Beneficial Soil Fungi Isolated from Tropical Fruit Crop Systems for Enhancing Yield and Growth in Dragon Fruit in Ecuador
Abstract
1. Introduction
2. Materials and Methods
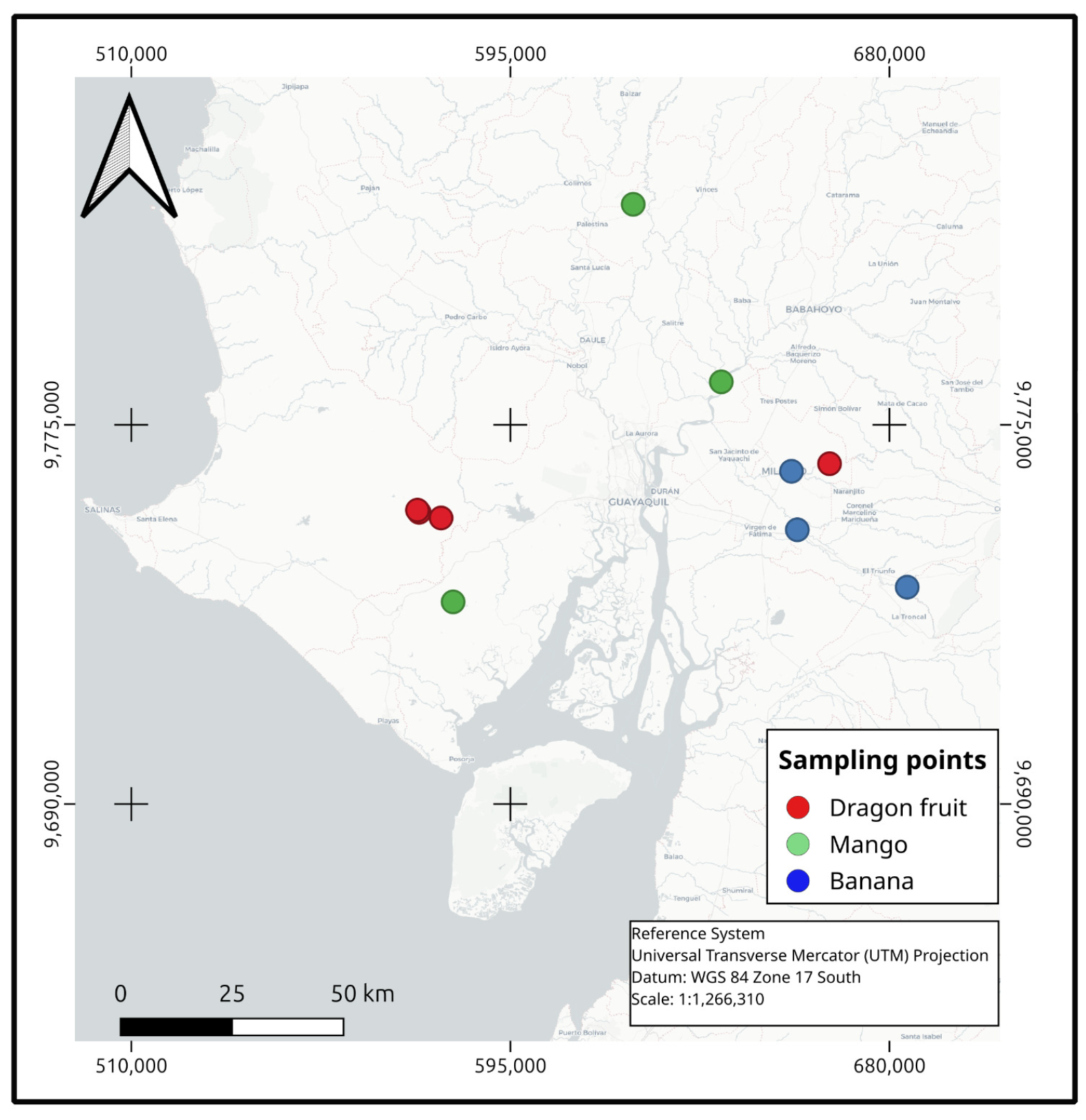
2.1. Description of the Study Site and Sampling
2.2. Fungal Isolation
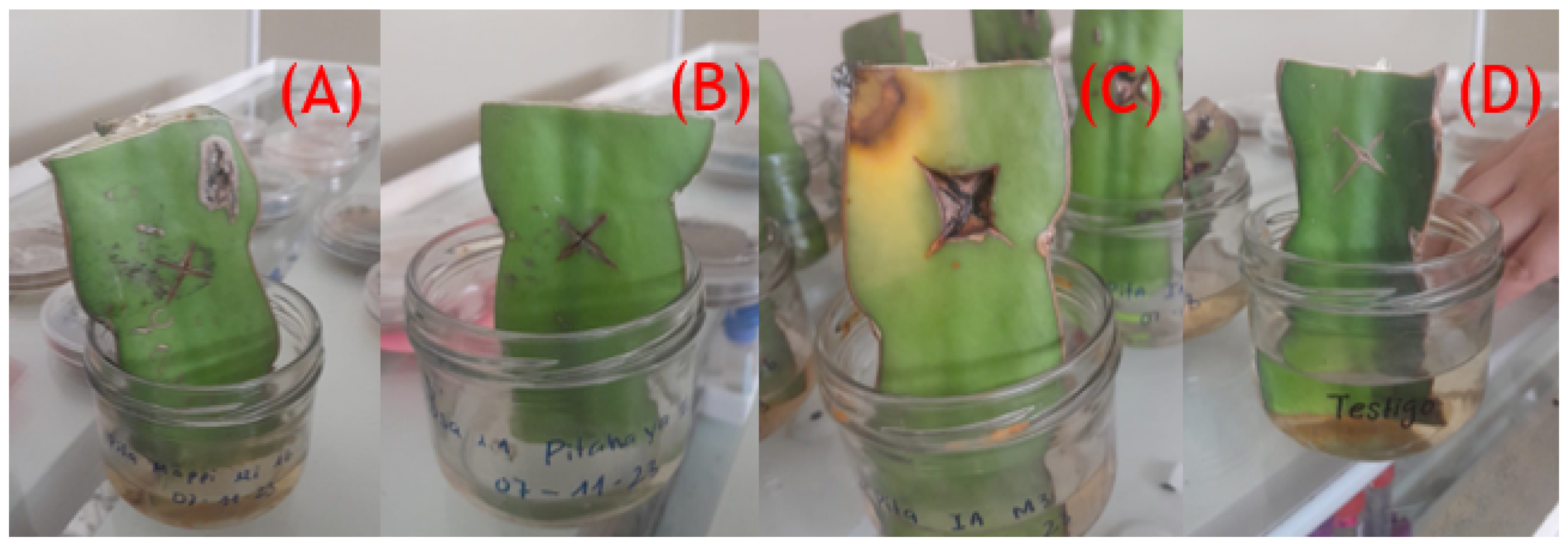
2.3. Detached Cladode Pathogenicity Testing
2.4. Molecular Identification of Fungal Strains
2.5. Evaluation of Beneficial Fungi in Commercial Dragon Fruit Production
3. Results
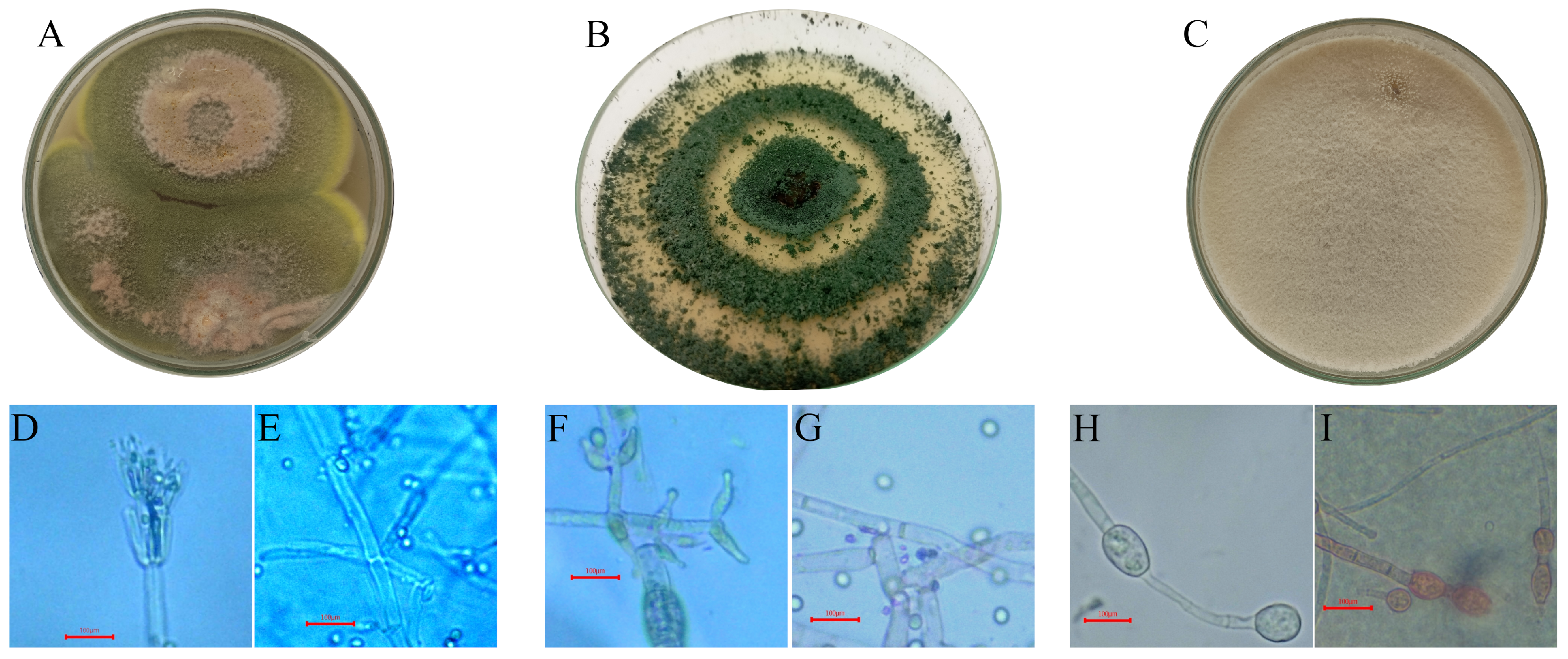
3.1. Beneficial Fungal Isolate Selection
3.2. Molecular Identification and Characterization
3.3. Pathogenicity Testing of the Fungal Isolates
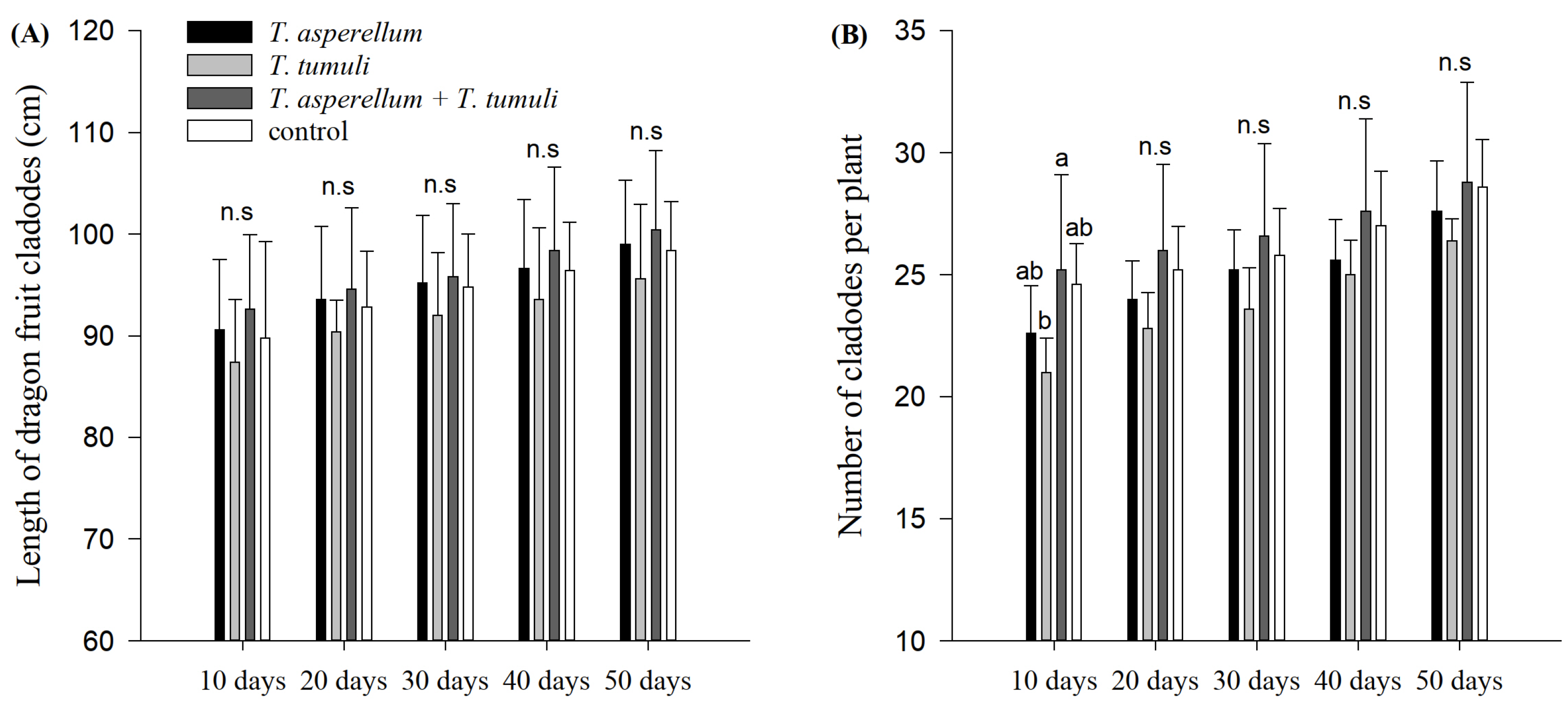
3.4. Evaluation of Beneficial Fungi in the Plants
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Monteiro, S.S.; Almeida, R.L.; Santos, N.C.; Pereira, E.M.; Silva, A.P.; Oliveira, H.M.L.; Pasquali, M.A.d.B. New functional foods with cactus components: Sustainable perspectives and future trends. Foods 2023, 12, 2494. [Google Scholar] [CrossRef] [PubMed]
- Diéguez-Santana, K.; Sarduy-Pereira, L.B.; Sablón-Cossío, N.; Bautista-Santos, H.; Sánchez-Galván, F.; Ruíz Cedeño, S.d.M. Evaluation of the circular economy in a pitahaya agri-food chain. Sustainability 2022, 14, 2950. [Google Scholar] [CrossRef]
- Mannino, G. A new era of sustainability: Plant biostimulants. Int. J. Mol. Sci. 2023, 24, 16329. [Google Scholar] [CrossRef] [PubMed]
- Intana, W.; Kumla, J.; Suwannarach, N.; Sunpapao, A. Biological control potential of a soil fungus Trichoderma asperellum K1-02 against Neoscytalidium dimidiatum causing stem canker of dragon fruit. Physiol. Mol. Plant Pathol. 2023, 128, 102151. [Google Scholar] [CrossRef]
- Baldi, E.; Gioacchini, P.; Montecchio, D.; Mocali, S.; Antonielli, L.; Masoero, G.; Toselli, M. Effect of biofertilizers application on soil biodiversity and litter degradation in a commercial apricot orchard. Agronomy 2021, 11, 1116. [Google Scholar] [CrossRef]
- Pozo, M.I.; Herrero, B.; Martín-García, J.; Santamaría, Ó.; Poveda, J. Evaluating potential side effects of Trichoderma as biocontrol agent: A two-edges sword? Curr. Opin. Environ. Sci. Health 2024, 41, 100566. [Google Scholar] [CrossRef]
- Mawarda, P.C.; Le Roux, X.; Dirk van Elsas, J.; Salles, J.F. Deliberate introduction of invisible invaders: A critical appraisal of the impact of microbial inoculants on soil microbial communities. Soil Biol. Biochem. 2020, 148, 107874. [Google Scholar] [CrossRef]
- Barquero, M.; Pastor-Buies, R.; Urbano, B.; González-Andrés, F. Challenges, regulations and future actions in biofertilizers in the european agriculture: From the lab to the field. In Microbial Probiotics for Agricultural Systems: Advances in Agronomic Use; Zúñiga-Dávila, D., González-Andrés, F., Ormeño-Orrillo, E., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 83–107. [Google Scholar] [CrossRef]
- Sundh, I.; Del Giudice, T.; Cembalo, L. Reaping the benefits of microorganisms in cropping systems: Is the regulatory policy adequate? Microorganisms 2021, 9, 1437. [Google Scholar] [CrossRef]
- Agrocalidad. Instructivo de la Normativa para Promover y Regular la Produccion Organica Ecologica Biologica en el Ecuador. 2013. Available online: https://www.agrocalidad.gob.ec/wp-content/uploads/2020/05/by3.pdf (accessed on 9 April 2025).
- Cruz, M.A.T.; Evallo, E.S.; Taguiam, J.D.W.; Bengoa, J.C.; Balendres, M.A.O. Identification of three Diaporthe species from Selenicereus sp. explants and their pathogenicity to three dragon fruit species. J. Phytopathol. 2022, 170, 791–801. [Google Scholar] [CrossRef]
- Emejas, D.P.J.; Jumao-As, C.M.; Salvaña, F.R.P. Identification of common fungal pathogens of dragon fruit (Hylocereus undatus) in Sarangani Province, Philippines. Environ. Exp. Biol. 2023, 21, 93–99. [Google Scholar] [CrossRef]
- Li, C.; Jia, Z.; Ma, S.; Liu, X.; Zhang, J.; Müller, C. Plant and native microorganisms amplify the positive effects of microbial inoculant. Microorganisms 2023, 11, 570. [Google Scholar] [CrossRef]
- Wang, M.; Sun, H.; Xu, Z. Characterization of Rhizosphere Microbial Diversity and Selection of Plant-Growth-Promoting Bacteria at the Flowering and Fruiting Stages of Rapeseed. Plants 2024, 13, 329. [Google Scholar] [CrossRef]
- Guardiola-Márquez, C.E.; Santos-Ramírez, M.T.; Figueroa-Montes, M.L.; Valencia-de los Cobos, E.O.; Stamatis-Félix, I.J.; Navarro-López, D.E.; Jacobo-Velázquez, D.A. Identification and characterization of beneficial soil microbial strains for the formulation of biofertilizers based on native plant growth-promoting microorganisms isolated from northern Mexico. Plants 2023, 12, 3262. [Google Scholar] [CrossRef] [PubMed]
- Evallo, E.; Taguiam, J.D.; Bengoa, J.; Maghirang, R.; Balendres, M.A. First report of Colletotrichum tropicale on dragon fruit and the response of three Selenicereus species to anthracnose. Int. J. Pest Manag. 2022, 70, 711–718. [Google Scholar] [CrossRef]
- Covacevich, F.; Consolo, F. Herramientas para el Estudio y Manipulación de Hongos Micorrícicos Arbusculares y Trichoderma, 1st ed.; Universidad Nacional de Mar del Plata: Mar del Plata, Argentina, 2014. [Google Scholar]
- Pringle, A.; Taylor, J.W. The fitness of filamentous fungi. Trends Microbiol. 2002, 10, 474–481. [Google Scholar] [CrossRef] [PubMed]
- Schoustra, S.; Punzalan, D. Correlation of mycelial growth rate with other phenotypic characters in evolved genotypes of Aspergillus nidulans. Fungal Biol. 2012, 116, 630–636. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, W.; Zhao, Z.; Long, Y.; Fan, R. Biocontrol potential and growth-promoting effect of endophytic fungus Talaromyces muroii SD1-4 against potato leaf spot disease caused by Alternaria alternata. BMC Microbiol. 2024, 24, 255. [Google Scholar] [CrossRef]
- Vinale, F.; Sivasithamparam, K.; Ghisalberti, E.L.; Marra, R.; Woo, S.L.; Lorito, M. Trichoderma–plant–pathogen interactions. Soil Biol. Biochem. 2008, 40, 1–10. [Google Scholar] [CrossRef]
- Harman, G.E.; Howell, C.R.; Viterbo, A.; Chet, I.; Lorito, M. Trichoderma species—Opportunistic, avirulent plant symbionts. Nat. Rev. Microbiol. 2004, 2, 43–56. [Google Scholar] [CrossRef]
- Daniel, M.C.; Lisbeth, E.; Harmon, P.F.; Chaparro, J.X. Botryosphaeriaceae infections on prunus germplasm: Evaluation of pathogenicity, resistance loci, and detached assays of southeastern United States isolates. HortScience 2024, 59, 1700–1707. [Google Scholar]
- Martin, K.J.; Rygiewicz, P.T. Fungal-specific PCR primers developed for analysis of the ITS region of environmental DNA extracts. BMC Microbiol. 2005, 5, 28. [Google Scholar] [CrossRef] [PubMed]
- Einax, E.; Voigt, K. Oligonucleotide primers for the universal amplification of β-tubulin genes facilitate phylogenetic analyses in the regnum Fungi. Org. Divers. Evol. 2003, 3, 185–194. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Sehim, A.E.; Hewedy, O.A.; Altammar, K.A.; Alhumaidi, M.S.; Abd Elghaffar, R.Y. Trichoderma asperellum empowers tomato plants and suppresses Fusarium oxysporum through priming responses. Front. Microbiol. 2023, 14, 1140378. [Google Scholar] [CrossRef]
- Oiuphisittraiwat, T.; Dethoup, T.; Jantasorn, A. Biological control of chili Anthracnose Disease using Talaromyces flavus Bodhi001. Indian J. Agric. Res. 2024, 58, 525–531. [Google Scholar] [CrossRef]
- Zhang, Z.K.; Wang, X.C.; Zhuang, W.Y.; Cheng, X.H.; Zhao, P. New species of Talaromyces (Fungi) isolated from soil in southwestern China. Biology 2021, 10, 745. [Google Scholar] [CrossRef]
- Sun, X.R.; Xu, M.Y.; Kong, W.L.; Wu, F.; Zhang, Y.; Xie, X.L.; Li, D.W.; Wu, X.Q. Fine identification and classification of a novel beneficial Talaromyces fungal species from masson pine rhizosphere soil. J. Fungi 2022, 8, 155. [Google Scholar] [CrossRef]
- Osuna-Enciso, T.; Valdez-Torres, J.B.; Sañudo-Barajas, J.A.; Muy-Rangel, M.D.; Hernández-Verdugo, S.; Villarreal-Romero, M.; Osuna-Rodríguez, J.M.; Osuna-Enciso, T.; Valdez-Torres, J.B.; Sañudo-Barajas, J.A.; et al. Fenología reproductiva, rendimiento y calidad del fruto de pitahaya Hylocereus undatus (How.) Britton and Rose) en el valle de Culiacán, Sinaloa, México. Agrociencia 2016, 50, 61–78. [Google Scholar]
- Xiao, Y.; Chen, R.; Chen, L.; Yang, B.; Jiang, L.; Fang, J. Endophytic Fungus Talaromyces sp. MR1 Promotes the Growth and Cadmium Uptake of Arabidopsis thaliana L. Under Cadmium Stress. Curr. Microbiol. 2023, 80, 346. [Google Scholar] [CrossRef]
- Chauhan, P.; Sharma, N.; Tapwal, A.; Kumar, A.; Verma, G.S.; Meena, M.; Seth, C.S.; Swapnil, P. Soil microbiome: Diversity, benefits and interactions with plants. Sustainability 2023, 15, 14643. [Google Scholar] [CrossRef]
- Peterson, S.W.; Jurjević, Ž. The Talaromyces pinophilus species complex. Fungal Biol. 2019, 123, 745–762. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.R.; Gong, L.Q.; Jin, M.Y.; Wang, R.; Liu, R.; Gao, J.; Liu, M.D.; Huang, L.; Wang, G.Z.; Wang, D.; et al. Research advances in the structures and biological activities of secondary metabolites from Talaromyces. Front. Microbiol. 2022, 13, 984801. [Google Scholar] [CrossRef] [PubMed]
- Kazerooni, E.A.; Rethinasamy, V.; Al-Sadi, A.M. Talaromyces pinophilus inhibits Pythium and Rhizoctonia-induced damping-off of cucumber. J. Plant Pathol. 2019, 101, 377–383. [Google Scholar] [CrossRef]
- Patel, D.; Patel, A.; Patel, M.; Goswami, D. Talaromyces pinophilus strain M13: A portrayal of novel groundbreaking fungal strain for phytointensification. Environ. Sci. Pollut. Res. 2021, 28, 8758–8769. [Google Scholar] [CrossRef]
- Zhou, X.; Chen, S.; Qiu, L.; Liao, L.; Lu, G.; Yang, S. How rhizosphere microbial assemblage is influenced by dragon fruits with white and red flesh. Plants 2024, 13, 1346. [Google Scholar] [CrossRef]
- GBIF.Org User. GBIF Occurrence Download. 2024. Available online: https://www.gbif.org/occurrence/download/0003842-241007104925546 (accessed on 9 October 2024).
- Cai, R.; Chen, S.; Long, Y.; Li, C.; Huang, X.; She, Z. Depsidones from Talaromyces stipitatus SK-4, an endophytic fungus of the mangrove plant Acanthus ilicifolius. Phytochem. Lett. 2017, 20, 196–199. [Google Scholar] [CrossRef]
- Zang, Y.; Genta-Jouve, G.; Escargueil, A.E.; Larsen, A.K.; Guedon, L.; Nay, B.; Prado, S. Antimicrobial oligophenalenone dimers from the soil fungus Talaromyces stipitatus. J. Nat. Prod. 2016, 79, 2991–2996. [Google Scholar] [CrossRef]
- El-Nagar, D.; Salem, S.H.; El-Zamik, F.I.; El-Basit, H.M.I.A.; Galal, Y.G.M.; Soliman, S.; Aziz, H.A.; Rizk, M.A.; El-Sayed, E.S.R. Bioprospecting endophytic fungi for bioactive metabolites with seed germination promoting potentials. BMC Microbiol. 2024, 24, 200. [Google Scholar] [CrossRef]
- Vishwakarma, S.; Chaudhry, V.; Chand, S.; Sagar, K.; Gupta, K.K.; Bhardwaj, N.; Prasad, R.; Kumar, P.; Chandra, H. The potential of fungal Endophytes in plants: Sources of bioactive compounds. Indian J. Microbiol. 2024. [Google Scholar] [CrossRef]
- Halo, B.A.; Al-Yahyai, R.A.; Al-Sadi, A.M. An endophytic Talaromyces omanensis enhances reproductive, physiological and anatomical characteristics of drought-stressed tomato. J. Plant Physiol. 2020, 249, 153163. [Google Scholar] [CrossRef]
- Wang, M.; Wang, Z.; Guo, M.; Qu, L.; Biere, A. Effects of arbuscular mycorrhizal fungi on plant growth and herbivore infestation depend on availability of soil water and nutrients. Front. Plant Sci. 2023, 14, 1101932. [Google Scholar] [CrossRef]
- Samuels, G.J.; Lieckfeldt, E.; Nirenberg, H. Trichoderma asperellum, a new species with warted conidia, and redescription of T. viride. Sydowia 1999, 57, 71–88. [Google Scholar]
- Li, Y.T.; Hwang, S.G.; Huang, Y.M.; Huang, C.H. Effects of Trichoderma asperellum on nutrient uptake and Fusarium wilt of tomato. Crop Prot. 2018, 110, 275–282. [Google Scholar] [CrossRef]
- Stracquadanio, C.; Quiles, J.M.; Meca, G.; Cacciola, S.O. Antifungal activity of bioactive metabolites produced by Trichoderma asperellum and Trichoderma atroviride in Liquid Medium. J. Fungi 2020, 6, 263. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.L.; Hermosa, R.; Lorito, M.; Monte, E. Trichoderma: A multipurpose, plant-beneficial microorganism for eco-sustainable agriculture. Nat. Rev. Microbiol. 2023, 21, 312–326. [Google Scholar] [CrossRef]
- Rigobelo, E.C.; Nicodemo, D.; Babalola, O.O.; Desoignies, N. Purpureocillium lilacinum as an agent of nematode control and plant growth-promoting fungi. Agronomy 2024, 14, 1225. [Google Scholar] [CrossRef]
- Abbas, A.; Fu, Y.; Qu, Z.; Zhao, H.; Sun, Y.; Lin, Y.; Xie, J.; Cheng, J.; Jiang, D. Isolation and evaluation of the biocontrol potential of Talaromyces spp. against rice sheath blight guided by soil microbiome. Environ. Microbiol. 2021, 23, 5946–5961. [Google Scholar] [CrossRef]
- Silva, L.I.d.; Pereira, M.C.; Carvalho, A.M.X.d.; Buttrós, V.H.; Pasqual, M.; Dória, J. Phosphorus-solubilizing microorganisms: A key to sustainable Agriculture. Agriculture 2023, 13, 462. [Google Scholar] [CrossRef]
- Pruksaphon, K.; Nosanchuk, J.D.; Ratanabanangkoon, K.; Youngchim, S. Talaromyces marneffei infection: Virulence, intracellular lifestyle and host defense mechanisms. J. Fungi 2022, 8, 200. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, F.; Zhang, Y.; Zhang, J. Involvement of Trichoderma asperellum strain T6 in regulating iron acquisition in plants. J. Basic Microbiol. 2014, 54, S115–S124. [Google Scholar] [CrossRef]
- Philpott, C.C. Iron uptake in fungi: A system for every source. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2006, 1763, 636–645. [Google Scholar] [CrossRef] [PubMed]
- Vylkova, S. Environmental pH modulation by pathogenic fungi as a strategy to conquer the host. PLoS Pathog. 2017, 13, e1006149. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Gavíra, A.; Huertas, V.; Diánez, F.; Sánchez-Montesinos, B.; Santos, M. Paecilomyces and its importance in the biological control of agricultural pests and diseases. Plants 2020, 9, 1746. [Google Scholar] [CrossRef] [PubMed]

| Characteristic | Result | Characteristic | Result |
|---|---|---|---|
| pH | 6.60 | Sulfur (ppm) | 11.00 |
| Organic matter (%) | 1.70 | Boron (ppm) | 1.14 |
| CEC (meq 100 g−1) | 33.30 | Copper (ppm) | 7.90 |
| Phosphorus (ppm) | 11.00 | Iron (ppm) | 485.00 |
| Potassium (meq 100 g−1) | 0.79 | Manganese (ppm) | 38.00 |
| Calcium (meq 100 g−1) | 19.66 | Molybdenum (ppm) | 0.03 |
| Magnesium (meq 100 g−1) | 5.80 | Zinc (ppm) | 2.20 |
| Sodium (meq 100 g−1) | 0.41 | Microbial biomass (ppm) | 668.00 |
| Exchangeable Al (meq 100 g−1) | <0.01 | Potentially mineralizable nitrogen (kg N ha−1) | 19.00 |
| Texture | Loamy Clay |
| Location | Total Fungal Isolates | Discarded Mycelial Growth Rate < 5 mm/day | Phytopathogenic Isolates * | Selected Beneficial Isolates |
|---|---|---|---|---|
| Finca La Fernandita | 12 | 11 | 1 | 0 |
| Finca Maxpri | 9 | 8 | 0 | 1 |
| Finca PitaSpot | 8 | 8 | 0 | 0 |
| UAE Station | 16 | 15 | 0 | 1 |
| Organism | Location (WGS84 UTM 17S) | % Identity | Accession No. * |
|---|---|---|---|
| Paecilomyces lagunculariae | 579517, 9754071 | 99.55 | NR145144.1 |
| Talaromyces tumuli | 666594, 9766293 | 99.61 | NR_165528.1 |
| Trichoderma asperellum | 574169, 9755778 | 100.00 | NR_130668.1 |
| Treatments | Fruit Weight at 40 Days (g) |
|---|---|
| T. tumuli | a |
| T. asperellum | b |
| T. asperellum + T. tumuli | c |
| control | c |
| p-value | 1.395 × 10−6 |
| Coefficient of variation (%) | 7.71 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcia, Y.; Valdez, D.; Ponce de Leon, D.; Urjilez, H.; Santos-Pinargote, J.; Mancero-Castillo, D. Beneficial Soil Fungi Isolated from Tropical Fruit Crop Systems for Enhancing Yield and Growth in Dragon Fruit in Ecuador. Int. J. Plant Biol. 2025, 16, 62. https://doi.org/10.3390/ijpb16020062
Garcia Y, Valdez D, Ponce de Leon D, Urjilez H, Santos-Pinargote J, Mancero-Castillo D. Beneficial Soil Fungi Isolated from Tropical Fruit Crop Systems for Enhancing Yield and Growth in Dragon Fruit in Ecuador. International Journal of Plant Biology. 2025; 16(2):62. https://doi.org/10.3390/ijpb16020062
Chicago/Turabian StyleGarcia, Yoansy, Danilo Valdez, Daniel Ponce de Leon, Hypatia Urjilez, Jaime Santos-Pinargote, and Daniel Mancero-Castillo. 2025. "Beneficial Soil Fungi Isolated from Tropical Fruit Crop Systems for Enhancing Yield and Growth in Dragon Fruit in Ecuador" International Journal of Plant Biology 16, no. 2: 62. https://doi.org/10.3390/ijpb16020062
APA StyleGarcia, Y., Valdez, D., Ponce de Leon, D., Urjilez, H., Santos-Pinargote, J., & Mancero-Castillo, D. (2025). Beneficial Soil Fungi Isolated from Tropical Fruit Crop Systems for Enhancing Yield and Growth in Dragon Fruit in Ecuador. International Journal of Plant Biology, 16(2), 62. https://doi.org/10.3390/ijpb16020062






