Abstract
The lotus (Nelumbo nucifera Gaertn.) is an ornamental aquatic plant, highly valued in Asian cultures for its religious symbolism, culinary uses, and medicinal properties. However, the lotus exhibits low genetic diversity in nature, which limits the genetic resources available for breeding programs. Gamma irradiation is an effective method for inducing genetic variation in lotus breeding. The present study examines the gamma sensitivity of lotus seedlings, along with the morphological and anatomical changes induced by various gamma dosages. The results showed that high-dose gamma irradiation (≥100 Gy) significantly inhibited seedling growth and altered most anatomical parameters, each exhibiting distinct dose–response patterns except for midrib diameter. The 100 Gy treatment resulted in the maximum stem diameter, while root diameter peaked at 500 Gy, and the highest dose (600 Gy) produced the largest petioles. Gamma irradiation also triggered tannin accumulation and reduced aerenchyma formation in the leaves. The obtained results demonstrate organ-specific responses to gamma irradiation in the lotus, with leaves being the most sensitive, while petioles, stems, and roots exhibited more variable dose-dependent effects.
1. Introduction
The lotus (Nelumbo nucifera Gaertn.) is an important aquatic plant classified within the Nelumbonaceae family, which comprises only two extant species [1]. It is widely distributed across Asia, where it has been extensively utilized and has become an integral part of daily life. In Chinese culture, seeds and roots are commonly consumed in traditional cuisine [2]. Moreover, the renowned lotus silk is also produced in Myanmar [3]. The striking characteristics of the lotus flower make it a dominant feature in aquatic landscape decoration. However, lotus showed low genetic diversity because of low gene flow levels and widespread clonal propagation [4]. In order to increase genetic variation, mutation breeding using gamma irradiation has played an important role in the cultivation of new varieties [5].
Gamma irradiation, an ionizing irradiation, is widely employed in plant mutation breeding due to its high penetration ability and effectiveness in inducing heritable changes at both phenotypic and genotypic levels [6]. A number of ornamental plants, such as roses [7], chrysanthemums [8], and tulips [9], have been genetically improved through gamma irradiation-induced mutation breeding. Generally, gamma rays can exert varying effects on plants depending on the dose. Low doses of gamma irradiation (below 100 Gy) have been shown to enhance the germination rate and growth of okra and cucumber [10], increase the number of fruits and total yield in tomato [11], and elevate photosynthetic pigment concentrations in lettuce [12]. Moreover, it has been reported that gamma irradiation at 10–60 Gy reduced shoot regeneration in Gerbera jamesonii and callus formation in Chrysanthemum morifolium, with both studies reporting significant declines in regeneration rates and nuclear DNA content at higher doses [13,14]. While the effects of low to moderate doses of gamma irradiation on plant growth and development have been relatively well studied, the impacts of high-dose gamma irradiation on structural traits, particularly anatomical features, remain largely unexplored.
In the lotus, a study involving acute gamma irradiation showed that seeds exposed to doses between 0 and 1000 Gy had limited germination, and none of the resulting seedlings survived [15]. In contrast, rhizomes irradiated with doses between 0 and 50 Gy showed a dose-dependent decline in germination rates, with an LD50 estimated at approximately 12 Gy [15]. In vitro propagation and mutation breeding have also been applied to improve lotus varieties. Previous studies have reported that young lotus plants derived from in vitro culture exhibited LD50 values of 26.138 Gy for pink lotus and 32.03 Gy for white lotus when exposed to gamma irradiation [16]. These findings highlight the sensitivity of lotus to gamma irradiation and its potential for inducing phenotypic variation.
The lotus, as an aquatic plant, often shows distinct anatomical adaptations that enable it to thrive in submerged or waterlogged environments. One of the most notable features is the development of aerenchyma, a specialized tissue with large intercellular air spaces that facilitate internal aeration and buoyancy [17]. Therefore, presenting data related to aerenchyma structure, as well as other anatomical characteristics, can be useful in predicting the growth potential of the lotus seedlings. This study investigated the impact of high-dose gamma irradiation on the morphological and anatomical characteristics of young lotus plants derived from tissue culture exposed to acute irradiation. The findings will provide an early means of assessing dose-dependent effects on plant development and predicting potential physiological or phenotypic outcomes, which will be valuable for optimizing mutation breeding conditions in lotus.
2. Materials and Methods
2.1. Plant Material and Culture Medium
Four-month-old seeds from the pink perianth of the lotus were harvested and utilized as plant material. The full-strength MS (Murashige and Skoog) medium [18], containing 3% (wv−1) sucrose, was supplemented with 2 mg L−1 of 6-Benzylaminopurine (Sigma-Aldrich®, St. Louis, MO, USA) and 0.2% (wv−1) Gellan gum (Sigma-Aldrich®, St. Louis, MO, USA) as the basal medium. The pH of the medium was adjusted to 5.7–5.8 using 1 N NaOH or HCl prior to autoclaving at 121 °C and 100 kPa for 20 min. For the gamma irradiation medium, the basal medium was supplemented with 0.2% (wv−1) Gellan gum to facilitate solidification, then aliquoted, and 10 mL of medium was poured into 50 mL sterile plastic centrifuge tubes (Avantor®, Radnor, PA, USA) under aseptic conditions. The liquid basal medium was used as the culture medium for the post-gamma irradiated material.
2.2. Plant Material Preparation and Gamma Irradiation
Fresh lotus fruits with a green pericarp were extracted from the torus. The collected fruit samples were thoroughly washed with tap water, followed by immersion in 70% ethanol and flame sterilization in a laminar airflow cabinet, with the process repeated twice. The embryos were excised longitudinally, and both the pericarp and endosperm were removed. The isolated embryos were cultured on Murashige and Skoog medium (1962) supplemented with 2 mg L−1 of 6-Benzylaminopurine (BAP) (BMS) for 7 days to monitor for contamination and ensure uniform growth before exposure to gamma rays. Afterward, explants were transferred to a centrifuge tube containing solidified BMS with 10 healthy seedlings per tube.
Gamma irradiation was performed using a Gamma Chamber GC5000 (Board of Radiation & Isotope Technology, BRIT, Navi Mumbai, India), applying doses ranging from 0 to 1000 Gy (dose rate: 1.16 kGy−1h) at the Thailand Institute of Nuclear Technology. Three replicates, each consisting of 10 seedlings, were used for each treatment. Each replicate was transferred to 35 mL of liquid basal medium in a 200 mL cylindrical glass bottle (5.2 cm width × 10.5 cm height × 4.8 cm diameter, Union Glass, Samut Prakan, Thailand), which was sealed with a transparent plastic lid. All bottles were placed on an orbital agitator at 120 rpm. The culture conditions included a 16 h photoperiod with a photosynthetic photon flux density (PPFD) of 23 μmol m−2s−1, provided by 18 W cool white LED lights (Philips, Bangkok, Thailand), and a temperature of 25 ± 2 °C throughout the experiment. The survivability of the seedlings was examined after 30 days of gamma ray exposure for LD50(30d) calculation.
2.3. Morphological and Anatomical Examination
To compare the morphological and anatomical alterations between control and gamma-irradiated lotus seedlings, data were collected from three replicates of surviving seedlings from each treatment group. The morphological characteristics examined included leaf number, leaf length, petiole length, root number, root length, and node number. Tissue samples from one-month-old seedlings derived from gamma-irradiated seeds (0–600 Gy) were collected by excision from the central region of the leaf, petiole, stem, and root (Figure 1). The aerenchyma ratio of leaves, petioles, stems, and roots was determined by the formula below.
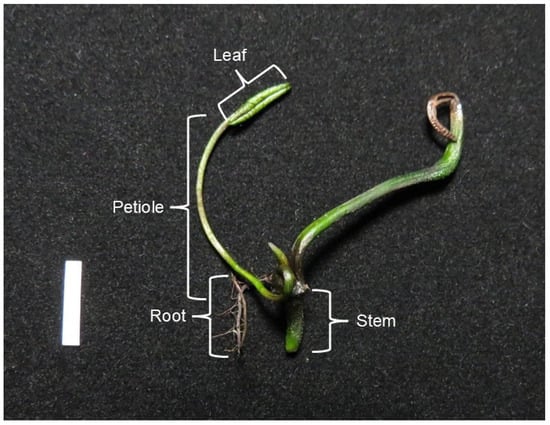
Figure 1.
Different tissue structures collected for anatomical examination. Scale bar = 1 cm.
The samples were fixed in FAAII (formaldehyde (VMR Chemicals, Fontenay-sous-Bois, France), glacial acetic acid (Sigma-Aldrich®, Burlington, MA, USA), and 70% ethyl alcohol (Sigma-Aldrich®, Burlington, MA, USA); 5:5:90 v−1v−1v−1) for 48 h, following the protocol outlined by Ruzin (1999) [19]. Samples were randomly collected from three seedlings in each treatment. Anatomical characteristics were then analyzed both quantitatively and qualitatively.
2.4. Statistical Analysis
All experiments were designed using a completely randomized design (CRD), which contained 10 replicates in each treatment. The means of each parameter tested (leaf length, leaf numbers, petiole length, root length, and root numbers) were compared using a one-way analysis of variance (ANOVA), followed by Duncan’s multiple range test (DMRT) at a significance level of p ≤ 0.05. Statistical analysis was performed using SPSS® statistics software version 26.0 (IBM Corporation, Armonk, NY, USA).
The research framework is illustrated in Figure 2.
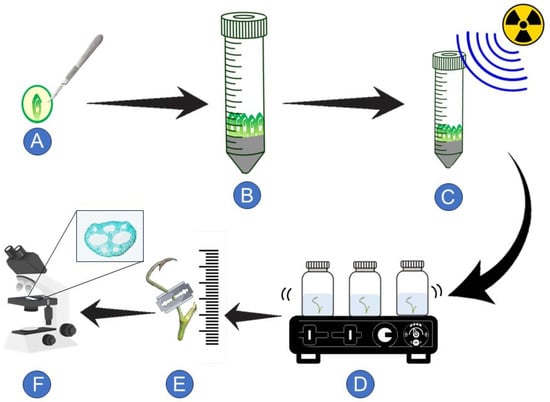
Figure 2.
Conceptual diagram of the study of anatomical investigation of gamma irradiated lotus seedling (Nelumbo nucifera Gaertn.). (A) Embryos were isolated from flamed fruit. (B) Lotus embryos were cultured on solidified basal medium (BMS; Murashige and Skoog medium (1962)) supplemented with 2 mg L−1 of 6-Benzylaminopurine (BAP) for 7 days. (C) Lotus seedlings (M1V0) were acute gamma irradiation at 0–1100 Gy. (D) Gamma irradiated lotus seedlings (M1V1) were transferred into liquefied BMS medium for 30 days on 120 rpm orbital agitator. (E) Morphological and anatomical alteration investigations were observed. (F) Anatomical alteration affected by gamma irradiation observed under a microscope.
3. Results
3.1. Morphological Observation
Lotus seedlings (M1V0) were subjected to acute gamma irradiation at doses ranging from 0 to 1100 Gy. The results indicated that no M1V1 seedlings survived at high gamma doses between 700 and 1100 Gy before reaching 30 days of post-irradiation. The LD50(30d) was calculated at approximately 570 Gy. Adverse effects on the morphological characteristics of the lotus seedlings were observed. Gamma-irradiated seedlings exhibited reductions in leaf number, leaf length, petiole length, root number, root length, and node number compared to the control group (Table 1). Specifically, leaf number decreased from 6.9 (control) to 1.77 leaves, and petiole length was reduced from 38.60 cm (control) to 1.85 cm at 600 Gy. Gamma irradiation at doses between 100 and 600 Gy also strongly hindered root formation and completely suppressed node development (Figure 3).

Table 1.
Effect of gamma rays on morphological character of M1V1 lotus seedlings after 30 days of irradiation.

Figure 3.
Morphology of lotus seedling after exposure to acute gamma irradiation at 0–600 Gy. Scale bar = 1 cm.
3.2. Anatomical Observation
The anatomical characteristics of lotus (Nelumbo nucifera Gaertn.) embryos were examined following exposure to varying doses of gamma irradiation to assess potential structural changes. The effect of gamma irradiation on the diameter of midribs, petioles, stems, and roots of lotus seedlings is summarized in Table 2 and Figure 4, Figure 5, Figure 6 and Figure 7. The general anatomy of the plants without treatment (0 Gy) was presented as the control.

Table 2.
Effect of gamma irradiation on diameter of midribs, petioles, stems, and roots of lotus seedlings.
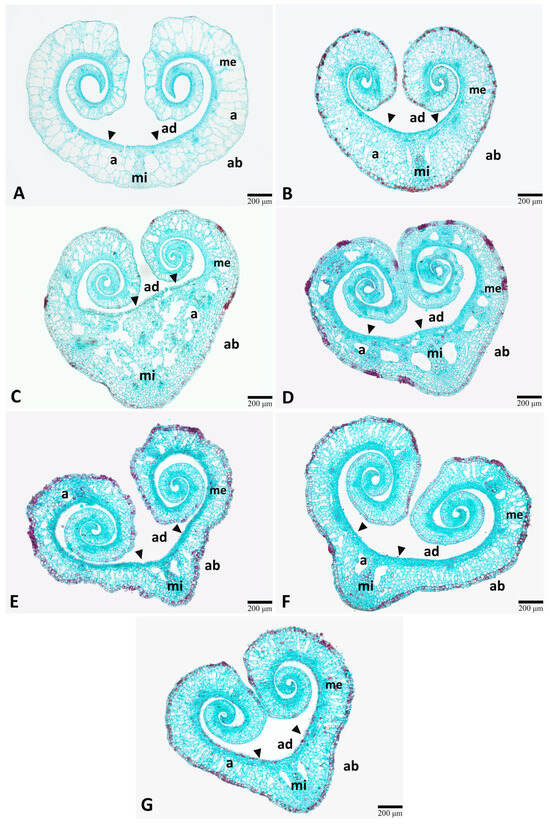
Figure 4.
Comparison of the leaf anatomical structure of lotus (Nelumbo nucifera Gaertn.) under various gamma irradiation doses (Gy). (A) 0 Gy (control). (B) 100 Gy. (C) 200 Gy. (D) 300 Gy. (E) 400 Gy. (F) 500 Gy. (G) 600 Gy. a, air space; ab, abaxial side; ad, adaxial side; me, mesophyll; mi, midrib.
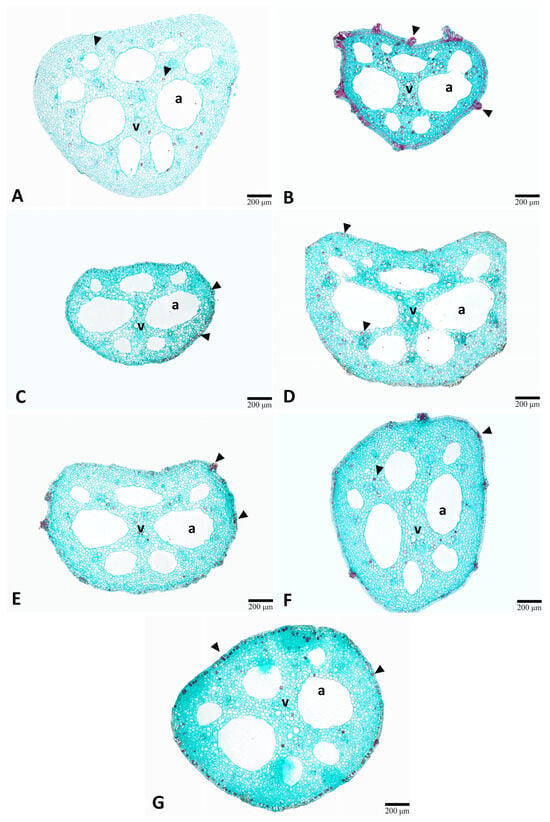
Figure 5.
Comparison of the petiole anatomical structure of lotus (Nelumbo nucifera Gaertn.) under various gamma irradiation doses (Gy). (A) 0 Gy (control). (B) 100 Gy. (C) 200 Gy. (D) 300 Gy. (E) 400 Gy. (F) 500 Gy. (G) 600 Gy. a, air space; v, vascular bundle.
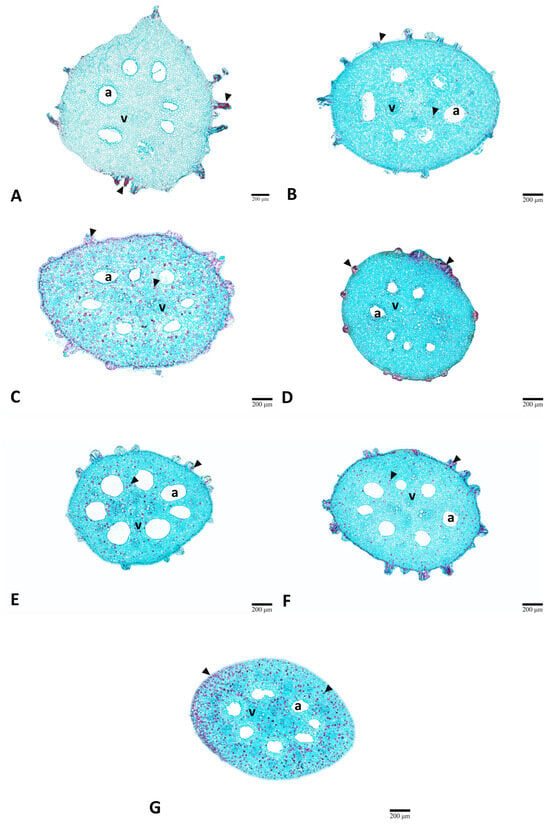
Figure 6.
Comparison of the stem anatomical structure of lotus (Nelumbo nucifera Gaertn.) under various gamma irradiation doses (Gy). (A) 0 Gy (control). (B) 100 Gy. (C) 200 Gy. (D) 300 Gy. (E) 400 Gy. (F) 500 Gy. (G) 600 Gy. a, air space; v, vascular bundle.
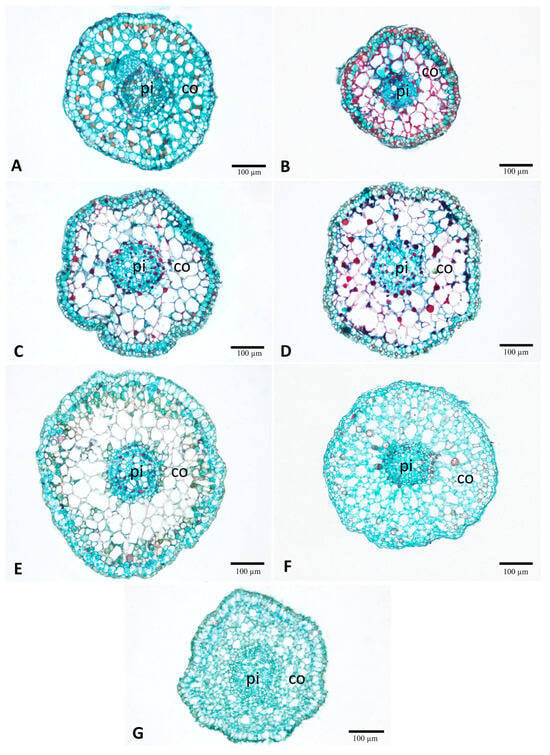
Figure 7.
Comparison of the root anatomical structure of lotus (Nelumbo nucifera Gaertn.) under various gamma irradiation doses (Gy). (A) 0 Gy (control). (B) 100 Gy. (C) 200 Gy. (D) 300 Gy. (E) 400 Gy. (F) 500 Gy. (G) 600 Gy. co, cortex; pi, pith.
As the leaf blades were still in the early stage, they remained curled inward, resulting in a heart-shaped appearance when sectioned transversely (Figure 4). The epidermal cells of the adaxial side formed papillae (Figure 4A–G, arrowheads). The mesophyll was not distinctly differentiated into palisade and spongy parenchyma layers. Aerenchyma was predominantly observed, with extensive air spaces extending through the mesophyll. According to the comparative anatomy, the results showed no significant differences in the midrib diameter between treatments (Table 2). Moreover, tannins were prominent in the leaf blade, appearing as red to dark red cells due to safranin staining. Quantitative anatomical analysis showed that no tannin accumulation was detected in the epidermis of the leaf blade of control plants (0 Gy) (Figure 4A), whereas all treated plants exhibited tannin accumulation in the epidermis (Figure 4B–G).
The petiole was characterized by an outline that was rounded to semi-circular (Figure 5). The epidermis was uniseriate with oval or polygonal epidermal cell shape. Parenchyma constituted the main ground tissue, and the vascular bundles were scattered throughout the petiole. Prominent aerenchyma, characterized by large intercellular air spaces, was observed within the petiole structure of all treatments (Figure 5). However, no tannin accumulation was observed in the epidermal layer, but tannins were present in the parenchyma throughout the petiole cross-section (Figure 5A). In contrast, the treated plants exhibited tannin deposition in the epidermis and parenchyma (Figure 5B–G, arrowheads). Meanwhile, gamma irradiation treatment significantly influenced petiole size in the treated plants, with sizes ranging from 1.13 mm to 1.86 mm across different irradiation doses. Low-dose irradiation at 100 Gy resulted in a slight reduction, while 200 Gy treatment caused the most significant reduction in petiole size (Table 2). Interestingly, higher irradiation doses (300–600 Gy) did not follow the same inhibitory pattern. Plants treated with 300 Gy showed recovery with petiole sizes, which were not significantly different from the control. Similarly, treatments at 400 Gy and 500 Gy maintained petiole sizes comparable to the control group. The highest dose tested (600 Gy) produced the largest petioles (Table 2).
Stems were usually round in shape (Figure 6). Vascular bundles were irregularly distributed and embedded in the parenchymatous tissue. Extensive air spaces were radially arranged, and tannins were distributed throughout the cross-section of all treatments (Figure 6A–G, arrowheads). Stem diameter displayed the most pronounced stimulatory response to low-dose irradiation. The 100 Gy treatment produced the maximum diameter, representing a 30% increase over the control (Table 2). Meanwhile, higher doses (200–600 Gy) showed a gradual decline (Table 2).
The roots were almost round in shape (Figure 7). Parenchyma served as the primary ground tissue, while aerenchyma was also present throughout the cortical zone of roots (Figure 7). Tannins were detected in the epidermis and some parenchyma in the cortex and pith of all treatments (Figure 7A–G). According to comparative anatomy, root diameter showed a different pattern compared to the petioles. The 100 Gy treatment resulted in a significant reduction compared with the control (0 Gy). However, doses from 200 to 500 Gy showed a progressive increase, reaching the maximum at 500 Gy, before decreasing again at 600 Gy (Table 2).
Since aerenchyma formation was a notable anatomical feature, the aerenchyma ratios were further quantified, and the comparative data are summarized in Table 3. The results showed that gamma irradiation significantly affected the percentage of aerenchyma in different organs of lotus plants, exhibiting a dose-dependent response. In leaves, the percentage of aerenchyma was substantially reduced in all gamma-irradiated treatments (6.13–9.12%) compared to the control group (40.23%), with statistically significant differences observed (Table 3).

Table 3.
Effect of gamma irradiation on the % aerenchyma ratio of leaves, petioles, stems, and roots of lotus seedlings.
In the petiole, the highest aerenchyma percentages were found in the control (36.11%) and 200 Gy (37.59%) treatments, with no significant differences between these groups, while higher doses resulted in lower but not significantly different values, ranging from 25.15 to 31.69%.
In stems, the highest aerenchyma percentage occurred at 400 Gy (14.17%), significantly greater than the control (7.20%) and most other treatments. Other doses showed no significant differences relative to the control, with values ranging from 5.82% to 9.39%.
In roots, aerenchyma percentages at low to moderate doses (100–400 Gy) ranged from 27.23% to 36.98%, showing no significant reduction compared to the control (31.26%). However, higher doses (500 and 600 Gy) caused a marked decrease, with values of 11.54% and 20.24%, respectively, the former being significantly lower than all other treatments.
4. Discussion
Based on our results, gamma irradiation at doses ≥ 100 Gy exhibited inhibitory effects on lotus seedlings, as evidenced by reductions in all measured morphometric parameters, which may indicate exposure to excessive levels of gamma irradiation (Table 1). A decrease in plant regeneration has frequently been observed with increasing gamma dosage. For instance, exposure to gamma irradiation ranging from 10 to 60 Gy resulted in a reduction in shoot regeneration in Gerbera jamesonii, with the number of shoots decreasing from 7.5 to 2.5 in petiole explants and from 8.7 to 4.1 in plantlets [13]. A similar observation was reported by Yamaguchi et al. (2008), where exposure to gamma irradiation ranging from 10 to 60 Gy led to a decline in callus regeneration from leaf explants of Chrysanthemum morifolium cv. ‘Taihei’ from 80% to 10%, along with a reduction in nuclear DNA content at higher irradiation doses [14].
However, lower doses of gamma irradiation significantly enhanced vegetative traits, whereas higher doses had a suppressive effect. A similar pattern was observed in reproductive traits. Lokesha et al. (1994) reported that shoot length, leaf number, node number, and root length decreased with increasing gamma doses (100–300 Gy) in seeds of Bambusa arundinacea [20]. Similarly, Salvia uliginosa cv. ‘Ballon Azul’ showed reduced root length and quality in M1V1 plants after exposure to 10–50 Gy. However, root growth showed recovery in M1V2 plants [21]. High doses of gamma irradiation have been shown to retard plant growth in various species, including bougainvillea [22], chrysanthemum [23], and hybrid tea rose [7].
According to the comparative anatomical analysis, based on both qualitative and quantitative data, the results showed that gamma irradiation treatment significantly affected all anatomical parameters measured, except for the midrib diameter. Each parameter displayed a distinct dose-response pattern, providing insights into the differential sensitivity of plant organs to irradiation stress. Several studies have reported that gamma irradiation, at lower doses, can lead to alterations in vegetative traits [24,25]. For example, Rosmala et al. (2016) found that gamma irradiation at 75, 90, and 105 Gy significantly increased the thickness of the palisade mesophyll, spongy tissue, and upper epidermis in Handeuleum (Graptophyllum pictum L. Griff) [26]. Moreover, Sakr et al. (2013) stated that gamma-ray irradiation at doses of 5, 10, and 15 Gy altered the size of the epidermis, mesophyll, and the diameter of vascular tissues in leaves, stems, and roots in Dracaena surculosa (L.) [27]. However, the midrib diameter of lotus showed no significant differences under high-dose gamma irradiation treatments. The unaltered midrib diameter also indicates that not all anatomical traits are equally responsive to mutagenic treatment, emphasizing the importance of evaluating multiple parameters when assessing irradiation effects in plant mutation studies.
Moreover, visible tannin accumulation was observed in the epidermis of the leaf blade in all gamma-irradiated plants, whereas no such accumulation was present in the control. This suggests that gamma irradiation may induce metabolic changes without necessarily altering anatomical dimensions. Supporting this observation, Janiak et al. (2017) reported that gamma irradiation preserved or even enhanced both antioxidant activity and tannin content in various Bulgarian herbal teas [28]. Generally, gamma rays can cause oxidative stress in plant cells, which often triggers the production of phenolic compounds such as tannins to mitigate damage [29]. The enhanced tannin levels observed in the leaves suggest that gamma irradiation not only affects primary growth processes but also stimulates protective mechanisms.
Besides tannin accumulation, the formation of aerenchyma is also distinctly notable. The results of the aerenchyma ratio revealed that different parts of the lotus respond differently to gamma irradiation. Leaves are the most sensitive, while roots and stems react in varying ways depending on the irradiation dose. Our results showed that gamma irradiation appeared to reduce aerenchyma formation in leaves. Aerenchyma, a specialized tissue that facilitates internal gas exchange, is commonly formed in response to hypoxia or other stress conditions, and its development involves programmed cell death (PCD) and cell wall degradation [17]. Gamma irradiation, particularly at high doses, generates reactive oxygen species (ROS) and induces oxidative stress, which may disrupt the delicate balance of signaling pathways that regulate PCD. This aligns with previous findings in rice, where ROS accumulation has been shown to play a key role in aerenchyma formation in leaf sheaths and internodes [17]. Ethylene, a gaseous plant hormone, rapidly accumulates in roots under waterlogged conditions and plays a crucial role in inducing lysigenous aerenchyma formation in crops like maize and rice [30,31]. The disruption of aerenchyma formation observed in lotus exposed to high doses of gamma irradiation may be linked to interference with ethylene signaling or production. Such stress could suppress ethylene synthesis or disrupt its signaling pathways, thereby inhibiting the programmed cell death process necessary for aerenchyma development. Consequently, the formation of aerenchyma is impaired under high irradiation stress. The disruption not only affects the anatomical structure but may also compromise the plant’s ability to tolerate low-oxygen environments. These findings highlight the importance of carefully selecting irradiation doses in mutation breeding programs to avoid compromising essential anatomical traits.
Overall, the morphological and anatomical alterations observed under high-dose gamma irradiation may be closely associated with changes in plant traits relevant to both productivity and ornamental value. The inhibition of seedling growth and reduction of most anatomical dimensions indicate impaired tissue development, which could reduce biomass accumulation and overall vigor. The reduced formation of aerenchyma suggests a diminished capacity for gas exchange and internal aeration, potentially limiting stress tolerance in hypoxic or waterlogged conditions. Conversely, the accumulation of tannins in the leaves represents a protective adaptation, enhancing defense against herbivory and pathogen attack, which could improve survivability under field conditions. Changes in vascular tissues, although variable across doses, may influence water and nutrient transport efficiency; this could affect leaf robustness, floral development, or overall plant architecture, thereby contributing to variation in decorativeness. Taken together, these anatomical responses reflect both inhibitory and adaptive processes that may generate novel phenotypic traits under mutagenic treatments, linking structural modifications at the tissue level to visible characteristics at the whole-plant level. However, the effects on physiological responses, genetic changes, and the performance of the next generation still need to be investigated in future studies.
5. Conclusions
Gamma irradiation at doses of 100 Gy or higher inhibited lotus seedling growth and significantly altered most anatomical traits, with each showing a unique dose-response pattern, except for midrib diameter. Gamma irradiation also triggered tannin accumulation and reduced aerenchyma formation in the leaves. The results demonstrate organ-specific responses to gamma irradiation in the lotus, with leaves being the most sensitive, while petioles, stems, and roots exhibited more variable dose-dependent effects. These findings could serve as a foundation for selecting an appropriate gamma dose for inducing mutations in Nelumbo nucifera Gaertn. without causing adverse anatomical alterations that could negatively affect physiological functions essential for survival and growth, factors that are critical for successful lotus breeding programs. Moreover, it can be applied to the modification of anatomical and physiological traits for improved stress tolerance or specific agricultural purposes.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/ijpb16030101/s1; Table S1: Normal distribution was tested using a Shapiro–Wilk test and Kruskal–Wallis test was performed to determine differences among the treatments (0–600 Gy); Table S2: Summary of one-way ANOVAs of anatomical parameters of midribs, petioles, roots, and stem diameter of gamma irradiated lotus seedlings; Table S3: Summary of one-way ANOVAs of anatomical parameters of the leaf, petiole, root, and stem aerenchyma ratio of gamma irradiated lotus seedlings.
Author Contributions
Conceptualization, P.S., P.O., and S.S.; methodology, P.S., P.O., S.S., and O.D.; software, P.S., P.O., S.Y., and P.K.; validation, P.S., P.O., S.Y., P.K., and S.S.; formal analysis, P.S., P.O., and S.S.; investigation, P.S. and S.S.; resources, S.S., P.S., and P.O.; data curation, S.S., P.O., O.D., and P.K.; writing—original draft preparation, P.S. and S.S.; writing—review and editing, P.S. and S.S.; visualization, P.S. and S.S.; supervision, S.S.; project administration, S.S. and P.O.; funding acquisition, S.S., P.S., and P.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Thailand Institute of Nuclear Technology (Public Organization) under the TINT to University program, and the APC was funded by the International SciKU Branding (ISB), the Faculty of Science, Kasetsart University, Thailand.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author(s).
Acknowledgments
S.S. acknowledges the Faculty of Natural Resources and Agro-Industry, Kasetsart University, Chalermphrakiat Sakon Nakhon Province Campus, for their support with facilities. P.O. thanks the Thailand Institute of Nuclear Technology for their support with the facilities of gamma irradiation. P.S. gratefully acknowledges the kind assistance of Imee Poorahong and Anawat Padpaiboon.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| BAP | 6-Benzylaminopurine |
| BMS | Murashige and Skoog medium (1962) supplemented with 2 mg L−1 of 6-Benzylaminopurine |
| PCD | Programmed cell death |
| ROS | Reactive oxygen species |
References
- Zhu, F. Structures, Properties, and Applications of Lotus Starches. Food Hydrocoll. 2017, 63, 332–348. [Google Scholar] [CrossRef]
- Yang, H.; He, S.; Feng, Q.; Liu, Z.; Xia, S.; Zhou, Q.; Wu, Z.; Zhang, Y. Lotus (Nelumbo nucifera): A Multidisciplinary Review of Its Cultural, Ecological, and Nutraceutical Significance. Bioresour. Bioprocess. 2024, 11, 18. [Google Scholar] [CrossRef]
- Aishwariya, S.; Thamima, S. Sustainable textiles from lotus. Asian Text. J. 2019, 28, 56–59. [Google Scholar]
- Mekbib, Y.; Huang, S.X.; Ngarega, B.K.; Li, Z.Z.; Shi, T.; Ou, K.F.; Liang, Y.T.; Chen, J.M.; Yang, X.Y. The Level of Genetic Diversity and Differentiation of Tropical Lotus, Nelumbo nucifera Gaertn. (Nelumbonaceae) from Australia, India, and Thailand. Bot. Stud. 2020, 61, 15. [Google Scholar] [CrossRef]
- Ma, L.; Kong, F.; Sun, K.; Wang, T.; Guo, T. From Classical Radiation to Modern Radiation: Past, Present, and Future of Radiation Mutation Breeding. Front. Public Health 2021, 9, 768071. [Google Scholar] [CrossRef] [PubMed]
- United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR). Sources and Effects of Ionizing Radiation: UNSCEAR 2000 Report to the General Assembly, with Scientific Annexes. Volume I: Sources; United Nations: New York, NY, USA, 2000. [Google Scholar]
- Bala, M.; Singh, K.P. In Vitro Mutagenesis of Rose (Rosa hybrida L.) Explants Using Gamma-Radiation to Induce Novel Flower Colour Mutations. J. Hortic. Sci. Biotechnol. 2013, 88, 462–468. [Google Scholar] [CrossRef]
- Puripunyavanich, V.; Boonsirichai, K. Effect of gamma irradiation in ‘jongkolnee’ waterlily. In Proceedings of the 31st Congress on Science and Technology of Thailand, Bangkok, Thailand, 18–20 October 2005. [Google Scholar]
- Li, Y.; Chen, L.; Zhan, X.; Liu, L.; Feng, F.; Guo, Z.; Wang, D.; Chen, H. Biological Effects of Gamma-Ray Radiation on Tulip (Tulipa gesneriana L.). PeerJ 2022, 10, e12792. [Google Scholar] [CrossRef] [PubMed]
- Jaipo, N.; Kosiwikul, M.; Panpuang, N.; Prakrajang, K. Low dose gamma radiation effects on seed germination and seedling growth of cucumber and okra. J. Phys. Conf. Ser. 2019, 1380, 012106. [Google Scholar] [CrossRef]
- Wiendl, T.A.; Wiendl, F.W.; Arthur, P.B.; Franco, S.S.H.; Franco, J.G.; Arthur, V. Effects of Gamma Radiation in Tomato Seeds. In Proceedings of the 2013 International Nuclear Atlantic Conference, Recife, Brazil, 24–29 November 2013; Assciação Brasileira de Energia Nuclear-Aben: Rio de Janeiro, Brazil, 2013. ISBN 978-85-99141-05-2. [Google Scholar]
- Marcu, D.; Cristea, V.; Daraban, L. Dose-Dependent Effects of Gamma Radiation on Lettuce (Lactuca sativa var. capitata) Seedlings. Int. J. Radiat. Biol. 2013, 89, 219–223. [Google Scholar] [CrossRef]
- Hasbullah, N.A.; Taha, R.M.; Saleh, A.; Mahmad, N. Irradiation effect on in vitro organogenesis, callus growth and plantlet development of Gerbera jamesonii. Hortic. Bras. 2012, 30, 252–257. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Shimizu, A.; Degi, K.; Morishita, T. Effects of Dose and Dose Rate of Gamma Ray Irradiation on Mutation Induction and Nuclear DNA Content in Chrysanthemum. Breed. Sci. 2008, 58, 331–335. [Google Scholar] [CrossRef]
- Soontornyatara, S.; Singhavorachai, P.; Puripunyavanich, V.; Taywiya, P. Effect of Gamma Ray on Morphological Characteristic of Nelumbo nucifera (Roseum Plenum Lotus). ISHS Acta Hortic. 2017, 1167, 217–220. [Google Scholar] [CrossRef]
- Pikulthong, V.; Hongjan, N.; Ariya, S.; Dechkla, M.; Boonman, N.; Wanna, C.; Wongwiwat, P.; Phakpaknam, S. In Vitro Acute Gamma Radiation on Tissue of Pink and White Lotus (Nelumbo nucifera Gaertn.) in Thailand. Plant Sci. Today 2024, 11, 306–313. [Google Scholar] [CrossRef]
- Takahashi, H.; Yamauchi, T.; Colmer, T.D.; Nakazono, M. Aerenchyma Formation in Plants. Plant Cell Monogr. 2014, 21, 247–265. [Google Scholar] [CrossRef]
- Murasnige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Ruzin, S.E. Plant Microtechnique and Microscopy, 3rd ed.; Oxford University Press: New York, NY, USA, 1999; pp. 154–196. [Google Scholar]
- Lokesha, R.; Vasudeva, R.; Shashidhar, H.E.; Reddy, A.N.Y. Radio-sensitivity of Bambusa arundinacea to gamma rays. J. Trop. For. Sci. 1994, 6, 444–450. [Google Scholar]
- Maynard, R.C.I.; Ruter, J.M. Co60 Gamma Irradiation Reduces Rooting Ability in M1V1 Salvia uliginosa While Inducing Leaf Variegation. Int. J. Radiat. Biol. 2024, 100, 663–668. [Google Scholar] [CrossRef]
- Chatse, D.; Gajbhiye, R.; Kedar, D.; Ningot, E.P.; Shende, P. Impact of Gamma Radiation on Bougainvillea Varieties Root Parameters in the VM1 and VM2 Generation. Int. J. Adv. Biochem. Res. 2024, 8, 521–524. [Google Scholar] [CrossRef]
- Puripunyavanich, V.; Piriyaphattarakit, A.; Chanchula, N.; Taychasinpitak, T. Mutation induction of in vitro chrysanthemum by gamma irradiation. Chiang Mai J. Sci. 2019, 46, 609–617. [Google Scholar]
- Jan, S.; Parween, T.; Siddiqi, T.O.; Mahmooduzzafar. Gamma Radiation Effects on Growth and Yield Attributes of Psoralea corylifolia L. with Reference to Enhanced Production of Psoralen. Plant Growth Regul. 2011, 64, 163–171. [Google Scholar] [CrossRef]
- Widiastuti, A.; Sibir, S.; Suhartanto, M.R. Diversity Analysis of Mangosteen (Garcinia mangostana) Irradiated by Gamma-Ray Based on Morphological and Anatomical Characteristics. Nusant. Biosci. 1970, 2, 1. [Google Scholar] [CrossRef]
- Rosmala, A.; Khumaida, N.; Sukma, D. Alteration of Leaf Anatomy of Handeuleum (Graptophyllum pictum L. Griff) Due to Gamma Irradiation. HAYATI J. Biosci. 2016, 23, 138–142. [Google Scholar] [CrossRef]
- Sakr, S.S.; El-Khateeb, M.A.; Taha, H.S.; Esmail, S.A. Effects of Gamma Irradiation on In Vitro Growth, Chemical Composition and Anatomical Structure of Dracaena surculosa (L.). J. Appl. Sci. Res. 2013, 9, 3795–3801. [Google Scholar]
- Janiak, M.A.; Slavova-Kazakova, A.; Karamać, M.; Kancheva, V.; Terzieva, A.; Ivanova, M.; Tsrunchev, T.; Amarowicz, R. Effects of Gamma-Irradiation on the Antioxidant Potential of Traditional Bulgarian Teas. Nat. Prod. Commun. 2017, 12, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Behgar, M.; Ghasemi, S.; Naserian, A.; Borzoie, A.; Fatollahi, H. Gamma Radiation Effects on Phenolics, Antioxidants Activity and In Vitro Digestion of Pistachio (Pistachia vera) Hull. Radiat. Phys. Chem. 2011, 80, 963–967. [Google Scholar] [CrossRef]
- Visser, E.J.W.; Voesenek, L.A.C.J. Acclimation to Soil Flooding-Sensing and Signal-Transduction. Plant Soil. 2005, 274, 197–214. [Google Scholar] [CrossRef]
- Jung, J.; Lee, S.C.; Choi, H.-K. Anatomical patterns of aerenchyma in aquatic and wetland plants. J. Plant Biol. 2008, 51, 428–439. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).