Impacts of Foliar Application of Se and TiO2 Nanoparticles on Growth, Development, and Flowering in Lilium Sunny Oriental
Abstract
1. Introduction
2. Materials and Methods
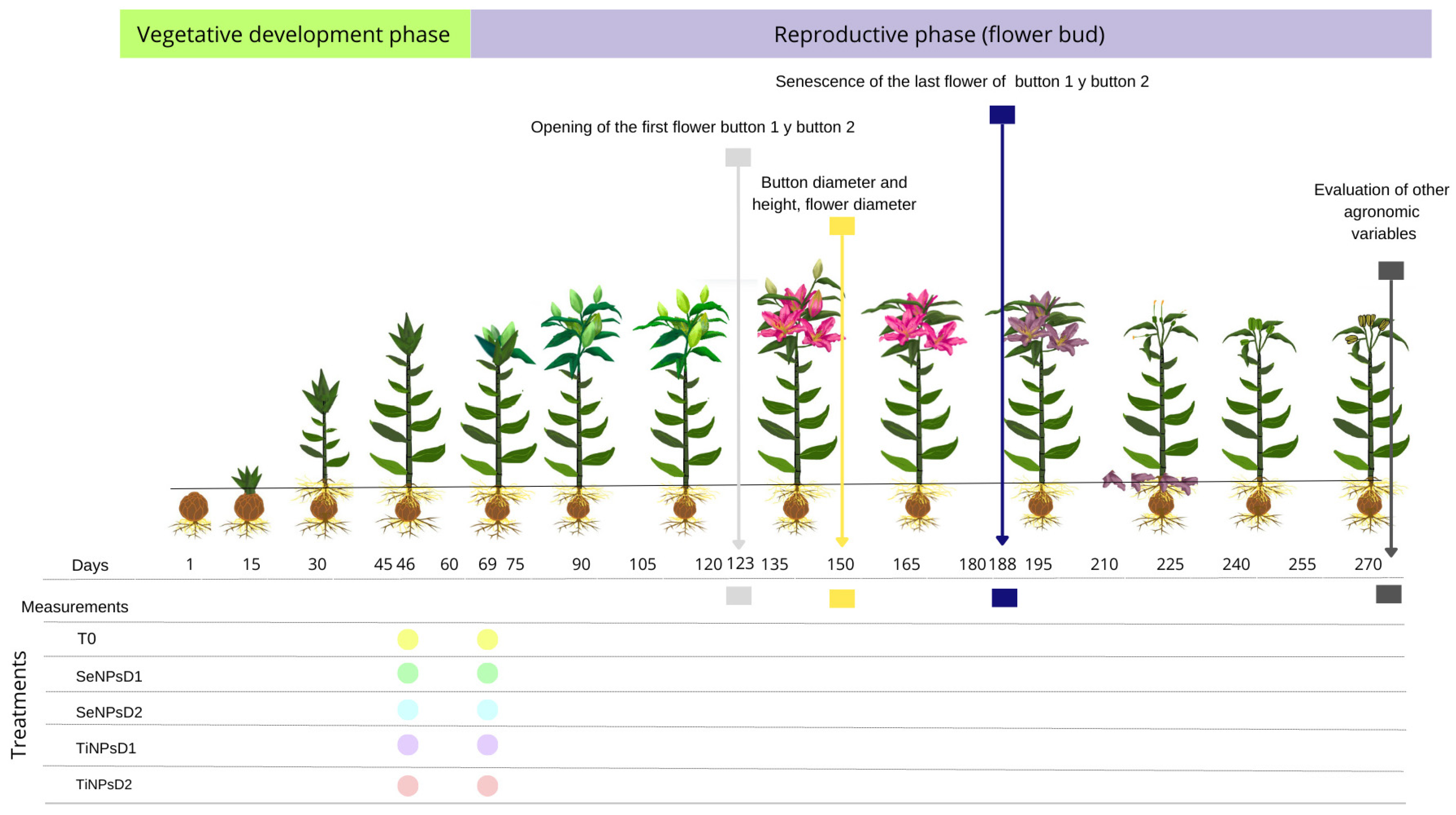
2.1. Crop Establishment and Management
2.2. Application of Treatments
2.3. Agronomic Variables
2.4. Scanning Electron Microscopy and Energy Dispersive X-Ray Spectroscopy
2.5. Statistical Analysis
3. Results
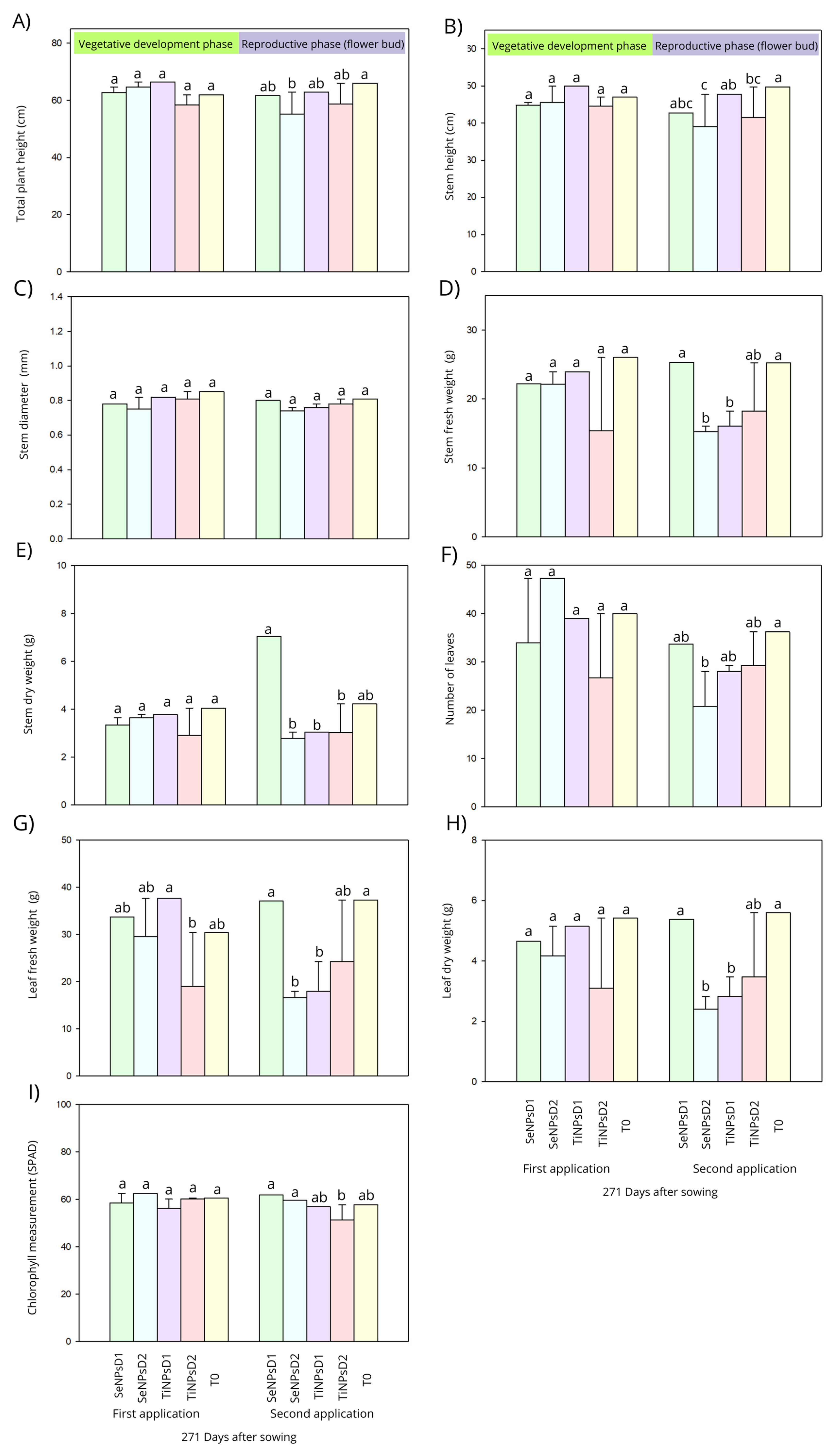
3.1. Vegetative Parameters
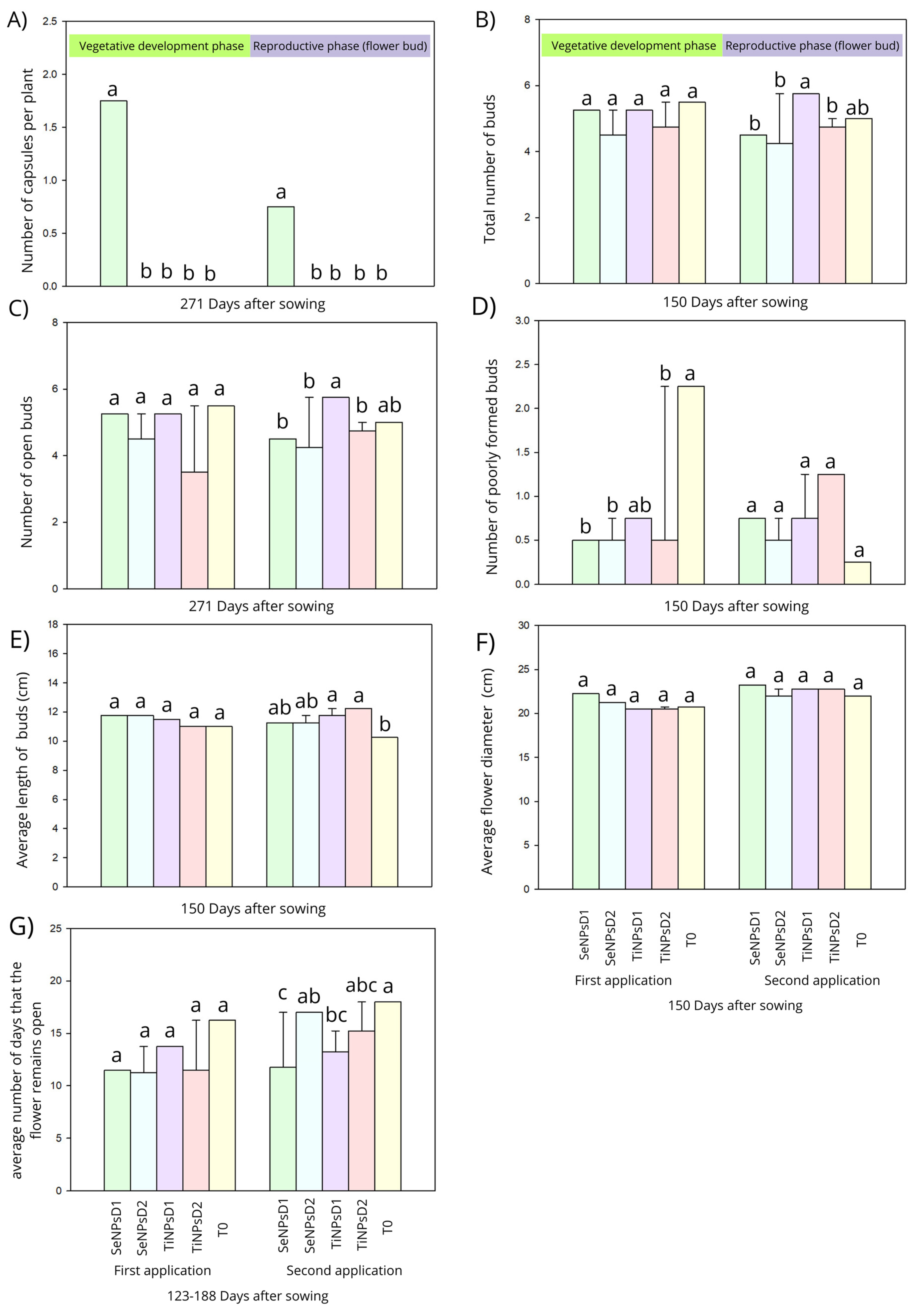
3.2. Reproductive Parameters
3.3. Scanning Electron Microscopy and Energy Dispersive X-Ray Spectroscopy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yan, Y.; Zhu, X.; Yu, Y.; Li, C.; Zhang, Z.; Wang, F. Nanotechnology Strategies for Plant Genetic Engineering. Adv. Mater. 2021, 34, 2106945. [Google Scholar] [CrossRef]
- Wei, L.; Wei, S.; Hu, D.; Feng, L.; Liu, Y.; Liu, H.; Liao, W. Comprehensive Flavor Analysis of Volatile Components During the Vase Period of Cut Lily (Lilium spp. ‘Manissa’) Flowers by HS-SPME/GC–MS Combined With E-Nose Technology. Front. Plant Sci. 2022, 13, 822956. [Google Scholar] [CrossRef]
- Jiang, F.; Zhou, L.; Zhou, W.; Zhong, Z.; Yu, K.; Xu, J.; Zou, L.; Liu, W. Effect of modified atmosphere packaging combined with plant essential oils on preservation of fresh-cut lily bulbs. LWT 2022, 162, 113513. [Google Scholar] [CrossRef]
- González-García, Y.; Juárez-Maldonado, A. Nanomaterials on Plant Growth and Stress Adaptation. Plants 2025, 14, 1651. [Google Scholar] [CrossRef]
- Zhu, Z.; Chen, Y.; Shi, G.; Zhang, X. Selenium delays tomato fruit ripening by inhibiting ethylene biosynthesis and enhancing the antioxidant defense system. Food Chem. 2017, 219, 179–184. [Google Scholar] [CrossRef]
- González-García, Y.; Juárez-Maldonado, A.; Benavides-Mendoza, A.; Ojeda-Barrios, D.L.; Tortella Fuentes, G.; Seabra, A.B. Improving Nutritional Quality of Food Crops Using Nanomaterials and Nanostimulants. In Plant Biostimulation with Nanomaterials; Smart Nanomaterials Technology; Springer: Singapore, 2025; pp. 187–221. [Google Scholar] [CrossRef]
- Song, J.; Yu, S.; Yang, R.; Xiao, J.; Liu, J. Opportunities for the use of selenium nanoparticles in agriculture. NanoImpact 2023, 31, 100478. [Google Scholar] [CrossRef]
- Zhang, T.; Qi, M.; Wu, Q.; Xiang, P.; Tang, D.; Li, Q. Recent research progress on the synthesis and biological effects of selenium nanoparticles. Front. Nutr. 2023, 10, 1183487. [Google Scholar] [CrossRef]
- Reyes-Pérez, J.J.; Llerena-Ramos, L.T.; Torres-Rodríguez, J.A.; Rodríguez-Rodríguez, S.; Sariñana-Navarrete, M.A. Respuesta Morfológica y Fenológica de Plantas de Chile Pimiento a la Suplementación de Nanopartículas de Selenio. Terra Latinoam. 2025, 43, 1–12. [Google Scholar] [CrossRef]
- Gamage, A.; Fernando, N.; Mani, S.; Manamperi, A.; Madhujith, T. Biodegradable Bionanocomposites in Agriculture Applications. In Nanoformulations for Agricultural Applications; Radhakrishnan, E.K., Aswani, R., Visakh, P.M., Eds.; Scrivener Publishing LLC.: Beverly, MA, USA, 2025; pp. 203–230. [Google Scholar] [CrossRef]
- Treviño López, E.A.; Sandoval-Rangel, A.; Benavides Mendoza, A.; Benavides Mendoza, A.; Ortega Ortiz, H.; Cadenas Pliego, G.; Cabrera de la Fuente, M. Nanopartículas de selenio absorbidas en hidrogeles de quitosán-polivinil alcohol en la producción de pepino injertado. Rev. Mex. Cienc. Agríc. 2021, 26, 159–169. [Google Scholar] [CrossRef]
- Kolenčík, M.; Ernst, D.; Urík, M.; Ďurišová, Ľ.; Bujdoš, M.; Šebesta, M.; Kratošová, G. Foliar application of low concentrations of titanium dioxide and zinc oxide nanoparticles to the common sunflower under field conditions. Nanomaterials 2020, 10, 1619. [Google Scholar] [CrossRef]
- Ur Rahim, H.; Qaswar, M.; Uddin, M.; Giannini, C.; Herrera, M.L.; Rea, G. Nano-enable materials promoting sustainability and resilience in modern agriculture. Nanomaterials 2021, 11, 2068. [Google Scholar] [CrossRef]
- INEGI (Instituto Nacional de Estadística, Geografía e Informática). Anuario Estadístico y Geográfico del Estado de Hidalgo, México; Gobierno del Estado de Hidalgo: Higalgo, Mexico, 2017; Volume 1, p. 1674. Available online: https://www.inegi.org.mx/contenido/productos/prod_serv/contenidos/espanol/bvinegi/productos/nueva_estruc/anuarios_2017/702825095093.pdf (accessed on 1 March 2025).
- González-Lemus, U.; Tapia-Zayago, F.A.; Pérez-Ríos, S.R.; Zaldívar-Ortega, A.K.; Rueda-Puente, E.O.; Hernández-Pérez, A.; Hernández-Soto, I. Lentil Biorooting Agents: An Ecological Alternative to Improve the Growth and Development of Italian Zucchini in Sustainable Production Systems. Horticulturae 2025, 11, 332. [Google Scholar] [CrossRef]
- Larue, C.; Veronesi, G.; Flank, A.M.; Surble, S.; Herlin-Boime, N.; Carrière, M. Comparative uptake and impact of TiO2 nanoparticles in wheat and rapeseed. J. Toxicol. Environ. Health A 2012, 75, 722–734. [Google Scholar] [CrossRef] [PubMed]
- Satti, S.H.; Raja, N.I.; Javed, B.; Akram, A.; Mashwani, Z.U.; Ahmad, M.S.; Ikram, M. Titanium dioxide nanoparticles elicited agro-morphological and physicochemical modifications in wheat plants to control Bipolaris sorokiniana. PLoS ONE 2021, 11, e0246880. [Google Scholar] [CrossRef]
- Flores-Pérez, S.; Castillo-González, A.M.; Valdez-Aguilar, L.A.; Avítia-García, E. Uso de diferentes proporciones de led rojos y azules para mejorar el crecimiento de Lilium spp. Rev. Mex. Cienc. Agric. 2021, 12, 5. [Google Scholar] [CrossRef]
- Ontiveros-Capurata, R.E.; Juárez-López, P.; Mendoza-Tafolla, R.O.; Alia-Tejacal, I.; Villegas-Torres, O.G.; Guillén-Sánchez, D.; Cartmill, A.D. Relationship between chlorophyll and nitrogen concentration, and fresh matter production in basil ‘Nufar’ (Ocimum basilicum) with three handheld chlorophyll meter readings: SPAD, atLEAF and MC-100. Rev. Chapingo Ser. Hortic. 2022, 28, 189–202. [Google Scholar] [CrossRef]
- Velasco-Lara, D.; De La Cruz-Guzmán, G.H.; Mandujano-Piña, M.; Arriaga-Frías, A.; Ramírez-Santiago, D. Dosis de paclobutrazol para modificar el aspecto visual de Lilium cv. Litouwen. Rev. Mex. Cienc. Agric. 2024, 15, 1–10. [Google Scholar] [CrossRef]
- Rios-Florida, L.G.; De La Cruz-Guzmán, G.H.; Arriaga-Frías, A.; Mandujano-Piña, M. Effect of paclobutrazol and Glomus intraradices on the crop of Lilium cv. Armandale and Tresor. Siembra 2022, 9, 1–9. [Google Scholar] [CrossRef]
- Soriano Melgar, L.A.A.; López-Guerrero, A.; Cortéz-Mazatan, G.; Mendoza-Mendoza, E.; Peralta-Rodríguez, R.D. Zinc Oxide and Zinc Oxide/Graphene Nanoparticles Used in Vase Solutions on Lisianthus (Eustoma grandiflorum) Postharvest Life. Agroproductividad 2018, 11, 137–144. [Google Scholar] [CrossRef]
- Bazzicalupo, M.; Calevo, J.; Adamo, M.; Giovannini, A.; Copetta, A.; Cornara, L. Seed Micromorphology, In Vitro Germination, and Early-Stage Seedling Morphological Traits of Cattleya purpurata (Lindl. & Paxton) Van den Berg. Horticulturae 2021, 7, 480. [Google Scholar] [CrossRef]
- Yang, C.; Wang, C.; Khan, Z.; Duan, S.; Li, Z.; Shen, H. Algal polysaccharides–Selenium nanoparticles regulate the uptake and distribution of selenium in rice plants. Front. Plant Sci. 2023, 14, 1135080. [Google Scholar] [CrossRef]
- Juárez-Maldonado, A.; Benavides-Mendoza, A.; Ojeda-Barrios, D.L.; Fuentes, G.T.; Seabra, A.B. Plant Biostimulation with Nanomaterials; Springer Nature: Berlin/Heidelberg, Germany, 2025; pp. 21–43. [Google Scholar] [CrossRef]
- Nogales, F.; Pajuelo, E.; Romero-Herrera, I.; Carreras, O.; Merchán, F.; Carrasco López, J.A.; Ojeda, M.L. Uncovering the Role of Selenite and Selenium Nanoparticles (SeNPs) in Adolescent Rat Adipose Tissue beyond Oxidative Balance: Transcriptomic Analysis. Antioxidants 2024, 13, 750. [Google Scholar] [CrossRef]
- Samynathan, R.; Venkidasamy, B.; Ramya, K.; Muthuramalingam, P.; Shin, H.; Kumari, P.S.; Sivanesan, I. A recent update on the impact of nano-selenium on plant growth, metabolism, and stress tolerance. Plants 2023, 12, 853. [Google Scholar] [CrossRef]
- Sánchez-Navarro, J.F.; González-García, Y.; Benavides-Mendoza, A.; Morales-Díaz, A.B.; González-Morales, S.; Cadenas-Pliego, G.; Juárez-Maldonado, A. Silicon nanoparticles improve the shelf life and antioxidant status of lilium. Plants 2021, 10, 2338. [Google Scholar] [CrossRef]
- Gong, H.Z.; Li, S.; Wang, F.Y.; Zhu, Y.; Jiang, Q.L.; Zhu, X.L.; Jiang, J. Titanium dioxide nanoparticles Disrupt ultrastructure and function of Rat thyroid tissue via oxidative stress. Heliyon 2024, 10, e34722. [Google Scholar] [CrossRef]
- Hossain, A.; Skalicky, M.; Brestic, M.; Maitra, S.; Sarkar, S.; Ahmad, Z.; Laing, A.M. Selenium biofortification: Roles, mechanisms, responses and prospects. Molecules 2021, 26, 881. [Google Scholar] [CrossRef] [PubMed]
- Li, C.C.; Jhou, S.M.; Li, Y.C.; Ciou, J.W.; Lin, Y.Y.; Hung, S.C.; Chang, H.H. Exposure to low levels of photocatalytic TiO2 nanoparticles enhances seed germination and seedling growth of amaranth and cruciferous vegetables. Sci. Rep. 2022, 12, 18228. [Google Scholar] [CrossRef] [PubMed]
- Azmat, R.; Altaf, I.; Moin, S. The reflection of the photocatalytic properties of TiO2 nanoparticles on photosynthetic activity of Spinacia oleracea plants. Pakistan J. Bot. 2020, 52, 1229–1234. [Google Scholar] [CrossRef] [PubMed]
- Verma, D.K.; Patel, S.; Kushwah, K.S. Synthesis of titanium dioxide (TiO2) nanoparticles and impact on morphological changes, seed yield and phytotoxicity of Phaseolus vulgaris L. Trop. Plant Res. 2020, 7, 158–170. [Google Scholar] [CrossRef]
- Kamali, M.; Shoor, M.; Feizi, H. Impacts of Nanosized and Bulk Titanium Dioxide on Flowering and Morpho-physiological Traits of Petunia (Petunia hybrida) under Salinity Stress. J. Hortic. Sci. 2018, 32, 199–212. [Google Scholar] [CrossRef]
- Juárez-Maldonado, A.; Ortega-Ortíz, H.; Morales-Díaz, A.B.; González-Morales, S.; Morelos-Moreno, Á.; Cabrera-De la Fuente, M.; Sandoval-Rangel, A.; Cadenas-Pliego, G.; Benavides-Mendoza, A. Nanoparticles and Nanomaterials as Plant Biostimulants. Int. J. Mol. Sci. 2019, 20, 162. [Google Scholar] [CrossRef]
- Kinraide, T.B.; Wang, P. The surface charge density of plant cell membranes (sigma): An attempt to resolve conflicting values for intrinsic sigma. J. Exp. Bot. 2010, 61, 2507–2518. [Google Scholar] [CrossRef] [PubMed]
- Wiley, D.; Weihs, G.F. Surface Charge Density. In Encyclopedia of Membranes; Springer: Berlin/Heidelberg, Germany, 2016; pp. 1864–1866. [Google Scholar] [CrossRef]
- Canellas, L.P.; Olivares, F.L.; Aguiar, N.O.; Jones, D.L.; Nebbioso, A.; Mazzei, P.; Piccolo, A. Humic and fulvic acids as biostimulants in horticulture. Sci. Hortic. 2015, 196, 15–27. [Google Scholar] [CrossRef]
- Almutairi, K.F.; Górnik, K.; Awad, R.M.; Ayoub, A.; Abada, H.S.; Mosa, W.F.A. Influence of Selenium, Titanium, and Silicon Nanoparticles on the Growth, Yield, and Fruit Quality of Mango under Drought Conditions. Horticulturae 2023, 9, 1231. [Google Scholar] [CrossRef]
- Djanaguiraman, M.; Anbazhagan, V.; Dhankher, O.P.; Prasad, P.V. Uptake, Translocation, Toxicity, and Impact of Nanoparticles on Plant Physiological Processes. Plants 2024, 13, 3137. [Google Scholar] [CrossRef]
- Bhat, M.A.; Mishra, A.K.; Shah, S.N.; Bhat, M.A.; Jan, S.; Rahman, S.; Jan, A.T. Soil and mineral nutrients in plant health: A prospective study of iron and phosphorus in the growth and development of plants. Curr. Issues Mol. Biol. 2024, 46, 5194–5222. [Google Scholar] [CrossRef]
- Sperotto, R.A.; Ricachenevsky, F.K.; Williams, L.E.; Vasconcelos, M.W.; Menguer, P.K. From soil to seed: Micronutrient movement into and within the plant. Front. Plant Sci. 2014, 5, 438. [Google Scholar] [CrossRef]
- Grillet, L.; Mari, S.; Schmidt, W. Iron in seeds–loading pathways and subcellular localization. Front. Plant Sci. 2014, 4, 535. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Wang, Y.; Li, K.; Wan, Y.; Wang, Q.; Zhuang, Z.; Li, H. Uptake, translocation and biotransformation of selenium nanoparticles in rice seedlings (Oryza sativa L.). J. Nanobiotechnol. 2020, 18, 103. [Google Scholar] [CrossRef]
- Diehl, D.; Knott, M.; Schaumann, G.E. Purification effects show seed and root mucilage’s ability to respond to changing rhizosphere conditions. Biopolymers 2023, 114, e23561. [Google Scholar] [CrossRef] [PubMed]
- Haughn, G.W.; Western, T.L. Arabidopsis seed coat mucilage is a specialized cell wall that can be used as a model for genetic analysis of plant cell wall structure and function. Front. Plant Sci. 2012, 3, 64. [Google Scholar] [CrossRef] [PubMed]
- Sigala-Aguilar, N.A.; Juárez-Altamirano, R.; Flores-Rentería, D.; Torres-Gómez, A.; Fernández-Luqueño, F. Physiological and Morphological Responses of Crops When Applying Nanostimulant. In Plant Biostimulation with Nanomaterial; Springer: Singapore, 2025; Volume 1, pp. 1–22. [Google Scholar] [CrossRef]
- Hernandez-Perez, H.; Fabián, F.L.; Juárez-Maldonado, A. Assessment of Iron Oxide Engineered Nanoparticles in the Accumulation in Endogeic Earthworms, and Ferns Under Natural Forest Soil Conditions. Water Air Soil Pollut. 2025, 236, 517. [Google Scholar] [CrossRef]
- Sigala-Aguilar, N.A.; Torres-Gómez, A.P.; Pérez-Hernández, H.; Fernández-Luqueño, F. Beneficial and toxicological impact of nanotechnology in agriculture as the basis for a regulatory framework. In Nano-Bioinoculants; Academic Press: Cambridge, MA, USA, 2025; Volume 1, pp. 375–390. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Granados, N.T.; Pérez-Ríos, S.R.; González-García, Y.; Fernández-Luqueño, F.; Aquino-Torres, E.; Saucedo-García, M.; Zaldívar-Ortega, A.K.; Reyes-Santamaria, M.I.; Hernández-Soto, I. Impacts of Foliar Application of Se and TiO2 Nanoparticles on Growth, Development, and Flowering in Lilium Sunny Oriental. Int. J. Plant Biol. 2025, 16, 103. https://doi.org/10.3390/ijpb16030103
Sánchez-Granados NT, Pérez-Ríos SR, González-García Y, Fernández-Luqueño F, Aquino-Torres E, Saucedo-García M, Zaldívar-Ortega AK, Reyes-Santamaria MI, Hernández-Soto I. Impacts of Foliar Application of Se and TiO2 Nanoparticles on Growth, Development, and Flowering in Lilium Sunny Oriental. International Journal of Plant Biology. 2025; 16(3):103. https://doi.org/10.3390/ijpb16030103
Chicago/Turabian StyleSánchez-Granados, Nayla Tamara, Sergio Rubén Pérez-Ríos, Yolanda González-García, Fabian Fernández-Luqueño, Eliazar Aquino-Torres, Mariana Saucedo-García, Ana Karen Zaldívar-Ortega, Ma Isabel Reyes-Santamaria, and Iridiam Hernández-Soto. 2025. "Impacts of Foliar Application of Se and TiO2 Nanoparticles on Growth, Development, and Flowering in Lilium Sunny Oriental" International Journal of Plant Biology 16, no. 3: 103. https://doi.org/10.3390/ijpb16030103
APA StyleSánchez-Granados, N. T., Pérez-Ríos, S. R., González-García, Y., Fernández-Luqueño, F., Aquino-Torres, E., Saucedo-García, M., Zaldívar-Ortega, A. K., Reyes-Santamaria, M. I., & Hernández-Soto, I. (2025). Impacts of Foliar Application of Se and TiO2 Nanoparticles on Growth, Development, and Flowering in Lilium Sunny Oriental. International Journal of Plant Biology, 16(3), 103. https://doi.org/10.3390/ijpb16030103






