Abstract
Multiple myeloma is a hematological cancer depicted by the proliferation of plasma cells within the bone marrow, causing immune dysfunction and other abnormalities. The gut microbiome, the microbial community in the gastrointestinal tract, was found to modulate systemic immunity, inflammation, and metabolism. Although the interplay between gut microbiome and multiple myeloma has been found in recent research, there is a gap in knowledge linking the effect of the microbiome on the pathogenesis and treatment of multiple myeloma. The imbalance in the gut microbiome, dysbiosis, may influence multiple myeloma pathogenesis through immune modulation and inflammation. Certain microbial species have been associated with multiple myeloma progression, complications, and therapeutic responses to treatment. Moreover, microbiome-derived metabolites, short-chain fatty acids, can influence the immune circuits associated with multiple myeloma progression. Understanding the bidirectional relationship between multiple myeloma and gut microbiota may provide insights into enhanced treatment and the development of new microbiome-based interventions. The current review provides a comprehensive highlight of current evidence linking the gut microbiome with multiple myeloma, demonstrating its significant roles in the development, progression, and treatment of multiple myeloma. Additionally, it focuses on the therapeutic potential of modulating the gut microbiome as a novel adjunct strategy in multiple myeloma management.
1. Introduction
1.1. Multiple Myeloma
Multiple myeloma (MM) is a hematologic neoplastic disease characterized by clonal proliferation of plasma cells in the bone marrow []. It is the second most common hematological cancer of the plasma B cells, with significant heterogeneity in the bone marrow microenvironment, and the most common primary bone malignancy [,]. According to the International Myeloma Working Group (IMWG), MM is defined as a clonal plasma cell malignancy characterized by clonal bone marrow plasma cells ≥ 10% or extramedullary plasmacytoma and evidence of end-organ damage attributable to the plasma cell disorder (the classic CRAB features), including hypercalcemia (serum calcium > 0.25 mmol/L above the upper limit of normal), renal insufficiency (serum creatinine > 2 mg/dL or creatinine clearance < 40 mL/min), anemia (hemoglobin value of >20 g/L below the lowest limit of normal, or a hemoglobin value < 100 g/L), and bone lesions (one or more osteolytic lesions) [,].
It exerts severe sequelae of consequences, including severe bone pain, bone loss, and pathological fractures that considerably reduce patients’ quality of life and affect their prognosis. This bone dysfunction includes persistent inhibition of osteoblastic bone formation and excessive osteoclast resorption that result in incurable osteolytic lesions even when patients are in complete and prolonged remission [,].
MM has been studied as part of a disease of plasma cell disorders. The earliest stage is monoclonal gammopathy of undetermined significance (MGUS), diagnosed by serum monoclonal protein < 3 g/dL, <10% clonal bone marrow plasma cells, and absence of end-organ damage, with an average risk of progression to MM of ~1% per year. Smoldering multiple myeloma (SMM) represents the intermediate stage. SMM is characterized by serum M-protein ≥ 3 g/dL and/or 10–60% clonal plasma cells in the bone marrow, without CRAB features, but with a higher risk of progression to MM (~10% per year). Symptomatic or active MM is defined by ≥10% bone marrow plasma cells or extramedullary plasmacytoma, in addition to CRAB features. Plasma cell leukemia and extramedullary plasmacytoma represent the most aggressive progression of MM relapsed/refractory states [].
The etiology of MM remains largely unclear. However, the pathophysiology of MM is complicated, with high heterogeneity underpinned by a multistep process of initiation and progression implicated to be triggered by environmental and genetic aberrations. Nevertheless, MM may develop without known risk factors and be promoted by multiple factors. The initiation of the asymptomatic premalignant stage is recognized as MGUS. In MGUS, the clonal plasma cells produce light chain immunoglobulin (Ig) and the absence of monoclonal heavy chain Ig expression. Light chain MGUS, a precursor to clinical MM, is thus characterized by an abnormal κ/λ FLC ratio, and patients with MGUS IgG or IgA progress to MM [,]. A more advanced asymptomatic stage is SMM, which may progress to active MM within 5 years. It is shrouded in genetic alterations, mutation, chromosomal translocation, aneuploidy and epigenetic aberrations []. The levels and patterns of these genetic and epigenetic changes are related to patients’ survival, the progression time from MGUS through SMM to symptomatic MM, the time between initiation and progression, and response to standard treatment. Globally, variation in the levels of DNA methylation is common among patients, and progression to MM is associated with hypomethylation when compared to premalignant stages []. However, the perturbation of intricate signaling pathways follows the genetic aberrations. Some of these pivotal signaling pathways include the mitogen-activated protein kinase (MAPK) pathway, nuclear factor kappa B (NF-κB) pathway, and cell cycle pathway, contributing to the development of MM []. NF-κB is considered the key player of inflammation in human bodies that represents the connection between different signaling axes [,]. The role of the NF-κB pathway and its regulator kinase and receptor CD40 in lymphoid neoplasms and MM is well reported in the existing literature []. In addition, the stroma cells in the bone marrow microenvironment (BMM) contribute to the pathogenesis of MM. Bone marrow stroma cells (BMSCs) orchestrate binding to a number of proteins expressed on MM cells to enhance their retention in the BMM. Further interactions of MM cells with BMM induce secretion of soluble growth factors and cytokines, including insulin-like growth factor (IGF-1), vascular endothelial growth factor (VEGF), B-cell activating factor (BAFF), a proliferation-inducing ligand (APRIL), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF- α) []. The integrated secretion of these proteins in the BMM activates intracellular signals that regulate the growth, proliferation, migration, and drug resistance of malignant MM cells [,,].
These malignant bone marrow plasma cells secrete excessive amounts of monoclonal immunoglobulin paraproteins, leading to hematopoietic dysfunction, bone deterioration, and end-organ failure []. Of importance, the preponderance of monoclonal immunoglobulin (paraprotein or M-protein) secreted by malignant plasma cells causes hyperviscosity, amyloidosis, fatigue, and recurrent infections. The monoclonal immunoglobulin infiltrates vital organs, causing renal dysfunction [].
The global incidence of MM has steadily increased by 143% since 1975, making it the second most prevalent blood cancer following leukemia, with the attendant high morbidity and mortality [,]. Global deaths due to neoplasms increased by 94% from 1990 to 2016. According to the Global Cancer Observatory (GLOBOCAN) statistics, there were 160,000 cases of MM globally in 2018, accounting for 0.9% of all cancer diagnoses, and about 90,000 of those cases were male and 70,000 were female []. In the latest GLOBOCAN estimates, there were 176,404 new cases of MM globally, with 117,077 new deaths from MM in 2020 []. These estimates represent 0.9% newly diagnosed cases and 1.2% new deaths of all reported cancer types globally. MM contributes to up to 10% of hematologic neoplasms. Less than two-thirds of people under 40 seem to experience it more regularly than people over 40, and the median age of diagnosis is 65 years [].
Currently, MM is an insidious and intractable disease; relapses and drug resistance still remain significant challenges and an undaunting source of worry to clinicians. However, recent advancements in understanding the molecular and cellular background of MM have led to the development of effective chemotherapy, stem cell transplantation, proteasome inhibitors (PI), immunomodulatory drugs (IMiDs), monoclonal antibodies, bispecific antibodies, and chimeric antigen receptor T-cell immunotherapy (CAR-T) [,]. The main goals of treatment of MM are to block disease progression, suppress malignancy and metastasis, mitigate MM-related complications, and increase survival. The approval of multiple active agents in the treatment of MM has resulted in a number of drug combination regimens that can be used first-line and in relapsed settings. A triplet combination regimen may be considered for MM patients ineligible for stem cell transplantation and then followed with maintenance therapy until toxicity limits the use. The foremost frontline triplet therapy is RVd (lenalidomide, bortezomib, and dexamethasone), which is the current standard for initial treatment [,]. Other proteasome inhibitors can be used for MM treatment, including carfilzomib []. However, carfilzomib treatment has been associated with several adverse effects []. The new generation proteasome inhibitors and IMiDs, including ixazomib, pomalidomide, isatuximab-containing regimens, and chimeric antigen receptor (CAR) T-cell therapy, are being used to manage relapses with evident efficacy [,]. Despite significant improvements in the clinical treatment strategies and increased survival rates of MM patients, MM remains incurable due to inevitable relapses and drug resistance during maintenance therapy.
1.2. The Gut Microbiome
Human microbiota is the ecological community of commensal, symbiotic, and pathogenic microorganisms colonizing the human body, including the GIT, respiratory system, oral cavity, skin, and female reproductive system [,]. It offers many health benefits to the host, including immunity regulation and response, reaction to infection, energy metabolism and nutrient metabolite uptake, hematopoiesis and neurobehavioral traits, GIT maintenance and integrity, and biosynthesis of steroids, vitamins, neurotransmitters, and hormones [,,,]. The human gut is a natural habitat that harbors billions of microorganisms, which form a complex ecosystem known as the gut microbiota []. Although the composition of microbiota is compositionally dynamic throughout life, infancy and early childhood shape the microbial population, which is still vulnerable to changes by diseases and antibiotics. Bacteria in the phyla of Actinobacteria, Bacteroidetes, Firmicutes, and Proteobacteria are the most studied microorganisms in the gut microbiota []. The genomic content (microbiome) of microbiota is about 100 times the human genome [,]. However, growing evidence indicates the role of human microbiota in the development of cancers, infections, inflammatory, and immune-mediated disorders [,]. The gut microbiota was associated with cancer for the first time in 1970 when germ-free mice subject to 1,2-dimethylhydrazine had less tumor load []. Later studies show that tumorigenesis is ameliorated in germ-free or antibiotic-treated mice [,,]. Studies have shown that the microbiota can affect immune responses related to the innate and adaptive immune system via microbial signals, immune cells, cytokines, chemokines and T-cell homeostasis [,]. The pathogenesis of hematological neoplasms, including lymphoma, acute leukemia, and MM, has been associated with human microbiota. The increasing research effort to understand the pathophysiological machinery underlying the development of MM implicates the gut microbiome []. The emerging mechanistic and signaling cascades for this crucial role implicate gut microbiome taxonomic shift, influence of gut microbiome on the host’s adaptive and innate immune systems, inflammatory pathways and bone marrow microenvironment [,]. Gut microbiome dysbiosis, that is, imbalanced composition and diversity of the gut microbiome, may provoke activation of plasma cells in the bone marrow, leading to clonal selection, genetic translocation and oncogenesis. Microbiome-synthesized bioactive compounds, such as short-chain fatty acids, are capable of modulating NF-κB, inflammatory cytokines and T-helper cells to affect MM pathogenesis []. Activation of T-helper 17 (Th17) cells in the gut may migrate to bone marrow and promote myeloma progression []. The gut microbiome affects the differentiation and migration of Th17 cells in the bone marrow. Alterations in short-chain fatty acids occasioned by altered gut microbiota composition are associated with MM progression []. The findings that the gut microbiome may promote inflammation and/or stimulate the release of immunosuppressive interleukins indicate its pivotal role in the development and progression of MM and therapeutic options, including transplantation []. The development of gut microbiota-targeted immunotherapy, surfacing in the innovative approach to treating MM, underscores the immunological connections between the gut and the transition from asymptomatic MGUS to symptomatic MM.
Therefore, this review is targeted at uncovering the role of the gut microbiome in the pathophysiology of MM, immunological responses, potential mechanisms of gut microbiome-mediated perturbations to the progression of MM and the predominance of certain bacterial taxa in the gut microbiome implicated in driving MM carcinogenesis in recent studies. In addition, we present emerging novel therapeutic strategies, including probiotics, prebiotics, and personalized medicine.
2. Methods
The literature added in the current review was identified through searches in PubMed and Scopus. Articles published between 2020 and 2025 were considered, with emphasis on experimental and clinical studies. Additional references were identified from the bibliographies of relevant papers. The search terms used were Multiple myeloma, Microbiome, Microbiota, Clinical, and Preclinical. We focused our search nearly on the last 5 years. The number of articles screened may be around 40 to 50 articles.
3. Microbiome and Hematologic Cancers
This section discusses how gut microbiome alterations can contribute to the pathogenesis and treatment outcomes of hematologic cancers, including multiple myeloma. Also, how gut microbiota can affect systemic immunity and inflammation. This could affect the disease progression and treatment of hematologic malignancies.
3.1. Role of the Microbiome in Hematological Cancers
The gut microbiome plays a crucial role in pathogenesis and the therapeutic response of hematologic malignancies. Alteration to microbial diversity, or dysbiosis, has been correlated with disease progression and poor clinical outcomes. That is, microbial signatures can influence not only the incidence of hematologic cancers but also treatment tolerance and relapses. For instance, the gut microbiome has been proven as a biomarker for clinical outcomes in allogeneic hematopoietic stem cell transplantation and its manipulation has also been shown to boost therapeutic responses in animal models []. The role of the microbiome composition has also been highlighted in a study that aimed to explore the effects of alterations in the gut microbiome on infections and immunological sequelae during treatment of Korean children, adolescents and young adults with hematologic malignancies. 16S rRNA gene sequencing of 26 oncohematological patients showed a lower abundance of Lachnospiraceae and a higher abundance of Enterococcaceae in their gut microbiome than their healthy counterparts, demonstrating a relationship between microbial configuration and hematologic cancers [].
3.2. Systemic Immunity and Inflammation
The gut microbiome has a well-established impact on systemic immunity and inflammatory processes. Alterations in the gut microbial composition have the capacity to stimulate inflammatory signaling pathways that extend beyond the scope of the gut, impacting systemic immunity as a whole. That is, dysbiosis of the microbiome has been associated with multiple inflammatory and autoimmune diseases such as IBD, multiple sclerosis, type I diabetes, and rheumatoid arthritis, suggesting a role of the microbiome in inducing inflammatory changes on a systemic level [].
In addition to pro-inflammatory effects, alterations in the gut microbiome interfere with the immune system function, susceptibility to infection, and vaccine response impairment. Studies utilizing germ-free (GF) mice showed an impaired immune system in GF mice compared to their gnotobiotic counterparts. Specifically, GF mice lacked proper maturation of the gut-associated lymphoid tissues and isolated lymphoid follicles and developed relatively fewer and smaller Peyer’s patches and mesenteric lymph nodes. Additionally, the intestines of GF mice had immature and defective IgA-producing B cells, intestinal epithelial cells with a lower regenerative rate, and a thinner protective intestinal mucosal layer than gnotobiotic mice, which subjected them to a higher susceptibility to pathogen infections. On a molecular level, GF mice had decreased levels of interleukin-17-producing Th17 cells and defects in regulatory T cells. Of note, some of the immunologic developmental impairment, namely the defective IgA-producing B cells and improperly developed lymphoid structures were, however, reversed with the sustained exposure of GF mice to commensal bacteria, further reinforcing the role of the microbiome in the proper development of the immune system and suggesting a dynamic relationship between them [,].
The gut microbiome activated the innate immune system. It constantly triggers the release of IL-10 from resident macrophages, leading to the stimulation of T cells and the inhibition of Th17 cells, which maintains the function of the intestinal innate immune system. An alternative mechanism through which intestinal commensals regulate innate immunity is through boosting the bone marrow neutrophil response against certain pathogens, such as Streptococcus pneumoniae and Staphylococcus aureus [].
3.3. The Effect of Gut Microbiome on the Immune System
There appears to be a myriad of mechanisms through which the microbiome influences the immune system, potentially leading to carcinogenesis. While the formation of a local chronic inflammatory state is regarded as the most implicated carcinogenic mechanism, some bacteria, such as Helicobacter pylori, induce DNA damage and disrupt essential intracellular signaling pathways involved in regulating mucosal cell growth and proliferation. Helmink et al. further explored the different mechanisms underlying dysbiosis-mediated colorectal cancer, ranging from the production of pro-inflammatory toxins and the induction of reactive oxygen species to interference with signaling pathways and the impairment of antitumor immune functions.
Bacteroides fragilis has been shown to induce colitis in mice, interfere with E-cadherin junctions, and promote β-catenin signaling and IL-8 release in epithelial cells of the colon through its metalloprotease B. fragilis toxin []. Fusobacterium nucleatum has been shown to promote tumorigenesis by aiding tumors in evading the antitumor innate immune system due to the interaction between the Fusobacterium nucleatum Fap2 protein and the NK cell inhibitory receptor TIGIT []. Furthermore, Fusobacterium nucleatum was shown to induce small intestinal and colonic carcinogenesis, increase infiltration of myeloid cell subsets into tumors and activate NF-kB pro-inflammatory signaling in genetically predisposed mice []. Apart from immune dysregulation and alterations in signaling pathways, Escherichia coli and Campylobacter jejuni have proven to promote carcinogenesis through the production of directly genotoxic metabolites colibactin and cytolethal distending toxin, respectively.
Other molecular mechanisms have been elucidated by Honda et al., showing a relationship between the microbiome and the adaptive immune system, namely Th17 and regulatory T-cells. Specifically, the epithelium-adhering microbial species promotes Th17 differentiation and polarization through binding to the intestinal epithelial cells and presenting its antigens on APCs to naïve CD4+ T cells. Differentiated Th17 cells can then release cytokines such as IL-17, which plays a role in epithelial barrier integrity against pathogens. However, certain microbes like segmented filamentous bacteria can trigger Th17-mediated autoimmune responses by inducing epithelial expression of serum amyloid A. Serum amyloid A promotes the release of IL-23 from CX3CR1-expressing cells that can lead to the conversion of differentiated TH17 into pathogenic TH17 that can migrate to the draining lymph nodes, inducing autoimmune disease (Figure 1) [,].
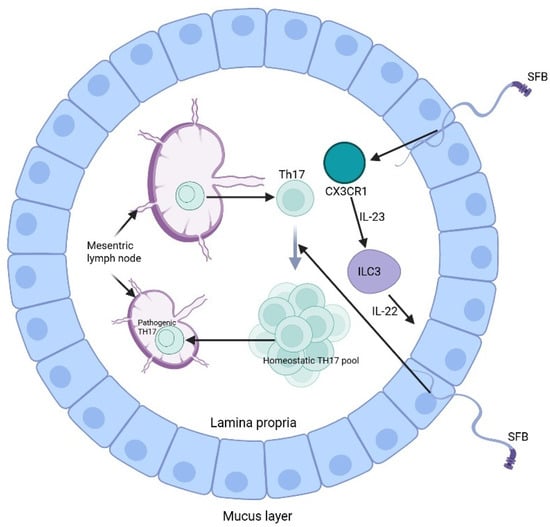
Figure 1.
Induction of Th17 by segmented filamentous bacteria (SFB) (Created in https://BioRender.com).
Furthermore, the gut microbiome plays a crucial role in establishing immune tolerance through its ability to promote regulatory T cell development in the intestinal lamina propria. The colon contains a specific subset of Treg cells, which express the RORγt transcription factor and need microbiota signals for their development []. The absence of microbiota in germ-free mice results in a substantial decrease in peripherally induced Treg cells, which demonstrates the essential role of microbial communities in their development. The RORγt-expressing Treg cells show restricted T cell receptor (TCR) diversity and develop from specific microbial antigens instead of thymic selection processes. Research on TCRs from colonic Treg cells shows that their development and maturation process happens within the colon and needs microbial stimulation. The research demonstrates how the microbiome creates an immune system that adapts to maintain Treg cell populations essential for intestinal health and inflammation prevention [].
3.4. Link Between the Gut Microbiota and Other Cancers
The relationship between microbiome and carcinogenesis has been witnessed in a broad range of cancers. For instance, H. pylori has been implicated in both gastric adenocarcinoma and mucosa-associated lymphoid tissue (MALT lymphoma), which earned it a classification by the World Health Organization as a class I carcinogen []. Colon carcinogenesis has also been found to be heavily influenced by different species of the microbiome. A study conducted by He et al., for example, highlighted the role of Campylobacter jejuni in the formation of colorectal cancer by altering the microbial composition through the DNAse activity of the genotoxin cytolethal distending toxin []. A large body of evidence has also reported an association between the aforementioned Fusobacterium nucleatum and both colorectal carcinoma and adenoma [,,]. In a study conducted by McCoy et al., a higher abundance of Fusobacterium in the normal rectal mucosa was significantly associated with a higher likelihood of the presence of adenomas, and greater local cytokine gene expression, hinting at a potential role of mucosal inflammation underlying the process [].
Other cancers that were proven to be associated with alterations in the microbiome include hepatocellular carcinoma (HCC), biliary tract, and pancreatic cancers []. Helicobacter hepaticus was shown to induce the development of HCC through promoting tumor growth and stimulating the WNT and NF-kB signaling pathways in mice []. Helicobacter species has also been associated with HCC in clinical studies that were able to find Helicobacter in the liver of patients with primary liver carcinoma and not in their healthy counterparts []. The fecal abundance of another microbial species—Escherichia coli—was also found to be higher in the presence of HCC, hinting at a potential link between Escherichia coli and carcinogenesis of the liver and further supporting the role of the microbiome in carcinogenesis in general [].
Gallbladder carcinoma is another cancer where an alteration in the microbiome has been demonstrated to play a role in its pathogenesis. Salmonella typhi infection has been proven as a causative agent of gallbladder carcinoma, as it induces tumorigenesis of predisposed cells with TP53 mutations and c-MYC amplification through stimulating MAPK and AKT pathways []. Higher risk of biliary tract cancers has been associated with multiple alterations in different microbial species, such as higher abundance of Helicobacter species, Methylophilaceae, Fusobacterium, Prevotella, Actinomyces, and Novosphingobium [].
The effect of microbiomes on cancer development extends to include pancreatic cancer. A comprehensive comparison between the salivary microbiota of patients with pancreatic cancer and healthy controls demonstrated a higher prevalence of Neisseria elongata and Streptococcus mitis in the saliva in the pancreatic cancer cohort []. Similarly, a case–control study showed that a higher abundance of salivary Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans incurs the same association [].
4. Interplay Between the Gut Microbiome and Multiple Myeloma
Recently, attention has shifted to the microbiota’s potential role in MM. Research shows that symbiotic microorganisms have impacts beyond the gastrointestinal tract, not only modifying local immune responses through metabolites and interacting with innate immunity, but also affecting systemic immunity by affecting the function and differentiation of immune cells, suggesting the role of the microbiome in regulating the immunological environment linked to the MM pathophysiology []. The main microbial groups associated with MM have been reported in Table 1.

Table 1.
Microbial groups associated with multiple myeloma (MM).
4.1. Microbiota Alteration in Multiple Myeloma
Investigation on mouse models showed that the microbiota may contribute to the development of MM. In humans, four bacterial phyla are the most abundant, which are Firmicutes, Bacteroidetes, Actinobacteria, and Proteobacteria []. The microbiota of MM patients differs from that of healthy people, which is known as microbiota dysbiosis [,]. Patients with MM have demonstrated an increased abundance of Proteobacteria and a decrease in Actinobacteria. Moreover, stool samples of MM subjects showed increased levels of Streptococcus, Klebsiella, and Pseudomonas aeruginosa []. Anaerostipes hadrus, Clostridium butyricum, and Clostridium saccharobutylicum were found to be substantially less prevalent in MM patients than in healthy individuals [,,]. Additionally, adding Clostridium butyricum to an MM mouse model resulted in tumor progression remission. These alterations in microbial composition may affect the microbiota-derived metabolites, which are important in signaling and are considered metabolic substrates. Such alterations can disrupt immune regulation and metabolic processes, hence resulting in disease progression [].
4.2. Microbiota-Derived Metabolites in Multiple Myeloma
4.2.1. Short-Chain Fatty Acids
The metabolites generated by the gut microbiota from the fermentation of insoluble dietary fibers are the short-chain fatty acids (SCFAs), mainly consisting of acetate, propionate, and butyrate []. They are important for intestinal homeostasis and immune regulation via influencing signaling pathways []. SCFAs can activate NF-kB and produce pro-inflammatory cytokines, which include IL-6 and tumor necrosis factor alpha (TNF-α), which contribute to Th17 cell development. Moreover, SCFAs also increase IL-10 levels and forkhead box P3 (FoxP3) expression, causing CD4+ T cell differentiation to Treg cells []. Butyrate, which has been known for its anti-inflammatory effect, can hinder NF-κB activation by lipopolysaccharide (LPS) and also inhibit neutrophils induced by LPS from releasing pro-inflammatory cytokines, such as cytokine-induced neutrophil chemoattractant-2, TNF-α, and nitric oxide []. After myeloma treatment, researchers discovered that negative microscopic residual lesions were linked to butyrate, generated by Eubacterium halii or Faecalibacterium prausnitzii [].
4.2.2. L-Glutamine
A metabolite that is also linked to gut microbiota is L-glutamine. Glutamine can enhance intracellular energy production and also fulfill the increased demand for nucleotide biosynthesis in cancer cells to promote growth, since it is utilized as a nitrogen or carbon source [,]. The metabolism of MM cells consumes an excess amount of glutamine, and so their growth is hampered by the depletion of glutamine [,]. It was found that stool samples from MM subjects had considerably higher levels of bacteria associated with nitrogen utilization and recycling, such as Klebsiella and Streptococcus, compared to healthy individuals. After transplanting Klebsiella pneumoniae into a mouse model of MM, researchers found that bacteria contribute to disease progression through glutamine de novo synthesis. The investigation demonstrated that mice treated with ammonium or urea had substantially higher levels of glutamine concentrations in their serum and feces. The accumulation of abnormal amino acids and blood urea in the host bone marrow microenvironment supported the proliferation of nitrogen-cycling microbiota, leading to the degradation of urea and glutamine synthesis []. These findings suggest that gut microbiota may offer multiple myeloma cells a supply of nitrogen to fulfill their energy needs for rapid proliferation and glutamine production, which would boost the progression of the disease.
4.3. Microbiota and Immune Regulation in Multiple Myeloma
Intestinal dendritic cells detect and present antigens from colonized bacteria in immune tissues such as the Peyer’s patches and mesenteric lymph nodes. Moreover, this cell also differentiates T cells in lymph nodes into Treg, Th17, Th1, and Th2 cells, producing pro- or anti-inflammatory cytokines [,]. The interaction between the gut microbiome and the immune system affects both immune regulation and tumor growth. Excessive Th17 cell inflammation or Treg-induced immune suppression can promote cancer progression []. The intestinal Th17 cell differentiation aids in protecting the mucosal barrier from pathogens. In turn, commensal bacteria in the gut assist in Th17 cell differentiation []. These cells are distinguished by the generation of cytokines, including IL-17A, IL-17F, and IL-22, which are inflammatory mediators. Among them, IL-17 can accelerate multiple myeloma progression by promoting the growth of tumors by influencing the tumor microenvironment [,].
Interestingly, a preclinical investigation has shown that the gut commensal bacterium Prevotella heparinolytica stimulates Th17 cell differentiation in the intestine, which may then migrate to the bone marrow, contributing to multiple myeloma progression. In genetically engineered Vk*MYC mice, disease progression was substantially delayed when either the gut microbiota was disrupted or IL-17 was deficient [,]. This study suggested that there is an immunological axis between IL-17 and eosinophils. Mechanistically, it was discovered that IL-17 in plasma cells stimulates signal transducer and activator of transcription 3 (STAT3) signaling and activates eosinophils, thus boosting the pro-tumor environment. It was found that administering antibodies that target IL-17, its receptor IL-17RA, and IL-5 can disrupt this axis and significantly decrease the number of Th17 cells and eosinophils, hence preventing the progression of multiple myeloma and indicating a promising therapeutic approach (Figure 2) [].
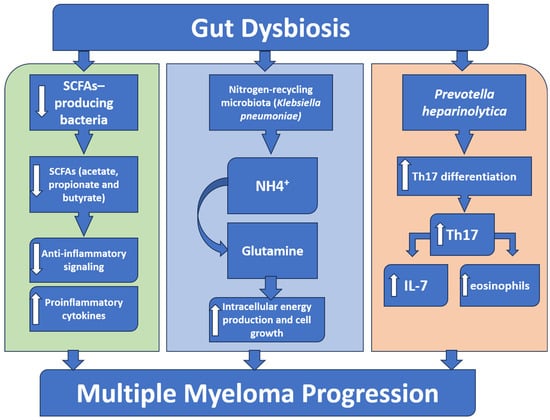
Figure 2.
A schematic diagram showing the role of gut microbiota in multiple myeloma progression. Gut dysbiosis results in a reduced abundance of the beneficial short-chain fatty acids (SCFAs)-producing bacteria, which leads to a decrease in the anti-inflammatory SCFAs, permitting an overactivation of inflammatory signaling. Simultaneously, an increase in nitrogen-recycling bacteria (e.g., Klebsiella pneumoniae) increases glutamine synthesis that boosts the intracellular energy and tumor growth. Furthermore, a specific bacterium like Prevotella heparinolytica supports the intestinal Th17 cell differentiation and migration to the bone marrow, where they generate IL-17, stimulate eosinophils, and trigger additional IL-6 release. Together, these pathways all lead to the progression of multiple myeloma.
5. Role of the Microbiome in Multiple Myeloma Treatment
Owing to their dual mechanisms, IMiDs (i.e., lenalidomide and pomalidomide) are highly considered for MM treatment []. Their multifaceted mechanisms of action involve direct tumoricidal effects, modulation of the bone marrow microenvironment, and enhancement of host immune surveillance. IMiDs have the ability to enhance the immune system to attack cancer cells by binding to the E3 ubiquitin ligase complex, causing the breakdown of the transcription factors important for myeloma plasma cell survival. Inhibition of the survival of myeloma cells can lead to apoptosis, inhibition of pro-myeloma cytokines such as IL-6, and the prevention of cancer progression. In addition, IMiDs promote IL-2 release and T cell activation, inducing significant immunostimulatory effects []. Additionally, IMiDs exert anti-angiogenic effects and disrupt stromal support within the bone marrow niche, further limiting MM cell growth.
In addition, IMiDs were found to contribute to an essential role in immune modulation, enhancing T-cell and natural killer (NK) cell activation while reducing regulatory T-cell function. Their integration into frontline and relapsed/refractory MM regimens, often in combination with proteasome inhibitors and monoclonal antibodies, has significantly improved overall survival. There is a large body of evidence that the gut microbiome can contribute to the immune system of cancer patients; thus, it can affect the IMiDs’ action []. Some microbiota species, such as Lactobacillus and Akkermansia, may produce a synergistic effect with IMiDs through the regulation of T cell differentiation and modulation of inflammation. On the other hand, dysbiosis could impair IMiDs’ action by preventing T cell function and enhancing inflammation. This could lead to shutting down the immune system’s ability to fight myeloma cells [].
CAR-T cells and BiTEs are immunotherapies used in relapsed or refractory MM. CAR-T cells are engineered autologous T cells to target specific tumor antigens, such as B-cell maturation antigen (BCMA). BCMA-directed CAR-T cells recognize and eliminate MM cells through direct cytotoxicity, production of inflammatory cytokines, and induction of apoptosis. CAR-T cells have the ability to modify the T cell antigenic receptors, which in turn could target myeloma cells [,].
Moreover, BiTEs can bind the CD3 protein on T cells and tumor-associated antigens such as BCMA, GPRC5D, or FcRH5 on malignant plasma cells, enhancing the anti-cancer effect. As presented, CAR-T cells and BiTEs rely heavily on T cell function and activity. Therefore, the use of broad-spectrum antibiotics can lead to dysbiosis, compromising the immune cells of the patient and hindering the effectiveness of CAR-T cells and BiTEs [].
5.1. Microbiome-Based Therapies in Multiple Myeloma
Recent scientific evidence supports a significant role for the gut microbiome in shaping immune responses and influencing cancer progression, including hematological malignancies like MM. Alterations in microbial diversity and composition (dysbiosis) have been associated with disease severity, treatment toxicity, and immune dysfunction in MM patients [,]. Consequently, microbiome-based therapies, including fecal microbiota transplantation (FMT), probiotics, and prebiotics, are emerging as novel adjuncts to conventional MM treatments, particularly in the era of precision medicine. The microbiome modulation therapies and their outcomes in MM management have been listed in Table 2.
5.1.1. Fecal Microbiota Transplantation (FMT)
FMT involves the transfer of stool from a healthy donor into a recipient to restore a beneficial microbial composition. Initially established for the treatment of recurrent Clostridioides difficile infection, its application in oncology is growing due to its ability to reverse treatment-related dysbiosis and modulate anti-tumor immunity [].
In MM, the gut microbiome is significantly disrupted following high-dose chemotherapy and autologous stem cell transplantation (ASCT) []. Dysbiosis in this context is linked with increased risk of bloodstream infections, mucosal barrier injury, and impaired immune reconstitution []. Preclinical and early clinical observations suggest that beneficial taxa such as Eubacterium hallii and Faecalibacterium prausnitzii enhance short-chain fatty acid (SCFA) production, promote immune regulation, and support T-cell and natural killer (NK) cell activation, thereby strengthening anti-myeloma immunity. Additionally, preclinical models suggest that FMT can restore microbial diversity and enhance CD8+ T cell function, leading to improved tumor control [,]. A study by Daillère et al. demonstrated that the efficacy of cyclophosphamide in MM-bearing mice was partially dependent on gut microbiota, particularly Enterococcus hirae and Barnesiella intestinihominis, which promoted immune-mediated tumor regression []. In another study, germ-free or antibiotic-treated mice showed inferior responses to immunotherapy unless they received FMT from responder patients []. These results support the immunomodulatory potential of FMT in MM.

Table 2.
The microbiome modulation therapies and the associated outcomes in multiple myeloma (MM).
Table 2.
The microbiome modulation therapies and the associated outcomes in multiple myeloma (MM).
| Therapy/Exposure Type | Type (Clinical/Preclinical) | Design | Sample Size (n) | Population/Model | Intervention/Exposure | Outcomes Measured | Key Findings | Adverse Events/Safety | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Autologous FMT | Clinical | Prospective feasibility study | 7 | Adults undergoing HSCT | Re-infusion of the patient’s own pre-treatment stool (autologous FMT) | Feasibility, gut microbiota restoration, incidence of infections, GVHD, engraftment | Auto-FMT was feasible, safe, and restored gut microbiota diversity with early clinical benefits. | No FMT-related serious adverse events; overall well-tolerated | [] |
| Fecal microbiota diversity changes after auto-HCT | Clinical | Prospective, multicenter observational cohort study | 1325 | Adults undergoing autologous HCT for hematologic malignancies (incl. multiple myeloma, lymphoma) | Fecal microbiome profiling (16S rRNA sequencing) before and after auto-HCT | Microbiota diversity (Shannon index), associations with overall survival, relapse, infectious complications | Gut microbiota diversity loss after auto-HCT predicted poorer survival and higher non-relapse mortality. | Not applicable (observational, sequencing only) | [] |
| Gut microbiome perturbation and ASCT outcomes | Clinical | Prospective observational pilot study | 30 | Patients with multiple myeloma undergoing ASCT | Longitudinal fecal microbiome profiling (16S rRNA sequencing) during ASCT | Microbial diversity, engraftment, infectious complications, treatment response | Post-ASCT microbial diversity loss delayed engraftment, increased infections, and altered treatment response. | Not applicable (observational, sequencing only) | [] |
| Gut microbiome alterations in MM | Clinical | Cross-sectional case–control study | 37 | Newly diagnosed MM patients vs. age-matched controls | Stool microbiome sequencing (16S rRNA & metagenomics) | Microbiome composition, metabolic pathways, and nitrogen metabolism | MM patients showed nitrogen-recycling bacteria enrichment, potentially accelerating disease progression. | Not applicable (observational, sequencing only) | [] |
| Gut microbiome diversity and HSCT outcomes | Clinical | Prospective observational cohort study | 80 | Adults undergoing allogeneic HSCT (incl. MM) | Longitudinal fecal microbiome profiling (16S rRNA sequencing) | GVHD incidence, overall survival, and relapse rates | Higher gut microbial diversity post-HSCT predicted lower mortality and reduced GVHD incidence. | Not applicable (observational, sequencing only) | [] |
| Microbiota diversity and allo-HSCT survival | Clinical | Multicenter observational cohort study (US, EU) | 1362 | Hematologic malignancies (incl. MM) undergoing allo-HSCT | Fecal microbiome profiling (16S rRNA sequencing) | Overall survival, GVHD, infections | Microbial diversity loss strongly predicted increased mortality post-HSCT. | Not applicable (observational, sequencing only) | [] |
Hematopoietic stem cell transplantation (HSCT), Fecal microbiota transplantation (FMT), Graft versus host disease (GVHD), Autologous stem cell transplantation (ASCT).
Clinical trials are now underway to assess FMT’s safety and efficacy in MM. For instance, the phase I trial NCT03772840 investigates FMT in patients undergoing ASCT and its impact on microbiome recovery and immune reconstitution []. Although promising, challenges such as donor standardization, risk of pathogen transmission, and long-term safety remain.
5.1.2. Probiotics and Prebiotics
Probiotics and prebiotics offer non-invasive alternatives to FMT for modulating the gut microbiome. Probiotics are live microorganisms that confer health benefits, while prebiotics are non-digestible food components that selectively support the growth of beneficial microbes.
In MM, specific probiotic strains such as Bifidobacterium longum and Collinsella have shown promise in enhancing gut barrier function, reducing inflammation, and promoting anti-tumor immunity []. A. muciniphila, in particular, has been linked with enhanced responses to PD-1 blockade in solid tumors, potentially through stimulation of IL-12-dependent immune pathways [].
Prebiotics like inulin and fructooligosaccharides (FOS) support SCFA-producing bacteria, which play critical roles in reducing inflammation and maintaining gut homeostasis [,]. SCFAs such as butyrate exert immunomodulatory effects by promoting regulatory Treg differentiation and enhancing mucosal integrity, features especially valuable in MM patients with compromised immunity [].
However, caution is necessary. Case reports of Lactobacillus rhamnoses bacteremia in immunocompromised patients have raised concerns about probiotic safety in MM []. Therefore, strain-specific safety evaluations are crucial before recommending probiotics in routine practice. Ongoing clinical trials assess the effects of microbiota-directed dietary interventions in MM, which may help identify safe, evidence-based recommendations for probiotic/prebiotic use (Figure 3).
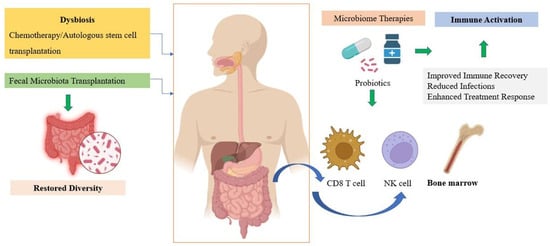
Figure 3.
The microbiome-immune-myeloma cells interplay. Created in https://BioRender.com.
5.1.3. Personalized Medicine
Individual variations in microbiome composition influence treatment response, toxicity, and prognosis, making microbiome profiling a key component of personalized oncology. In MM, studies have shown that specific microbial taxa correlate with disease burden, progression, and immune competence. For example, a higher abundance of Blautia and Faecalibacterium was associated with favorable outcomes, whereas increased Escherichia coli and Klebsiella pneumoniae correlated with worse survival and elevated inflammatory markers [].
Metagenomic sequencing enables the identification of patient-specific dysbiotic signatures, allowing for tailored microbial interventions. Personalized probiotics, selected based on an individual’s gut ecosystem, are under investigation for their ability to restore balance without causing harm. Similarly, individualized dietary regimens rich in specific prebiotics may help promote beneficial SCFA-producing bacteria.
Precision FMT, using defined bacterial consortia rather than whole fecal samples, is another emerging concept. This approach minimizes the risk of pathogen transmission and allows for standardized microbial therapy. For instance, defined microbial cocktails enriched in Clostridiales and Lachnospiraceae families could be engineered to modulate Tregs and anti-inflammatory cytokines [,].
In addition, microbiome-based biomarkers are being developed to predict treatment outcomes. For example, low alpha diversity and abundance of Proteobacteria were associated with increased risk of bloodstream infections post-ASCT in MM patients []. Early identification of such high-risk profiles could allow for proactive microbiome modulation.
By integrating microbiome data with host genomics, transcriptomics, and immune signatures, a systems biology approach can be developed for MM management. This precision medicine model holds the potential to optimize immunotherapy, minimize toxicity, and improve patient-specific outcomes. Microbiome-based therapies are transforming our approach to managing multiple myeloma. Through FMT, probiotics, prebiotics, and personalized modulation strategies, it is possible to recalibrate the gut-immune axis to support better immune recovery, reduce complications, and enhance therapeutic efficacy. Although challenges regarding safety, standardization, and regulatory approval persist, accumulating clinical and translational evidence strongly supports the integration of microbiome-targeted interventions into MM care.
5.2. Safety, Regulation, and Interactions of Microbiota with Standard MM Therapies
As previously discussed, gut microbiota has been found to affect MM disease progression, response to treatment (especially immunotherapies), and toxicity. Therefore, practical integration strategies such as using specific diets, prebiotics and probiotics, and FMT to modulate the gut ecosystem are encouraged. These strategies could significantly enhance efficiency and prevent treatment-related toxicities. Regarding safety, FMT induced some risk, including the possibility of infections, adverse reactions, and non-compliance; therefore, clinical trials and standardized procedures are required to address these issues.
Current microbiota-modulating interventions are generally considered safe in case of short-term treatment, but long-term safety data in MM patients remain scarce. Thus, regulatory protocols are rising, highlighting the safety and quality control standards of microbiota-based therapies to enable clinical adoption.
Moreover, inclusion of microbiota modulation into MM clinical management requires careful consideration of its interactions with standard MM therapies. The gut microbiota can affect the response and toxicity of standard MM treatments, including proteasome inhibitors (e.g., bortezomib, carfilzomib), immunomodulatory drugs (e.g., lenalidomide), corticosteroids (e.g., dexamethasone), monoclonal antibodies, and autologous hematopoietic stem cell transplantation (HSCT) []. Some SCFA–producing bacteria (i.e., Faecalibacterium prausnitzii) have been associated with decreased gastrointestinal toxicity and enhanced treatment efficacy; on the other hand, disruption of microbiota with the use could negatively affect the therapeutic responses of MM therapies [].
The integration of microbiome modulation into clinical management of MM requires optimal timing of treatment cycles, ensuring safety, especially with MM patients’ immunocompromised status, and taking into consideration the patient’s microbiome variability.
6. Limitations of Current Studies
Current studies linking the role of the microbiome in MM disease progression and treatment showed some limitations. Many clinical studies on MM involved a relatively small number of MM patients. This could limit the statistical power of the study; therefore, studies with a larger number of patients are encouraged to validate observed associations and determine robust microbiota signatures relevant to MM treatment outcomes. Moreover, many studies depend on the mechanistic pathways suggested from murine/animal models of MM. These models provide valuable mechanistic insights, but there are differences in microbiota composition, immune system function, and tumor biology compared to humans. Another limitation is the lack of longitudinal data. Cross-sectional or retrospective clinical studies provide a single and limited time point regarding the microbiota. Longitudinal studies are required to study the microbiota dynamics throughout disease progression and treatment cycles of MM. In addition, MM patients frequently receive antibiotics and chemotherapy, both of which profoundly alter the gut microbiota. The microbiota composition changes in case of disease with or without treatment. Tracing these changes is important. Therefore, standardized data regarding the concomitant antibiotic/chemotherapy use is needed to reduce confounding influences.
7. Conclusions and Future Directions
There is a large body of evidence demonstrating the interplay between the gut microbiome and MM. The gut microbial ecosystem plays an essential role not only in immune regulation and inflammation but also in disease onset, progression, and treatment outcomes. Microbial dysbiosis and changes in the microbial-derived metabolites have been linked to critical changes in the tumor microenvironment and resistance to therapy in MM patients. Modulation of the gut microbiome through probiotics, prebiotics, or fecal microbiota transplantation represents a promising avenue that may improve MM treatment. However, despite ongoing preclinical and preliminary clinical findings, there is still a gap of knowledge in the link between the microbiome and MM. The exact mechanistic pathways linking the gut microbiome to MM onset and progression remain incompletely understood. There is an urgent need for established preclinical mechanistic studies and longitudinal clinical trials to better understand this interplay and develop personalized microbiome-based interventions. Longitudinal clinical trials with a larger number of patients are required to validate the findings. Also, the development of effective microbiota-based therapies requires a personalized approach due to individual microbiome variability. Furthermore, the multi-omics approach can be included in future studies to study microbiome composition differences and enhance clinical outcomes.
Author Contributions
M.Y.G.: Conceptualization, Literature review, Writing—Review and Editing, Data Collection. N.K.G.: Literature review, Data collection, Writing—original draft. D.E.M.: Literature review, Data collection, Writing—original draft. A.C.F.: Literature review, Data collection, Writing—original draft. D.B.: Literature review, Data collection, Writing—original draft. P.A.M.: Literature review, Data collection, Writing—original draft. C.C.: Conceptualization, Writing—Review and Editing, Supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Shi, J.; Lu, Y.; Wei, W.; Ma, G.; Li, C.; Li, L.; Wang, Y.; Wang, Y.; Xu, R.; Cui, S. Ferroptosis: A novel pharmacological mechanism against multiple myeloma. Front. Pharmacol. 2025, 16, 1606804. [Google Scholar] [CrossRef]
- Liu, J.; Xie, F.; Yi, Z.G.; Ma, T.; Tie, W.T.; Li, Y.H.; Bai, J.; Zhang, L.S. Gut microbiota deficiency ameliorates multiple myeloma and myeloma-related bone disease by Th17 cells in mice models. J. Cancer 2023, 14, 3191–3202. [Google Scholar] [CrossRef]
- Parrondo, R.D.; Ailawadhi, S.; Cerchione, C. Bispecific antibodies for the treatment of relapsed/refractory multiple myeloma: Updates and future perspectives. Front. Oncol. 2024, 14, 1394048. [Google Scholar] [CrossRef]
- van de Donk, N.; Pawlyn, C.; Yong, K.L. Multiple myeloma. Lancet 2021, 397, 410–427. [Google Scholar] [CrossRef]
- Cerchione, C.; Usmani, S.Z.; Stewart, A.K.; Kaiser, M.; Rasche, L.; Kortum, M.; Mateos, M.V.; Spencer, A.; Sonneveld, P.; Anderson, K.C. Gene Expression Profiling in Multiple Myeloma: Redefining the Paradigm of Risk-Adapted Treatment. Front. Oncol. 2022, 12, 820768. [Google Scholar] [CrossRef]
- Malard, F.; Neri, P.; Bahlis, N.J.; Terpos, E.; Moukalled, N.; Hungria, V.T.M.; Manier, S.; Mohty, M. Multiple myeloma. Nat. Rev. Dis. Primers 2024, 10, 45. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, S.V. Multiple myeloma: 2022 update on diagnosis, risk stratification, and management. Am. J. Hematol. 2022, 97, 1086–1107. [Google Scholar] [CrossRef] [PubMed]
- Abduh, M.S. An overview of multiple myeloma: A monoclonal plasma cell malignancy’s diagnosis, management, and treatment modalities. Saudi J. Biol. Sci. 2024, 31, 103920. [Google Scholar] [CrossRef]
- Kyle, R.A.; Larson, D.R.; Therneau, T.M.; Dispenzieri, A.; Kumar, S.; Cerhan, J.R.; Rajkumar, S.V. Long-Term Follow-up of Monoclonal Gammopathy of Undetermined Significance. N. Engl. J. Med. 2018, 378, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Qu, Y.; Wang, M.; Chu, B.; Chen, W.; Zheng, Y.; Niu, T.; Qian, Z. Pathogenesis and treatment of multiple myeloma. MedComm 2022, 3, e146. [Google Scholar] [CrossRef]
- Al-Mansoori, L.; Al-Jaber, H.; Prince, M.S.; Elrayess, M.A. Role of Inflammatory Cytokines, Growth Factors and Adipokines in Adipogenesis and Insulin Resistance. Inflammation 2022, 45, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Habib, C.N.; Ali, A.E.; Anber, N.H.; George, M.Y. Lactoferrin ameliorates carfilzomib-induced renal and pulmonary deficits: Insights to the inflammasome NLRP3/NF-kappaB and PI3K/Akt/GSK-3beta/MAPK axes. Life Sci. 2023, 335, 122245. [Google Scholar] [CrossRef]
- Zakaria, N.; Menze, E.T.; Elsherbiny, D.A.; Tadros, M.G.; George, M.Y. Lycopene mitigates paclitaxel-induced cognitive impairment in mice; Insights into Nrf2/HO-1, NF-kappaB/NLRP3, and GRP-78/ATF-6 axes. Prog. Neuropsychopharmacol. Biol. Psychiatry 2025, 137, 111262. [Google Scholar] [CrossRef]
- Morgan, G.J.; He, J.; Tytarenko, R.; Patel, P.; Stephens, O.W.; Zhong, S.; Deshpande, S.; Bauer, M.; Weinhold, N.; Schinke, C.; et al. Kinase domain activation through gene rearrangement in multiple myeloma. Leukemia 2018, 32, 2435–2444. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, S.; Ali, T.A.; Faiyaz, A.; Khan, O.S.; Raza, S.S.; Kulinski, M.; Omri, H.E.; Bhat, A.A.; Uddin, S. Cytokine-Mediated Dysregulation of Signaling Pathways in the Pathogenesis of Multiple Myeloma. Int. J. Mol. Sci. 2020, 21, 5002. [Google Scholar] [CrossRef]
- Garcia-Ortiz, A.; Rodriguez-Garcia, Y.; Encinas, J.; Maroto-Martin, E.; Castellano, E.; Teixido, J.; Martinez-Lopez, J. The Role of Tumor Microenvironment in Multiple Myeloma Development and Progression. Cancers 2021, 13, 217. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Liao, M.; Bai, J.; Li, Y.; Chen, Y.; Zhang, L.; Guo, X.; Li, L.; Zhang, L. Exploring the causal relationship between gut microbiota and multiple myeloma risk based on Mendelian randomization and biological annotation. Front. Microbiol. 2024, 15, 1310444. [Google Scholar] [CrossRef]
- Padala, S.A.; Barsouk, A.; Barsouk, A.; Rawla, P.; Vakiti, A.; Kolhe, R.; Kota, V.; Ajebo, G.H. Epidemiology, Staging, and Management of Multiple Myeloma. Med. Sci. 2021, 9, 3. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Zhang, L.; Xiang, Y.; Li, Y.; Zhang, J. Gut microbiome in multiple myeloma: Mechanisms of progression and clinical applications. Front. Immunol. 2022, 13, 1058272. [Google Scholar] [CrossRef]
- Rosinol, L.; Oriol, A.; Rios, R.; Sureda, A.; Blanchard, M.J.; Hernandez, M.T.; Martinez-Martinez, R.; Moraleda, J.M.; Jarque, I.; Bargay, J.; et al. Bortezomib, lenalidomide, and dexamethasone as induction therapy prior to autologous transplant in multiple myeloma. Blood 2019, 134, 1337–1345. [Google Scholar] [CrossRef]
- Botta, C.; Martino, E.A.; Conticello, C.; Mendicino, F.; Vigna, E.; Romano, A.; Palumbo, G.A.; Cerchione, C.; Martinelli, G.; Morabito, F.; et al. Treatment of Lenalidomide Exposed or Refractory Multiple Myeloma: Network Meta-Analysis of Lenalidomide-Sparing Regimens. Front. Oncol. 2021, 11, 643490. [Google Scholar] [CrossRef] [PubMed]
- George, M.Y.; Dabour, M.S.; Rashad, E.; Zordoky, B.N. Empagliflozin Alleviates Carfilzomib-Induced Cardiotoxicity in Mice by Modulating Oxidative Stress, Inflammatory Response, Endoplasmic Reticulum Stress, and Autophagy. Antioxidants 2024, 13, 671. [Google Scholar] [CrossRef]
- Dabour, M.S.; George, M.Y.; Grant, M.K.O.; Zordoky, B.N. Canagliflozin differentially modulates carfilzomib-induced endoplasmic reticulum stress in multiple myeloma and endothelial cells. Arch. Toxicol. 2025, 99, 729–744. [Google Scholar] [CrossRef]
- Durer, C.; Durer, S.; Lee, S.; Chakraborty, R.; Malik, M.N.; Rafae, A.; Zar, M.A.; Kamal, A.; Rosko, N.; Samaras, C.; et al. Treatment of relapsed multiple myeloma: Evidence-based recommendations. Blood Rev. 2020, 39, 100616. [Google Scholar] [CrossRef] [PubMed]
- Group, N.H.W.; Peterson, J.; Garges, S.; Giovanni, M.; McInnes, P.; Wang, L.; Schloss, J.A.; Bonazzi, V.; McEwen, J.E.; Wetterstrand, K.A.; et al. The NIH Human Microbiome Project. Genome Res. 2009, 19, 2317–2323. [Google Scholar] [CrossRef]
- Muguerza-Rodriguez, L.; Mier, A.; Ponce-Gonzalez, J.G.; Casals, C.; Corral-Perez, J. Systematic Review on the Importance of Gut Microbiota in the Regulation of Type 2 Diabetes Through Physical Activity and Exercise. Curr. Issues Mol. Biol. 2025, 47, 505. [Google Scholar] [CrossRef] [PubMed]
- Thursby, E.; Juge, N. Introduction to the human gut microbiota. Biochem. J. 2017, 474, 1823–1836. [Google Scholar] [CrossRef]
- Gamal, N.K.; El-Naga, R.N.; Ayoub, I.M.; George, M.Y. Neuromodulatory effect of troxerutin against doxorubicin and cyclophosphamide-induced cognitive impairment in rats: Potential crosstalk between gut-brain and NLRP3 inflammasome axes. Int. Immunopharmacol. 2025, 149, 114216. [Google Scholar] [CrossRef]
- Xu, K.; Motiwala, Z.; Corona-Avila, I.; Makhanasa, D.; Alkahalifeh, L.; Khan, M.W. The Gut Microbiome and Its Multifaceted Role in Cancer Metabolism, Initiation, and Progression: Insights and Therapeutic Implications. Technol. Cancer Res. Treat. 2025, 24, 1–15. [Google Scholar] [CrossRef]
- Fattizzo, B.; Cavallaro, F.; Folino, F.; Barcellini, W. Recent insights into the role of the microbiome in malignant and benign hematologic diseases. Crit. Rev. Oncol. Hematol. 2021, 160, 103289. [Google Scholar] [CrossRef]
- Reddy, B.S.; Narisawa, T.; Wright, P.; Vukusich, D.; Weisburger, J.H.; Wynder, E.L. Colon carcinogenesis with azoxymethane and dimethylhydrazine in germ-free rats. Cancer Res. 1975, 35, 287–290. [Google Scholar] [PubMed]
- Dapito, D.H.; Mencin, A.; Gwak, G.Y.; Pradere, J.P.; Jang, M.K.; Mederacke, I.; Caviglia, J.M.; Khiabanian, H.; Adeyemi, A.; Bataller, R.; et al. Promotion of hepatocellular carcinoma by the intestinal microbiota and TLR4. Cancer Cell 2012, 21, 504–516. [Google Scholar] [CrossRef]
- Pushalkar, S.; Hundeyin, M.; Daley, D.; Zambirinis, C.P.; Kurz, E.; Mishra, A.; Mohan, N.; Aykut, B.; Usyk, M.; Torres, L.E.; et al. The Pancreatic Cancer Microbiome Promotes Oncogenesis by Induction of Innate and Adaptive Immune Suppression. Cancer Discov. 2018, 8, 403–416. [Google Scholar] [CrossRef]
- Round, J.L.; Mazmanian, S.K. The gut microbiota shapes intestinal immune responses during health and disease. Nat. Rev. Immunol. 2009, 9, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.B.; Zhou, Y.L.; Fang, J.Y. Gut Microbiota in Cancer Immune Response and Immunotherapy. Trends Cancer 2021, 7, 647–660. [Google Scholar] [CrossRef]
- Ahmed, N.; Ghannoum, M.; Gallogly, M.; de Lima, M.; Malek, E. Influence of gut microbiome on multiple myeloma: Friend or foe? J. Immunother. Cancer 2020, 8, e000576. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Gu, J.; Liu, J.; Huang, B.; Li, J. Fecal Microbiota Taxonomic Shifts in Chinese Multiple Myeloma Patients Analyzed by Quantitative Polimerase Chain Reaction (QPCR) and 16S rRNA High-Throughput Sequencing. Med. Sci. Monit. 2019, 25, 8269–8280. [Google Scholar] [CrossRef]
- Calcinotto, A.; Brevi, A.; Chesi, M.; Ferrarese, R.; Garcia Perez, L.; Grioni, M.; Kumar, S.; Garbitt, V.M.; Sharik, M.E.; Henderson, K.J.; et al. Microbiota-driven interleukin-17-producing cells and eosinophils synergize to accelerate multiple myeloma progression. Nat. Commun. 2018, 9, 4832. [Google Scholar] [CrossRef]
- Rodriguez-Garcia, A.; Arroyo, A.; Garcia-Vicente, R.; Morales, M.L.; Gomez-Gordo, R.; Justo, P.; Cuellar, C.; Sanchez-Pina, J.; Lopez, N.; Alonso, R.; et al. Short-Chain Fatty Acid Production by Gut Microbiota Predicts Treatment Response in Multiple Myeloma. Clin. Cancer Res. 2024, 30, 904–917. [Google Scholar] [CrossRef]
- D’Angelo, C.R.; Sudakaran, S.; Callander, N.S. Clinical effects and applications of the gut microbiome in hematologic malignancies. Cancer 2021, 127, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Jung, J.; Lee, J.A.; Lee, E.; Lee, H.; Eom, H.S.; Park, H.J. Understanding gut Microbiome changes in Korean children, adolescents, and young adults with hematologic malignancies. Ann. Hematol. 2025, 104, 2947–2961. [Google Scholar] [CrossRef]
- Helmink, B.A.; Khan, M.A.W.; Hermann, A.; Gopalakrishnan, V.; Wargo, J.A. The microbiome, cancer, and cancer therapy. Nat. Med. 2019, 25, 377–388. [Google Scholar] [CrossRef]
- Kim, D.; Zeng, M.Y.; Núñez, G. The interplay between host immune cells and gut microbiota in chronic inflammatory diseases. Exp. Mol. Med. 2017, 49, e339. [Google Scholar] [CrossRef]
- Deshmukh, H.S.; Liu, Y.; Menkiti, O.R.; Mei, J.; Dai, N.; O’Leary, C.E.; Oliver, P.M.; Kolls, J.K.; Weiser, J.N.; Worthen, G.S. The microbiota regulates neutrophil homeostasis and host resistance to Escherichia coli K1 sepsis in neonatal mice. Nat. Med. 2014, 20, 524–530. [Google Scholar] [CrossRef]
- Purcell, R.V.; Pearson, J.; Aitchison, A.; Dixon, L.; Frizelle, F.A.; Keenan, J.I. Colonization with enterotoxigenic Bacteroides fragilis is associated with early-stage colorectal neoplasia. PLoS ONE 2017, 12, e0171602. [Google Scholar] [CrossRef]
- Gur, C.; Ibrahim, Y.; Isaacson, B.; Yamin, R.; Abed, J.; Gamliel, M.; Enk, J.; Bar-On, Y.; Stanietsky-Kaynan, N.; Coppenhagen-Glazer, S.; et al. Binding of the Fap2 Protein of Fusobacterium nucleatum to Human Inhibitory Receptor TIGIT Protects Tumors from Immune Cell Attack. Immunity 2015, 42, 344–355. [Google Scholar] [CrossRef]
- Kostic, A.D.; Chun, E.; Robertson, L.; Glickman, J.N.; Gallini, C.A.; Michaud, M.; Clancy, T.E.; Chung, D.C.; Lochhead, P.; Hold, G.L.; et al. Fusobacterium nucleatum Potentiates Intestinal Tumorigenesis and Modulates the Tumor-Immune Microenvironment. Cell Host Microbe 2013, 14, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Honda, K.; Littman, D.R. The microbiota in adaptive immune homeostasis and disease. Nature 2016, 535, 75–84. [Google Scholar] [CrossRef]
- Wu, H.-J.; Ivanov, I.I.; Darce, J.; Hattori, K.; Shima, T.; Umesaki, Y.; Littman, D.R.; Benoist, C.; Mathis, D. Gut-Residing Segmented Filamentous Bacteria Drive Autoimmune Arthritis via T Helper 17 Cells. Immunity 2010, 32, 815–827. [Google Scholar] [CrossRef] [PubMed]
- Ohnmacht, C.; Park, J.-H.; Cording, S.; Wing, J.B.; Atarashi, K.; Obata, Y.; Gaboriau-Routhiau, V.; Marques, R.; Dulauroy, S.; Fedoseeva, M.; et al. The microbiota regulates type 2 immunity through RORγt+T cells. Sciences (Am. Assoc. Adv. Sci.) 2015, 349, 989–993. [Google Scholar] [CrossRef]
- He, Z.; Gharaibeh, R.Z.; Newsome, R.C.; Pope, J.L.; Dougherty, M.W.; Tomkovich, S.; Pons, B.; Mirey, G.; Vignard, J.; Hendrixson, D.R.; et al. Campylobacter jejuni promotes colorectal tumorigenesis through the action of cytolethal distending toxin. Gut 2019, 68, 289–300. [Google Scholar] [CrossRef]
- Castellarin, M.; Warren, R.L.; Freeman, J.D.; Dreolini, L.; Krzywinski, M.; Strauss, J.; Barnes, R.; Watson, P.; Allen-Vercoe, E.; Moore, R.A.; et al. Fusobacterium nucleatum infection is prevalent in human colorectal carcinoma. Genome Res. 2012, 22, 299–306. [Google Scholar] [CrossRef]
- Rubinstein, M.R.; Wang, X.; Liu, W.; Hao, Y.; Cai, G.; Han, Y.W. Fusobacterium nucleatum Promotes Colorectal Carcinogenesis by Modulating E-Cadherin/β-Catenin Signaling via its FadA Adhesin. Cell Host Microbe 2013, 14, 195–206. [Google Scholar] [CrossRef] [PubMed]
- McCoy, A.N.; Araújo-Pérez, F.; Azcárate-Peril, A.; Yeh, J.J.; Sandler, R.S.; Keku, T.O. Fusobacterium Is Associated with Colorectal Adenomas. PLoS ONE 2013, 8, e53653. [Google Scholar] [CrossRef] [PubMed]
- Mima, K.; Nakagawa, S.; Sawayama, H.; Ishimoto, T.; Imai, K.; Iwatsuki, M.; Hashimoto, D.; Baba, Y.; Yamashita, Y.-i.; Yoshida, N.; et al. The microbiome and hepatobiliary-pancreatic cancers. Cancer Lett. 2017, 402, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Ward, J.M.; Fox, J.G.; Anver, M.R.; Haines, D.C.; George, C.V.; Collins, M.J.; Gorelick, P.L.; Nagashima, K.; Gonda, M.A.; Gilden, R.V.; et al. Chronic Active Hepatitis and Associated Liver Tumors in Mice Caused by a Presistent Bacterial Infection With a Novel Helicobacter Species. JNCI J. Natl. Cancer Inst. 1994, 86, 1222–1227. [Google Scholar] [CrossRef]
- Huang, Y.; Fan, X.G.; Wang, Z.M.; Zhou, J.H.; Tian, X.F.; Li, N. Identification of helicobacter species in human liver samples from patients with primary hepatocellular carcinoma. J. Clin. Pathol. 2004, 57, 1273–1277. [Google Scholar] [CrossRef]
- Grąt, M.; Wronka, K.M.; Krasnodębski, M.; Masior, Ł.; Lewandowski, Z.; Kosińska, I.; Grąt, K.; Stypułkowski, J.; Rejowski, S.; Wasilewicz, M.; et al. Profile of Gut Microbiota Associated with the Presence of Hepatocellular Cancer in Patients with Liver Cirrhosis. Transplant. Proc. 2016, 48, 1687–1691. [Google Scholar] [CrossRef]
- Scanu, T.; Spaapen, R.M.; Bakker, J.M.; Pratap, C.B.; Wu, L.-E.; Hofland, I.; Broeks, A.; Shukla, V.K.; Kumar, M.; Janssen, H.; et al. Salmonella Manipulation of Host Signaling Pathways Provokes Cellular Transformation Associated with Gallbladder Carcinoma. Cell Host Microbe 2015, 17, 763–774. [Google Scholar] [CrossRef]
- Farrell, J.J.; Zhang, L.; Zhou, H.; Chia, D.; Elashoff, D.; Akin, D.; Paster, B.J.; Joshipura, K.; Wong, D.T.W. Variations of oral microbiota are associated with pancreatic diseases including pancreatic cancer. Gut 2012, 61, 582–588. [Google Scholar] [CrossRef]
- Fan, X.; Alekseyenko, A.V.; Wu, J.; Peters, B.A.; Jacobs, E.J.; Gapstur, S.M.; Purdue, M.P.; Abnet, C.C.; Stolzenberg-Solomon, R.; Miller, G.; et al. Human oral microbiome and prospective risk for pancreatic cancer: A population-based nested case-control study. Gut 2018, 67, 120–127. [Google Scholar] [CrossRef]
- Pianko, M.J.; Golob, J.L. Host-microbe interactions and outcomes in multiple myeloma and hematopoietic stem cell transplantation. Cancer Metastasis Rev. 2022, 41, 367–382. [Google Scholar] [CrossRef]
- Jian, X.; Zhu, Y.; Ouyang, J.; Wang, Y.; Lei, Q.; Xia, J.; Guan, Y.; Zhang, J.; Guo, J.; He, Y.; et al. Alterations of gut microbiome accelerate multiple myeloma progression by increasing the relative abundances of nitrogen-recycling bacteria. Microbiome 2020, 8, 74. [Google Scholar] [CrossRef]
- Konishi, H.; Saito, T.; Takahashi, S.; Tanaka, H.; Okuda, K.; Akutsu, H.; Dokoshi, T.; Sakatani, A.; Takahashi, K.; Ando, K.; et al. The butyrate derived from probiotic Clostridium butyricum exhibits an inhibitory effect on multiple myeloma through cell death induction. Sci. Rep. 2025, 15, 11919. [Google Scholar] [CrossRef] [PubMed]
- Pianko, M.J.; Devlin, S.M.; Littmann, E.R.; Chansakul, A.; Mastey, D.; Salcedo, M.; Fontana, E.; Ling, L.; Tavitian, E.; Slingerland, J.B.; et al. Minimal residual disease negativity in multiple myeloma is associated with intestinal microbiota composition. Blood Adv. 2019, 3, 2040–2044. [Google Scholar] [CrossRef]
- Khanna, S.; Tosh, P.K. A Clinician’s Primer on the Role of the Microbiome in Human Health and Disease. Mayo Clin. Proc. 2014, 89, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.; Chen, D.; Zhang, K.; Zhang, W.; Liu, T.; Wang, S.; Dai, X.; Wang, B.; Zhong, W.; Cao, H. Gut microbiota-derived short-chain fatty acids and colorectal cancer: Ready for clinical translation? Cancer Lett. 2022, 526, 225–235. [Google Scholar] [CrossRef]
- Mirzaei, R.; Afaghi, A.; Babakhani, S.; Sohrabi, M.R.; Hosseini-Fard, S.R.; Babolhavaeji, K.; Khani Ali Akbari, S.; Yousefimashouf, R.; Karampoor, S. Role of microbiota-derived short-chain fatty acids in cancer development and prevention. Biomed. Pharmacother. 2021, 139, 111619. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H.; Park, J.; Kim, M. Gut Microbiota-Derived Short-Chain Fatty Acids, T Cells, and Inflammation. Immune Netw. 2014, 14, 277. [Google Scholar] [CrossRef]
- Vinolo, M.A.R.; Rodrigues, H.G.; Hatanaka, E.; Sato, F.T.; Sampaio, S.C.; Curi, R. Suppressive effect of short-chain fatty acids on production of proinflammatory mediators by neutrophils. J. Nutr. Biochem. 2011, 22, 849–855. [Google Scholar] [CrossRef]
- Kodama, M.; Nakayama, K.I. A second Warburg-like effect in cancer metabolism: The metabolic shift of glutamine-derived nitrogen. BioEssays 2020, 42, 2000169. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Bai, C.; Ruan, Y.; Liu, M.; Chu, Q.; Qiu, L.; Yang, C.; Li, B. Coordinative metabolism of glutamine carbon and nitrogen in proliferating cancer cells under hypoxia. Nat. Commun. 2019, 10, 201. [Google Scholar] [CrossRef] [PubMed]
- Bolzoni, M.; Chiu, M.; Accardi, F.; Vescovini, R.; Airoldi, I.; Storti, P.; Todoerti, K.; Agnelli, L.; Missale, G.; Andreoli, R.; et al. Dependence on glutamine uptake and glutamine addiction characterize myeloma cells: A new attractive target. Blood 2016, 128, 667–679. [Google Scholar] [CrossRef]
- Chiu, M.; Toscani, D.; Marchica, V.; Taurino, G.; Costa, F.; Bianchi, M.G.; Andreoli, R.; Franceschi, V.; Storti, P.; Burroughs-Garcia, J.; et al. Myeloma Cells Deplete Bone Marrow Glutamine and Inhibit Osteoblast Differentiation Limiting Asparagine Availability. Cancers 2020, 12, 3267. [Google Scholar] [CrossRef]
- Niess, J.H.; Reinecker, H.-C. Dendritic cells in the recognition of intestinal microbiota. Cell. Microbiol. 2006, 8, 558–564. [Google Scholar] [CrossRef]
- Rescigno, M. Intestinal Dendritic Cells. In Advances in Immunology; Elsevier: Amsterdam, The Netherlands, 2010; pp. 109–138. [Google Scholar] [CrossRef]
- Knochelmann, H.M.; Dwyer, C.J.; Bailey, S.R.; Amaya, S.M.; Elston, D.M.; Mazza-McCrann, J.M.; Paulos, C.M. When worlds collide: Th17 and Treg cells in cancer and autoimmunity. Cell. Mol. Immunol. 2018, 15, 458–469. [Google Scholar] [CrossRef] [PubMed]
- Sano, T.; Kageyama, T.; Fang, V.; Kedmi, R.; Martinez, C.S.; Talbot, J.; Chen, A.; Cabrera, I.; Gorshko, O.; Kurakake, R.; et al. Redundant cytokine requirement for intestinal microbiota-induced Th17 cell differentiation in draining lymph nodes. Cell Rep. 2021, 36, 109608. [Google Scholar] [CrossRef]
- Chang, S.H. T helper 17 (Th17) cells and interleukin-17 (IL-17) in cancer. Arch. Pharmacal Res. 2019, 42, 549–559. [Google Scholar] [CrossRef]
- Rossi, M.; Altomare, E.; Botta, C.; Gallo Cantafio, M.E.; Sarvide, S.; Caracciolo, D.; Riillo, C.; Gaspari, M.; Taverna, D.; Conforti, F.; et al. miR-21 antagonism abrogates Th17 tumor promoting functions in multiple myeloma. Leukemia 2021, 35, 823–834. [Google Scholar] [CrossRef]
- Arumugam, M.; Raes, J.; Pelletier, E.; Le Paslier, D.; Yamada, T.; Mende, D.R.; Fernandes, G.R.; Tap, J.; Bruls, T.; Batto, J.-M.; et al. Enterotypes of the human gut microbiome. Nature 2011, 473, 174–180. [Google Scholar] [CrossRef]
- Holstein, S.A.; McCarthy, P.L. Immunomodulatory Drugs in Multiple Myeloma: Mechanisms of Action and Clinical Experience. Drugs 2017, 77, 505–520. [Google Scholar] [CrossRef] [PubMed]
- Colley, A.; Brauns, T.; Sluder, A.E.; Poznansky, M.C.; Gemechu, Y. Immunomodulatory drugs: A promising clinical ally for cancer immunotherapy. Trends Mol. Med. 2024, 30, 765–780. [Google Scholar] [CrossRef]
- Nobels, A.; van Marcke, C.; Jordan, B.F.; Van Hul, M.; Cani, P.D. The gut microbiome and cancer: From tumorigenesis to therapy. Nat. Metab. 2025, 7, 895–917. [Google Scholar] [CrossRef]
- Rastogi, S.; Singh, A. Gut microbiome and human health: Exploring how the probiotic genus Lactobacillus modulate immune responses. Front. Pharmacol. 2022, 13, 1042189. [Google Scholar] [CrossRef]
- Martino, M.; Canale, F.A.; Alati, C.; Vincelli, I.D.; Moscato, T.; Porto, G.; Loteta, B.; Naso, V.; Mazza, M.; Nicolini, F.; et al. CART-Cell Therapy: Recent Advances and New Evidence in Multiple Myeloma. Cancers 2021, 13, 2639. [Google Scholar] [CrossRef]
- Nicolini, F.; Bravaccini, S.; Mazza, M.; Gruszka, A.M.; Tazzari, M.; MartIn-Antonio, B.; Juan, M.; Ibrahim, T.; Maltoni, R.; Martinelli, G.; et al. CAR T cells targeting options in the fight against multiple myeloma. Panminerva Med. 2021, 63, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Swan, D.; Madduri, D.; Hocking, J. CAR-T cell therapy in Multiple Myeloma: Current status and future challenges. Blood Cancer J. 2024, 14, 206. [Google Scholar] [CrossRef]
- Schoultz, I.; Claesson, M.J.; Dominguez-Bello, M.G.; Fak Hallenius, F.; Konturek, P.; Korpela, K.; Laursen, M.F.; Penders, J.; Roager, H.; Vatanen, T.; et al. Gut microbiota development across the lifespan: Disease links and health-promoting interventions. J. Intern. Med. 2025, 297, 560–583. [Google Scholar] [CrossRef]
- Shen, Y.; Fan, N.; Ma, S.X.; Cheng, X.; Yang, X.; Wang, G. Gut Microbiota Dysbiosis: Pathogenesis, Diseases, Prevention, and Therapy. MedComm (2020) 2025, 6, e70168. [Google Scholar] [CrossRef] [PubMed]
- Vinterberg, J.E.; Oddsdottir, J.; Nye, M.; Pinton, P. Management of Recurrent Clostridioides difficile Infection (rCDI): A Systematic Literature Review to Assess the Feasibility of Indirect Treatment Comparison (ITC). Infect. Dis. Ther. 2025, 14, 327–355. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.S.; Kang, Z.R.; Chen, Y.X.; Fang, J.Y. The gut microbiome modulate response to immunotherapy in cancer. Sci. China Life Sci. 2025, 68, 381–396. [Google Scholar] [CrossRef]
- Lei, W.; Zhou, K.; Lei, Y.; Li, Q.; Zhu, H. Gut microbiota shapes cancer immunotherapy responses. npj Biofilms Microbiomes 2025, 11, 143. [Google Scholar] [CrossRef] [PubMed]
- Daillere, R.; Vetizou, M.; Waldschmitt, N.; Yamazaki, T.; Isnard, C.; Poirier-Colame, V.; Duong, C.P.M.; Flament, C.; Lepage, P.; Roberti, M.P.; et al. Enterococcus hirae and Barnesiella intestinihominis Facilitate Cyclophosphamide-Induced Therapeutic Immunomodulatory Effects. Immunity 2016, 45, 931–943. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Chirlaque, C.; Aranda, C.J.; Ocon, B.; Capitan-Canadas, F.; Ortega-Gonzalez, M.; Carrero, J.J.; Suarez, M.D.; Zarzuelo, A.; Sanchez de Medina, F.; Martinez-Augustin, O. Germ-free and Antibiotic-treated Mice are Highly Susceptible to Epithelial Injury in DSS Colitis. J. Crohns Colitis 2016, 10, 1324–1335. [Google Scholar] [CrossRef]
- Li, A.; Bowen, J.M.; Ball, I.A.; Wilson, S.; Yong, A.; Yeung, D.T.; Lee, C.H.; Bryant, R.V.; Costello, S.P.; Ryan, F.J.; et al. Autologous Faecal Microbiota Transplantation to Improve Outcomes of Haematopoietic Stem Cell Transplantation: Results of a Single-Centre Feasibility Study. Biomedicines 2023, 11, 3274. [Google Scholar] [CrossRef]
- Khan, N.; Lindner, S.; Gomes, A.L.C.; Devlin, S.M.; Shah, G.L.; Sung, A.D.; Sauter, C.S.; Landau, H.J.; Dahi, P.B.; Perales, M.A.; et al. Fecal microbiota diversity disruption and clinical outcomes after auto-HCT: A multicenter observational study. Blood 2021, 137, 1527–1537. [Google Scholar] [CrossRef] [PubMed]
- Taur, Y.; Coyte, K.; Schluter, J.; Robilotti, E.; Figueroa, C.; Gjonbalaj, M.; Littmann, E.R.; Ling, L.; Miller, L.; Gyaltshen, Y.; et al. Reconstitution of the gut microbiota of antibiotic-treated patients by autologous fecal microbiota transplant. Sci. Transl. Med. 2018, 10, 460. [Google Scholar] [CrossRef]
- Peled, J.U.; Gomes, A.L.C.; Devlin, S.M.; Littmann, E.R.; Taur, Y.; Sung, A.D.; Weber, D.; Hashimoto, D.; Slingerland, A.E.; Slingerland, J.B.; et al. Microbiota as Predictor of Mortality in Allogeneic Hematopoietic-Cell Transplantation. N. Engl. J. Med. 2020, 382, 822–834. [Google Scholar] [CrossRef]
- Routy, B.; Lenehan, J.G.; Miller, W.H., Jr.; Jamal, R.; Messaoudene, M.; Daisley, B.A.; Hes, C.; Al, K.F.; Martinez-Gili, L.; Puncochar, M.; et al. Fecal microbiota transplantation plus anti-PD-1 immunotherapy in advanced melanoma: A phase I trial. Nat. Med. 2023, 29, 2121–2132. [Google Scholar] [CrossRef]
- Ding, Y.; Hou, Y.; Lao, X. The Role of Akkermansia muciniphila in Disease Regulation. Probiotics Antimicrob. Proteins 2025, 17, 2027–2038. [Google Scholar] [CrossRef]
- Al-Adham, I.S.I.; Agha, A.; Al-Akayleh, F.; Al-Remawi, M.; Jaber, N.; Al Manasur, M.; Collier, P.J. Prebiotics Beyond the Gut: Omics Insights, Artificial Intelligence, and Clinical Trials in Organ-Specific Applications. Probiotics Antimicrob. Proteins 2025, 17, 2500–2521. [Google Scholar] [CrossRef]
- Hughes, R.L.; Alvarado, D.A.; Swanson, K.S.; Holscher, H.D. The Prebiotic Potential of Inulin-Type Fructans: A Systematic Review. Adv. Nutr. 2022, 13, 492–529. [Google Scholar] [CrossRef]
- Mann, E.R.; Lam, Y.K.; Uhlig, H.H. Short-chain fatty acids: Linking diet, the microbiome and immunity. Nat. Rev. Immunol. 2024, 24, 577–595. [Google Scholar] [CrossRef]
- Eze, U.J.; Lal, A.; Elkoush, M.I.; Halytska, M.; Atif, S. Recurrent Lactobacillus Rhamnoses Bacteremia and Complications in an Immunocompromised Patient with History of Probiotic Use: A Case Report. Cureus 2024, 16, e54879. [Google Scholar] [CrossRef]
- Devi, L.S.; Broor, S.; Rautela, R.S.; Grover, S.S.; Chakravarti, A.; Chattopadhya, D. Increasing Prevalence of Escherichia coli and Klebsiella pneumoniae Producing CTX-M-Type Extended-Spectrum Beta-Lactamase, Carbapenemase, and NDM-1 in Patients from a Rural Community with Community Acquired Infections: A 3-Year Study. Int. J. Appl. Basic Med. Res. 2020, 10, 156–163. [Google Scholar] [CrossRef]
- Aghamajidi, A.; Maleki Vareki, S. The Effect of the Gut Microbiota on Systemic and Anti-Tumor Immunity and Response to Systemic Therapy against Cancer. Cancers 2022, 14, 3563. [Google Scholar] [CrossRef]
- Zaplana, T.; Miele, S.; Tolonen, A.C. Lachnospiraceae are emerging industrial biocatalysts and biotherapeutics. Front. Bioeng. Biotechnol. 2023, 11, 1324396. [Google Scholar] [CrossRef] [PubMed]
- Kriss, M.; Hazleton, K.Z.; Nusbacher, N.M.; Martin, C.G.; Lozupone, C.A. Low diversity gut microbiota dysbiosis: Drivers, functional implications and recovery. Curr. Opin. Microbiol. 2018, 44, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Alkharabsheh, O.; Sidiqi, M.H.; Aljama, M.A.; Gertz, M.A.; Frankel, A.E. The Human Microbiota in Multiple Myeloma and Proteasome Inhibitors. Acta Haematol. 2020, 143, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Wei, Y.; Zhu, Y.; Guo, J.; Zhang, J.; He, Y.; Li, X.; Liu, J.; Zhou, W. The Interaction between Gut Microbiota and Host Amino Acids Metabolism in Multiple Myeloma. Cancers 2023, 15, 1942. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).