Abstract
Hemoglobin (Hb) Agrinio is a rare non-deletional a-globin mutation observed almost exclusively in Greek, Spanish or other Mediterranean families. The clinical manifestations of a carrier of a single Hb Agrinio mutation (single heterozygosity) depend on the concomitant presence or absence of other mutations or variants in the beta, alpha or other modifying genes. We present a Greek patient harboring a Hb Agrinio variant plus the - -Med alpha deletional allele, having an infrequent severe form of alpha thalassemia, in contrast to the typical alpha thalassemic patient and requiring regular red blood cell (RBC) transfusions and chelation treatment. We also provide a concise literature review regarding alpha thalassemic hemoglobin variants and their molecular and clinical combinations. A phase 2, double-blind, randomized, placebo-controlled, multicenter clinical trial to determine the efficacy and safety of luspatercept (BMS-986346/ACE-536) for the treatment of anemia in adults with alpha thalassemia with the participation of our center is currently recruiting patients (NCT05664737).
1. Introduction
There are mainly two types of the alpha globin gene mutations. Deletional mutations (reduced (α+) or absent (α0)) are more frequently encountered and lead to the incomplete and defective production of the alpha globin chains (quantitative disorders), whereas point mutations (non-deletional) are not as common, but still exist, causing dysfunctional, irregular hemoglobin (Hb) molecules (qualitative disorders). Both of the abovementioned molecular lesions, lead to various clinical manifestations towards a distinct phenotype based on the combination of genetic defects [1]. Frequently, non-deletional mutations cause a more severe clinical syndrome, when compared with deletional molecular defects.
Clinically significant forms of alpha thalassemia are hemoglobinopathy H (HbH) disease and hydrops fetalis syndrome. HbH disease derives from the presence of only one residual functioning active alpha globin gene out of four genes. The three other remaining alpha genes are impaired. Thus, the consequent excess of beta globin chains results in moderate to severe hemolysis. There is a wide variability in clinical and hematological severity. Hypochromic anemia, hepatosplenomegaly and jaundice are the most common clinical manifestations. Usually, the anemia of HbH disease is mild without requiring regular red blood cell (RBC) transfusions, in comparison to severe transfusion dependent beta-thalassemia [1,2,3,4]. Patients with HbH disease are usually transfused in pregnancy or in rare emergency cases, like a surgery. Nevertheless, there are exceptions to this rule for HbH, especially when deletional and rare non-deletional mutations are combined to form HbH disease. Co-inheritance of unstable alpha chain variants with deletional mutations (α0 or α+ thalassemia) may also lead to a more severe than expected clinical phenotype [2,4]. In this work, focus will be given to the alpha gene mutations. However, the presence of beta gene mutations affects the clinical manifestations and modifies HbH disease phenotype, and thus these beta mutations will also be discussed in the co-existence of alpha lesions.
Hb Agrinio is considered a highly unstable Hb variant, whose hallmark is the conversion of leucine to proline at the codon 29 of the a-globin chain (CTG→CCG), which forms Hb Agrinio. It is a rare non-deletional a-globin mutation observed almost exclusively in Greek or Greek-Cypriot families. The mutated amino acid 29 is located on the beta helix of the a-globin in the B10 location. The result of this alteration is probably the reason for this highly form of unstable Hb [5,6,7].
It has been observed that the homozygous state for the Hb Agrinio (Hb Agrinio/Hb Agrinio, defective Agrinio variants in both alleles, albeit extremely rare) correlates with a more severe hypochromic, microcytic and hemolytic anemia, blood transfusions since infancy, low levels of HbH (<2.5%) and rare HbH inclusions [5,8,9].
Conversely, the clinical manifestations of a carrier of a single Hb Agrinio mutation (single heterozygosity) depend on the concomitant presence or absence of other mutations or variants in the beta, alpha or other modifying genes. The anemia is usually milder in this case, compared to the homozygous state [6,8,10].
The standard identification of an HbH disease carrier might be easily overlooked in cases with a single Hb Agrinio heterozygosity or other alpha molecular defects in one allele. These carriers can have children with HbH disease or even hydrops fetalis. The difference between the latter and the Hb Bart’s hydrops has been extensively described. Hb Bart’s hydrops are the result of a homozygous alpha thalassemia (a0-thal, alpha deletional thalassemia) with the HbF and HbA being around 65% and Hb Bart over 35% [6,8,10].
Finally, the co-inheritance of Hb Agrinio in one allele and other deletional a-thalassemia variants in the other allele (double heterozygosity state) causes a phenotype with a more severe form of HbH disease and chronic hemolytic anemia. Even though the latter is an alpha thalassemia, in this rare case, the patient will express the whole spectrum of the β-thalassemia major phenotype (regular transfusions, chelation therapy) [6,11,12].
2. Materials and Methods
We present a Greek patient harboring Hb Agrinio variant plus the - -Med alpha deletional allele, having an infrequent severe form of alpha thalassemia and requiring regular RBC transfusions and chelation treatment. We also provide a concise literature review regarding the molecular and clinical combinations of the alpha thalassemic hemoglobin variants.
The study was conducted under the declaration of Helsinki. A written signed consent form of the patient is available and the blank form of the consent used without any identification is accessible. The Scientific Ethics Committee of the General Hospital of Larissa is responsible for the approval of the study and the identification code of the submitted project is 11858.
3. Case Presentation
The male patient was born in October 2003 and was diagnosed at the age of 8 months (June 2004) due to growth retardation. DNA analysis for alpha genes (αAgrα/- -Μed) showed the concomitant presence of Hb Agrinio, a rare mutated hemoglobin form, inherited from the father (αAgrα/αα) and common - -Med alpha deletional mutation (- -Μed/αα), inherited from the mother. Both parents had normal forms of the other alpha allele, thereby they were carriers of HbH without clinical manifestations.
Ever since, he started regular RBC transfusions. The characteristic inclusion bodies were evident inside the RBCs by microscopy. Without transfusions, the levels of Hb were as low as 6.5 gr/dL, significantly worse than the expected hematocrit for HbH. After monthly RBC transfusions, Hb initially reached 8–9 gr/dL and then was stabilized to 10 gr/dL. Chelation treatment was initiated at 2 years (June 2006) with deferasirox at a dose of 15 mg/kg with the previous oral tablet dissolved in liquid. From 2004 until late 2017, he would receive two RBC units every month, whereas since 2018 up to the present, due to the gain of height and weight, he receives three to four units every month, because of physical development (usually two units every 15 days). Since 2017 (at the age of 14), the new film-coated tablets of deferasirox have been the chelation choice of treatment, at a dose initially around 14 mg/kg/day and increasing to 17.5–21 mg/kg/day in recent years.
The levels of lactate dehydrogenase (LDH) were increased all these years (range 676–1851 (NR 170–480 U/L)), due to excessive chronic hemolysis, caused by the combination of the unstable Hb Agrinio and the alpha mutation - -Med. Median LDH value for the last 5 years was 1188 U/L. In the last year, due to stabilization to higher levels of Hb (around 10.5 gr/dL), chronic hemolysis tends to be less and LDH is usually below 700 U/L. The main hematological and laboratory data of the patient are shown in Table 1.

Table 1.
Main hematological and biochemical laboratory data of the patient.
Hepatomegaly (15 cm) and splenomegaly (18 cm) were evident, as a result of the hemoglobinopathy. Splenectomy was recommended in order to lower the transfusion burden. However, due to the lack of hypersplenism, the patient decided to increase the frequency of transfusions plus chelation treatment. After initiation of more intensive chelation, the MRI T2* showed a lack of significant iron levels both in the heart and the liver (T2* heart: 31.48 msec, T2* liver: 6.99 msec, LIC: 4.20 mg/g dw). Previous evaluations of liver iron showed mild to medium hepatic hemosiderosis, as measured by MRI T2* from 2010–2017. Hepatic, splenic and bone marrow hemosiderosis are shown in Figure 1 and Figure 2, respectively.
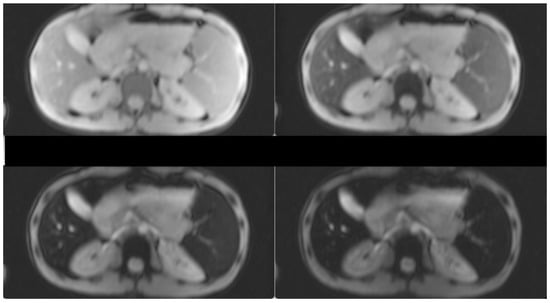
Figure 1.
T2-star (T2*) Multi-Echo Gradient Echo images for iron quantification (the first two and the last two, out of the 12-echo images are shown). T2* Signal drop is shown in the liver and spleen at the longest echo images indicative of iron deposition. Liver T2* = 6 ms(N > 16)and LIC = 4.28 mg/gr dw (N < 1.8). Spleen T2* = 7.1 ms.
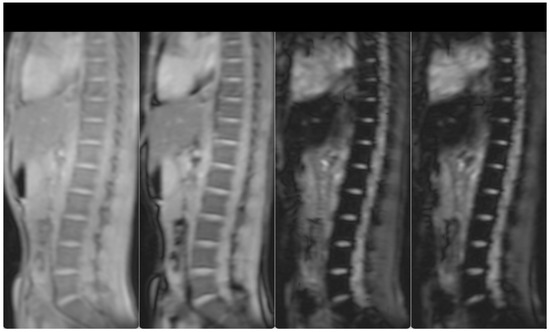
Figure 2.
T2-star (T2*) Multi-Echo Gradient Echo images for iron quantification (the first two and the last two, out of the 12-echo images are shown). T2* Signal drop is shown in the spine bone marrow at the longest echo images indicative of iron deposition. Bone marrow T2* = 3.7 ms (N > 16).
Up to the present, he remains under frequent RBC transfusions (clinical similarity with homozygous beta thalassemia) with concomitant chelation with deferasirox. The Hb target is 11 mg/dL. Splenectomy has been deferred (due to the known complications of thrombotic events), and will take place only in the presence of possible hypersplenism, which is not the case until recently.
4. Discussion and Literature Review
HbH in general is a mild disease. Such patients are usually transfused in pregnancy or in emergency cases, such as a surgery. Their follow-up includes an abdominal and heart ultrasound every year, along with the necessary laboratory investigations. However, the concomitant presence of Hb Agrinio plus the - -Med alpha deletional mutation, has been associated with very severe clinical manifestations, resembling these encountered in beta thalassemia major patients and requiring frequent RBC transfusions plus chelation therapy. We gave focus on the severe clinical presentation of our patient and we described the clinical evolution in time. We finally posed the dilemmas in treating the patient, especially in the era of novel therapies for thalassemia.
Hb Agrinio was first identified in Agrinio, Greece in 1995 and named after the respective city [13]. The final clinical manifestations depend on the co-existence of this rare severe qualitative defect with other alpha or beta globin mutations. Other cases have been reported in North Macedonia [12], Cyprus [7], Spain [1,9,14] and Bulgaria [9]. A concise literature review is shown in Table 2, entitled ‘alpha thalassemic hemoglobin variants and their molecular and clinical combinations’, where the concomitant molecular defects with Hb Agrinio, along with the corresponding clinical manifestations are presented.

Table 2.
Alpha thalassemic hemoglobin variants and their molecular and clinical combinations.
Interestingly, other defective alpha hemoglobinopathies such as Hb Icaria [15] or Hb Adana [16] have been described. Three cases of compound heterozygosity for Hb Icaria plus - -Med have clinically expressed a severe form of HbH disease, which correlated with a regular need for blood transfusions from an early age, splenomegaly and growth retardation [15] (Table 2). Furthermore, the combination of Hb Adana plus the –a3.7 kb deletion (a+ thalassemia) has been associated with chronic hemolytic anemia, significant splenomegaly and thrombocytopenia [16] (Table 2).
On the other hand, luspatercept is an agent used in the recent years for the treatment of anemia of transfusion-dependent beta thalassemia (TDT) (BELIEVE study) [17] and non-transfusion dependent beta thalassemia (NTDT) (BEYOND study) [18]. The drug inhibits Smad 2/3 signaling and enhances erythroid maturation. It exhibits its action as an activin receptor ligand trap, which prevents detrimental molecules for the RBC fate from reaching the surface of the RBC. Even though the efficacy of luspatercept is much higher in beta NTDT (thalassemia intermedia) and modest in beta TDT or beta thalassemia major, little is known regarding its value in alpha thalassemia. A phase 2, double-blind, randomized, placebo-controlled, multicenter clinical trial to determine the efficacy and safety of luspatercept (BMS-986346/ACE-536) for the treatment of anemia in adults with alpha thalassemia with the participation of our center is currently recruiting patients (NCT05664737).
In the clinical phase III trial, luspatercept was granted approval in the States and the European Union, due to the clinical finding of significantly lowering the transfusion burden by at least 33% in a series of 336 thalassemic patients [17]. It is estimated that the mechanism of action of luspatercept is not restricted to beta chains, but might involve alpha chains as well. Hence, there is molecular reasoning and there is postulation that luspatercept might work also in these rare clinical manifestations, encountered in alpha thalassemia. The forthcoming clinical trial (NCT05664737) will address the issue. Nevertheless, there is no certainty that the drug luspatercept will be effective in alpha thalassemia. It might be proven effective, but it might not. There may also be different degrees of response among individual patients and that is why the clinical trial will be conducted. The patient described has not participated in the clinical trial by the submission date of this work. He has decided to wait for the results of the trial, instead of participating.
Author Contributions
M.D.D. was in charge of the study, conducted the research, treated the patient, wrote and corrected the manuscript, performed literature review and assisted in making the tables; S.P. wrote parts of the manuscript, helped in forming and writing the results of the study, made the tables and performed literature review; O.Z., I.A. and K.K. (Konstantinos Karapiperis) assisted in making the tables and performed literature review; C.C., A.M. and E.T. performed literature review and assisted in forming part of the tables and the manuscript; E.A. provided the radiological images of the patient; K.K. (Konstantinos Karakoussis) provided consultation for parts of the manuscript, performed literature review and corrected the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted under the declaration of Helsinki. The study was approved by the Scientific and Ethics Committee of the General Hospital of Larissa; Approval Number: 62/11858.
Informed Consent Statement
Written informed consent from the patient has been obtained.
Data Availability Statement
All data of the study are available in our department (DNA Result, laboratory results and radiological images) with the initial of the patient upon request. No identifiable information is provided for the patient, due to personal data.
Acknowledgments
The authors would like to thank Joanne Traeger-Synodinos for the DNA analysis of the patient (Laboratory of Medical Genetics, National and Kapodistrian Athens University, Athens, Greece). Moreover, the authors would like to thank Antonis Kattamis (“Agia Sophia” Children Hospital, First Department of Pediatrics, National and Kapodistrian University of Athens, Athens, Greece) for providing consultation for the patient.
Conflicts of Interest
All authors declare they have no conflict of interest.
References
- Galanello, R.; Cao, A. Gene test review. Alpha-thalassemia. Genet. Med. Off. J. Am. Coll. Med. Genet. 2011, 13, 83–88. [Google Scholar] [CrossRef]
- Piel, F.B.; Weatherall, D.J. The α-thalassemias. N. Engl. J. Med. 2014, 371, 1908–1916. [Google Scholar] [CrossRef]
- Viprakasit, V.; Ekwattanakit, S. Clinical Classification, Screening and Diagnosis for Thalassemia. Hematol./Oncol. Clin. N. Am. 2018, 32, 193–211. [Google Scholar] [CrossRef] [PubMed]
- Farashi, S.; Harteveld, C.L. Molecular basis of α-thalassemia. Blood Cells Mol. Dis. 2018, 70, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Traeger-Synodinos, J.; Metaxotou-Mavromati, A.; Kanavakis, E.; Vrettou, C.; Papassotiriou, I.; Michael, T.; Kattamis, C. An α-thalassemic hemoglobinopathy: Homozygosity for the HB Agrinio α 2-globin chain variant. Hemoglobin 1998, 22, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Traeger-Synodinos, J.; Douna, V.; Papassotiriou, I.; Stamoulakatou, A.; Ladis, V.; Siahanidou, T.; Fylaktou, I.; Kanavakis, E. Variable and often severe phenotypic expression in patients with the α-thalassemic variant Hb Agrinio [α29(B10)Leu→Pro (α2)]. Hemoglobin 2010, 34, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Felekis, X.; Phylactides, M.; Drousiotou, A.; Christou, S.; Kyrri, A.; Kyriakou, K.; Kalogerou, E.; Christopoulos, G.; Kleanthous, M. Hb Agrinio [α29(B10)Le-->uPro (α2)] in combination with --MED I. Results in a severe form of Hb H disease. Hemoglobin 2008, 32, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Wajcman, H.; Traeger-Synodinos, J.; Papassotiriou, I.; Giordano, P.C.; Harteveld, C.L.; Baudin-Creuza, V.; Old, J. Unstable and thalassemic α chain hemoglobin variants: A cause of Hb H disease and thalassemia intermedia. Hemoglobin 2008, 32, 327–349. [Google Scholar] [CrossRef] [PubMed]
- Szepetowski, S.; Berger, C.; Joly, P.; Baron-Joly, S.; Huguenin, Y.; Cantais, A.; Brun, S.; Ged, C.; Badens, C.; Thuret, I.; et al. Homozygosity for the hyperunstable hemoglobin variant Hb Agrinio (HBA2:c.89T>C) leads to severe antenatal anemia: Eight new cases in three families. Am. J. Hematol. 2022, 97, E393–E395. [Google Scholar] [CrossRef] [PubMed]
- Kanavakis, E.; Papassotiriou, I.; Karagiorga, M.; Vrettou, C.; Metaxotou-Mavrommati, A.; Stamoulakatou, A.; Kattamis, C.; Traeger-Synodinos, J. Phenotypic and molecular diversity of haemoglobin H disease: A Greek experience. Br. J. Haematol. 2000, 111, 915–923. [Google Scholar] [CrossRef] [PubMed]
- Douna, V.; Papassotiriou, I.; Stamoulakatou, A.; Metaxotou-Mavrommati, A.; Kanavakis, E.; Traeger-Synodinos, J. Association of mild and severely unstable α chain variants: The first observation of a compound heterozygote with Hb Setif [α94(G1)Asp-->Tyr (α2)] and Hb Agrinio [α29(B10)Leu-->Pro (α2)] in a Greek family. Hemoglobin 2008, 32, 592–595. [Google Scholar] [CrossRef] [PubMed]
- Dimishkovska, M.; Kuzmanovska, M.; Kocheva, S.; Martinova, K.; Karanfilski, O.; Stojanoski, Z.; Plaseska-Karanfilska, D. First Cases of Hb Agrinio Described in Patients from the Republic of Macedonia. Hemoglobin 2017, 41, 308–310. [Google Scholar] [CrossRef] [PubMed]
- Papadakis, M.; Drakoulakou, O.; Papapanagiotou, E.; Pessini, D.; Loutradi-Anagnostou, A. Hb A2-Agrinio [δ43(CD2)Glu-->Gly(GAG-->GGG)]: A new δ chain variant detected in a Greek family. Hemoglobin 1995, 19, 295–299. [Google Scholar] [CrossRef] [PubMed]
- de la Fuente-Gonzalo, F.; Baiget, M.; Badell, I.; Ricard, P.; Vinuesa, L.; Martínez-Nieto, J.; Ropero, P.; Villegas, A.; González, F.A.; Díaz-Mediavilla, J.; et al. Study of three families with Hb Agrinio [α29(B10)Leu→Pro, CTG>CCG (α2)] in the Spanish population: Three homozygous cases. Hemoglobin 2012, 36, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Kanavakis, E.; Traeger-Synodinos, J.; Papasotiriou, I.; Vrettou, C.; Metaxotou-Mavromati, A.; Stamoulakatou, A.; Lagona, E.; Kattamis, C. The interaction of α zero thalassaemia with Hb Icaria: Three unusual cases of haemoglobinopathy H. Br. J. Haematol. 1996, 92, 332–335. [Google Scholar] [CrossRef] [PubMed]
- Tampaki, A.; Theodoridou, S.; Apostolou, C.; Delaki, E.E.; Vlachaki, E. A case of late diagnosis of compound heterozygosity for Hb Adana (HBA2:c.179G>A) in trans to an α+- thalassemia deletion: Guilty or innocent. Hippokratia 2020, 24, 43–45. [Google Scholar] [PubMed]
- Cappellini, M.D.; Viprakasit, V.; Taher, A.T.; Georgiev, P.; Kuo, K.H.M.; Coates, T.; Voskaridou, E.; Liew, H.K.; Pazgal-Kobrowski, I.; Forni, G.L.; et al. A Phase 3 Trial of Luspatercept in Patients with Transfusion-Dependent β-Thalassemia. N. Engl. J. Med. 2020, 382, 1219–1231. [Google Scholar] [CrossRef] [PubMed]
- Taher, A.T.; Cappellini, M.D.; Kattamis, A.; Voskaridou, E.; Perrotta, S.; Piga, A.G.; Filosa, A.; Porter, J.B.; Coates, T.D.; Forni, G.L.; et al. Luspatercept for the treatment of anaemia in non-transfusion-dependent β-thalassaemia (BEYOND): A phase 2, randomised, double-blind, multicentre, placebo-controlled trial. Lancet. Haematol. 2022, 9, e733–e744. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).