Abstract
Ocular complications can occur in up to 90% of patients with blood malignancies. Such complications range from direct infiltration to local hemostatic imbalance and treatment-related toxicity. This narrative review is based on a systematic computerized search of the literature conducted until January 2024 and examines the common ocular complications associated with blood cancers. Ocular complications from primary disease include mass effects from ocular adnexal lymphomas and intraocular lymphomas, with B-cell lymphomas accounting for 95% of primary ocular presentations. Secondary disease involvement from systemic hematological malignancies can lead to a wide range of ocular manifestations, such as leukemic retinopathy. Furthermore, toxicity from antineoplastic therapies and ocular graft versus host disease (oGVHD) after hematopoietic stem cell transplantation present additional risks to ocular health. In conclusion, ocular complications in blood cancer patients are an integral part of patient management, requiring regular ophthalmic evaluations and close collaboration between oncologists and ophthalmologists. Advances in therapy and an increased focus on early symptom recognition are essential for preserving vision and enhancing patient quality of life.
1. Introduction
Hematological malignancies represent a set of blood disorders affecting the lymphoid or myeloid lineage. The most frequently reported manifestations of the disease include weight loss, fatigue, and fever; however, eye changes may be observed in up to 90% of affected individuals, depending on the underlying condition [1,2]. All ocular structures may be affected due to a variety of pathological mechanisms, including direct infiltration, local hemostatic imbalance, and treatment-related direct toxicity [3]. Ocular graft versus host disease (GVHD) after hematopoietic stem cell transplantation (HSCT) represents another important complication of cancer therapy, requiring prompt ophthalmological evaluation and treatment [3,4]. Finally, lymphoproliferative diseases may affect the eye primarily as extranodal lymphomas of the orbit, ocular adnexa, and vitreoretinal tissue [5,6,7].
Despite a growing number of reported cases, eye manifestations of blood cancers are relatively unknown for the clinician and may masquerade as common disorders. This narrative review outlines the most common ocular complications observed in patients with hematological malignancies.
2. Primary Disease Involvement of the Eye
Ophthalmologic complications related to hematologic diseases often arise due to primary or secondary involvement of the eye or the ocular adnexa. Reasonably, the primary manifestation of such involvement is directly correlated with the presence of the disease, and the symptoms vary depending on the anatomical structure affected.
Primary or secondary involvement of the eye is considered rare in leukemias as well as an uncommon occurrence in lymphomas. Regardless of the rarity of such lymphomas, a stable increase in incidence during the last decades has been observed, with an annual change of 4.8% from 1973 to 2009 [8]. Approximately 95% of blood tumors primarily affecting the eye are B-cell lymphomas, with only a few cases of T-cell lymphomas, of which the majority are NK T-cell lymphomas [9]. Most cases of ocular lymphomas are of primary involvement of the eye structures, and in only 10–30% of cases, there is a secondary involvement from diffuse lymphomas [10].
Based on their anatomical localization, ocular lymphomas can be classified into ocular adnexal lymphomas and intraocular lymphomas. The first category includes extranodal marginal zone lymphomas (EMZL) (37–68%), follicular lymphomas (FL) (10–23%), diffuse large B-cell lymphomas (DLBCL) (10–15%), and mantle cell lymphomas (MCL) (7–8%) [5]. Rarely also other types, such as Hodgkin lymphoma, Burkitt lymphoma, chronic lymphocytic leukemia, or plasma cell disorders, may involve the eye [5]. Intraocular lymphomas include vitreoretinal lymphomas and secondary intraocular lymphomas.
2.1. Ocular Adnexal Lymphomas
Ocular adnexal lymphomas primarily present symptoms associated with their mass effect. These include proptosis, reduced eye motility, pain, ptosis, changes in visual acuity, diplopia, and a palpable mass. Rarely, symptoms are related to the local invasion of adjacent anatomical structures [5,10]. Symptom duration and rapidity of onset are related to the disease nature. Diagnosis is always performed on histological specimens, preferably from excisional biopsy [11].
The selection of a treatment plan is primarily guided by factors including the type of lymphoma and its molecular features, the presence of symptoms related to eye involvement, and the extent of disease spread. Typically, in cases of localized disease, radiotherapy is frequently employed, utilizing radiation doses between 20 and 40 Gy to achieve optimal disease control [12,13].
Regarding higher-stage diseases, systemic treatments are preferred owing to the increased risk of disease recurrence. Options include chemotherapy, immunotherapy, or combinations of both, with variable results according to the disease characteristics [14,15,16].
2.2. Intraocular Lymphomas
Primary intraocular lymphomas are considered a subset of primary central nervous system lymphomas (PCNS) and have been reported in the literature as vitreoretinal lymphoma or PCNS lymphoma ocular variant [7]. While relatively uncommon, intraocular lymphomas pose unique challenges in terms of diagnosis and management due to their intricate connection with the eye and central nervous systems [17].
Primary vitreoretinal lymphoma (PVRL) is a rare form of DLBCL that initially exclusively involves the posterior structures of the eye, i.e., the vitreous body, retina, and rarely the optic nerve, without concomitant central nervous system (CNS) involvement at diagnosis [18,19]. PVRL is often referred to as masquerade syndrome, as its symptoms closely mimic those of posterior uveitis, and it initially responds to steroid treatment [20]. This condition typically presents with bilateral eye involvement in approximately 70% of cases. Common initial symptoms include blurred vision, painless loss of vision, and floaters. Upon examination, a variable degree of vitritis is observed, characterized by the presence of lymphoma cells in the anterior vitreous [21]. In detail, the distribution of malignant cells along the vitreal fibrils gives rise to a characteristic “aurora borealis” appearance [22]. At the level of the retina, lymphoma infiltrates may be evident by multifocal cream-colored retinal spots, mimicking drusen [23]. Anterior chamber findings are uncommon in PVRL; however, cases of occurrences like keratic precipitates, iris or angle infiltration, and, very rarely, pseudohypopyon can also be observed [24,25]. Optic nerve infiltration in systemic metastatic retinal lymphoma may have initial occult signs but with profound visual loss [26].
Diagnostic procedures include magnetic resonance imaging (MRI), optical coherence tomography (OCT), fluorescein angiography, positron emission tomography (PET) scan, and assessment of interleukin (IL)-10 levels in the vitreous, but ultimately the diagnosis is made on the histological specimen by vitrectomy [27]. Treatment is heterogeneous, with patients being treated either locally with intravitreal injections of chemotherapies, immunotherapies, or ocular irradiation [17].
As PVRL involves the posterior structures of the eye, very few cases of aggressive and indolent lymphomas affect the uvea, most commonly EMZLs involving the choroid, while even rarer cases have been reported of DLBCL affecting the iris [28,29]. The clinical presentation of choroidal lymphoma is characterized by blurred vision or metamorphopsia, as well as salmon-colored subconjunctival patches when transscleral infiltration occurs [30]. Ciliary lymphomas, on the other hand, have a clinical presentation resembling that of anterior uveitis [31]. Notwithstanding the type of lymphoma, involvement of the iris and angle may lead to secondary glaucoma [31]. Prognosis and treatment depend on the histologic type, which has a very good prognosis for EMZL [28].
3. Secondary Disease Involvement
Hematological malignancies that may affect the eye include leukemias and lymphomas, as well as multiple myeloma, myeloproliferative neoplasms, myelodysplastic syndromes, and Waldenstrom’s macroglobulinaemia, potentially affecting all eye structures [1,32,33,34,35,36].
Retinal tissue is commonly involved in leukemias, occurring in up to 30–50% of affected individuals [37,38,39]. Leukemic retinopathy is more frequently observed in acute leukemias and is the most common ophthalmic manifestation in affected patients [40,41]. Primary leukemic retinopathy involves direct infiltration of the retina by cancerous leukocytes, while secondary retinopathy results from hematological complications of leukemia, such as thrombocytopenia, anemia, and hyperviscosity [3,42]. The hallmark of leukemic retinopathy is retinal hemorrhages, stemming from both direct infiltration by leukemic cells and the broader impacts of secondary leukemic retinopathy. Hemorrhages may vary in appearance, presenting as dots, Roth spots (white-centered hemorrhages), or flame-shaped patterns, and may extend into the subretinal tissue or vitreous.
Cotton wool spots are also frequent and result from nerve fiber layer infarcts or localized accumulations of leukemic cells [43]. Moreover, peripheral microaneurysms and neovascularization are significant ocular signs of chronic leukemia, with up to 50% of affected individuals showing these manifestations in their peripheral retina [44].
Nodular retinal infiltrates occur in association with elevated leukocyte counts and are the result of leukostasis and direct retinal infiltration in both acute and chronic pathologies [45,46]. Infiltrates are often described as grayish-white and may involve the foveal area, affecting vision [47,48].
Bilateral or mono-lateral serous detachment of the retina is associated with diffuse infiltration of leukemic cells within and surrounding choroidal vessels [49,50]. Possibly, blood stagnation or mechanical compression of choroidal vessels results in ischemia of the overlying retinal pigment epithelium and disruption of the intercellular tight junctions [50,51].
Central retinal vein and/or artery occlusion is a rare manifestation of leukemia and often presents with optic disc edema [39]. Most likely, it is caused by a state of hypercoagulability and leukocytosis, although direct leukemic infiltration could also be involved in its pathogenesis [37].
Optic nerve infiltration can be observed in acute leukemias and, more rarely in chronic forms [52,53]. The optic disc presents as pale and swollen with blurred margins, and may be accompanied by hemorrhages [52]. Optic nerve infiltration is frequent in children affected by acute leukemias and should rain concern for CNS involvement [54,55,56].
In patients with lymphocytic leukemia, iris infiltration is rare but may be the first sign of relapse [57,58]. Patients may present with blurred vision, conjunctival injection, anterior chamber reaction, pseudohypopyon, thickening of the iris stroma, change in iris shape and color are common clinical signs in leukemic iris infiltration [59].
More unusual ocular manifestations of leukemia include chronic conjunctivitis with redness, discharge and follicle-like lesions of the upper and lower palpebral conjunctiva in lymphocytic leukemia [60], corneal ring ulcer in acute monocytic leukemia [61], Sjogren’s syndrome in chronic lymphocytic leukemia [62] anterior segment ischemia in chronic myelogenous leukemia [63].
Secondary intraocular lymphomas affect the eye from a lymphoma originating outside the CNS. Most cases involve orbital lymphomas that extend directly into intraocular structures or non-Hodgkin lymphomas (NHL) that spread to the eye through the bloodstream, affecting the uvea, ocular adnexa, orbit, lacrimal gland, and conjunctiva [64]. Intraocular NHL can produce diffuse or multiple vitreoretinal foci as well as unifocal or multifocal lesions to the uvea, with variable presentations [65]. Iris lymphoma, for instance, tends to be high-grade and usually develops in patients with known aggressive systemic disease [66].
4. Ocular GVHD
Ocular GVHD (oGVHD) is a form of chronic GVHD predominantly affecting the ocular surface. It impacts approximately 40–60% of individuals who undergo allogeneic HSCT [67]. The incidence of oGVHD is higher in cases of chronic GVHD affecting other organs, with 50–90% of patients experiencing systemic GVHD also presenting with symptoms of oGVHD [68,69].
The pathophysiology of oGVHD is rapidly progressive, with inflammatory and donor T cell-mediated immune dysregulatory mechanisms leading to fibrosis of the lacrimal gland, conjunctiva and meibomian glands, possibly with corneal involvement [70].
The lacrimal gland is the principal target of oGVHD. Donor CD4+ T cells and CD8+ T cells infiltrate the preductal area of the gland, generating a pro-inflammatory environment and thus recruiting macrophages, antigen-presenting cells (APCs), and CD34+ stromal fibroblasts [71,72]. Recent evidence suggests that recruited fibroblasts may either derive from circulating donor-derived precursors or may arise from local epithelial to mesenchymal transition [73,74]. Regardless of their source, the accumulation of abnormal collagen fibers and extracellular matrix components by fibroblasts results in the fibrosis of the glandular interstitium [5,75]. These alterations ultimately result in lacrimal gland dysfunction, with consequent aqueous deficient dry eye.
Meibomian gland dysfunction (MGD) is observed in 47.8% of oGVHD, indicating that affected patients may present both evaporative and aqueous-deficient forms of dry eye [76]. The pathophysiological mechanisms leading to MGD after HSCT remain elusive, and recent data highlights that some degree of glandular dysfunction may already exist prior to the transplantation process [77,78]. The main pathologic features observed in affected patients include obstruction of the gland orifice and cystic dilations of the ducts with atrophy. This process is mediated by lymphocyte infiltration and fibrosis around the glandular structures, resulting in altered meibum secretion and consequent tear film instability (Figure 1A) [79,80].
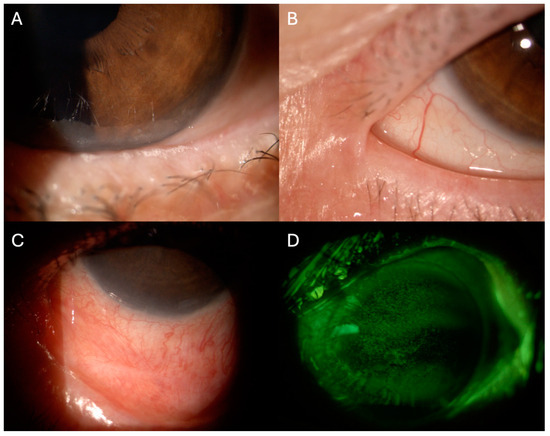
Figure 1.
Slit-lamp photographs of clinical findings of ocular graft versus host disease. (A). Foamy secretion from meibomian gland dysfunction. (B) Ankyloblepharon, (C). Symblepharon. (D) Corneal punctate epithelial defects were highlighted using fluorescein staining with cobalt blue and yellow filters.
Conjunctival tissue involvement is observed in approximately 10% of oGVHD and is considered a negative prognostic factor for overall survival, being an indicator of severe systemic involvement [69,81]. Histopathological studies have evidenced the presence of lymphocytic infiltrating of the subconjunctival stroma, as well as neutrophils and neutrophil extracellular traps [82,83,84]. The complex interaction of these players contributes to the changes observed in the conjunctival tissue of oGVHD, such as fibrosis with goblet cell metaplasia, shortening, and reduced density of microvilli [85]. Clinically, these findings translate into conjunctival hyperemia, chemosis, and cicatricial conjunctivitis with consequent lagophthalmos, punctal stenosis, subtarsal fibrosis, symblepharon, and ankyloblepharon (Figure 1B,C) [86,87]. Superior limbal keratoconjunctivitis-like inflammation may also be present [88].
Such ocular surface alterations may induce corneal suffering. Corneal in vivo confocal microscopy findings have revealed increased density of dentritic epithelial cells and globular immune cells with altered morphology of corneal sub-basal nerves [89,90]. Clinically, superficial punctate keratopathy is the most frequent finding (Figure 1D) but neovascularization and sterile ulceration have been reported in severe cases [86,91]. Patients report pain, photophobia and decreased vision.
To date, diagnosis of oGVHD relies on two international criteria: National Institutes of Health (NIH) guidelines and the International Consensus Group of ophthalmologists’ criteria (Table 1) [92,93].

Table 1.
Elements for ocular GVHD diagnosis according to the NIH diagnostic criteria and to the International Consensus Group of ophthalmologists’ criteria.
Treatment of oGVHD has different objectives: (i) lubrication, (ii) control of drainage, (iii) control of evaporation, and (iv) decrease of ocular surface inflammation. The main therapeutic tools include permanent or reversible punctual occlusion, eyelid hygiene with warming compresses, and lid margin cleansing [94]. In severe cases, topical antibiotic ointments, corticosteroids, or cyclosporin may help obtain disease remission, as well as systemic fatty acid supplementation and systemic tetracyclines [4].
5. Toxicity to Antineoplastic Therapies
Systemic chemotherapy and targeted therapies used in leukemia treatment may cause significant ocular morbidity. Frequent findings include posterior subcapsular cataracts and subconjunctival hemorrhages (Figure 2A,B). Table 2 represents the most frequent ocular findings in patients treated with systemic agents.
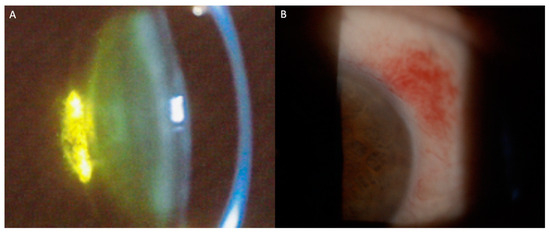
Figure 2.
Slit-lamp photographs of signs of ocular toxicities to antineoplastic agents. (A) Posterior subcapsular cataract; (B) Conjunctival hemorrhage.
Eye radiation therapy may cause self-limiting acute toxicity as periorbital erythema, eyelash loss, conjunctival injection, excessive tearing, and swelling, with a higher incidence in dosages >25 Gy [95,96]. Chronic toxicity is observed in 50% of treated patients and includes the development of cataracts, dry eye disease, retinopathy, and keratitis [97].
PVRL may sometimes be treated using intravitreal injections of chemotherapies or immunotherapies. The most commonly reported complications include elevations in intraocular pressure and epithelial keratopathy [17].

Table 2.
Ocular toxicity from systemic anti-neoplastic therapy.
Table 2.
Ocular toxicity from systemic anti-neoplastic therapy.
| Agent | Sign/Symptom | Underlying Mechanism |
|---|---|---|
| Busulfan [98,99,100] | Posterior subcapsular cataracts | Inhibition of nucleic acid formation in lens epithelium |
| Vincristine [101] | Temporary or permanent loss of vision | Axonal damage due to microtubule disruption |
| Dexamethasone [102] | Increased intraocular pressure; cataract | Increased resistance to aqueous outflow; unclear, likely non-enzymatic formation of Schiff base intermediates |
| Fludarabine [103,104] | Rapid progressive loss of vision | Direct damage to retinal bipolar and ganglion cells; gray and white matter |
| Cytarabine [105,106] | Reversible corneal toxicity and conjunctivitis | Unknown, likely inhibition of DNA synthesis corneal and conjunctival epithelium |
| Imatinib [107] | Periorbital edema, conjunctival hemorrhage | Periocular soft tissue expression of molecular targets for Imatinib |
| Immune checkpoint inhibitors [108] | Uveitis | Activation of complement cascade, recruitment of innate immunity cells in cerebrospinal fluid, loss of immune-privilege |
6. Conclusions
Ocular complications are a critical yet often underrecognized aspect of managing hematological malignancies. The eye can be a barometer for systemic disease activity and adverse effects of treatment. As such, routine ophthalmic assessments should be integrated into the standard of care for these patients. Interdisciplinary collaboration between hematology and ophthalmology services is vital for prompt identification and management of ocular issues. Advances in targeted cancer therapies hold promise for reducing ocular side effects and enhancing patient quality of life. Awareness of ocular symptoms, both within medical teams and amongst patients, is key to safeguarding vision and ensuring comprehensive care in the context of blood cancers.
Author Contributions
Conceptualization, G.G., C.R., V.S., M.B., A.C., A.T. and G.A.; Methodology, G.G., A.B., C.R., M.B. and A.L.; Validation, A.B., M.B., G.C.S., A.C., A.L. and A.T.; Investigation, A.B., C.R., G.A., G.C.S. and G.G.; Writing—Original Draft Preparation, C.R., A.B., G.A., A.C., M.B. and G.G.; Writing—Review and Editing, G.G., G.C.S., M.B., G.A., A.C., A.L. and V.S.; Supervision, G.G, A.C, A.T., M.B., G.A. and C.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
No new data were created in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Talcott, K.E.; Garg, R.J.; Garg, S.J. Ophthalmic manifestations of leukemia. Curr. Opin. Ophthalmol. 2016, 27, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Buchan, J.; McKibbin, M.; Burton, T. The prevalence of ocular disease in chronic lymphocytic leukaemia. Eye 2003, 17, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Sharma, T.; Grewal, J.; Gupta, S.; Murray, P.I. Ophthalmic manifestations of acute leukaemias: The ophthalmologist’s role. Eye 2004, 18, 663–672. [Google Scholar] [CrossRef]
- Giannaccare, G.; Pellegrini, M.; Bernabei, F.; Scorcia, V.; Campos, E. Ocular surface system alterations in ocular graft-versus-host disease: All the pieces of the complex puzzle. Graefe’s Arch. Clin. Exp. Ophthalmol. 2019, 257, 1341–1351. [Google Scholar] [CrossRef] [PubMed]
- Olsen, T.G.; Heegaard, S. Orbital lymphoma. Surv. Ophthalmol. 2019, 64, 45–66. [Google Scholar] [CrossRef]
- Tanenbaum, R.E.; Galor, A.; Dubovy, S.R.; Karp, C.L. Classification, diagnosis, and management of conjunctival lymphoma. Eye Vis. 2019, 6, 22. [Google Scholar] [CrossRef] [PubMed]
- Witmer, M.T. Primary Vitreoretinal Lymphoma: Management of Isolated Ocular Disease. Cancer Control 2016, 23, 110–116. [Google Scholar] [CrossRef]
- Hassan, W.M.; Bakry, M.S.; Hassan, H.M.; Alfaar, A.S. Incidence of orbital, conjunctival and lacrimal gland malignant tumors in USA from Surveillance, Epidemiology and End Results, 1973–2009. Int. J. Ophthalmol. 2016, 9, 1808. [Google Scholar]
- Valenzuela, J.; Echegaray, J.J.; Dodds, E.; Kurup, S.K.; Lowder, C.; Ondrejka, S.L.; Singh, A.D. Ophthalmic Manifestations of Hodgkin Lymphoma: A Review. Ocul. Oncol. Pathol. 2021, 7, 381–389. [Google Scholar] [CrossRef]
- Olsen, T.G.; Holm, F.; Mikkelsen, L.H.; Rasmussen, P.K.; Coupland, S.E.; Esmaeli, B.; Finger, P.T.; Graue, G.F.; Grossniklaus, H.E.; Honavar, S.G.; et al. Orbital Lymphoma—An International Multicenter Retrospective Study. Am. J. Ophthalmol. 2019, 199, 44–57. [Google Scholar] [CrossRef]
- WHO. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues; WHO: Geneva, Switzerland, 2009. [Google Scholar]
- Goda, J.S.; Le, L.W.; Lapperriere, N.J.; Millar, B.-A.; Payne, D.; Gospodarowicz, M.K.; Wells, W.; Hodgson, D.C.; Sun, A.; Simpson, R.; et al. Localized Orbital Mucosa-Associated Lymphoma Tissue Lymphoma Managed with Primary Radiation Therapy: Efficacy and Toxicity. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, e659–e666. [Google Scholar] [CrossRef]
- Kharod, S.M.; Herman, M.P.; Morwith, C.G.; Lightsey, J.; Mendenhall, W.M.; Mendenhall, N.P. Radiotherapy in the Management of Orbital Lymphoma. Am. J. Clin. Oncol. Cancer Clin. Trials 2015, 41, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Qualls, D.; Imber, B.S.; Okwali, M.; Hamlin, P.A.; Kumar, A.; Lahoud, O.B.; Matasar, M.J.; Noy, A.; Owens, C.; Zelenetz, A.D.; et al. Long-term outcomes of patients with limited-stage ocular adnexal DLBCL treated with combined modality therapy in the rituximab era. Br. J. Haematol. 2023, 200, 524–527. [Google Scholar] [CrossRef]
- Salar, A.; Domingo-Domenech, E.; Panizo, C.; Nicolás, C.; Bargay, J.; Muntañola, A.; Canales, M.; Bello, J.L.; Sancho, J.M.; Tomás, J.F.; et al. Long-term results of a phase 2 study of rituximab and bendamustine for mucosa-associated lymphoid tissue lymphoma. Blood 2017, 130, 1772–1774. [Google Scholar] [CrossRef]
- Zucca, E.; Conconi, A.; Martinelli, G.; Bouabdallah, R.; Tucci, A.; Vitolo, U.; Martelli, M.; Pettengell, R.; Salles, G.; Sebban, C.; et al. Final results of the IELSG-19 randomized trial of mucosa-associated lymphoid tissue lymphoma: Improved event-free and progression-free survival with rituximab plus chlorambucil versus either chlorambucil or rituximab monotherapy. J. Clin. Oncol. 2017, 35, 1905–1912. [Google Scholar] [CrossRef]
- Soussain, C.; Malaise, D.; Cassoux, N. Primary vitreoretinal lymphoma: A diagnostic and management challenge. Blood 2021, 138, 1519–1534. [Google Scholar] [CrossRef] [PubMed]
- Soussain, C.; Houillier, C.; Ghesquieres, H.; Soubeyran, P.; Chinot, O.; Taillandier, L.; Houot, R.; Ahle, G.; Gyan, E.; Chabrot, C.; et al. The French LOC Network for Primary CNS Lymphoma (PCNSL) Patients: What Can We Learn from a Large National Database? Blood 2016, 128, 926. [Google Scholar] [CrossRef]
- Levasseur, S.D.; Wittenberg, L.A.; White, V.A. Vitreoretinal lymphoma: A 20-year review of incidence, clinical and cytologic features, treatment, and outcomes. JAMA Ophthalmol. 2013, 131, 50–55. [Google Scholar] [CrossRef]
- Reichstein, D. Primary vitreoretinal lymphoma: An update on pathogenesis, diagnosis and treatment. Curr. Opin. Ophthalmol. 2016, 27, 177–184. [Google Scholar] [CrossRef]
- Kalogeropoulos, D.; Vartholomatos, G.; Mitra, A.; Elaraoud, I.; Ch’ng, S.W.; Zikou, A.; Papoudou-Bai, A.; Moschos, M.M.; Kanavaros, P.; Kalogeropoulos, C. Primary vitreoretinal lymphoma. Saudi J. Ophthalmol. 2019, 33, 66–80. [Google Scholar] [CrossRef]
- Chan, C.C.; Rubenstein, J.L.; Coupland, S.E.; Davis, J.L.; Harbour, J.W.; Johnston, P.B.; Cassoux, N.; Touitou, V.; Smith, J.R.; Batchelor, T.T.; et al. Primary vitreoretinal lymphoma: A report from an International Primary Central Nervous System Lymphoma Collaborative Group symposium. Oncologist 2011, 16, 1589–1599. [Google Scholar] [CrossRef] [PubMed]
- Sagoo, M.S.; Mehta, H.; Swampillai, A.J. Primary intraocular lymphoma. Surv. Ophthalmol. 2014, 59, 503–516. [Google Scholar] [CrossRef] [PubMed]
- Coupland, S.E.; Damato, B. Understanding intraocular lymphomas. Clin. Exp. Ophthalmol. 2008, 36, 564–578. [Google Scholar] [CrossRef]
- Gill, M.K.; Jampol, L.M. Variations in the presentation of primary intraocular lymphoma: Case reports and a review. Surv. Ophthalmol. 2001, 45, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Mohd Fauzi Yap, M.F.B.; Mohd Zain, A.; Tumian, N.R.; Palaniappan, S.; Nasaruddin, R.A.; Md Din, N. Optic Nerve Infiltration in Systemic Metastatic Retinal Lymphoma (SMRL): Multimodal Imaging and Challenges in Diagnosis. Ocul. Immunol. Inflamm. 2021, 29, 479–484. [Google Scholar] [CrossRef] [PubMed]
- Costopoulos, M.; Touitou, V.; Golmard, J.L.; Darugar, A.; Fisson, S.; Bonnemye, P.; Le Lez, M.L.; Soussain, C.; Cassoux, N.; Lamy, T.; et al. ISOLD: A New Highly Sensitive Interleukin Score for Intraocular Lymphoma Diagnosis. Ophthalmology 2016, 123, 1626–1628. [Google Scholar] [CrossRef]
- Gao, X.; Li, B.; You, Q.; Peng, X. Primary extranodal marginal zone B-cell lymphoma with diffuse uveal involvement and focal infiltration of the trabecular meshwork: A case report and review of literature. BMC Ophthalmol. 2015, 15, 48. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kakkassery, V.; Coupland, S.E.; Heindl, L.M. Iris lymphoma—A systematic guide for diagnosis and treatment. Surv. Ophthalmol. 2021, 66, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Mashayekhi, A.; Shukla, S.Y.; Shields, J.A.; Shields, C.L. Choroidal lymphoma: Clinical features and association with systemic lymphoma. Ophthalmology 2014, 121, 342–351. [Google Scholar] [CrossRef]
- Valenzuela, J.; Yeaney, G.A.; Hsi, E.D.; Azzato, E.M.; Peereboom, D.M.; Singh, A.D. Large B-cell lymphoma of the uvea: Histopathologic variants and clinicopathologic correlation. Surv. Ophthalmol. 2020, 65, 361–370. [Google Scholar] [CrossRef]
- Sen, H.N.; Chan, C.C.; Caruso, R.C.; Fariss, R.N.; Nussenblatt, R.B.; Buggage, R.R. Waldenström’s macroglobulinemia-associated retinopathy. Ophthalmology 2004, 111, 535–539. [Google Scholar] [CrossRef] [PubMed]
- Shoji, M.K.; Chen, Y.; Topilow, N.J.; Abou Khzam, R.; Dubovy, S.R.; Johnson, T.E. Orbital Involvement in Multiple Myeloma. Ophthalmic Plast. Reconstr. Surg. 2023, 39, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.C.; Liang, T.H.; Chang, H.N.; Lin, J.S.; Lin, H.Y. Behçet disease associated with myelodysplastic syndrome. J. Clin. Rheumatol. 2008, 14, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Ahn, B.Y.; Choi, K.D.; Choi, Y.J.; Jea, S.Y.; Lee, J.E. Isolated monocular visual loss as an initial manifestation of polycythemia vera. J. Neurol. Sci. 2007, 258, 151–153. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.L.; Burnham, J.M.; Pang, V.; Idowu, O.; Iyer, S. Ocular Manifestations of Primary Myelofibrosis. Retin. Cases Brief. Rep. 2016, 10, 364–367. [Google Scholar] [CrossRef] [PubMed]
- El Salloukh, N.A.; Hage, D.G.; Bashshur, A.Z.; Kheir, W.J. Early Ophthalmological Manifestations of Acute Myeloid Leukemia: Current Perspectives. Clin. Ophthalmol. 2022, 16, 2119–2127. [Google Scholar] [CrossRef] [PubMed]
- De Queiroz Mendonca, C.; Freire, M.V.; Viana, S.S.; Silva Tavares, M.K.G.; Almeida Silva, W.M.; Cipolotti, R. Ocular manifestations in acute lymphoblastic leukemia: A five-year cohort study of pediatric patients. Leuk. Res. 2019, 76, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Reddy, S.C.; Jackson, N.; Menon, B.S. Ocular involvement in leukemia—A study of 288 cases. Ophthalmologica 2003, 217, 441–445. [Google Scholar] [CrossRef] [PubMed]
- Green, W.; Rao, P.K.; Harocopos, G.J. Extramedullary Relapse of Acute Myelogenous Leukemia Presenting as a Large Serous Retinal Detachment. Ocul. Oncol. Pathol. 2017, 3, 95–100. [Google Scholar] [CrossRef]
- Russo, V.; Scott, I.U.; Querques, G.; Stella, A.; Barone, A.; Delle Noci, N. Orbital and ocular manifestations of acute childhood leukemia: Clinical and statistical analysis of 180 patients. Eur. J. Ophthalmol. 2008, 18, 619–623. [Google Scholar] [CrossRef]
- Vishnevskia-Dai, V.; Sella King, S.; Lekach, R.; Fabian, I.D.; Zloto, O. Ocular Manifestations of Leukemia and Results of Treatment with Intravitreal Methotrexate. Sci. Rep. 2020, 10, 1994. [Google Scholar] [CrossRef] [PubMed]
- Reddy, S.C.; Jackson, N. Retinopathy in acute leukaemia at initial diagnosis: Correlation of fundus lesions and haematological parameters. Acta Ophthalmol. Scand. 2004, 82, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Yan, X.; Zhang, X.; Yang, H. Leukostasis retinopathy: An uncommon visual threatening complication of chronic myeloid leukemia with severe hyperleukocytosis—A case report and review of the literature. Indian J. Ophthalmol. 2018, 66, 1871–1874. [Google Scholar] [PubMed]
- Mandava, N.; Costakos, D.; Bartlett, H.M. Chronic myelogenous leukemia manifested as bilateral proliferative retinopathy. Arch. Ophthalmol. 2005, 123, 576–577. [Google Scholar] [PubMed]
- Johnson, J.S.; Lopez, J.S.; Kavanaugh, A.S.; Liang, C.; Mata, D.A. A 25-Year-Old Man with Exudative Retinal Detachments and Infiltrates without Hematological or Neurological Findings Found to Have Relapsed Precursor T-Cell Acute Lymphoblastic Leukemia. Case Rep. Ophthalmol. 2015, 6, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Kumawat, D.; Dhakal, S. Leukemic retinopathy and foveal infiltrates. Int. Ophthalmol. 2018, 38, 1301–1303. [Google Scholar] [CrossRef] [PubMed]
- Vicini, G.; Nicolosi, C.; Malandrino, D.; Tozzetti, C.; Rizzo, S.; Sodi, A. Leukostasis retinopathy with leukemic infiltrates as onset manifestation of chronic myeloid leukemia: A case report. Eur. J. Ophthalmol. 2021, 31, NP116–NP121. [Google Scholar] [CrossRef] [PubMed]
- Sayadi, J.; Gouider, D.; Allouche, Y.; Choura, R.; Cherni, I.; Sayadi, M.; Benneji, H.; Zghal, I.; Malek, I.; Nacef, L. Ophthalmic Manifestations of Newly Diagnosed Acute Leukemia Patients in a Tunisian Cohort. Clin. Ophthalmol. 2022, 16, 3425–3435. [Google Scholar] [CrossRef]
- Veerappan Pasricha, M.; Callaway, N.F.; Nguyen, Q.D.; Do, D.V. Serous retinal detachment as a presenting sign of acute lymphoblastic leukemia: A case report and literature review. Am. J. Ophthalmol. Case Rep. 2021, 23, 101142. [Google Scholar] [CrossRef]
- Patel, A.V.; Miller, J.B.; Nath, R.; Shih, H.A.; Yoon, M.K.; Freitag, S.K.; Papaliodis, G.; Chen, T.C.; Eliott, D.; Kim, I.K. Unilateral Eye Findings: A Rare Herald of Acute Leukemia. Ocul. Oncol. Pathol. 2016, 2, 166–170. [Google Scholar] [CrossRef]
- Lin, Y.C.; Wang, A.G.; Yen, M.Y.; Hsu, W.M. Leukaemic infiltration of the optic nerve as the initial manifestation of leukaemic relapse. Eye 2004, 18, 546–550. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Hadyah, S.; Park, A.; Akhtari, M.; Scott, J.; Ran-Castillo, D.; Chong, E.; Koh, H.; Oregel, K.; Khandelwal, K.; et al. Leukemic infiltration of the optic nerve in chronic lymphocytic leukemia: A case report and review of literature. Leuk. Res. Rep. 2023, 20, 100391. [Google Scholar] [CrossRef] [PubMed]
- Dini, G.; Capolsini, I.; Cerri, C.; Massei, M.S.; Mastrodicasa, E.; Perruccio, K.; Gorello, P.; Caniglia, M.; Verrotti, A.; Arcioni, F. Acute lymphoblastic leukemia relapse presenting with optic nerve infiltration. SAGE Open Med. Case Rep. 2023, 11, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Kuan, H.C.; Mustapha, M.; Oli Mohamed, S.; Abdul Aziz, R.A.; Loh, C.K.; Mohammed, F.; Naffi, A.A.; Othman, O.; Nasaruddin, R.A.; Alias, H. Isolated Infiltrative Optic Neuropathy in an Acute Lymphoblastic Leukemia Relapse. Cureus 2022, 14, e25625. [Google Scholar] [CrossRef] [PubMed]
- Lee, V.; Farooq, A.V.; Shah, H.A. Leukemic and Lymphomatous Optic Neuropathy: A Case Series. J. Neuroophthalmol. 2021, 41, e796–e802. [Google Scholar] [CrossRef] [PubMed]
- Gruenewald, R.L.; Perry, M.C.; Henry, P.H. Leukemic iritis with hypopyon. Cancer 1979, 44, 1511–1513. [Google Scholar] [CrossRef] [PubMed]
- Ploysangam, P.; Reynolds, A.L. Iris Infiltration as a Sign of Relapse in a Child with Chronic Myeloid Leukemia. Ophthalmology 2021, 128, 780. [Google Scholar] [CrossRef] [PubMed]
- Türkoğlu, E.B.; Öcal, O. Anterior segment optical coherence tomography findings in leukemic iris infiltration. Photodiagn. Photodyn. Ther. 2022, 37, 102578. [Google Scholar] [CrossRef] [PubMed]
- Hakim, F.; Farooq, A. Conjunctival chronic lymphocytic leukemia presenting as bilateral chronic conjunctivitis. Am. J. Ophthalmol. Case Rep. 2022, 27, 101670. [Google Scholar] [CrossRef]
- Wood, W.J.; Nicholson, D.H. Corneal ring ulcer as the presenting manifestation of acute monocytic leukemia. Am. J. Ophthalmol. 1973, 76, 69–72. [Google Scholar] [CrossRef]
- Hodgson, K.; Ferrer, G.; Montserrat, E.; Moreno, C. Chronic lymphocytic leukemia and autoimmunity: A systematic review. Haematologica 2011, 96, 752–761. [Google Scholar] [CrossRef] [PubMed]
- Cullis, C.M.; Hines, D.R.; Bullock, J.D. Anterior segment ischemia: Classification and description in chronic myelogenous leukemia. Ann. Ophthalmol. 1979, 11, 1739–1744. [Google Scholar] [PubMed]
- Chan, C.C.; Wallace, D.J. Intraocular lymphoma: Update on diagnosis and management. Cancer Control 2004, 11, 285–295. [Google Scholar] [CrossRef]
- Jahnke, K.; Korfel, A.; Komm, J.; Bechrakis, N.E.; Stein, H.; Thiel, E.; Coupland, S.E. Intraocular lymphoma 2000–2005: Results of a retrospective multicentre trial. Graefe’s Arch. Clin. Exp. Ophthalmol. 2006, 244, 663–669. [Google Scholar]
- Mashayekhi, A.; Shields, C.L.; Shields, J.A. Iris involvement by lymphoma: A review of 13 cases. Clin. Exp. Ophthalmol. 2013, 41, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Pathak, M.; Diep, P.P.; Lai, X.; Brinch, L.; Ruud, E.; Drolsum, L. Ocular findings and ocular graft-versus-host disease after allogeneic stem cell transplantation without total body irradiation. Bone Marrow Transplant. 2018, 53, 863–872. [Google Scholar] [CrossRef] [PubMed]
- Dietrich-Ntoukas, T.; Cursiefen, C.; Westekemper, H.; Eberwein, P.; Reinhard, T.; Bertz, H.; Nepp, J.; Lawitschka, A.; Heiligenhaus, A.; Seitz, B.; et al. Diagnosis and treatment of ocular chronic graft-versus-host disease: Report from the German-Austrian-Swiss Consensus Conference on Clinical Practice in chronic GVHD. Cornea 2012, 31, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Nassiri, N.; Eslani, M.; Panahi, N.; Mehravaran, S.; Ziaei, A.; Djalilian, A.R. Ocular graft versus host disease following allogeneic stem cell transplantation: A review of current knowledge and recommendations. J. Ophthalmic Vis. Res. 2013, 8, 351–358. [Google Scholar] [PubMed]
- Wolff, D.; Radojcic, V.; Lafyatis, R.; Cinar, R.; Rosenstein, R.K.; Cowen, E.W.; Cheng, G.S.; Sheshadri, A.; Bergeron, A.; Williams, K.M.; et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: IV. The 2020 Highly morbid forms report. Transplant. Cell. Ther. 2021, 27, 817–835. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, Y.; Kuwana, M.; Yamazaki, K.; Mashima, Y.; Yamada, M.; Mori, T.; Okamoto, S.; Oguchi, Y.; Kawakami, Y. Periductal area as the primary site for T-cell activation in lacrimal gland chronic graft-versus-host disease. Investig. Ophthalmol. Vis. Sci. 2003, 44, 1888–1896. [Google Scholar] [CrossRef]
- Ogawa, Y.; Yamazaki, K.; Kuwana, M.; Mashima, Y.; Nakamura, Y.; Ishida, S.; Toda, I.; Oguchi, Y.; Tsubota, K.; Okamoto, S.; et al. A significant role of stromal fibroblasts in rapidly progressive dry eye in patients with chronic GVHD. Investig. Ophthalmol. Vis. Sci. 2001, 42, 111–119. [Google Scholar]
- Ogawa, Y.; Kodama, H.; Kameyama, K.; Yamazaki, K.; Yasuoka, H.; Okamoto, S.; Inoko, H.; Kawakami, Y.; Kuwana, M. Donor fibroblast chimerism in the pathogenic fibrotic lesion of human chronic graft-versus-host disease. Investig. Ophthalmol. Vis. Sci. 2005, 46, 4519–4527. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, Y.; Shimmura, S.; Kawakita, T.; Yoshida, S.; Kawakami, Y.; Tsubota, K. Epithelial mesenchymal transition in human ocular chronic graft-versus-host disease. Am. J. Pathol. 2009, 175, 2372–2381. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Huang, R.; Huang, S.; Fan, W.; Yuan, R.; Wang, X.; Zhang, X. Recent advances in ocular graft-versus-host disease. Front. Immunol. 2023, 14, 1092108. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, Y.; Okamoto, S.; Wakui, M.; Watanabe, R.; Yamada, M.; Yoshino, M.; Ono, M.; Yang, H.Y.; Mashima, Y.; Oguchi, Y.; et al. Dry eye after haematopoietic stem cell transplantation. Br. J. Ophthalmol. 1999, 83, 1125–1130. [Google Scholar] [CrossRef] [PubMed]
- Giannaccare, G.; Bonifazi, F.; Sebastiani, S.; Sessa, M.; Pellegrini, M.; Arpinati, M.; Moscardelli, F.; Versura, P.; Campos, E. Meibomian Gland Dropout in Hematological Patients Before Hematopoietic Stem Cell Transplantation. Cornea 2018, 37, 1264–1269. [Google Scholar] [CrossRef] [PubMed]
- Giannaccare, G.; Bonifazi, F.; Sessa, M.; Fresina, M.; Arpinati, M.; Bandini, G.; Versura, P. Dry Eye Disease Is Already Present in Hematological Patients Before Hematopoietic Stem Cell Transplantation. Cornea 2016, 35, 638–643. [Google Scholar] [CrossRef] [PubMed]
- Appenteng Osae, E.; Steven, P. Meibomian Gland Dysfunction in Ocular Graft vs. Host Disease: A Need for Pre-Clinical Models and Deeper Insights. Int. J. Mol. Sci. 2021, 22, 3516. [Google Scholar]
- Perez, V.L.; Mousa, H.M.; Soifer, M.; Beatty, C.; Sarantopoulos, S.; Saban, D.R.; Levy, R.B. Meibomian Gland Dysfunction: A Route of Ocular Graft-Versus-Host Disease Progression That Drives a Vicious Cycle of Ocular Surface Inflammatory Damage. Am. J. Ophthalmol. 2023, 247, 42–60. [Google Scholar] [CrossRef]
- Jabs, D.A.; Wingard, J.; Green, W.R.; Farmer, E.R.; Vogelsang, G.; Saral, R. The eye in bone marrow transplantation. III. Conjunctival graft-vs-host disease. Arch. Ophthalmol. 1989, 107, 1343–1348. [Google Scholar]
- Eberwein, P.; Issleib, S.; Böhringer, D.; Mittelviefhaus, H.; Schwartzkopff, J.; Finke, J.; Reinhard, T. Conjunctival HLA-DR and CD8 expression detected by impression cytology in ocular graft versus host disease. Mol. Vis. 2013, 19, 1492–1501. [Google Scholar] [PubMed]
- Sonawane, S.; Khanolkar, V.; Namavari, A.; Chaudhary, S.; Gandhi, S.; Tibrewal, S.; Jassim, S.H.; Shaheen, B.; Hallak, J.; Horner, J.H.; et al. Ocular surface extracellular DNA and nuclease activity imbalance: A new paradigm for inflammation in dry eye disease. Investig. Ophthalmol. Vis. Sci. 2012, 53, 8253–8263. [Google Scholar] [CrossRef]
- An, S.; Raju, I.; Surenkhuu, B.; Kwon, J.E.; Gulati, S.; Karaman, M.; Pradeep, A.; Sinha, S.; Mun, C.; Jain, S. Neutrophil extracellular traps (NETs) contribute to pathological changes of ocular graft-vs.-host disease (oGVHD) dry eye: Implications for novel biomarkers and therapeutic strategies. Ocul. Surf. 2019, 17, 589–614. [Google Scholar] [CrossRef]
- Tatematsu, Y.; Ogawa, Y.; Shimmura, S.; Dogru, M.; Yaguchi, S.; Nagai, T.; Yamazaki, K.; Kameyama, K.; Okamoto, S.; Kawakami, Y.; et al. Mucosal microvilli in dry eye patients with chronic GVHD. Bone Marrow Transplant. 2012, 47, 416–425. [Google Scholar] [CrossRef] [PubMed]
- Tabbara, K.F.; Al-Ghamdi, A.; Al-Mohareb, F.; Ayas, M.; Chaudhri, N.; Al-Sharif, F.; Al-Zahrani, H.; Mohammed, S.Y.; Nassar, A.; Aljurf, M. Ocular findings after allogeneic hematopoietic stem cell transplantation. Ophthalmology 2009, 116, 1624–1629. [Google Scholar] [CrossRef] [PubMed]
- Kheirkhah, A.; Coco, G.; Satitpitakul, V.; Dana, R. Subtarsal Fibrosis Is Associated with Ocular Surface Epitheliopathy in Graft-Versus-Host Disease. Am. J. Ophthalmol. 2018, 189, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Sivaraman, K.R.; Jivrajka, R.V.; Soin, K.; Bouchard, C.S.; Movahedan, A.; Shorter, E.; Jain, S.; Jacobs, D.S.; Djalilian, A.R. Superior Limbic Keratoconjunctivitis-like Inflammation in Patients with Chronic Graft-Versus-Host Disease. Ocul. Surf. 2016, 14, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Kheirkhah, A.; Qazi, Y.; Arnoldner, M.A.; Suri, K.; Dana, R. In Vivo Confocal Microscopy in Dry Eye Disease Associated with Chronic Graft-Versus-Host Disease. Investig. Ophthalmol. Vis. Sci. 2016, 57, 4686–4691. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Ogawa, Y.; Mukai, S.; Saijo-Ban, Y.; Kamoi, M.; Uchino, M.; Yamane, M.; Ozawa, N.; Fukui, M.; Mori, T.; et al. In Vivo Confocal Microscopy Evaluation of Ocular Surface with Graft-Versus-Host Disease-Related Dry Eye Disease. Sci. Rep. 2017, 7, 10720. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Farooq, A.V.; Harocopos, G.J.; Sollenberger, E.L.; Hou, J.H.; Bouchard, C.S.; Shieh, C.; Tran, U.L.; Lubniewski, A.J.; Huang, A.J.W.; et al. Corneal perforation in ocular graft-versus-host disease. Am. J. Ophthalmol. Case Rep. 2021, 24, 101224. [Google Scholar] [CrossRef] [PubMed]
- Jagasia, M.H.; Greinix, H.T.; Arora, M.; Williams, K.M.; Wolff, D.; Cowen, E.W.; Palmer, J.; Weisdorf, D.; Treister, N.S.; Cheng, G.S.; et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group report. Biol. Blood Marrow Transplant. 2015, 21, 389–401.e1. [Google Scholar] [CrossRef]
- Ogawa, Y.; Kim, S.K.; Dana, R.; Clayton, J.; Jain, S.; Rosenblatt, M.I.; Perez, V.L.; Shikari, H.; Riemens, A.; Tsubota, K. International Chronic Ocular Graft-vs-Host-Disease (GVHD) Consensus Group: Proposed diagnostic criteria for chronic GVHD (Part I). Sci. Rep. 2013, 3, 3419. [Google Scholar] [CrossRef]
- Singh, R.B.; Yung, A.; Coco, G.; Sinha, S.; Dohlman, T.H.; Yin, J.; Dana, R. Efficacy and retention of silicone punctal plugs for treatment of dry eye in patients with and without ocular graft-versus-host-disease. Ocul. Surf. 2020, 18, 731–735. [Google Scholar] [CrossRef]
- Le, Q.T.; Eulau, S.M.; George, T.I.; Hildebrand, R.; Warnke, R.A.; Donaldson, S.S.; Hoppe, R.T. Primary radiotherapy for localized orbital MALT lymphoma. Int. J. Radiat. Oncol. Biol. Phys. 2002, 52, 657–663. [Google Scholar] [CrossRef]
- Stafford, S.L.; Kozelsky, T.F.; Garrity, J.A.; Kurtin, P.J.; Leavitt, J.A.; Martenson, J.A.; Habermann, T.M. Orbital lymphoma: Radiotherapy outcome and complications. Radiother. Oncol. 2001, 59, 139–144. [Google Scholar] [CrossRef]
- Finger, P.T. Radiation therapy for orbital tumors: Concepts, current use, and ophthalmic radiation side effects. Surv. Ophthalmol. 2009, 54, 545–568. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Tripathi, R.C.; Tripathi, B.J. Drug-induced ocular disorders. Drug Saf. 2008, 31, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Kaida, T.; Ogawa, T.; Amemiya, T. Cataract induced by short-term administration of large doses of busulfan: A case report. Ophthalmologica 1999, 213, 397–399. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Ando, R.; Ohira, T.; Hoshiya, T.; Tamura, K. Ocular lesions induced in infant rats by busulfan. Histol. Histopathol. 2015, 30, 321–330. [Google Scholar]
- Lee, W.H.; You, S.K.; Lee, Y.H. Bilateral optic neuropathy following vincristine chemotherapy: A case report with description of multimodal imaging findings. Medicine 2021, 100, e24706. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, M.; Terashita, Y.; Hara, K.; Cho, Y.; Iguchi, A.; Chin, S.; Manabe, A. Corticosteroid-induced glaucoma in pediatric patients with hematological malignancies. Pediatr. Blood Cancer 2019, 66, e27977. [Google Scholar] [CrossRef]
- Bishop, R.J.; Ding, X.; Heller, C.K., 3rd; Illei, G.; Caruso, R.; Cunningham, D.; Pavletic, S.; Chan, C.C. Rapid vision loss associated with fludarabine administration. Retina 2010, 30, 1272–1277. [Google Scholar] [CrossRef]
- Ding, X.; Herzlich, A.A.; Bishop, R.; Tuo, J.; Chan, C.C. Ocular toxicity of fludarabine: A purine analog. Expert. Rev. Ophthalmol. 2008, 3, 97–109. [Google Scholar] [CrossRef][Green Version]
- Hollander, D.A.; Aldave, A.J. Drug-induced corneal complications. Curr. Opin. Ophthalmol. 2004, 15, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.; Kato, J.; Yamane, A.; Aisa, Y.; Kawata, Y.; Ichimura, M.; Tsuneyama, C.; Yashima, T.; Ogawa, Y.; Tsubota, K.; et al. Prevention of cytarabine-induced kerato-conjunctivitis by eye rinse in patients receiving high-dose cytarabine and total body irradiation as a conditioning for hematopoietic stem cell transplantation. Int. J. Hematol. 2011, 94, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Ho, W.L.; Wong, H.; Yau, T. The ophthalmological complications of targeted agents in cancer therapy: What do we need to know as ophthalmologists? Acta Ophthalmol. 2013, 91, 604–609. [Google Scholar] [CrossRef]
- Zhang, H.; Houadj, L.; Wu, K.Y.; Tran, S.D. Diagnosing and Managing Uveitis Associated with Immune Checkpoint Inhibitors: A Review. Diagnostics 2024, 14, 336. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).