Impact of Quantitative Computed Tomography-Based Analysis of Abdominal Adipose Tissue in Patients with Lymphoma
Abstract
1. Introduction
2. Methods
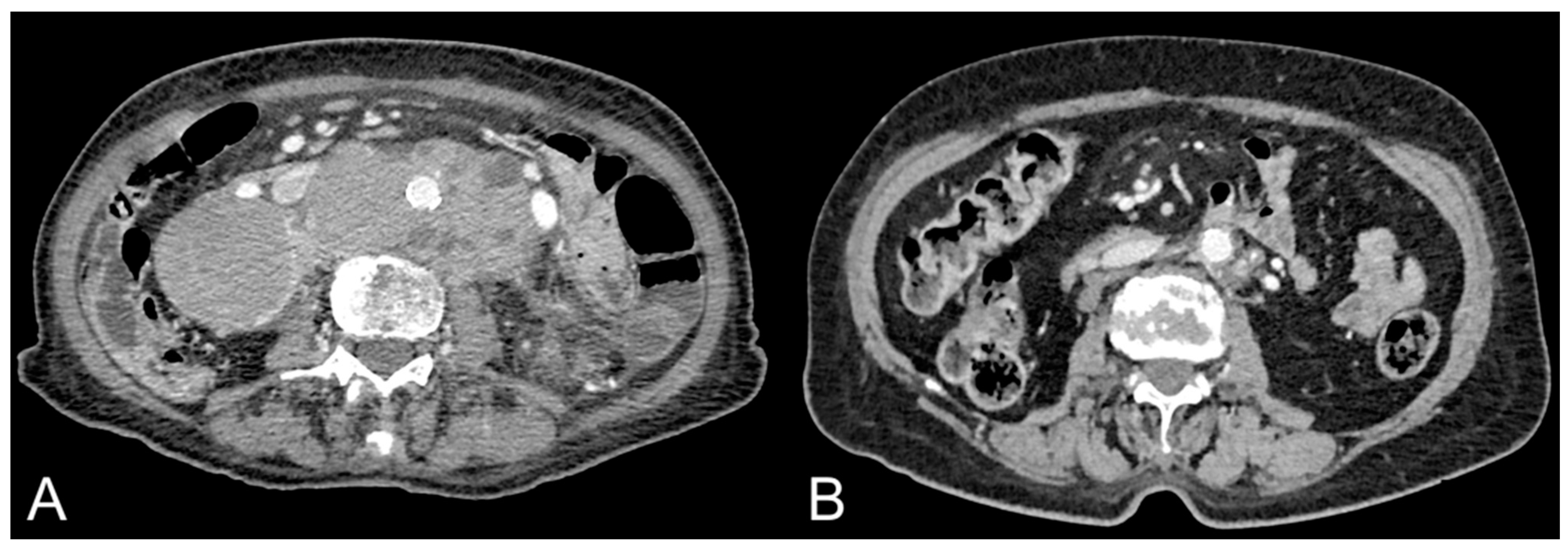
3. Abdominal Adipose Tissue Quantification and Distribution in Patients with Lymphoma
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Louie, S.M.; Roberts, L.S.; Nomura, D.K. Mechanisms linking obesity and cancer. Biochim. Biophys. Acta 2013, 1831, 1499–1508. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S.C.; Wolk, A. Body mass index and risk of non-Hodgkin’s and Hodgkin’s lymphoma: A meta-analysis of prospective studies. Eur. J. Cancer 2011, 47, 2422–2430. [Google Scholar] [CrossRef]
- Murphy, F.; Kroll, M.E.; Pirie, K.; Reeves, G.; Green, J.; Beral, V. Body size in relation to incidence of subtypes of haematological malignancy in the prospective Million Women Study. Br. J. Cancer 2013, 108, 2390–2398. [Google Scholar] [CrossRef]
- Lichtman, M.A. Obesity and the risk for a hematological malignancy: Leukemia, lymphoma, or myeloma. Oncologist 2010, 15, 1083–1101. [Google Scholar] [CrossRef] [PubMed]
- Matos, A.; Marinho-Dias, J.; Ramalheira, S.; Oliveira, M.J.; Bicho, M.; Ribeiro, R. Mechanisms underlying the association between obesity and Hodgkin lymphoma. Tumor Biol. 2016, 37, 13005–13016. [Google Scholar] [CrossRef]
- Carlsen, H.; Haugen, F.; Zadelaar, S.; Kleemann, R.; Kooistra, T.; Drevon, C.A.; Blomhoff, R. Diet-induced obesity increases NF-kappaB signaling in reporter mice. Genes Nutr. 2009, 4, 215–222. [Google Scholar] [CrossRef]
- Jost, P.J.; Ruland, J. Aberrant NF-kappaB signaling in lymphoma: Mechanisms, consequences, and therapeutic implications. Blood 2007, 109, 2700–2707. [Google Scholar] [CrossRef]
- Nagel, D.; Vincendeau, M.; Eitelhuber, A.C.; Krappmann, D. Mechanisms and consequences of constitutive NF-kappaB activation in B-cell lymphoid malignancies. Oncogene 2014, 33, 5655–5665. [Google Scholar] [CrossRef]
- Jiménez-Cortegana, C.; Hontecillas-Prieto, L.; García-Domínguez, D.J.; Zapata, F.; Palazón-Carrión, N.; Sánchez-León, M.L.; Tami, M.; Pérez-Pérez, A.; Sánchez-Jiménez, F.; Vilariño-García, T.; et al. Obesity and Risk for Lymphoma: Possible Role of Leptin. Int. J. Mol. Sci. 2022, 23, 15530. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.H.; Lee, J.K.; Kim, K.M.; Kook, H.R.; Lee, H.; Kim, K.B.; Lee, S.; Byun, S.S.; Lee, S.E. Visceral obesity in predicting oncologic outcomes of localized renal cell carcinoma. J. Urol. 2014, 192, 1043–1049. [Google Scholar] [CrossRef]
- Ibrahim, M.M. Subcutaneous and visceral adipose tissue: Structural and functional differences. Obes. Rev. 2010, 11, 11–18. [Google Scholar] [CrossRef]
- Despres, J.P.; Lemieux, I. Abdominal obesity and metabolic syndrome. Nature 2006, 444, 881–887. [Google Scholar] [CrossRef]
- Zhang, H.P.; Zou, J.; Xu, Z.Q.; Ruan, J.; Yang, S.D.; Yin, Y.; Mu, H.J. Association of leptin, visfatin, apelin, resistin and adiponectin with clear cell renal cell carcinoma. Oncol. Lett. 2017, 13, 463–468. [Google Scholar] [CrossRef][Green Version]
- Mallio, C.A.; Greco, F.; Pacella, G.; Schena, E.; Beomonte Zobel, B. Gender-based differences of abdominal adipose tissue distribution in non-small cell lung cancer patients. Shanghai Chest 2018, 2, 20. [Google Scholar] [CrossRef]
- Greco, F.; Cirimele, V.; Mallio, C.A.; Beomonte Zobel, B.; Grasso, R.F. Increased visceral adipose tissue in male patients with clear cell renal cell carcinoma. Clin. Cancer Investig. J. 2018, 7, 132–136. [Google Scholar] [CrossRef]
- Greco, F.; Mallio, C.A. Relationship between visceral adipose tissue and genetic mutations (VHL and KDM5C) in clear cell renal cell carcinoma. Radiol. Med. 2021, 126, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Karmali, R.; Alrifai, T.; Fughhi, I.A.M.; Ng, R.; Chukkapalli, V.; Shah, P.; Basu, S.; Nathan, S.; Szymanski-Grant, K.; Gordon, L.I.; et al. Impact of cachexia on outcomes in aggressive lymphomas. Ann. Hematol. 2017, 96, 951–956. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.G.; Gomes-Marcondes, M.C. Metformin treatment modulates the tumour-induced wasting effects in muscle protein metabolism minimising the cachexia in tumour-bearing rats. BMC Cancer 2016, 16, 418. [Google Scholar] [CrossRef]
- Albano, D.; Camoni, L.; Rinaldi, R.; Tucci, A.; Zilioli, V.R.; Muzi, C.; Ravanelli, M.; Farina, D.; Coppola, A.; Camalori, M.; et al. Comparison between skeletal muscle and adipose tissue measurements with high-dose CT and low-dose attenuation correction CT of 18F-FDG PET/CT in elderly Hodgkin lymphoma patients: A two-centre validation. Br. J. Radiol. 2021, 94, 20200672. [Google Scholar] [CrossRef] [PubMed]
- Albano, D.; Dondi, F.; Treglia, G.; Tucci, A.; Ravanelli, M.; Farina, D.; Bertagna, F. Longitudinal Body Composition Changes Detected by [18F]FDG PET/CT during and after Chemotherapy and Their Prognostic Role in Elderly Hodgkin Lymphoma. Cancers 2022, 14, 5147. [Google Scholar] [CrossRef]
- Lucijanic, M.; Huzjan Korunic, R.; Ivic, M.; Fazlic Dzankic, A.; Kusec, R.; Pejsa, V. Perirenal and subcutaneous fat differently affect outcomes in newly diagnosed classical Hodgkin lymphoma patients. Hematol. Oncol. 2021, 39, 575–579. [Google Scholar] [CrossRef]
- Hinnerichs, M.; Ferraro, V.; Zeremski, V.; Mougiakakos, D.; Omari, J.; Pech, M.; Bär, C.; Wienke, A.; Saalfeld, S.; Strobel, A.; et al. Prognostic Impact of Quality and Distribution of Adipose Tissue in Patients with Primary Central Nervous System Lymphoma. Vivo 2022, 36, 2828–2834. [Google Scholar] [CrossRef]
- Camus, V.; Lanic, H.; Kraut, J.; Modzelewski, R.; Clatot, F.; Picquenot, J.M.; Contentin, N.; Lenain, P.; Groza, L.; Lemasle, E.; et al. Prognostic impact of fat tissue loss and cachexia assessed by computed tomography scan in elderly patients with diffuse large B-cell lymphoma treated with immunochemotherapy. Eur. J. Haematol. 2014, 93, 9–18. [Google Scholar] [CrossRef]
- Xiao, D.Y.; Luo, S.; O'Brian, K.; Sanfilippo, K.M.; Ganti, A.; Riedell, P.; Lynch, R.C.; Liu, W.; Kahl, B.S.; Cashen, A.F.; et al. Longitudinal Body Composition Changes in Diffuse Large B-cell Lymphoma Survivors: A Retrospective Cohort Study of United States Veterans. J. Natl. Cancer Inst. 2016, 108, djw145. [Google Scholar] [CrossRef] [PubMed]
- Wadhwa, A.; Adams, K.M.; Dai, C.; Richman, J.S.; McDonald, A.M.; Williams, G.R.; Bhatia, S. Association between body composition and chemotherapy-related toxicity in children with lymphoma and rhabdomyosarcoma. Cancer 2022, 128, 1302–1311. [Google Scholar] [CrossRef] [PubMed]
- Tram, N.K.; Chou, T.H.; Ettefagh, L.N.; Deep, K.; Bobbey, A.J.; Audino, A.N.; Stacy, M.R. Quantification of chemotherapy-induced changes in body composition in pediatric, adolescent, and young adult lymphoma using standard of care CT imaging. Eur. Radiol. 2022, 32, 7270–7277. [Google Scholar] [CrossRef]
- Daniele, A.; Guarini, A.; Summa, S.D.; Dellino, M.; Lerario, G.; Ciavarella, S.; Ditonno, P.; Paradiso, A.V.; Divella, R.; Casamassima, P.; et al. Body Composition Change, Unhealthy Lifestyles and Steroid Treatment as Predictor of Metabolic Risk in Non-Hodgkin’s Lymphoma Survivors. J. Pers. Med. 2021, 11, 215. [Google Scholar] [CrossRef]
- Hidayat, K.; Du, X.; Shi, B.M. Body fatness at a young age and risks of eight types of cancer: Systematic review and meta-analysis of observational studies. Obes. Rev. 2018, 19, 1385–1394. [Google Scholar] [CrossRef]
- Mallio, C.A.; Napolitano, A.; Castiello, G.; Giordano, F.M.; D’Alessio, P.; Iozzino, M.; Sun, Y.; Angeletti, S.; Russano, M.; Santini, D.; et al. Deep Learning Algorithm Trained with COVID-19 Pneumonia Also Identifies Immune Checkpoint Inhibitor Therapy-Related Pneumonitis. Cancers 2021, 13, 652. [Google Scholar] [CrossRef] [PubMed]
- Marano, M.; Vespasiani Gentilucci, U.; Altamura, C.; Siotto, M.; Squitti, R.; Bucossi, S.; Quintiliani, L.; Migliore, S.; Greco, F.; Scarciolla, L.; et al. Altered metal metabolism in patients with HCV-related cirrhosis and hepatic encephalopathy. Metab. Brain Dis. 2015, 30, 1445–1452. [Google Scholar] [CrossRef]
- Lambin, P.; Rios-Velazquez, E.; Leijenaar, R.; Carvalho, S.; van Stiphout, R.G.; Granton, P.; Zegers, C.M.; Gillies, R.; Boellard, R.; Dekker, A.; et al. Radiomics: Extracting more information from medical images using advanced feature analysis. Eur. J. Cancer 2012, 48, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Malone, E.R.; Sim, H.W.; Stundzia, A.; Pierre, S.; Metser, U.; O'Malley, M.; Sacher, A.G.; Sridhar, S.S.; Hansen, A.R. Predictive radiomics signature for treatment response to nivolumab in patients with advanced renal cell carcinoma. Can. Urol. Assoc. J. 2022, 16, E94–E101. [Google Scholar] [CrossRef]
- Greco, F.; Mallio, C.A. Artificial intelligence and abdominal adipose tissue analysis: A literature review. Quant. Imaging Med. Surg. 2021, 11, 4461–4474. [Google Scholar] [CrossRef] [PubMed]
- Greco, F.; Salgado, R.; Van Hecke, W.; Del Buono, R.; Parizel, P.M.; Mallio, C.A. Epicardial and pericardial fat analysis on CT images and artificial intelligence: A literature review. Quant. Imaging Med. Surg. 2022, 12, 2075–2089. [Google Scholar] [CrossRef] [PubMed]
| Authors | Purpose | Adipose Tissue Compartments | Number of Patients | Results |
|---|---|---|---|---|
| Albano et al. (2021) [19] | To compare HDCT and LDCT of 18F-FDG PET)/CT in elderly patients with HL for evaluation of abdominal adipose tissue compartments in HL patients | VAT SAT IMAT | 90 | VAT (r = 0.942, p < 0.0001) SAT (r = 0.894, p < 0.0001) IMAT: good correlation but less significant (r = 0.742) |
| Lucijanic et al. (2021) [20] | To evaluate relationship between abdominal adipose tissue compartments and clinical outcomes in HL patients | Perirenal adipose tissue SAT | 82 | Higher minimum thickness of perirenal adipose tissue and a lower thickness of SAT (both p < 0.05) in patients with advanced disease International Prognostic Score stage disease for perirenal adipose tissue (Rho = 0.34, p = 0.002) and SAT (Rho = −0.27, p = 0.013) ROC curve analysis points for survival for minimal perirenal adipose tissue thickness (>2 mm; 33/82 (40.2%) patients), maximal perirenal adipose tissue thickness (>25 mm; 29/82 (35.4%) patients) and SAT thickness (≤22 mm; 54/82 (65.9%) patients) Univariate analysis showed higher minimal perirenal adipose tissue thickness (HR = 8.4; p < 0.001), higher maximal perirenal adipose tissue thickness (HR = 3.15; p = 0.049) and lower SAT thickness (HR = 3.57; p = 0.033) were significantly associated with inferior OS |
| Hinnerichs et al. (2022) [21] | To evaluate relationship between abdominal adipose tissue compartments and clinical outcomes in PCNSL patients | VAT SAT | 74 | No correlations |
| Camus et al. (2014) [22] | To evaluate body composition in elderly patients treated with immunochemotherapy in DLBCL patients | VAT SAT | 90 | The median PFS was 13.6 months in the adipopenic group and 49.4 months in the non-adipopenic group (hazard ratio (HR) = 2.27; 95% confidence interval (CI): 1.3–4; p = 0.0042) The median OS was 25.7 months in the adipopenic group and 57.1 months in the non-adipopenic group (HR = 1.93; 95% CI: 1.05–3.55; p = 0.0342) |
| Xiao et al. (2016) [23] | To evaluate longitudinal body composition changes and identified clinical variables related with development of sarcopenia and visceral obesity in DLBCL patients | VAT SAT | 343 | SAT increased from baseline of 6.5% during therapy (95% confidence interval (CI) = 2.6% to 10.5%) and of 21.4% by 24 months after therapy (95% CI = 15.7% to 27.2%) VAT increased from baseline of 4.5% during therapy (95% CI = −0.9% to 9.9%) and of 21.6% by 24 months after therapy (95% CI = 14.8% to 28.4%) |
| Wadhwa et al. (2022) [24] | To evaluate body composition in association with chemotherapy toxicity in patients with HL, NHL and rhabdomyosarcoma | height-adjusted TAT | 107 | No correlations |
| Tram et al. (2022) [25] | To evaluate body composition changes in pediatric, adolescent and young adult patients with lymphoma | VAT SAT | 110 | Male patients with NHL with stage 3 or 4 disease younger than 12 years of age showed significantly greater adipose tissue after the first treatment cycle |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Greco, F.; Beomonte Zobel, B.; Mallio, C.A. Impact of Quantitative Computed Tomography-Based Analysis of Abdominal Adipose Tissue in Patients with Lymphoma. Hematol. Rep. 2023, 15, 474-482. https://doi.org/10.3390/hematolrep15030049
Greco F, Beomonte Zobel B, Mallio CA. Impact of Quantitative Computed Tomography-Based Analysis of Abdominal Adipose Tissue in Patients with Lymphoma. Hematology Reports. 2023; 15(3):474-482. https://doi.org/10.3390/hematolrep15030049
Chicago/Turabian StyleGreco, Federico, Bruno Beomonte Zobel, and Carlo Augusto Mallio. 2023. "Impact of Quantitative Computed Tomography-Based Analysis of Abdominal Adipose Tissue in Patients with Lymphoma" Hematology Reports 15, no. 3: 474-482. https://doi.org/10.3390/hematolrep15030049
APA StyleGreco, F., Beomonte Zobel, B., & Mallio, C. A. (2023). Impact of Quantitative Computed Tomography-Based Analysis of Abdominal Adipose Tissue in Patients with Lymphoma. Hematology Reports, 15(3), 474-482. https://doi.org/10.3390/hematolrep15030049








