J.L.; Ein, L.J. The Role of the Cross Face Nerve Graft in Facial Reanimation and Endoscopic Harvest of the Sural Nerve. Atlas Oral Maxillofac. Surg. Clin. N. Am. 2023, 31, 25–31. [Google Scholar] [CrossRef]
- Yang, S.F.; Kim, J.C. Reinnervation with Selective Nerve Grafting from Multiple Donor Nerves. Facial Plast. Surg. Clin. N. Am. 2021, 29, 389–396. [Google Scholar] [CrossRef]
- Vos, D.J.; Fritz, M.A.; Genther, D.J.; Byrne, P.J.; Ciolek, P.J. Masseteric Atrophy Following Masseteric Nerve Transfer: Radiographic Findings of Asymmetry in the Paralyzed Face? Laryngoscope 2024, 134, 4514–4520. [Google Scholar] [CrossRef]
- Cassoni, A.; Catalano, C.; Di Giorgio, D.; Raponi, I.; Di Brino, M.; Perotti, S.; Valentini, V. Masseter-Facial Neurorrhaphy for Facial Palsy Reanimation: What Happens after Masseter Denervation? Histomorphometric and Stomatognathic Functional Analysis. J. Craniomaxillofac. Surg. 2020, 48, 680–684. [Google Scholar] [CrossRef] [PubMed]
- Yawn, R.J.; Wright, H.V.; Francis, D.O.; Stephan, S.; Bennett, M.L. Facial Nerve Repair after Operative Injury: Impact of Timing on Hypoglossal-Facial Nerve Graft Outcomes. Am. J. Otolaryngol. 2016, 37, 493–496. [Google Scholar] [CrossRef] [PubMed]
- Wada, S.-I.; Haginomori, S.-I.; Mori, A.; Ichihara, T.; Kanazawa, A.; Kawata, R.; Takubo, T.; Yorifuji, S. The Midline Electroneurography Method for Facial Palsy Reflects Total Nerve Degeneration. Acta Otolaryngol. 2013, 133, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Amer, T.A.; ElSharkawy, O.A. The Split Hypoglossal Nerve and Cross-Face Nerve Graft for Dual Innervation of the Functional Muscle Transfer in Facial Reanimation. J. Craniofac. Surg. 2022, 33, 2625–2630. [Google Scholar] [CrossRef]
- Alhumaidan, M.I.; alKarni, A.F.; Alrabiah, H.F.; Alkhathami, A.M.; Alhazmi, B.; Alqirnas, M.Q. Facial Reanimation Using Free Partial Latissimus Dorsi Muscle Transfer: Single Versus Dual Innervation Method, a Systematic Review. Eur. Arch. Otorhinolaryngol. 2025, 282, 5365–5374. [Google Scholar] [CrossRef]
- Biglioli, F.; Colombo, V.; Tarabbia, F.; Pedrazzoli, M.; Battista, V.; Giovanditto, F.; Dalla Toffola, E.; Lozza, A.; Frigerio, A. Double Innervation in Free-Flap Surgery for Long-Standing Facial Paralysis. J. Plast. Reconstr. Aesthet. Surg. 2012, 65, 1343–1349. [Google Scholar] [CrossRef]
- Cardenas-Mejia, A.; Covarrubias-Ramirez, J.V.; Bello-Margolis, A.; Rozen, S. Double Innervated Free Functional Muscle Transfer for Facial Reanimation. J. Plast. Surg. Hand Surg. 2015, 49, 183–188. [Google Scholar] [CrossRef]
- Crawford, K.L.; Stramiello, J.A.; Orosco, R.K.; Greene, J.J. Advances in Facial Nerve Management in the Head and Neck Cancer Patient. Curr. Opin. Otolaryngol. Head Neck Surg. 2020, 28, 235–240. [Google Scholar] [CrossRef]
- Ylä-Kotola, T.; Kauhanen, M.S.C.; Asko-Seljavaara, S. Facial Reanimation by Transplantation of a Microneurovascular Muscle: Long-Term Follow-Up. Scand. J. Plast. Reconstr. Surg. Hand Surg. 2004, 38, 272–276. [Google Scholar] [CrossRef]
- Park, H.; Hong, D.W.; Oh, T.S. Functional Outcome Comparison of Free Gracilis Muscle Anchoring Methods in Patients with Facial Paralysis: Upper Lip Red Line Incision versus Facelift Incision Approach. J. Reconstr. Microsurg. 2023, 39, 027–034. [Google Scholar] [CrossRef]
- Ferri, A.; Zito, F.; Menapace, G.; Zannoni, C.; Bergonzani, M.; Perlangeli, G.; Bianchi, B. Optimizing the Results of Facial Animation Surgery: Botulinum Toxin Injection into Free Functional Gracilis Flap Transfer. J. Plast. Reconstr. Aesthet. Surg. 2023, 83, 415–422. [Google Scholar] [CrossRef]
- Guntinas-Lichius, O. The Facial Nerve in the Presence of a Head and Neck Neoplasm: Assessment and Outcome after Surgical Management. Curr. Opin. Otolaryngol. Head Neck Surg. 2004, 12, 133–141. [Google Scholar] [CrossRef]
- Thielker, J.; Kouka, M.; Guntinas-Lichius, O. Erhalt, Rekonstruktion und Rehabilitation des N. facialis. HNO 2023, 71, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.S.; Choi, J.E.; Lim, J.H.; Cho, Y.-S. Management of Facial Nerve Schwannoma: When Is the Timing for Surgery. Eur. Arch. Otorhinolaryngol. 2022, 279, 1243–1249. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Rowlands, S. Masseteric Nerve for Reanimation: Does Age, Radiation and Duration of Facial Palsy Affect the Outcome? Indian J. Otolaryngol. Head Neck Surg. 2025, 77, 1800–1807. [Google Scholar] [CrossRef] [PubMed]
- Ibarra, G.; Lasso, J.M. Salvage Procedures for Facial Reanimation with Neurovascular Flaps When Previous Surgeries Failed. Ann. Plast. Surg. 2022, 89, 196–200. [Google Scholar] [CrossRef]
- Bos, R.; Reddy, S.G.; Mommaerts, M.Y. Lengthening Temporalis Myoplasty versus Free Muscle Transfer with the Gracilis Flap for Long-Standing Facial Paralysis: A Systematic Review of Outcomes. J. Craniomaxillofac. Surg. 2016, 44, 940–951. [Google Scholar] [CrossRef]
- Kanona, H.; Saeed, S.R.; Randhawa, P.; Kimber, R.; Rodger, A.; Khalil, S.; Andrews, P. Evaluation of the Patient with Facial Palsy: A Multidisciplinary Approach. Facial Plast. Surg. 2024, 40, 400–406. [Google Scholar] [CrossRef]
| Surgical Option | Criteria for Selection | Surgical Variants |
|---|---|---|
| Reanastomosis of the interrupted facial nerve | Iatrogenic injury, reanastomosis free of tension (rerouting) | |
| Cable graft | Intraoperatory interruption/resection of a section of the nerve, need for tension free anastomosis | |
| Nerve transfer | Proximal stump not available, lack of recovery with a cable graft, still viable facial musculature | Hypoglosso facial Masseterin facial (spinal accessory facial) |
| Muscle transfer | Long term facial paralysis/Facial musculature atrophy | Regional muscle transfer (Labbè operation) Free muscle transfer (gracilis, latissimus dorsi, pectoralis minor, etc.) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Luca, L.M.; Cannova, S.; Lai, S.; Accolla, M.; Barbazza, A.; Calò, L.; Rizzo, D.; Tramaloni, P.; Bonali, M.; Fernandez, I.J.; et al. Management of Facial Paralysis Following Skull Base Surgery: A Comprehensive Narrative Review. Audiol. Res. 2025, 15, 155. https://doi.org/10.3390/audiolres15060155
De Luca LM, Cannova S, Lai S, Accolla M, Barbazza A, Calò L, Rizzo D, Tramaloni P, Bonali M, Fernandez IJ, et al. Management of Facial Paralysis Following Skull Base Surgery: A Comprehensive Narrative Review. Audiology Research. 2025; 15(6):155. https://doi.org/10.3390/audiolres15060155
Chicago/Turabian StyleDe Luca, Laura Maria, Sergio Cannova, Sebastiana Lai, Marco Accolla, Alice Barbazza, Lea Calò, Davide Rizzo, Pierangela Tramaloni, Marco Bonali, Ignacio Javier Fernandez, and et al. 2025. "Management of Facial Paralysis Following Skull Base Surgery: A Comprehensive Narrative Review" Audiology Research 15, no. 6: 155. https://doi.org/10.3390/audiolres15060155
APA StyleDe Luca, L. M., Cannova, S., Lai, S., Accolla, M., Barbazza, A., Calò, L., Rizzo, D., Tramaloni, P., Bonali, M., Fernandez, I. J., & Bussu, F. (2025). Management of Facial Paralysis Following Skull Base Surgery: A Comprehensive Narrative Review. Audiology Research, 15(6), 155. https://doi.org/10.3390/audiolres15060155






