Abstract
Poplars and aspens (Populus L. spp.) are undervalued options for use in managed landscapes. The genus comprises a multitude of taxa often negatively associated with disease susceptibility and short lifespans; however, it also hosts a diverse range of abiotic stress tolerances. The objective of this study was to generate a relative scale of the predicted drought tolerance of Populus spp. to inform site and taxon selection in managed settings. Utilizing vapor pressure osmometry, this study examined seasonal osmotic adjustment and predicted leaf water potential at the turgor loss point (Ψpo) among several Populus taxa. All evaluated taxa demonstrated the ability to osmotically adjust (ΔΨπ100) throughout the growing season. Bigtooth aspen (P. grandidentata Michx.) exhibited the most osmotic adjustment (−1.1 MPa), whereas black cottonwood (P. trichocarpa Torr. & A. Gray ex Hook.) exhibited the least (−0.44 MPa). Across the taxa, the estimated mean Ψpo values in spring and summer were −1.8 MPa and −2.8 MPa, respectively. Chinese aspen (P. cathayana Rehder) exhibited the lowest Ψpo (−3.32 MPa), whereas black cottonwood exhibited the highest (−2.47 MPa). The results indicate that drought tolerance varies widely among these ten Populus species and hybrids; bigtooth aspen and Chinese aspen are the best suited to tolerating drought in managed landscapes.
1. Introduction
In recent years the plant palettes of managed landscapes have been significantly influenced by unseasonable weather conditions and the increased frequency of severe weather events, like droughts, driven by climatic shifts [1]. Landscapes comprising minimal species diversity are easily challenged by demanding conditions, including biotic and abiotic stresses, leading to broader and exacerbated negative impacts on landscape health, aesthetics, and the ecosystem services they provide [1]. As such, the diversification of tree species in managed landscapes remains a necessary objective for improving overall landscape resiliency [2,3]. As the impact of unpredictable and erratic abiotic stresses becomes increasingly apparent, green industry practitioners, including horticulturists, arborists, and urban foresters, seek information regarding species tolerances that can be used to inform future planting plans [4]. Among the species that can be used to bolster landscape diversity are poplars and aspens (Populus spp.), a group typically underrepresented in modern managed landscapes, especially within urban green spaces. However, their horticultural potential, ecological value, and utilitarian uses could rationalize the broader application of Populus spp. in appropriate sites within managed landscapes as one tool to further support the goal of species diversification.
Species belonging to the genus Populus (L.) are often negatively associated with traits considered unsuitable for application in landscapes and urban settings, including an overall tendency to exhibit short lifespans, susceptibility to breakage during storms due to reduced wood resistance, shallow roots capable of damaging infrastructure, a tendency to produce root suckers, and an overall susceptibility to insect infestation and fungal infection [5]. However, assumptions and generalizations across the species within a genus can often exclude desirable outliers with potential for broader use [4]. Among the reasons why Populus species have been considered appealing trees for use in horticultural settings are their fast rates of growth, ease of propagation, overall hardiness [6], and known tolerances to anthropogenic pressures [7]. In fact, poplars and aspens were historically found to be planted at a high frequency throughout urban green spaces in the northern regions of the world in the 20th century but were replaced over time in favor of species less prone to breakage and generally considered less messy [7]. Arguably, this trend of opting for a narrow band of species selections with certain desirable traits has contributed to the overall reduced species diversity observed in managed landscapes today. Thus, while these alternative trees have advantages and deserve their place in urban green spaces, their prominence and the scale of their application yields overall system weaknesses for urban forests.
According to Plants of the World Online [8], a resource generated using the World Checklist of Vascular Plants [9], the genus Populus comprises 60 accepted taxa; however, this taxonomy seemingly undergoes routine revision and is occasionally debated [10]. Both in the wild and in cultivation, this genus is distributed across North America, Europe, North Africa, and Asia [11]. Populus species are characteristically known for their large mature size and fast growth [6] and are planted in parks, wind breaks, fence rows, and canal plantings [11]. While disease susceptibility can limit the application of poplars and aspens in high-visibility locales, a multitude of cultivars exist [11] representing unique and ornamental forms for use in horticulture, including some with improved tolerances to biotic pressures. In addition to the unique cultivars used in horticulture, many members of the genus Populus are recognized by their ability to generate a characteristic quaking or trembling sound created by their leaves when wind moves through the branches, especially those offering long flattened petioles that lie in a plane dissimilar to that of the leaf blade [12]. This unique characteristic is often considered an attractive trait of these plants, along with their outstanding yellow fall colors, of which the quaking aspen (P. tremuloides L.) is known to have some of the best of any temperate deciduous tree found in the United States [11,13].
Aside from their horticultural value, Populus species offer extensive ecological benefits and ecosystem services, serving in a multitude of utilitarian applications [14]. Poplars and aspens have been used for erosion control, riparian buffer systems, phytoremediation and wastewater reuse, bioenergy [14], and restoration projects [10]. In urban centers, they are suited for use in modifying microclimates by shading and combatting noise pollution [7] as well as resisting climate change via carbon sequestration [14].
While Populus species are known for offering a range of adaptable traits, they are broadly considered drought-sensitive when compared to other woody plants [15,16,17]. Conversely, some evidence exists that demonstrates that drought tolerance can vary across and within species and populations [17,18]. Much attention has been given to clonal-level variation for improvement and use in silviculture; however, few studies characterize broad species-level differences, particularly when employing useful tolerance metrics [19] suitable for application in landscapes and urban settings, as opposed to drought avoidance metrics [20]. A relative ranking of drought tolerance for underutilized taxa could guide site and species selection while also bolstering demand for suitable nursery stock. Therefore, the objective of this study was to evaluate taxa belonging to the genus Populus for drought tolerance, as indicated by seasonal osmotic adjustment, leaf osmotic potential at full turgor, and predicted leaf turgor loss points, to develop a relative scale of tolerances useful for informing site selection and justifying the use of select species to diversify plant palettes and bolster landscape resiliency.
2. Materials and Methods
This study was conducted in 2022, with data collected in late May (spring) once leaves were fully expanded and again in August (summer) after trees experienced a growing season characterized by a severe drought. The taxa evaluated (Table 1) were represented by established landscape trees (in situ) of various age classes [approximated by the year the plants were accessioned into the collection (Table 1)] located on the property of the Minnesota Landscape Arboretum in Chaska, MN, USA (44°51’43.9” N 93°36’58.7” W).

Table 1.
The Populus taxa evaluated to predict drought tolerance capacity in the spring and summer of 2022 at the Minnesota Landscape Arboretum (MLA) in Carver County, MN, USA (lat. 44.5° N, long. 93.3° W). Mature, healthy poplars and aspens growing on comparable sites within the collections of the MLA and those that occur naturally on site (native and naturalized) were assessed.
From June through August 2022, the mean (°C) temperature (min. °C, max. °C) of the site where the trees were sampled was 22.3° (17.2°, 27.6°). During that timeframe, the site received approximately 17.6 cm total precipitation. Weather data were acquired from the nearest weather station [Chaska (Univ MN HRC), MN] in the Network for Environment and Weather Applications station network [21]. All the evaluated trees had their own roots and none were derived from grafted stock.
The day before measurements were taken, three branch samples from each tree were obtained. The number of individual trees sampled varied by their availability in the collections, with a minimum of three separate trees per taxon used for evaluation (Table 1). Branches were selected at random; however, careful attention was given to avoid branches that were shaded-out or exhibiting signs of disease, as well as those developing inflorescences or fruits [22]. Branches were detached using a long reach pruner (TP 3206 SwivelCUT, Corona®, Corona, CA, USA) and proximal, cut ends were placed in a bucket filled with water. A subsequent basal cut of each stem was performed under water (removing ~4 cm of the most proximal portion of the stem) to remove any vascular embolisms that may have formed when stems were detached.
Sample storage, transport, and processing were conducted precisely as in the methodology described by Miller and Bassuk [4]. In short, samples were transported to a laboratory and equilibrated overnight in the dark at room temperature to ensure that each sample was fully hydrated. The subsequent day, measurements were taken using leaves removed from the branches previously equilibrated. Leaf discs were created using a 6 mm cork borer, targeting leaf tissue without large leaf veins. The leaf discs were individually wrapped in tin foil and then placed in liquid nitrogen for a minimum of two minutes. The discs were subsequently removed from the flask of liquid nitrogen, unpacked from their tin foil wrapping, and perforated using sharp forceps [23] prior to loading into a Vapro 5600 Vapor Pressure Osmometer (Wescor, Logan, UT, USA). Samples were equilibrated within the 10 μL chamber of the Vapro 5600 for ten minutes. Next, the solute concentration (Cs) of each leaf disc was recorded continuously (using the process-delay mode) until three measurements within a range of 5 mmol·kg−1 were logged. For each of the three leaves analyzed (each representing unique stems per evaluated tree), three measurements were recorded, yielding nine total observations (n = 9) collected from each tree at every period of sampling. For each measurement, the temperature of the Vapro 5600 was recorded.
After measurements were completed, Van’t Hoff’s relation [Ψπ100 = −RTCs (where R equals a gas constant, T is the temperature (kelvins), and Cs is the solute concentration of the sample at full turgor)] was used to calculate the osmotic potential at full turgor (Ψπ100). A temperate-tree calibrated equation (ΨP0 = −0.2554 + 1.1243 × Ψπ100) circulated by Sjöman et al. [24] as a modification to the cosmopolitan, meta-analysis-derived equation originally published by Bartlett et al. [25] to predict the turgor loss point based on its observed relationship with the osmotic potential at full turgor, was used for this research.
Statistical Analysis
The spring and summer datasets for both osmotic potential at full turgor (Ψπ100) and predicted leaf turgor loss points (ΨP0) were subjected to a one-way analysis of variance. Each dataset was normally distributed and met the assumptions of the model without transformation. Post hoc mean separations were performed using Tukey’s honestly significant difference test. Osmotic adjustment (ΔΨπ100), calculated as the difference between the spring and summer mean osmotic potential, was analyzed using a paired T-test. Statistical analysis was conducted using JMP Pro 16 software (JMP Version 16, SAS Institute, Inc., Cary, NC, USA).
3. Results
3.1. Osmotic Potential at Full Turgor (Ψπ100)
Across the taxa, the mean Ψπ100 (±SE) was −1.45 (±0.01) MPa in spring and −2.29 (±0.02) MPa in summer. Differences across the taxa in both spring and summer (p < 0.0001) were observed (Table 2). In spring, P. ×canadensis exhibited the highest Ψπ100, whereas the lowest Ψπ100 was observed in P. cathayana. In summer, the highest Ψπ100 occurred in P. trichocarpa and the lowest in P. cathayana. Each taxon demonstrated a significant capacity for seasonal osmotic adjustment (Table 2). P. trichocarpa and P. grandidentata exhibited the least and most seasonal osmotic adjustment, respectively (Table 2).

Table 2.
The osmotic potential at full turgor [Ψπ100 (±SE)] and seasonal osmotic adjustment (ΔΨπ100) for the 10 Populus taxa evaluated in 2022 at the Minnesota Landscape Arboretum using vapor pressure osmometry. The spring and summer means (Ψπ100) were subject to a one-way ANOVA (p < 0.0001) indicating differences between the taxa.
3.2. Predicted Leaf Turgor Loss Point (ΨP0)
The overall spring and summer ΨP0 means (±SE) across species were −1.8 (±0.02) MPa and −2.8 (±0.02) MPa, respectively. Differences across species (p < 0.0001) were detected in both spring and summer (Figure 1). The taxa exhibiting the highest and lowest ΨP0 in spring and summer matched the previous pattern observed for Ψπ100 (Figure 1).
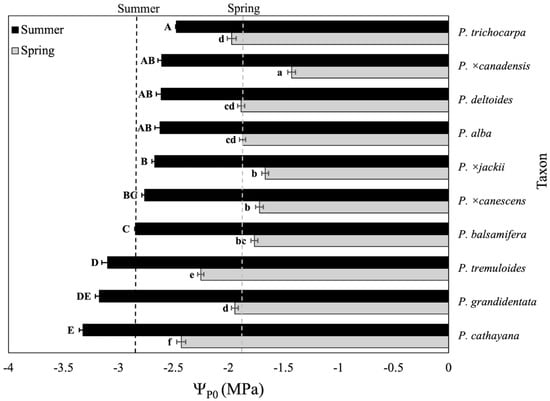
Figure 1.
Predicted leaf turgor loss points of species and interspecific hybrids of Populus in spring and summer, ranked by summer values. Seasonal means for spring and summer were compared separately. Means across taxa sharing letters for spring (lowercase) or summer (uppercase) are not significantly different according to Tukey’s honestly significant difference test (p 0.05). Error bars represent standard errors of means. Dotted vertical lines indicate overall means for spring (gray) and summer (black) across all taxa.
4. Discussion
Of the taxa evaluated in this study, all demonstrated an ability to osmotically adjust (ΔΨπ100) from spring to summer (Table 2). Bigtooth aspen (P. grandidentata) exhibited the most osmotic adjustment (−1.1 MPa), whereas black cottonwood (P. trichocarpa) exhibited the least (−0.44 MPa) (Table 2). This relatively small osmotic adjustment response of P. trichocarpa, as well as that of P. deltoides (W. Bartram ex Marshall) (0.65 MPa), generally aligns with the patterns observed by Gebre and colleagues [26], who found that osmotic adjustment rates were small for clones within a hybrid population of P. trichocarpa and P. deltoides. Their study, while different in its frequency of evaluation periods, interestingly found that the osmotic adjustment of the population, as well as that of a hybrid population of P. deltoides and P. nigra (L.), generally decreased throughout the growing season and was reduced with a corresponding increase in tree age. These factors were evaluated using techniques that fall outside of the scope of this study; however, tree age is of particular interest because many of the specimens used in this research were quite mature, established plants (Table 1). If tree age significantly influences osmotic adjustment capacity, future studies should evaluate this factor in more detail to determine its impact in managed landscapes.
This possibility is further underscored by findings from Deacon and colleagues, who used the same vapor pressure osmometry techniques as this study to evaluate the Ψπ100 of P. grandidentata, P. tremuloides, and their interspecific hybrid P. ×smithii (B.Boivin) in a common garden plot in central Minnesota in August 2016 [27]. Interestingly, they concluded that both P. grandidentata and P. tremuloides exhibited slightly less negative values than this study conducted at the Minnesota Landscape Arboretum. Although weather conditions differed across the years and likely contributed to the observed variations across studies, the age of the plant material is another major factor that merits further evaluation. They utilized recently propagated plants derived from root cuttings established for a few years in a common garden plot [27]. This is opposed to the much older plants evaluated and presented herein (Table 1). Multiple studies and comparisons suggest that tree age plays a unique role in the response of plants to drought stress and this phenomenon should be further evaluated to determine its role in applied contexts.
Across the taxa, the estimated mean Ψpo values in spring and summer were −1.8 MPa and −2.8 MPa, respectively (Figure 1). Chinese aspen (P. cathayana) exhibited the lowest Ψpo (−3.32 MPa), whereas black cottonwood exhibited the highest (−2.47 MPa) (Figure 1). These results indicate that drought tolerance varies widely among the ten Populus species and hybrids evaluated. However, relative to species belonging to other genera characterized using the same methodology [24,28,29,30,31], and according to the categorizations provided by Hirons and Sjöman, the species of Populus in this study appear to rank as either moderately sensitive (−2.5 MPa to −3 MPa) or moderately tolerant (−3 MPa to −3.5 MPa) [32]. This pattern aligns with the general trend that Populus species are typically more drought-sensitive relative to species belonging to other woody plant genera [17]. Further, it suggests that significant variation also exists across Populus species [17], demonstrating that thoughtful species selection could expand the use of poplars and aspens in managed landscapes.
In addition to tree age, other phenomena could be further explored in future studies. For example, multiyear and multisite analyses could be employed to further define the tolerance boundaries of the taxa included in this evaluation. In addition, vapor pressure osmometry evaluations could be coupled with other traditional plant physiological evaluation metrics like biomass measurement and stomatal conductance to further place these drought tolerance variables in the broader context of plant responses to drought stress. While this experiment employed a predictive model for determining the leaf water potential at the turgor loss point, other studies have compared the accuracy of this approach to traditional pressure–volume curves and have found a high degree of alignment between them [33]. Therefore, the values representing leaf water potential at the turgor loss point presented in this study, albeit predictive, should be considered to have a strong likelihood of accuracy compared to those derived from pressure–volume curves.
For both osmotic adjustment and predicted leaf turgor loss points, P. cathayana exhibited a unique capacity for bolstered tolerance relative to its counterparts (Table 2; Figure 1). This species is nearly nonexistent in mainstream horticulture within the United States and exists almost exclusively in botanic garden collections as well as regional plant material trials [34]. Like other studies that have employed botanical collections for screening to identify useful plant material [35], this research has also identified a unique taxon nearly completely unrepresented in landscape horticulture, and the experience serves to further support the role diverse botanical collections play in horticultural advancement.
This evaluation utilized mature plants at the Minnesota Landscape Arboretum, representing a variety of species and cultivars of poplars and aspens, to use plant biology to generate resources for the green industry that outline relative predictive drought tolerance rankings. The research embodies one part of a process aimed at driving interest in diverse tree selections suited to the harsh conditions of the managed landscape. Trees with exceptional ornamental traits and superior stress tolerances are in high demand, but plant palettes for managed landscapes are often limited. At a time when abiotic and biotic stresses, like more frequent droughts and invasive pests such as the emerald ash borer, test the resilience of managed landscapes, practitioners seek environmentally tolerant tree species, which nursery growers could supply [4]. The development of underutilized or neglected species bolsters crop diversity and increases the potential for economic return among liner nurseries, container growers, and field producers, as well as retailers. Based on these results, P. grandidentata and P. cathayana, both of which are underrepresented in managed landscapes throughout the United States, should be strongly considered for adoption and application to build more resilient landscapes. However, because P. cathayana is not endemic to North America and is minimally represented in public gardens, the species should be trialed more extensively in various regions to ensure that it does not exhibit invasiveness.
Literature reviews and data-driven evaluations suggest that some Populus taxa, such as P. ×berolinensis (K. Koch), are suited to the local conditions of various urban centers around the globe, and those taxa are more capable of adapting to climatic shifts than other staple tree genera used in urban settings, like Acer L. and Tilia L. [7]. The data generated from this study indicate that a variety of tolerance levels to water deficit exist within the genus Populus (Figure 1). While some Populus are simply not viable options for broader landscape applications due to their mature size or their susceptibility to a range of maladies [5], this research provides evidence that select taxa are more suitable as options for diversifying plant palettes and meeting the functional and aesthetic objectives of managed landscapes, thereby improving landscape resiliency. Given this information, the plant selection of poplars and aspens should be conducted on a case-by-case basis that considers local site constraints and matches those limitations to the capacity of a particular taxon, rather than excluding all Populus spp. due to generalizations incorrectly associated with the entire genus.
Funding
This research was supported in part by the University of Minnesota Landscape Arboretum Endowed Land Grant Chair fund and the University of Minnesota AGREETT program.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed towards the author.
Conflicts of Interest
The author declares no conflicts of interest. The funders had no role in the design of this study; in the collection, analysis, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| NEWA | Network for Environment and Weather Applications |
| POWO | Plants of the World Online |
References
- Pooler, M.; Contreras, R.C.; Criley, R.A.; Dosmann, M.S.; Galanti, R.; Hokanson, S.C.; Miller, B.M.; Peterson, B.J.; Nageswara-Rao, M.; Rounsaville, T.J.; et al. Seeing the Forest for the Trees: Threats, Vulnerabilities, and Opportunities for Woody Landscape Plant Genetic Resources. HortScience 2024, 59, 1497–1504. [Google Scholar] [CrossRef]
- Hooper, D.U.; Chapin, F.S.; Ewel, J.J.; Hector, A.; Inchausti, P.; Lavorel, S.; Lawton, J.H.; Lodge, D.M.; Loreau, M.; Naeem, S.; et al. Effects of biodiversity on ecosystem functioning: A consensus of current knowledge. Ecol. Monogr. 2005, 75, 3–35. [Google Scholar] [CrossRef]
- Alvey, A.A. Promoting and preserving biodiversity in the urban forest. Urban For. Urban Green. 2006, 5, 195–201. [Google Scholar] [CrossRef]
- Miller, B.M.; Bassuk, N.L. Carya Species for Use in the Managed Landscape: Predicted Drought Tolerance. HortScience 2022, 57, 1558–1563. [Google Scholar] [CrossRef]
- Avsar, M.D.; Ok, T. Using poplars (Populus L.) in urban afforestation: Kahramanmaras sample. Turk. J. For. 2010, 11, 127–135. [Google Scholar]
- Elias, T.S. The Complete Trees of North America: Field Guide and Natural History; Gramercy Publishing Company & Crown Publishers, Inc.: New York, NY, USA, 1987; pp. 457–458. [Google Scholar]
- Łukaszkiewicz, J.; Długoński, A.; Fortuna-Antoszkiewicz, B.; Fialová, J. The Ecological Potential of Poplars (Populus L.) for City Tree Planting and Management: A Preliminary Study of Central Poland (Warsaw) and Silesia (Chorzów). Land 2024, 13, 593. [Google Scholar] [CrossRef]
- Plants of the World Online. Available online: https://powo.science.kew.org/ (accessed on 4 February 2025).
- Govaerts, R.; Nic Lughadha, E.; Black, N.; Turner, R.; Paton, A. The World Checklist of Vascular Plants, a continuously updated resource for exploring global plant diversity. Sci. Data 2021, 8, 215. [Google Scholar] [CrossRef]
- Stobrawa, K. Poplars (Populus spp.): Ecological role, applications and scientific perspectives in the 21st century. Balt. For. 2014, 20, 204–213. [Google Scholar]
- Dirr, M. Manual of Woody Landscape Plants: Their Identification, Ornamental Characteristics, Culture, Propagation and Uses, 6th ed.; Stipes: Champaign, IL, USA, 2009; pp. 861–864. [Google Scholar]
- Peattie, D.C. A Natural History of Trees of Eastern and Central North America, 2nd ed.; Bonanza Books: New York, NY, USA, 1966; p. 88. [Google Scholar]
- Peattie, D.C. A Natural History of Western Trees; Bonanza Books: New York, NY, USA, 1950; p. 318. [Google Scholar]
- Isebrands, J.G.; Karnosky, D.F. Environmental benefits of poplar culture. In Poplar Culture in North America; Dickmann, D.I., Isebrands, J.G., Eckenwalder, J.E., Richardson, J., Eds.; NRC Research Press: Ottawa, ON, Canada, 2001; pp. 207–218. [Google Scholar]
- Demeritt, M.E. Populus L. Poplar hybrids. Salicaceae—Willow family. In Silvics of North America; Burns, R.M., Honkala, B.H., Eds.; USDA Forest Service: Washington, DC, USA, 1990; Volume 2, pp. 570–576. [Google Scholar]
- Tschaplinski, T.J.; Tuskan, G.A.; Gunderson, C.A. Water stress tolerance of black and eastern cottonwood clones and four hybrid progeny. I. Growth, water relations and gas exchange. Can. J. For. Res. 1994, 24, 364–371. [Google Scholar] [CrossRef]
- Silim, S.; Nash, R.; Reynard, D.; White, B.; Schroeder, W. Leaf gas exchange and water potential responses to drought in nine poplar (Populus spp.) clones with contrasting drought tolerance. Trees 2009, 23, 959–969. [Google Scholar] [CrossRef]
- Rood, S.B.; Bratney, J.H.; Hughes, F.M. Ecophysiology of riparian cottonwoods: Streamflow dependency, water relations and restoration. Tree Physiol. 2003, 23, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Grossman, J.J. Phenological physiology: Seasonal patterns of plant stress tolerance in a changing climate. New Phytol. 2023, 237, 1508–1524. [Google Scholar] [CrossRef] [PubMed]
- Hirons, A.D.; Thomas, P.A. Applied Tree Biology; John Wiley & Sons Ltd.: Oxford, UK, 2018; pp. 372–373. [Google Scholar]
- Network for Environment and Weather Applications. Available online: https://newa.cornell.edu/all-weather-data-query (accessed on 30 January 2025).
- Bartlett, M.K.; Scoffoni, C.; Ardy, R.; Zhang, Y.; Sun, S.; Cao, K.; Sack, L. Rapid determination of comparative drought tolerance traits: Using an osmometer to predict turgor loss point. Methods Ecol. Evol. 2012, 3, 880–888. [Google Scholar] [CrossRef]
- Kikuta, S.B.; Richter, H. Leaf discs or press saps? A comparison of techniques for the determination of osmotic potentials in freeze-thawed leaf material. J. Expt. Bot. 1992, 43, 1039–1044. [Google Scholar] [CrossRef]
- Sjöman, H.; Hirons, A.D.; Bassuk, N.L. Urban forest resilience through tree selection-Variation in drought tolerance in Acer. Urban For. Urban Green. 2015, 14, 858–865. [Google Scholar] [CrossRef]
- Bartlett, M.K.; Scoffoni, C.; Sack, L. The determinants of leaf turgor loss point and prediction of drought tolerance of species and biomes: A global meta-analysis. Ecol. Lett. 2012, 15, 393–405. [Google Scholar] [CrossRef]
- Gebre, G.M.; Tschaplinski, T.J.; Tuskan, G.A.; Todd, D.E. Clonal and seasonal differences in leaf osmotic potential and organic solutes of five hybrid poplar clones grown under field conditions. Tree Physiol. 1998, 18, 645–652. [Google Scholar] [CrossRef]
- Deacon, N.J.; Grossman, J.J.; Cavender-Bares, J. Drought and freezing vulnerability of the isolated hybrid aspen Populus ×. smithii relative to its parental species, P. tremuloides and P. grandidentata. Ecol. Evol. 2019, 9, 8062–8074. [Google Scholar] [CrossRef]
- Hirons, A.D.; Watkins, J.H.R.; Baxter, T.J.; Miesbauer, J.W.; Male-Muñoz, A.; Martin, K.W.; Bassuk, N.L.; Sjöman, H. Using botanic gardens and arboreta to help identify urban trees for the future. Plants People Planet 2021, 3, 182–193. [Google Scholar] [CrossRef]
- Schwartz Sax, M.; Bassuk, N.L.; Sjöman, H. Osmotic adjustment and gas exchange response during drought for two tree species (Quercus bicolor & Betula pendula) grown in containers with limited soil volume. In The Landscape Below Ground IV: Proceedings of the IV International Workshop on Tree Root Development in Urban Soils; Watson, G., Gilman, E., Miesbauer, J., Morgenroth, J., Scharenbroch, B., Eds.; International Society of Arboriculture: Champaign, IL, USA, 2020; pp. 742–762. [Google Scholar]
- Sjöman, H.; Hirons, A.D.; Bassuk, N.L. Improving confidence in tree species selection for challenging urban sites: A role for leaf turgor loss. Urban Ecosyst. 2018, 21, 1171–1188. [Google Scholar] [CrossRef]
- Sjöman, H.; Hirons, A.D.; Bassuk, N.L. Magnolias as urban trees-a preliminary evaluation of drought tolerance in seven magnolia species. Arboric. J. 2018, 40, 47–56. [Google Scholar] [CrossRef]
- Hirons, A.D.; Sjöman, H. Tree Species Selection for Green Infrastructure: A Guide for Specifiers, Trees & Design Action Group 2019, Issue 1.3. Available online: https://www.tdag.org.uk/tree-species-selection-for-green-infrastructure.html (accessed on 10 March 2025).
- Banks, J.M.; Hirons, A.D. Alternative methods of estimating the water potential at turgor loss point in Acer genotypes. Plant Methods 2019, 15, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Hoag, D.G. Trees and Shrubs for the Northern Plains; Lund Press, Inc.: Minneapolis, MN, USA, 1965; p. 272. [Google Scholar]
- Hannus, S.; Hirons, A.; Baxter, T.; McAllister, H.; Wiström, B.; Sjöman, H. Intraspecific drought tolerance of Betula pendula genotypes: An evaluation using leaf turgor loss in a botanical collection. Trees 2021, 35, 569–581. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).