Outcomes for Primary Central Nervous System Lymphoma from a Single Institution
Abstract
1. Introduction
1.1. Background
1.2. Rationale and Knowledge Gap
1.3. Objective
2. Materials and Methods
2.1. Patients
2.2. Induction and Consolidation
2.3. Statistical Analysis
2.4. Outcome Measures
3. Results
4. Discussion
4.1. Institution-Specific Factors Impacting Care in Our PCNSL Cohort
4.2. PCNSL Outcomes Stratified by Age
4.3. Impact of Racial and Ethnic Characteristics on PCNSL Outcomes
4.4. Treatment Toxicity and Performance Status as Predictors of PCNSL Outcomes in the Elderly
4.5. Maintenance Options for Elderly Patients with PCNSL
4.6. Role of Assessing Discharge Methotrexate Levels Post-Induction
4.7. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Green, K.; Munakomi, S.; Hogg, J.P. Central Nervous System Lymphoma. PubMed. Published 2021. Available online: https://www.ncbi.nlm.nih.gov/books/NBK545145/ (accessed on 5 May 2025).
- Morales-Martinez, A.; Lozano-Sanchez, F.; Duran-Peña, A.; Hoang-Xuan, K.; Houillier, C. Primary Central Nervous System Lymphoma in Elderly Patients: Management and Perspectives. Cancers 2021, 13, 3479. [Google Scholar] [CrossRef]
- Shiels, M.S.; Pfeiffer, R.M.; Besson, C.; Clarke, C.A.; Morton, L.M.; Nogueira, L.; Pawlish, K.; Yanik, E.L.; Suneja, G.; Engels, E.A. Trends in primary central nervous system lymphoma incidence and survival in the U.S. Br. J. Haematol. 2016, 174, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Villano, J.L.; Koshy, M.; Shaikh, H.; Dolecek, T.A.; McCarthy, B.J. Age, gender, and racial differences in incidence and survival in primary CNS lymphoma. Br. J. Cancer 2011, 105, 1414–1418. [Google Scholar] [CrossRef]
- Houillier, C.; Soussain, C.; Ghesquières, H.; Soubeyran, P.; Chinot, O.; Taillandier, L.; Lamy, T.; Choquet, S.; Ahle, G.; Damaj, G.; et al. Management and outcome of primary CNS lymphoma in the modern era. Neurology 2020, 94, e1027–e1039. [Google Scholar] [CrossRef] [PubMed]
- Ozkan, S.G. Primary Central Nervous System Lymphomas: A Single-Center Experience. SiSli Etfal Hastan. Tip Bul./Med. Bull. Sisli Hosp. 2025, 59, 98–105. [Google Scholar] [CrossRef]
- Siegal, T.; Bairey, O. Primary CNS Lymphoma in the Elderly: The Challenge. Acta Haematol. 2019, 141, 138–145. [Google Scholar] [CrossRef]
- Martinez-Calle, N.; Isbell, L.K.; Cwynarski, K.; Schorb, E. Treatment of elderly patients with primary CNS lymphoma. Ann. Lymphoma 2021, 5, 2. [Google Scholar] [CrossRef]
- Martinez-Calle, N.; Poynton, E.; Alchawaf, A.; Kassam, S.; Horan, M.; Rafferty, M.; Kelsey, P.; Scott, G.; Culligan, D.J.; Buckley, H.; et al. Outcomes of older patients with primary central nervous system lymphoma treated in routine clinical practice in the UK: Methotrexate dose intensity correlates with response and survival. Br. J. Haematol. 2020, 190, 394–404. [Google Scholar] [CrossRef]
- Schorb, E.; Fox, C.P.; Kasenda, B.; Linton, K.; Martinez-Calle, N.; Calimeri, T.; Ninkovic, S.; Eyre, T.A.; Cummin, T.; Smith, J.; et al. Induction therapy with the MATRix regimen in patients with newly diagnosed primary diffuse large B-cell lymphoma of the central nervous system—An international study of feasibility and efficacy in routine clinical practice. Br. J. Haematol. 2020, 189, 879–887. [Google Scholar] [CrossRef]
- Wismann, J.; Sommer-Sørensen, R.H.; Kofoed, M.S.; Halle, B.; Pedersen, C.B.; Schulz, M.K.; Grønhøj, M.H.; Larsen, T.S.; Møller, M.B.; Poulsen, F.R. Diagnosis, treatment, and outcome of primary CNS lymphoma—A single-center experience. Acta Neurochir. 2022, 164, 1365–1373. [Google Scholar] [CrossRef] [PubMed]
- Nilles, C.; Delgadillo, D.; Sarazin, M.; Nichelli, L.; Mokhtari, K.; Mathon, B.; Choquet, S.; Feuvret, L.; Alentorn, A.; Ribeiro, M.; et al. Primary CNS lymphoma of the corpus callosum: Presentation and neurocognitive outcomes. J. Neuro-Oncol. 2022, 158, 99–109. [Google Scholar] [CrossRef]
- CTCAE and AE Reporting. Cancer.gov. Published 2 September 2025. Available online: https://dctd.cancer.gov/research/ctep-trials/for-sites/adverse-events#ctcae (accessed on 15 October 2025).
- Kota, V.K.; Bradshaw, D.; Kota, D.; Mian, M.; Jimenez, S.; Corley, C.; Ashley, R.; Shishkina, I.; Brown, J.; Giddens, G.; et al. Academic Outreach and Collaboration with Community Oncology Practices to Enhance Referrals and Reduce Disparities in Hematological Malignancies. Blood 2023, 142 (Suppl. 1), 7238. [Google Scholar] [CrossRef]
- Illerhaus, G.; Ferreri, A.J.; Binder, M.; Borchmann, P.; Hasenkamp, J.; Stilgenbauer, S.; Roeth, A.; Weber, T.; Egerer, G.; Ernst, T.; et al. Effects on Survival of Non-Myeloablative Chemoimmunotherapy Compared to High-Dose Chemotherapy Followed By Autologous Stem Cell Transplantation (HDC-ASCT) As Consolidation Therapy in Patients with Primary CNS Lymphoma—Results of an International Randomized Phase III Trial (MATRix/IELSG43). Blood 2022, 140 (Suppl. 2), LBA-3. [Google Scholar] [CrossRef]
- Schorb, E.; Isbell, L.K.; Kerkhoff, A.; Mathas, S.; Braulke, F.; Egerer, G.; Röth, A.; Schliffke, S.; Borchmann, P.; Brunnberg, U.; et al. High-dose chemotherapy and autologous haematopoietic stem-cell transplantation in older, fit patients with primary diffuse large B-cell CNS lymphoma (MARTA): A single-arm, phase 2 trial. Lancet Haematol. 2024, 11, e196–e205. [Google Scholar] [CrossRef]
- Omid-Fard, N.; Puac-Polanco, P.; Torres, C.H.; Hamilton, L.; Nguyen, T.B. Imaging Features of Immunodeficiency-Associated Primary CNS Lymphoma: A Review. Can. Assoc. Radiol. J. 2024, 76, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Pulido, J.S.; Vierkant, R.A.; Olson, J.E.; Abrey, L.; Schiff, D.; O’NEill, B.P. Racial differences in primary central nervous system lymphoma incidence and survival rates. Neuro-Oncology 2009, 11, 318–322. [Google Scholar] [CrossRef] [PubMed]
- Rosas, D.; Velez, E.T.; Velez-Mejia, C.; Liu, Q.; Michalek, J.; Duque, A.E.D. Racial and Ethnic Disparities for Primary CNS Lymphoma: A National Cancer Database Analysis with Emphasis on Hispanics. Blood 2023, 142, 4508. [Google Scholar] [CrossRef]
- Ananth, S.; Martinez, M.J.; Garza, J.; Surapaneni, P.; Snedden, T.W.; Gregorio, D.; Kakarla, S.; Rawlings, J.; Michalek, J.; Liu, Q.; et al. Never too young: Retrospective study on age and racial disparity in the outcome of primary CNS Lymphoma in south Texas. J. Clin. Oncol. 2020, 38, e14553. [Google Scholar] [CrossRef]
- Schorb, E.; Kasenda, B.; Ihorst, G.; Scherer, F.; Wendler, J.; Isbell, L.; Fricker, H.; Finke, J.; Illerhaus, G. High-dose chemotherapy and autologous stem cell transplant in elderly patients with primary CNS lymphoma: A pilot study. Blood Adv. 2020, 4, 3378–3381. [Google Scholar] [CrossRef]
- Rubenstein, J.L.; Hsi, E.D.; Johnson, J.L.; Jung, S.-H.; Nakashima, M.O.; Grant, B.; Cheson, B.D.; Kaplan, L.D. Intensive Chemotherapy and Immunotherapy in Patients With Newly Diagnosed Primary CNS Lymphoma: CALGB 50202 (Alliance 50202). J. Clin. Oncol. 2013, 31, 3061–3068. [Google Scholar] [CrossRef]
- Grommes, C.; Pastore, A.; Palaskas, N.; Tang, S.S.; Campos, C.; Schartz, D.; Codega, P.; Nichol, D.; Clark, O.; Hsieh, W.-Y.; et al. Ibrutinib Unmasks Critical Role of Bruton Tyrosine Kinase in Primary CNS Lymphoma. Cancer Discov. 2017, 7, 1018–1029. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Liu, J. Bruton’s tyrosine kinase inhibitors in the treatment of primary central nervous system lymphoma: A mini-review. Front. Oncol. 2022, 12, 1034668. [Google Scholar] [CrossRef]
- Study of Tirabrutinib (ONO-4059) in Patients With Primary Central Nervous System Lymphoma (PROSPECT Study). Available online: https://clinicaltrials.gov/study/NCT04947319 (accessed on 16 October 2025).
- Zhang, Y.; Li, Y.; Zhuang, Z.; Wang, W.; Wei, C.; Zhao, D.; Zhou, D.; Zhang, W. Preliminary Evaluation of Zanubrutinib-Containing Regimens in DLBCL and the Cerebrospinal Fluid Distribution of Zanubrutinib: A 13-Case Series. Front. Oncol. 2021, 11, 760405. [Google Scholar] [CrossRef]
- Song, J.; Liu, H.; Jiao, Z.; Gao, D.; Ding, K.; Wang, Y.; Yu, H.; Shao, Y.; Jiang, H.; Gao, S.; et al. Zanubrutinib, Lenalidomide, Rituximab, Temozolomide and Methotrexate (RLZT±MTX) As First-Line Treatment for Newly Diagnosed PCNSL: A Prospective, Open-Lable, Multicenter Clinical Trial. Blood 2022, 140 (Suppl. 1), 3739–3740. [Google Scholar] [CrossRef]
- Ferreri, A.; Illerhaus, G.; Doorduijn, J.; Auer, D.; Bromberg, J.; Calimeri, T.; Cwynarski, K.; Fox, C.; Hoang-Xuan, K.; Malaise, D.; et al. Primary central nervous system lymphomas: EHA–ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2024, 35, 491–507. [Google Scholar] [CrossRef]
- Lin, Z.; Chen, B.; Ma, J.; Ma, Y.; Kang, H. Investigation of High Dose Cytarabine Plus Zanubrutinib in Relapsed/Refractory Primary Central Nervous System Lymphoma. Blood 2022, 140 (Suppl. 1), 12070. [Google Scholar] [CrossRef]
- Zanubrutinib Monotherapy in Relapsed/Refractory Central Nervous System Lymphoma. Available online: https://clinicaltrials.gov/study/NCT05117814 (accessed on 16 October 2025).
- Xing, Y.; Zhao, K.; Zhang, Y.; Wang, Y. BTK inhibition in primary central nervous system lymphoma: Mechanisms, clinical efficacy, and future perspectives. Front. Oncol. 2024, 14, 1463505. [Google Scholar] [CrossRef] [PubMed]
- Binder, A.F.; Burdette, S.; Galanis, P.; Birchmeier, K.; Handley, N.; Piddoubny, M. Decreasing Cost and Decreasing Length of Stay After Implementation of Updated High-Dose Methotrexate Discharge Criteria. JCO Oncol. Pract. 2020, 16, e791–e796. [Google Scholar] [CrossRef] [PubMed]
| N = 30 | CR/CRi/CRu | PR | PD | p-Value |
|---|---|---|---|---|
| N (%) | 15 (45) | 5 (16) | 10 (39) | |
| Age | 0.89 | |||
| <65 years | 6 (40%) | 2 (40%) | 5 (50%) | |
| ≥65 years | 9 (60%) | 3 (60%) | 5 (50%) | |
| Gender | 0.61 | |||
| Male | 7 (47%) | 3 (60%) | 6 (60%) | |
| Female | 8 (53%) | 2 (40%) | 4 (40%) | |
| Baseline LDH | 0.88 | |||
| >ULN | 5 (33%) | 2 (40%) | 5 (50%) | |
| <ULN | 9 (60%) | 2 (40%) | 5 (50%) | |
| Unavailable | 1 (7%) | 1 (20%) | 0 | |
| Baseline HIV status | 0.49 | |||
| Positive | 0 | 0 | 1 (10%) | |
| Negative | 15 (100%) | 5 (100%) | 9 (90%) | |
| Baseline creatinine clearance | 0.23 | |||
| ≥60 | 14 (93%) | 3 (60%) | 7 (70%) | |
| <60 | 1 (7%) | 2 (40%) | 3 (30%) | |
| Baseline CSF positive | 0.56 | |||
| Yes | 2 (13%) | 1 (20%) | 2 (20%) | |
| No | 8 (53%) | 1 (20%) | 5 (50%) | |
| Unavailable | 5 (33%) | 3 (60%) | 3 (30%) | |
| Seizures at presentation | <0.001 * | |||
| Yes | 0 | 0 | 4 (40%) | |
| No | 15 (100%) | 5 (100%) | 6 (60%) |
| N = 30 | CR/CRi/CRu | PR | PD | p-Value |
|---|---|---|---|---|
| Induction Regimen | 0.83 | |||
| HD-MTX Alone | 1 (7%) | 0 | 4 (40%) | |
| MTR | 11 (73%) | 5 (100%) | 4 (40%) | |
| R-MVP | 3 (20%) | 0 | 1 (10%) | |
| R | 0 | 0 | 1 (10%) | |
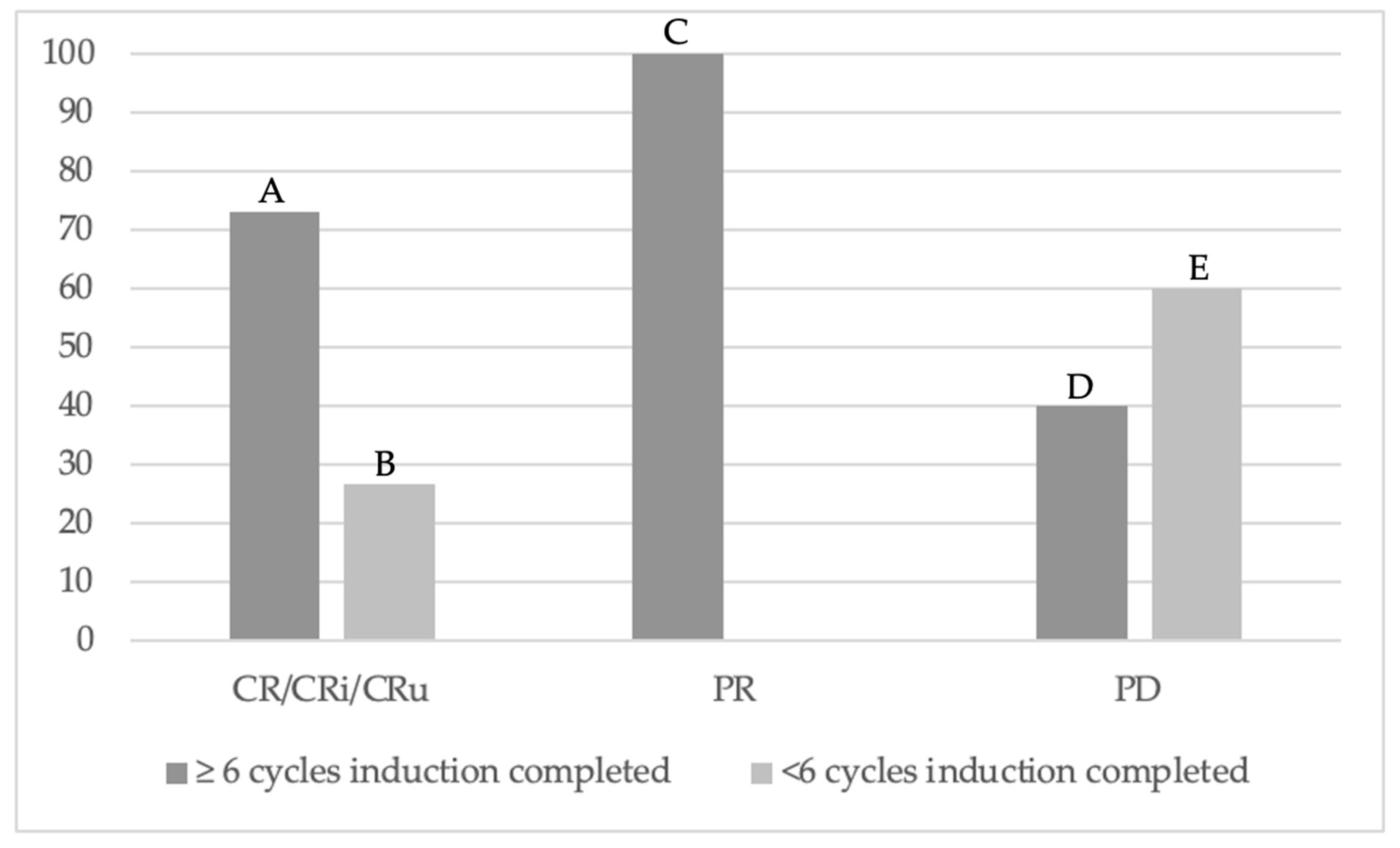
| Number of treatment cycles completed | 0.029 * | |||
| ≥6 | 11 (73%) | 5 (100%) | 4 (40%) | |
| <6 | 4 (27%) | 0 | 6 (60%) | |
| 24-h peak MTX level Median (Range) | 37.7 (1.88–490.9) | 33.99 (23–149.46) | 42.51 (0.99–212.34) | 0.31 |
| Mean Discharge MTX level | 0.013 * | |||
| <0.05 | 4 (27%) | 0 | 4 (40%) | |
| 0.05–0.1 | 5 (33%) | 4 (80%) | 5 (50%) | |
| >0.1 | 4 (27%) | 0 | 1 (10%) | |
| Unavailable | 2 (13%) | 1 (20%) | 0 | |
| End of induction PS (ECOG) | 0.002 * | |||
| 0–2 | 12 (80%) | 4 (80%) | 2 (20%) | |
| >2 | 3 (20%) | 1 (20%) | 8 (80%) | |
| Median follow-up (months) (range) | Total cohort: 12 months (0–66) | |||
| 19 | 30.5 | 4.5 | ||
| Median duration of remission (months) (range) | Total cohort: 16 months (0–103) | |||
| 25 | 40 | 1 | ||
| Status at last follow-up | ||||
| Remission | 13 (87%) | 3 (60%) | 8 (80%) | |
| Relapse | 2 (13%) | 2 (40%) | 2 (20%) | |
| Induction Toxicities | Total | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
|---|---|---|---|---|---|
| Number of individual below toxicities | 82 (100%) | 29 (35%) | 25 (31%) | 18 (22%) | 10 (12%) |
| Non-hematologic | 28 (34%) | 13 (16%) | 6 (7%) | 9 (11%) | 0 (0%) |
| Hepatotoxicity | 20 (24%) | 8 (10%) | 4 (5%) | 8 (10%) | 0 (0%) |
| Nephrotoxicity | 8 (10%) | 5 (6%) | 2 (2%) | 1 (1%) | 0 (0%) |
| Hematologic | 54 (66%) | 16 (20%) | 19 (23%) | 9 (11%) | 10 (12%) |
| Neutropenia | 15 (18%) | 2 (2%) | 5 (6%) | 2 (2%) | 6 (7%) |
| Thrombocytopenia | 16 (20%) | 9 (11%) | 4 (5%) | 1 (1%) | 2 (2%) |
| Anemia | 23 (28%) | 5 (6%) | 10 (12%) | 6 (7%) | 2 (2%) |
| Age ≥ 65 years | 51 (62%) | 19 (23%) | 13 (16%) | 10 (12%) | 9 (11%) |
| Age < 65 years | 31 (38%) | 10 (12%) | 12 (15%) | 8 (10%) | 1 (1%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dontu, S.; Boccucci, J.; Chahin, M.; Keruakous, A.; Jillella, A.; Cortes, J.; Kota, V.; Bryan, L.; Chauhan, A. Outcomes for Primary Central Nervous System Lymphoma from a Single Institution. Hematol. Rep. 2025, 17, 55. https://doi.org/10.3390/hematolrep17060055
Dontu S, Boccucci J, Chahin M, Keruakous A, Jillella A, Cortes J, Kota V, Bryan L, Chauhan A. Outcomes for Primary Central Nervous System Lymphoma from a Single Institution. Hematology Reports. 2025; 17(6):55. https://doi.org/10.3390/hematolrep17060055
Chicago/Turabian StyleDontu, Sruthi, Jacob Boccucci, Michael Chahin, Amany Keruakous, Anand Jillella, Jorge Cortes, Vamsi Kota, Locke Bryan, and Ayushi Chauhan. 2025. "Outcomes for Primary Central Nervous System Lymphoma from a Single Institution" Hematology Reports 17, no. 6: 55. https://doi.org/10.3390/hematolrep17060055
APA StyleDontu, S., Boccucci, J., Chahin, M., Keruakous, A., Jillella, A., Cortes, J., Kota, V., Bryan, L., & Chauhan, A. (2025). Outcomes for Primary Central Nervous System Lymphoma from a Single Institution. Hematology Reports, 17(6), 55. https://doi.org/10.3390/hematolrep17060055






