Abstract
The gut microbiome is essential for nutrient absorption, immune function, and overall metabolic health. A balanced microbial community allows for the breakdown of carbohydrates, proteins, fats, vitamins, and minerals into maximally absorbed nutrients and provides protection against inflammation. Dysbiosis, or microbial imbalance, disrupts these processes and leads to malabsorption, barrier dysfunction, and toxic metabolite production. These imbalances contribute to a wide variety of diseases, from obesity, diabetes, and cardiovascular disease to anemia, osteoporosis, and nervous system dysfunctions. Advances in sequencing, metabolomics, and functional assays have facilitated an enhanced understanding of the ecological and biochemical complexity of gut microbes. AI-based models are also providing new insights into personalized diet and therapeutic approaches. Through the redefinition of malnutrition and chronic disease within microbial ecology, science proves the potential for engineered probiotics, precision prebiotics, and gut-targeted therapies. These innovations hold the potential to improve global health and propel precision medicine in nutrition.
1. Introduction
Nutrition is pivotal for human survival, development, as well as for endurance, though its biological aspects are more sophisticated than just the diet taken. Even in an era of unprecedented food production, more than 3 billion people in the world are unable to afford a healthy diet, while nearly 45 million children aged under five are wasting, while 149 million are stunted [1]. A Lancet study further reports that, in 2022, more than one billion people were living with obesity. Since 1990, obesity has more than doubled among adults and quadrupled among children and adolescents, with 43% of adults classified as overweight [2]. This reflects a “double burden of malnutrition,” where undernutrition and overnutrition coexist within the same populations. This paradox speaks to a less well understood aspect, which is the variability and efficiency of human nutrient extraction, metabolism, and absorption, an undertaking profoundly influenced by gut microbial composition and function.
The recognition of the microbiome as a full-fledged partner in nutrition represents one of the most dramatic paradigm shifts in modern biomedicine comparable in scope to advances such as CRISPR-based gene editing and cancer immunotherapy because it redefines human physiology as an ecosystem process. For decades, the gut microbiota has been treated as a collection of commensals and opportunistic pathogens. The dogma began to change in the mid-twentieth century with the development of gnotobiotic animal models that allowed scientists to introduce specific microbial communities into germ-free hosts and observe how colonization influenced nutrient absorption and immune development [3]. Introduction of the Human Microbiome Project in 2007 [4] and subsequent metagenomic surveys established the concept of a “healthy” microbiome that is ecologically complex, functionally redundant, and metabolically complementary. Disturbance of this ecology, dysbiosis, has been labeled as a cause of malabsorption, micronutrient deficiency, and systemic disease. Unhealthy microbiome literature is not limited to the clinical environment. Western-style diets, higher in fat and lower in fiber, are associated with reduced fiber-fermenting bacteria like Prevotella and lower short-chain fatty acid (SCFA) levels, indicating diminished microbial fermentation and energy contribution [5]. Likewise, research in Malawian and Bangladeshi children with undernutrition delineated that microbiome immaturity—more than caloric insufficiency—co-conserves with stunting of growth [6,7]. These findings extend the domain of dysbiosis from gastrointestinal disease to global health disciplines and connect it with stunting, anemia, and micronutrient malnutrition in the developing world and obesity, diabetes, metabolic syndrome, liver disease, and even neurological disease in the developed world [8,9,10]. One of the strongest controls of such microbial profiles is nutrition, and either high-fat or high-fiber feeding has profound influences on both nutrient uptake and microbial diversity.
Mechanistically, dysbiosis poses multi-layered obstacles to nutrient absorption inducing “leaky gut” and sub-chronic inflammation. The obstacles restrict not just the absorption of nutrients but also deflect host energy to immune activation, creating a cycle of malabsorption and systemic illness.
Together, the convergence of revolutionary nutritional paradigms, historical milestones, and modern mechanistic understanding places the gut microbiome at once as mediator and barrier of human nutrition. This review integrates information on how individual microbial taxa and pathways control macronutrient and micronutrient metabolism, how dysbiosis disrupts such processes, and how emerging diagnostic and therapeutic approaches can break down gut-mediated obstacles to nutrient assimilation. Reframing malnutrition through the lens of microbial ecology, we create a new frontier in closing global health gaps and bringing precision nutrition.
2. The Healthy Gut Microbiome: Composition and Function
2.1. Dominant Phyla and Their Metabolic Signatures
The four phyla Firmicutes, Bacteroidetes, Actinomycetota, and Proteobacteria predominate in the healthy adult gut microbiome. Collectively, these groups account for over 90% of bacterial taxa [11,12]. From the initial culture-based studies in the early 20th century through the advent of sequencing technologies, these groups have been repeatedly found to be the structural and functional foundation pillars of the intestinal ecosystem [13,14].
Firmicutes [Roseburia, Ruminococcus, Faecalibacterium] are important producers of butyrate, synthesizing this short-chain fatty acid through the acetyl-CoA → butyryl-CoA → butyrate pathway. Butyrate has been long known to energize colonocytes and modulate mucosal immunity; the identification of Faecalibacterium prausnitzii as a potent anti-inflammatory commensal was a milestone in linking specific microbes to host immune modulation [15,16,17].
Members of the phylum Bacteroidetes, such as Bacteroides and Prevotella, are known for their high abundance of polysaccharide utilization loci (PULs), which enable them to predominantly digest a variety of glycans originating from both plant and host sources. These metabolic products are biased toward acetate [C2] and propionate [C3], which alter host gluconeogenesis and facilitate cross-feeding between various microbes. It was the comparative population studies that first systematically characterized dietary dependence of Bacteroides–Prevotella distributions. These studies highlighted the strong latent capability of diet as an ecological driver of gut composition [18,19].
Actinomycetota, especially Bifidobacterium, have a long-standing history in microbiology, since Henri Tissier first described them in 1899 in breast-fed infants, who dominated in colonization [20]. The capacity to digest human milk oligosaccharides [HMOs] in Bifidobacteria increased competitive advantages, particularly in the neonatal gut. Even beyond infancy, Bifidobacteria play a role in biosynthesis of B-vitamins and folate, as well as producing acetate and lactate to promote butyrate-producing Firmicutes [21,22]. Bifidobacterium’s role as early “probiotic” organisms has elevated and transformed scientific and therapeutic interest in their use for over 100 years.
Proteobacteria, often represented by Escherichia, are relatively rare in healthy microbiomes but increase disproportionately during dysbiosis [23]. Proteobacteria also produce siderophores to scavenge iron, increasing their competitive advantage, typically to the detriment of community stability [24]. Abundance of Proteobacteria has been linked with inflammatory disease [25], further establishing them as ecological and clinical sentinels of disturbance.
In addition to the most densely populated phyla, the gut microbiome comprises several secondary and sentinel populations. While fewer in number, these populations provide important ecological and metabolic services. Historically, the first non-‘big four’ commensal to be brought widely to attention was Akkermansia muciniphila [phylum Verrucomicrobiota], first cultured in 2004. It is now most famously known as a mucin-degrading specialist found in healthy adults, which can represent 1–4% of gut bacteria. Its ability to hydrolyse mucin into acetate and propionate—fostering mucin turnover and reinforcing epithelial junctions—has put A. muciniphila as a therapeutic agent and biomarker for the treatment of obesity, diabetes, and barrier dysfunction. Pasteurized Akkermansia is in the process of clinical trial as a next-generation probiotic [26].
Fusobacteriota, in contrast, illustrate the power of small taxa as disease sentinels. Although usually present at <0.5% of reads in normal stools, oral-derived Fusobacterium nucleatum has the ability to adhere to colonic mucosa, where it binds to epithelial cells using the FadA adhesin. Since the early 2010s, enrichment has been reproducibly identified in colorectal cancer tissue, linking rare commensals to oncogenesis [27,28].
Other frequent but quantitatively minor phyla are Synergistota and Tenericutes/Mollicutes, which ferment amino acids and carbohydrates, respectively, and have been sporadically cultured from human intestinal material since the later 20th century [29]. Spirochaeta remain common in non-Westernized populations such as the Hadza, where Treponema succinifaciens may contribute to fiber breakdown, but are exceedingly rare in urban populations—emphasizing the manner in which industrialization reduces phylogenetic diversity [30,31].
The so-called “rare biosphere” also includes Planctomycota, Elusimicrobiota, Lentisphaerae, and Saccharibacteria [TM7], each usually being <0.1% of gut taxa within large metagenomic datasets [11,12,32,33]. The next significant addition is the Melainabacteria, first described in 2013 as relatives of non-photosynthetic Cyanobacteria. Although rare, they also contain vitamin B biosynthesis and hydrogen metabolism genes, yet another degree of microbial cross-feeding [34].
Concomitant with bacteria, archaea make up a metabolically important but numerically minor component of the gut microbiome. The most common archaeon is Methanobrevibacter smithii, found in adults as high as 90% in some cohorts and typically making up 0.1–2% of total gut microbes. Through methane production from hydrogen and carbon dioxide, M. smithii interferes with efficiency of fermentation, impacts transit within the colon, and regulates caloric yield by the host [35,36]. Other archaea, such as Methanosphaera stadtmanae, are less common but induce vigorous immune responses, positioning them as immunomodulators in spite of their relative rarity [37].
The gut virome comes into play too. Bacteriophages, first visualized in the 19th century but deeply cataloged only with current metagenomics, are estimated to outnumber bacteria in the gut by an order of magnitude (up to 109 viral particles per gram of stool) [38,39]. They regulate bacterial population dynamics, enable horizontal gene transfer, and are “kill-the-winner” stabilizers of community structure [40]. The mycobiome, though sparse (usually <0.1% of microbial reads), contains fungi such as Candida albicans and Saccharomyces cerevisiae that can modulate local immunity and inflammation [41,42]. Similarly, unicellular eukaryotes like Blastocystis, are found today in 20–50% of healthy individuals worldwide, suggesting commensal or even beneficial roles contrary to earlier suppositions [43,44]. Overall, the gut microbiome is much more complex than just its lagoon-dwelling groups of bacteria. Large groups tend to cover most of the metabolic needs, with others possessing specialized domains, like maintaining the gut lining or handling gases. Viruses, fungi, and one-celled organisms play important roles as well. This list of both familiar and unfamiliar members establishes that the health of the gut is based on both quantity and diversity of microbes that interact. Because of this diversity, the gut microbiome is now considered to be a multi-kingdom system rather than as merely a collection of bacteria.
2.2. Spatial Biogeography of the Gut (Stomach to Colon)
The gut is not a single habitat, but an assembly of microbial niches defined by pH, oxygen, nutrient availability, and transit time gradients [45]. Spatial conditions impose strong selective pressures, which support varied microbial communities from stomach to colon and enable a functional division of labor along the gut axis.
In the upper tract (duodenum, stomach), there are severe conditions: low pH, high oxygen tension, rapid flow, and exposure to bile. Facultative anaerobes that are resistant to acid, such as Lactobacillus, Streptococcus, and Helicobacter, thrive there [45]. Microbial load is correspondingly low (~102–103 cells/g) [46]. Although present in low numbers, these taxa play important roles in initial digestion, lactate production, and host signaling, with certain species like Helicobacter pylori have been implicated in gastric pathophysiology for decades.
Continuing distally to the jejunum and ileum, microbial counts rise (104–107 cells/g) with declining oxygen tension and increasingly neutral pH [46]. Facultative anaerobes remain ubiquitous, but Enterobacteriaceae and other small intestine specialists subsist on simple sugars and bile-metabolized materials. The relatively fast transit time of the small intestine favors organisms that quickly grow and possess flexible metabolism [47].
It is in contrast to the colon, which harbors the most densely populated microbial population on Earth (>1011 cells/g). Its delayed transit, neutral pH, and unstressed anaerobiosis make it susceptible to obligate anaerobes such as Bacteroides, Prevotella, Ruminococcus, Roseburia, and Faecalibacterium [45]. They exist as saccharolytic consortia that ferment dietary fiber and resistant starches into short-chain fatty acids (SCFAs)—primarily acetate, propionate, and butyrate [48]. Aside from energy harvesting, they also regulate host immunity, gut barriers, and systemic metabolism. The colon’s significance in the host nutritional economy is further supported by the fact that it is a primary site for secondary bile acid conversions and the production of vitamins and cofactors (such as vitamin K, biotin, and folate) [49].
This longitudinal organization highlights the microbiome as a layered system: specialized upstream communities for adapting to stressors such as bile and oxygen, and downstream consortia that were attuned towards fermentation and biosynthesis. By connecting microbial metabolism and human physiology in a location-specific manner, this spatial compartmentalization ensures metabolic complementarity throughout the gut.
2.3. Core Functions in a Healthy State
Additionally, the gut microbiota does more than only facilitate digestion. It regulates the immune system, maintains the integrity of the intestinal lining, and controls the exchange of information between the body and microorganisms regarding metabolism. If this is affected, it may indicate an early digestive issue.
2.3.1. Barrier Integrity and Immune Regulation
Microbial metabolites, particularly short-chain fatty acids (SCFAs), tighten epithelial tight junctions, promote mucin production, and preferentially induce regulatory T cell (Treg) differentiation, thereby dampening inflammatory reactions. The mucus niche is self-sustaining by specialists such as Akkermansia muciniphila, which degrade mucins while inducing concomitant compensatory mucin regeneration and barrier reinforcement [50,51,52]. The interactions between mucosal and luminal microbes and the immune system are especially important, as they mediate tolerance and dampen unwanted inflammation. Mucosal microbes are those that adhere closely to the intestinal epithelium and mucus layer, directly influencing immune signaling and barrier integrity, whereas luminal microbes reside freely within the gut lumen, where they primarily function in nutrient fermentation and metabolite production.
2.3.2. Vitamin and Cofactor Biosynthesis
In addition to the diet of the host, colonic consortia produce B-group vitamins (folate, riboflavin, biotin) and vitamin K (menaquinones), directly contributing to micronutrient pools and bringing into focus the microbiome as an alternative source of necessary cofactors [53,54,55].
2.3.3. Detoxification and Metabolic Signaling
Microbes alter bile acids using certain enzymes and create new forms of bile acid that impact the body’s capacity to absorb fats and control metabolism [56,57]. Moreover, as microbes break down food’s choline, they create a substance that the body decouples into TMAO, which can affect heart and metabolic health [58,59].
To illustrate the functional diversity of key gut taxa, the following table [Table 1] summarizes representative organisms, their discovery history, ecological niches, metabolic enzymes, and nutritional outputs that shape host physiology.

Table 1.
Representative Gut Microorganisms: Discovery, Niche, Enzymes, and Nutritional Roles.
A well-functioning microbiome has a strong gut barrier, well-balanced immune responses, and micronutrient synthesis through diversity and function. Most critically, it degrades and processes the foods we eat. We will discuss below how microbes impact carbohydrate, protein, fat, vitamin, and mineral metabolism.
3. Gut Microbiota and Nutrient Metabolism
The gut microbiome also plays an important role in how the body utilizes nutrients, virtually acting as an additional set of tools for digesting foods. Microbes are involved in the processing of carbohydrates, fats, proteins, vitamins, and minerals in different mechanisms. The following sections will describe how these functions are accomplished and how our knowledge of them has evolved over time.
3.1. Carbohydrates
3.1.1. Sites and Organisms
Carbohydrate metabolism begins in the mouth, where initial studies by Clarke (1924) implicated Streptococcus mutans as the main etiology of dental caries, fermenting sucrose to yield lactic acid and insoluble glucans to construct plaque [60]. Later studies by Scardovi & Crociani (1974) extended such knowledge by characterizing Bifidobacterium dentium, which ferments several sugars and salivary glycoproteins in acidic oral conditions [61].
In the colon and intestine, research shifted to the degradation of complex polysaccharides. Bacteroides thetaiotaomicron, first discovered in the early 20th century and sequenced in 2003, revealed a stunning arsenal of glycoside hydrolases and polysaccharide utilization loci (PULs) [62]. These impart the ability to degrade hemicellulose, resistant starch, and host-derived glycans into acetate and propionate. Combined with this, Firmicutes such as Faecalibacterium prausnitzii and Eubacterium rectale were recognized as historic butyrate producers [64]. Resistant starch interventions also support this axis, consistently increasing butyrate and beneficial taxa in human trials, underlining the strong synthesis among diet, microbial metabolism, and host health [80].
3.1.2. Mechanistic Pathways and Enzymology
The starch utilisation system of B. thetaiotaomicron provided the initial mechanistic model of PULs: surface proteins (SusD) interact with oligosaccharides, SusC (a TonB-dependent transporter) imports them in, and periplasmic hydrolases and cytosolic glycolytic/phosphoketolase pathways funnel sugars into acetyl-CoA [19]. In butyrogenic Firmicutes, the most common terminal step is butyryl-CoA:acetate CoA-transferase (but/bcoA)-catalyzed, while a minority use the phosphotransbutyrylase/butyrate kinase (ptb/buk) pathway [64]. Butyrate also acts as an epigenetic regulator by inhibiting histone deacetylases (HDACs), enzymes that remove acetyl groups from histone proteins and thereby influence gene expression and signal molecules that activate GPR41/43 receptors, regulating barrier integrity and inflammation [81].
3.1.3. Systemic Fates
Among the major short-chain fatty acids (SCFAs), acetate, propionate, and butyrate play distinct systemic roles. Acetate, which is ubiquitously produced, contributes to hepatic lipogenesis and serves as an energy source for peripheral tissues [82]. Propionate, primarily derived from Bacteroidetes, enters the gluconeogenic pathway in the liver [83]. Butyrate, mainly produced by Firmicutes, provides energy to colonocytes, reinforces epithelial tight junctions, and exerts anti-inflammatory effects [16].
3.2. Proteins and Amino Acids
3.2.1. Sites and Organisms
Undigested proteins are carried to the colon and fermented there by Firmicutes and Bacteroidetes [84]. Sulfate-reducing bacteria such as Desulfovibrio reduce amino acids to hydrogen sulfide (H2S) [85], while pathobionts such as Clostridioides difficile metabolize tyrosine to p-cresol [86].
3.2.2. Mechanistic Pathways
Aromatic and sulfur-containing amino acids undergo diverse microbial transformations with significant physiological implications. As early as the 20th century, Clostridium sporogenes was shown to metabolize tryptophan into indole-3-propionic acid (IPA), a compound later identified as a potent antioxidant and anti-inflammatory ligand for the aryl hydrocarbon receptor (AhR) [87,88]. The microbial metabolism of tryptophan diverges into multiple pathways, yielding indoles and IPA, while the host concurrently channels tryptophan into kynurenine and serotonin pathways. Similarly, in tyrosine metabolism, the enzyme p-hydroxyphenylacetate decarboxylase (HpdBCA), reported in Clostridium difficile, catalyzes the conversion of tyrosine to p-cresol—a metabolite that disrupts microbial competition and imposes stress on host epithelial cells [89]. In the case of sulfur amino acids, Bilophila wadsworthia and other sulfate-reducing bacteria utilize glycyl radical enzymes to liberate hydrogen sulfide (H2S) from organosulfur compounds, generating a metabolite with both signaling and cytotoxic properties [90].
3.2.3. Host and By-Product Roles
While amino acid–derived compounds include desirable metabolites (IPA, indoles), others such as branched-chain fatty acids, ammonia, phenols, p-cresol, and H2S accumulate mostly in large quantities and can jeopardize epithelial and mitochondrial integrity [89]. However, hydrogen sulfide exerts a concentration-dependent duality. At low physiological levels it functions as a gasotransmitter that supports mucosal defense, immune regulation, and gut motility, whereas excessive accumulation during dysbiosis becomes cytotoxic.
3.3. Lipids
3.3.1. Bile Acids as Metabolic Levers
Microbial bile acid conversions play a central role in lipid and energy metabolism. Bile salt hydrolases (BSHs), first identified in Lactobacillus, Bifidobacterium, and Enterococcus, deconjugate taurine- and glycine-linked bile salts, thereby influencing micelle stability and cholesterol absorption [91]. By deconjugating bile salts, BSHs reduce micelle stability, thereby lowering the efficiency of dietary fat and fat-soluble vitamin absorption.
A landmark finding in the 1980s was the identification of Clostridium scindens as a key 7α-dehydroxylating species [92]. Its bai operon (BaiB/J/E) converts primary bile acids—cholic acid (CA) and chenodeoxycholic acid (CDCA)—into the secondary bile acids deoxycholic acid (DCA) and lithocholic acid (LCA). These metabolites act as ligands for the host receptors FXR (Farnesoid X Receptor) and TGR5 (Takeda G-protein-coupled Receptor 5), which regulate FGF19 signaling, hepatic bile acid synthesis, lipid absorption, and systemic glucose and energy balance [56].
This process is illustrated in Figure 1, which summarizes the microbial–host interactions governing bile acid metabolism.
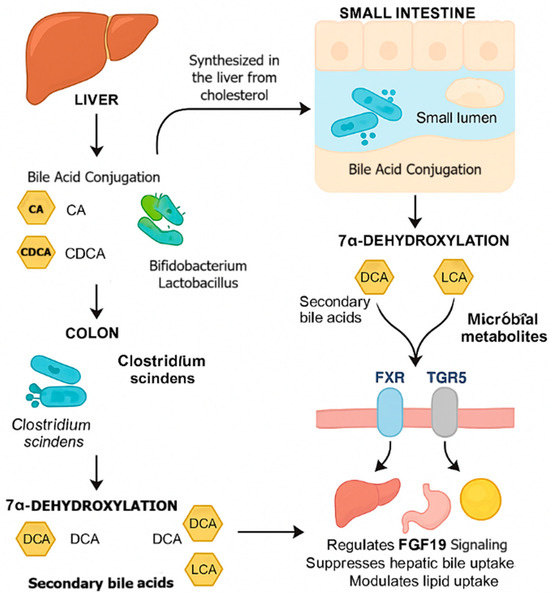
Figure 1.
Mechanistic overview of microbial bile acid metabolism and host signaling pathways. Primary bile acids (CA, CDCA) synthesized from cholesterol in the liver are secreted into the intestine for fat digestion. In the small intestine, bacteria such as Lactobacillus and Bifidobacterium express BSH enzymes that deconjugate bile salts. In the colon, Clostridium scindens performs 7α-dehydroxylation via the bai operon (BaiB/J/E), producing secondary bile acids (DCA, LCA), which activate FXR and TGR5 to regulate FGF19 signaling and metabolic homeostasis. Abbreviations: BSH—Bile Salt Hydrolase; FXR—Farnesoid X Receptor; TGR5—Takeda G-protein-coupled Receptor 5; FGF19—Fibroblast Growth Factor 19; CA—Cholic Acid; CDCA—Chenodeoxycholic Acid; DCA—Deoxycholic Acid; LCA—Lithocholic Acid. Arrows indicate the directional flow of bile acid synthesis, conjugation, microbial transformation, and associated signaling pathways.
3.3.2. Other Lipid–Microbe Axes
Dietary choline and phosphatidylcholine are metabolized by gut microbial TMA-lyases (cutC/D) into trimethylamine (TMA), which is subsequently oxidized by hepatic flavin-containing monooxygenases (FMO) to generate trimethylamine-N-oxide (TMAO), a compound strongly linked to cardiometabolic disorders [59,92]. Interestingly, interventions targeting the gut barrier have shown promise in mitigating these metabolic effects; for instance, pasteurized Akkermansia muciniphila has been demonstrated to improve metabolic health by enhancing insulin sensitivity, reducing adiposity, and strengthening gut barrier function in both mice and humans [93].
3.4. Vitamins and Cofactors
3.4.1. Representative Producers and Pathways
Several gut microorganisms contribute to the biosynthesis of essential vitamins. Bifidobacterium adolescentis harbors the fol and bio-operons responsible for the synthesis of folate and biotin, with increased luminal folate production demonstrated in vivo [94]. Similarly, Lactobacillus plantarum possesses the rib operon for riboflavin biosynthesis, and engineered strains have been shown to enhance riboflavin yield [73]. Escherichia coli synthesizes menaquinone (vitamin K2) through the men pathway, producing menaquinone-8 (MK-8) as its principal form [75]. In addition, Clostridium butyricum was among the first bacterial species identified as capable of de novo cobalamin (vitamin B12) synthesis via the cob/cbi operons under anaerobic conditions [77].
3.4.2. Chemistry of the Pathways
Microbial synthesis of essential vitamins involves distinct biochemical pathways. Folate is synthesized from GTP, which is converted through dihydroneopterin intermediates into tetrahydrofolate. Riboflavin biosynthesis proceeds from ribulose-5-phosphate, leading to the formation of riboflavin as the final product. Menaquinones (vitamin K2 compounds) are produced via chorismate-derived aromatic ring systems, while cobalamin (vitamin B12) is generated through an intricate, approximately 30-step corrinoid pathway that operates under anaerobic conditions. These vitamins are spread throughout microbial communities and absorbed by the host, further facilitating the microbiome as a micronutrient organ [53].
3.5. Minerals
Microbial phytases, such as those found in lactic acid bacteria and Bifidobacterium adolescentis, hydrolyze phytate to release bound minerals like iron, zinc, and calcium, while short-chain fatty acid (SCFA)-mediated acidification further enhances mineral solubility [94,95]. Oxalobacter formigenes specifically decarboxylates oxalate through oxalyl-CoA decarboxylase to produce formate and CO2, thereby preventing calcium oxalate precipitation and preserving dietary calcium for absorption [78]. Microbes also compete for trace elements through nutritional immunity, where siderophores produced by bacteria such as Proteobacteria provide a competitive advantage under iron-limiting conditions [24]. The gut microbiota also regulates systemic levels of redox-active small molecules via the nitrate–nitrite–NO axis, as oral–intestinal nitrate reductases in genera such as Streptococcus and Veillonella facilitate nitric oxide generation, influencing vascular tone and oxygen delivery [96]. Selenium-handling bacteria, including Clostridium sporogenes, incorporate selenium into selenoproteins under normal conditions and methylate excess selenite into less reactive forms [97]. Cross-feeding networks further illustrate microbial cooperation, where vitamin production supports both the host and other microbes—for example, B. adolescentis (biotin and folate) and L. plantarum (riboflavin) secrete vitamins beneficial to partner taxa in syntrophic consortia [73,93]. Moreover, metabolites such as lactate and acetate produced by primary fermenters like Bifidobacterium serve as substrates for secondary fermenters, including Eubacterium rectale and Faecalibacterium prausnitzii, to generate butyrate, forming a syntrophic energy chain that stabilizes the microbial ecosystem. Collectively, these interconnected processes demonstrate that the gut microbiome functions as a metabolic organ, aiding in the digestion and assimilation of carbohydrates, proteins, fats, vitamins, and minerals. Disruption of these coordinated activities leads to impaired nutrient processing and dysbiosis, contributing to various metabolic and inflammatory disorders.
4. Dysbiosis: Altered Microbial Ecology and Nutrient Malabsorption
4.1. Conditions and Exposures That Drive Dysbiosis
Dysbiosis does not arise spontaneously but is the manifestation of additive perturbations weakening microbial diversity, eroding keystone taxa, and enriching opportunists. The conditions causing dysbiosis can be categorized into following:
- (1)
- Dietary and lifestyle factors: Diets based on the West that are high in fat and low in fiber suppress saccharolytic fermentation and promote bile-tolerant organisms like Bilophila wadsworthia. Emulsifiers, sweeteners, alcohol deplete mucus layers and break glucose tolerance [98,99,100,101].
- (2)
- Iatrogenic exposures: While NSAIDs (Non-Steroidal Anti-Inflammatory Drugs), opioids, and chemotherapy disrupt mucosa or retard transit. Antibiotics cause deep changes reducing Faecalibacterium, Roseburia, and Bifidobacterium but allowing Enterobacteriaceae to grow [102]. Proton pump inhibitors elevate gastric pH, enabling oral taxa to colonize the distal gut [103].
- (3)
- Host-intrinsic processes: Host processes like cesarean section, formula feeding, early-life antibiotic exposure, aging, obesity, and chronic inflammation [e.g., IBD (Irritable Bowel Disease), NAFLD (Non-Alcoholic Fatty Liver Disease)] further reconfigure the microbiome [4,104,105,106,107,108]. Genetics and oxidative stress also have been demonstrated to be significant contributors to microbial imbalance [109,110].
Across these environments, the characteristic ecological change is loss of butyrate producers and mucin specialists, decrease in vitamin-synthesizing taxa, and rise in Proteobacteria and bile-adapted lineages.
4.2. Barrier Integrity and Immune Crosstalk
Butyrate-producing Firmicutes such as Faecalibacterium prausnitzii are depleted, depriving colonocytes of their main energy source, impairing ATP delivery, and reducing HDAC inhibition and GPR41/43 signaling [17,111]. At the same time, depletion of Akkermansia muciniphila reduces the mucin layer, while Proteobacteria blooms supply abundant lipopolysaccharides (LPS), major components of the outer membrane of Gram-negative bacteria that act as potent immune stimulators. [112]. The net result is epithelial leakiness, microbial translocation, and systemic inflammation—a state known as metabolic endotoxemia. Chronic barrier dysfunction not only drives immune activation but also reduces nutrient uptake by interrupting transport systems and facilitating protein loss. For example, the pro-inflammatory cytokine TNF-α—elevated during metabolic endotoxemia downregulates zinc transporter ZIP4 and iron transporters in intestinal epithelial cells, directly impairing mineral absorption [113]. Beyond immediate direct malabsorption of nutrients, dysbiosis creates ripple effects through oxidative damage, inflammation, and even epigenetic changes that lead to long-term physiological issues. This is represented in Figure 2.
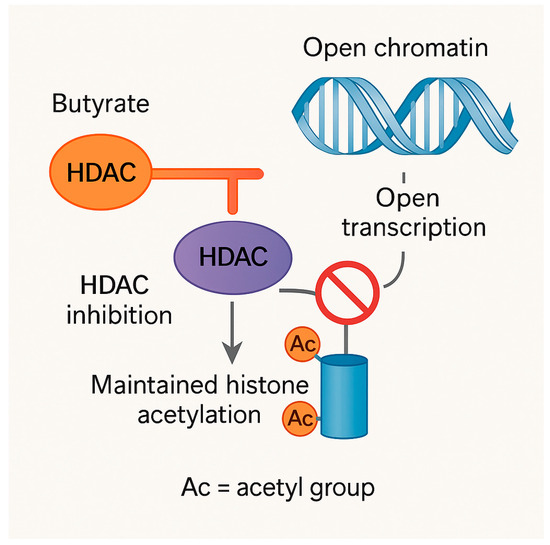
Figure 2.
Epigenetic regulation by butyrate through inhibition of histone deacetylases (HDACs). Dietary-derived butyrate acts as a histone deacetylase (HDAC) inhibitor in intestinal epithelial and host cells. HDACs normally remove acetyl groups (Ac) from histone tails, resulting in chromatin condensation and transcriptional repression. Butyrate binds to and inhibits HDAC activity (depicted by a blunt inhibitory line), preventing the enzyme from accessing acetylated histone tails. This inhibition prevents deacetylation, thereby maintaining histone hyperacetylation and promoting an open chromatin state that facilitates gene transcription. Ac = acetyl group. Orange line indicates HDAC inhibition by butyrate; grey arrows denote the directional flow of molecular processes.
4.3. Carbohydrate Metabolism
In general, Bacteroidetes employ polysaccharide utilization loci [PULs], as the example of the Sus system in Bacteroides thetaiotaomicron, to degrade resistant carbohydrates into oligosaccharides [19]. These are subsequently degraded by Firmicutes such as Eubacterium rectale and Roseburia into butyrate by the butyryl-CoA:acetate CoA-transferase pathway [64]. Dysbiosis breaks this metabolic chain: butyrate producers are lost, SCFA pools diminish, and colonocytes are starved for fuel. Communities shift to simple-sugar scavenging and proteolysis, increasing luminal pH and selecting against fiber degraders. This disruption not only eliminates host benefits mediated by SCFAs, epithelial energy, regulation of satiety hormones, gluconeogenic priming, and anti-inflammatory signaling but also destabilizes the microbial ecosystem itself [64,114,115].
4.4. Protein and Amino Acid Metabolism
Protein that is not digested proceeds normally under controlled fermentation, forming short indoles and aromatic metabolites with host-beneficial properties. For example, Clostridium sporogenes converts tryptophan into indole-3-propionic acid [IPA], an antioxidant, anti-inflammatory AhR ligand [67,87,88]. But in dysbiosis, the equilibrium is lost: Clostridioides difficile overexpresses p-hydroxyphenylacetate decarboxylase (HpdBCA), which converts tyrosine to p-cresol, a compound that represses rivals and damages epithelia [89]. Sulfide-producing bacteria such as Desulfovibrio and Bilophila wadsworthia overproduce hydrogen sulfide, which at excessive concentrations poisons mitochondria and destroys tight junctions [116]. The effect is an atmosphere replete with ammonia, branched-chain fatty acids, phenols, indoles, p-cresol, and H2S, which are pro-inflammatory, epitheliotoxic, and genotoxic. Uptake of amino acids is reduced, while diversion of tryptophan into kynurenine pathways depletes serotonin and indole reservoirs, linking dysbiosis with neuropsychiatric disease and intestinal inflammation [117]. At physiologic levels, however, microbially derived H2S acts as gasotransmitter supporting mucosal integrity and anti-inflammatory signaling; thus, the pathologic state in dysbiosis reflects its overproduction rather than its presence per se.
4.5. Lipid Metabolism and Bile Acid Remodeling
Bile acid metabolism is intricately linked to gut microbial composition. Species such as Lactobacillus, Bifidobacterium, and Enterococcus express bile salt hydrolases that deconjugate glycine- and taurine-conjugated bile salts [91], while Clostridium scindens carries out 7α-dehydroxylation via the bai operon, producing secondary bile acids that modulate FXR and TGR5 signaling [92]. Dysbiosis disrupts this balance: reduced bile salt hydrolase (BSH) activity destabilizes dietary fat micellar solubilization, and overabundant 7α-dehydroxylators and bile-tolerant taxa generate excessive deoxycholic and lithocholic acid, both epithelial stressors and carcinogens [72,92,118].
4.6. Folate and Cofactor Biosynthesis
The gut microbiome contributes importantly to vitamin supply, but this function degrades in dysbiosis. Folate and biotin are synthesized by Bifidobacterium species [73], riboflavin and niacin operons are contained in Lactobacillus [74], Escherichia coli supplies menaquinones (vitamin K2) [75], and Clostridium butyricum produces vitamin B12 de novo specifically [77]. Dysbiosis, causing direct host deficiencies, decreased vitamin exchange destabilizes microbial cross-feeding networks, locking into ecosystem collapse [54].
4.7. Mineral Handling and Nutritional Immunity
Functional communities facilitate mineral liberation and solubilization. Lactic acid bacteria and Bifidobacterium adolescentis secrete phytases that hydrolyze phytate, releasing bound iron, zinc, and calcium [95]. Acidification facilitated by short-chain fatty acids enhances solubility, and oxalate is degraded by Oxalobacter formigenes, keeping calcium out of precipitation [78]. Dysbiosis removes these facilitators, with minerals chelated and oxalate unmetabolized, while Proteobacteria flourish with siderophore mechanisms that competitively bind iron [24].
4.8. Micronutrient and Redox Pathways
Dysbiosis leads to a disruption in the nitrate–nitrite–nitric oxide (NO) pathway primarily through alterations in the microbial community composition. The loss or reduced activity of nitrate-reducing bacteria (NRB) within the oral cavity and gut such as Streptococcus, Veillonella, and Actinomycetota limits the microbial conversion of dietary nitrate (NO3−) to nitrite (NO2−). As a result, there is a diminished supply of nitrite available for subsequent reduction to bioactive nitric oxide. This decline in NO generation compromises essential physiological functions regulated by NO, including vascular tone, endothelial signaling, and metabolic homeostasis [119].
Similarly, dysbiosis interferes with selenium metabolism by decreasing selenium-reducing and methylating microbes such as Clostridium and Bacteroides. The subsequent decrease in microbial selenite reduction and selenoprotein synthesis allows for the accumulation of reactive selenium intermediates and diminution of antioxidant defense capacity, particularly by decreased activity of glutathione peroxidase and thioredoxin reductase [120]. Combined, these alterations diminish NO-mediated signaling and selenium-dependent antioxidant buffering, inclining the intestinal redox environment toward a pro-oxidative and inflammatory environment.
4.9. Consequences of Disease Resulting from Nutrient Malabsorption
The mechanistic derangements outlined in Section 4.3, Section 4.4, Section 4.5, Section 4.6, Section 4.7 and Section 4.8 collectively add up to a large range of systemic effects reaching outside the intestinal lumen. Deranged fermentation of dietary polysaccharides and butyrate-deficient short chain fatty acids (SCFAs) such as Faecalibacterium prausnitzii and Roseburia reduce the levels of butyrate, impairing the integrity of epithelia and triggering insulin resistance, obesity, and inflammatory bowel disease [9,14,113,121]. Overactive proteolytic fermentation cause DNA damage in epithelia, mitochondrial dysfunction, and colorectal carcinogenesis risk and neurobehavioral deficit linked to aberrant tryptophan metabolism [87,88,89,122]. Abnormal bile-salt-hydrolase (BSH) activity reduces micelle stability and lipid absorption, while secondary bile acids excess and trimethylamine-N-oxide (TMAO) formation cause mucosal injury and cardiometabolic disease [58,108,121]. Loss of vitamin-producing taxa such as Bifidobacterium and Lactobacillus undermines folate, riboflavin, biotin, cobalamin, and menaquinone synthesis that leads to hematologic, skeletal, and metabolic deficiencies [53,54,55]. Concurrent loss of phytase- and oxalate-degrading microbes reduces availability of iron, zinc, and calcium that leads to anemia, bone loss, and nephrolithiasis [20,24,78]. Finally, suppression of nitrate-reducing and selenium-consuming taxa diminishes nitric-oxide and antioxidant enzyme production, causing endothelial dysfunction, oxidative stress, and heightened cardiovascular and neurodegenerative disease risk [53]. Dysbiosis can therefore be viewed as a nutritional disease wherein deranged ecology is transformed into metabolic derangement and systemic disease. This is represented in Figure 3.
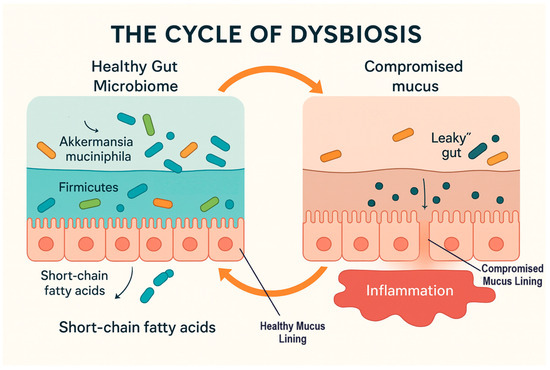
Figure 3.
The cycle of dysbiosis and its impact on gut barrier function. Orange arrows indicate the cyclical transition between healthy and dysbiotic gut states; grey arrows denote the directional flow of metabolic and barrier-related processes.
Cyclic representation of gut mucus dynamics and microbial balance during the transition between healthy and dysbiotic states. In the healthy gut, a thick and struc-tured mucus layer supports a balanced microbial community dominated by Akkerman-sia muciniphila, and Firmicutes, promoting short-chain fatty acid (SCFA) production and epithelial integrity. During dysbiosis, the mucus layer becomes thinned and discontin-uous, with increased gut permeability (“leaky gut”), and inflammation. Restoration of microbial diversity and mucus regeneration can reestablish barrier function, completing the cycle from dysbiosis back to a healthy state. To summarize these dysbiotic processes, the following table (Table 2) contrasts normal and disturbed microbial functions along axes of nutrients and associates each disturbance with nutritional endpoints and risks of disease.

Table 2.
Comparison of Nutrient Axes in Health vs. Dysbiosis and Associated Disease Outcomes.
These cascading disruptions illustrate how dysbiosis transforms a supportive ecosystem into a barrier to nutrient uptake. To truly grasp its impact, however, we need precise tools to measure both the quantitative shifts and qualitative functions of the gut microbiome.
5. Gut Microbiome Estimation: Quantification and Qualification Approaches
5.1. Estimation Concept in Gut Microbiome Research
Over the last two decades, new methods of “measuring” the human gut microbiome have revolutionized the field. Measurement of the microbiome is not simply counting microbes. It is a two-part process: first, how many microbes are there, and second, what kinds of microbes are there and what are they doing. Counting microbes tells us how many and naming them tells us who they are and what they are doing. Both are necessary to understand how microbes are involved in nutrient uptake. For instance, Clostridium scindens exemplifies why functional activity matters beyond abundance, it transforms primary into secondary bile acids, thereby influencing lipid absorption and host signaling. [56,72,91].
5.2. Measuring the Gut Microbiome
5.2.1. Relative Abundance by Sequencing
Traditional approaches, such as 16S rRNA gene amplicon sequencing and shotgun metagenomics, remain in widespread use for large-scale cohorts [123]. These technologies have provided insightful data on how the ratio of Firmicutes to Bacteroidetes varies in obesity or malnutrition, and how the loss of taxa, such as Faecalibacterium prausnitzii, predicts impaired butyrate production [64]. However, both approaches yield compositional data; a relative increase in Proteobacteria may be caused by a reduction in Firmicutes, without actual growth of Proteobacteria themselves. Sequencing bias and between-method variation are still limitations that researchers need to circumvent. Because these sequencing methods normalize read counts to total DNA, they reflect each taxon’s proportion rather than absolute cell numbers; a rise in one group necessarily lowers the relative share of others even without true growth.
5.2.2. Absolute Quantification
In order to circumvent this, methods enabling absolute quantitation are increasingly being targeted. Quantitative PCR (qPCR) of 16S rRNA genes or functional markers provides copy number estimates of specific organisms or pathways, e.g., tracking Oxalobacter formigenes abundance in calcium oxalate stone risk studies [78]. Flow cytometry combined with Fluorescence in situ hybridization (FISH) provides actual bacterial counts and can differentiate live from dead cells, an important distinction when linking microbiome biomass to metabolite production [124]. Even the less complex proxies, e.g., DNA yield/gram stool, are associated with overall biomass and have been correlated with caloric extraction efficiency in undernutrition [125]. Germ-free mouse experiments and pioneering microbiota transplant experiments also highlight how directly alterations in microbial abundance and composition affect nutrient absorption and host metabolism [9,126,127,128].
5.2.3. Advanced and Emerging Quantification Methods
Developments in the recent past are allowing quantitation to become more vigorous. Spike-in standards in sequence workflows enable conversion of relative read countsNovel strategies are making it easier to count microbes accurately. The inclusion of standard DNA at known amounts during sequencing enables the conversion of relative numbers to actual counts with fewer errors [129]. Digital droplet PCR (ddPCR) is a more sensitive and reproducible method of detecting rare but important microbes, e.g., O. formigenes or Desulfovibrio piger [130,131,132]. Such improvements pertain to the exploration of nutrient absorption because slight variation within rare microbes can have a profound influence on health. For example, F. prausnitzii strains vary in their ability to produce butyrate or anti-inflammatory metabolites [133]. Shotgun metagenomics and long-read sequencing now provide the resolution to distinguish between beneficial commensals and closely related pathobionts [134,135].
5.2.4. Functional Qualification
Predictive bioinformatics tools [PICRUSt2, HUMAnN, Tax4Fun] also infer the metabolic potential contained within metagenomic data [136,137]. However, the ultimate breakthrough is achieved with direct functional assays. Metatranscriptomics defines gene expression in vivo and thus whether polysaccharide utilization (PULs) or butyrate synthesis pathways are being actively transcribed in response to dietary fibers [138]. Metaproteomics identifies actual enzymes being present, i.e., butyryl-CoA:acetate CoA-transferase or BaiB (bile acid CoA ligase), thus establishing functional capacity at the protein level [139]. It is important to note that such predictions reflect the metabolic potential of the community based on gene content and may not equate to actual in situ gene expression or enzymatic activity.
5.2.5. Metabolic Qualification
Ultimately, the most direct evidence of function comes from metabolites. Metabolomic analyses employing GC–MS, LC–MS, and NMR provide comprehensive profiles of SCFAs, secondary bile acids, indoles, and vitamins, directly linking microbial activity to nutrient metabolism [140]. Stable isotope tracing [fluxomics] adds a causal element: by feeding 13C-labeled starch or 15N-labeled proteins, one can trace carbon and nitrogen flux into microbial fermentation end-products and track how this feed into host metabolic pools [141,142]. This has been particularly powerful in demonstrating how resistant starch supplementation alters butyrate flux, or how tryptophan catabolism partitions between host serotonin synthesis and microbial indole pathways.
5.3. Integrating Quantification and Qualification
The biggest challenge ahead for microbiome science is the integration of various types of data. By combining information on the microbes that are present, the genes that they are expressing, the proteins that they are producing, and the chemicals that they are creating, scientists can see how alterations in the microbiome lead to changes in health. It is a means of moving from pattern watching to actually viewing how things function.
Artificial intelligence (AI) is now being used to integrate heterogeneous microbiome data, enabling the creation of personalized models that predict how an individual’s microbiome will respond to different diets [143]. As these instruments become more refined, doctors will not only be able to diagnose gut-related disorders but also predict nutrient deficiencies and prescribe targeted therapies with greater accuracy. Moreover, integrating interactomics approaches which map protein–protein, metabolite microbe, and host-microbiota interaction networks—can enhance AI-driven predictions by revealing mechanistic links between microbial activity and host physiology [144].
The next table [Table 3] outlines major methods of microbiome estimation, the resolution afforded, their applications to nutrient metabolism, and their main limitations.

Table 3.
Overview of Quantitative and Qualitative Approaches in Gut Microbiome Research.
6. Future Scope and Market Value
The future of gut-restoring therapeutics is moving towards personalized, precision-based therapeutics, including engineered probiotics, synbiotics, and postbiotics that are designed to restore or augment individual microbial activities. Clinicians will increasingly depend on AI-powered platforms not only for the diagnosis of dysbiosis but also for the prediction of nutritional deficiencies and designing interventions based on each person’s microbiome.
From a market perspective, the potential is gigantic. The global market for microbiome therapeutics is expected to increase from $164.8 million in 2022 to $1.5 billion by 2027, at a compound annual growth rate (CAGR) of 54.8% from 2022 to 2027 [146]. Concurrently, the global nutritional supplements market size was USD 485.62 billion in 2024 and is projected to reach USD 704.28 billion by 2030, at a CAGR of 6.42% from 2025 to 2030 [147]. The combination of breakthrough microbiome science and supplement boom puts gut health in one of the most promising and fastest-growing segments in healthcare. With increasing interest in digestive health, increased prevalence of lifestyle-driven gut disease, and continued investment in microbiome science, gut-restoring therapies are likely to become a cornerstone of precision nutrition and preventive medicine.
7. Conclusions
The human gut microbiome is not a passive bystander but an active architect of nutritional health. Its intricate web of metabolic interactions determines whether the nutrients we consume are released, transformed, and absorbed or diverted into harmful byproducts that fuel disease. Dysbiosis is a breakdown in this symbiosis, converting the gut from a gateway of nourishment to a gatekeeper of malabsorption, systemic inflammation, and chronic disease.
By integrating evidence from across microbial taxa, nutrient pathways, and disease outcomes, this paper shows how ecological disbalances manifest as nutritional deficiencies and metabolic disorders. The intersection of mechanistic insights with new estimation tools highlights a key reality: gut health must be measured not only by diversity counts but by functional capacity and metabolic output. The microbiome science with personalized nutrition and therapeutics is a revolutionary opportunity. Engineered probiotics, precision prebiotics, and AI-targeted dietary interventions can potentially reclaim lost microbial activities and tailor nutrient supplementation to individual needs. In doing so, the therapeutic approach to dysbiosis is no longer one of simple microbial imbalance correction—it is one of transforming nutrition itself into a dialogue between host and microbiome, one that is potentially a key to reducing global malnutrition, preventing chronic disease, and advancing the frontier of precision medicine.
Author Contributions
Conceptualization, A.H.S. and A.R.; investigation, A.H.S. and A.R.; writing—original draft preparation, A.H.S.; writing—review and editing, A.R.; visualization, A.H.S.; supervision, A.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
Author Anna H. Sandhu was employed by the company Research and Development, SFG Biome, Inc.; Author Arun Radhakrishnan was employed by the company Research and Development, Sandhus Products, Inc. Furthermore, authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- World Health Organization. Malnutrition. Available online: https://www.who.int/news-room/fact-sheets/detail/malnutrition (accessed on 24 September 2025).
- Phelps, N.H.; Singleton, R.K.; Zhou, B.; Heap, R.A.; Mishra, A.; Bennett, J.E.; Paciorek, C.J.; Lhoste, V.P.; Carrillo-Larco, R.M.; Stevens, G.A.; et al. Worldwide trends in underweight and obesity from 1990 to 2022: A pooled analysis of 3663 population-representative studies with 222 million children, adolescents, and adults. Lancet 2024, 403, 1027–1050. [Google Scholar] [CrossRef]
- Wostmann, B.S. Germfree and Gnotobiotic Animal Models: Background and Applications; CRC Press: Boca Raton, FL, USA, 1996. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Hamady, M.; Fraser-Liggett, C.M.; Knight, R.; Gordon, J.I. The human microbiome project. Nature 2007, 449, 804–810. [Google Scholar] [CrossRef]
- De Filippo, C.; Cavalieri, D.; Di Paola, M.; Ramazzotti, M.; Poullet, J.B.; Massart, S.; Collini, S.; Pieraccini, G.; Lionetti, P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc. Natl. Acad. Sci. USA 2010, 107, 14691–14696. [Google Scholar] [CrossRef]
- Subramanian, S.; Huq, S.; Yatsunenko, T.; Haque, R.; Mahfuz, M.; Alam, M.A.; Benezra, A.; DeStefano, J.; Meier, M.F.; Muegge, B.D.; et al. Persistent gut microbiota immaturity in malnourished Bangladeshi children. Nature 2014, 510, 417–421. [Google Scholar] [CrossRef]
- Blanton, L.V.; Charbonneau, M.R.; Salih, T.; Barratt, M.J.; Venkatesh, S.; Ilkaveya, O.; Subramanian, S.; Manary, M.J.; Trehan, I.; Jorgensen, J.M.; et al. Gut bacteria that prevent growth impairments transmitted by microbiota from malnourished children. Science 2016, 351, aad3311. [Google Scholar] [CrossRef]
- Kau, A.L.; Ahern, P.P.; Griffin, N.W.; Goodman, A.L.; Gordon, J.I. Human nutrition, the gut microbiome and the immune system. Nature 2011, 474, 327–336. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef]
- Cryan, J.F.; Dinan, T.G. Mind-altering microorganisms: The impact of the gut microbiota on brain and behaviour. Nat. Rev. Neurosci. 2012, 13, 701–712. [Google Scholar] [CrossRef]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef]
- Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature 2012, 486, 207–214. [Google Scholar] [CrossRef]
- Eckburg, P.B.; Bik, E.M.; Bernstein, C.N.; Purdom, E.; Dethlefsen, L.; Sargent, M.; Gill, S.R.; Nelson, K.E.; Relman, D.A. Diversity of the human intestinal microbial flora. Science 2005, 308, 1635–1638. [Google Scholar] [CrossRef]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Microbial ecology: Human gut microbes associated with obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef]
- Zhu, L.B.; Zhang, Y.C.; Huang, H.H.; Lin, J. Prospects for clinical applications of butyrate-producing bacteria. World J. Clin. Pediatr. 2021, 10, 84–92. [Google Scholar] [CrossRef]
- Singh, V.; Lee, G.; Son, H.; Koh, H.; Kim, E.S.; Unno, T.; Shin, J.H. Butyrate producers, “The Sentinel of Gut”: Their intestinal significance with and beyond butyrate, and prospective use as microbial therapeutics. Front. Microbiol. 2023, 13, 1103836. [Google Scholar] [CrossRef]
- Sokol, H.; Pigneur, B.; Watterlot, L.; Lakhdari, O.; Bermúdez-Humarán, L.G.; Gratadoux, J.J.; Blugeon, S.; Bridonneau, C.; Furet, J.P.; Corthier, G.; et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc. Natl. Acad. Sci. USA 2008, 105, 16731–16736. [Google Scholar] [CrossRef]
- Wu, G.D.; Chen, J.; Hoffmann, C.; Bittinger, K.; Chen, Y.Y.; Keilbaugh, S.A.; Bewtra, M.; Knights, D.; Walters, W.A.; Knight, R.; et al. Linking long-term dietary patterns with gut microbial enterotypes. Science 2011, 334, 105–108. [Google Scholar] [CrossRef]
- Martens, E.C.; Koropatkin, N.M.; Smith, T.J.; Gordon, J.I. Complex glycan catabolism by the human gut microbiota: The Bacteroidetes Sus-like paradigm. J. Biol. Chem. 2009, 284, 24673–24677. [Google Scholar] [CrossRef]
- Lee, J.H.; O’Sullivan, D.J. Genomic insights into bifidobacteria. Microbiol. Mol. Biol. Rev. 2010, 74, 378–416. [Google Scholar] [CrossRef]
- Turroni, F.; Milani, C.; Duranti, S.; Mahony, J.; van Sinderen, D.; Ventura, M. Glycan utilization and cross-feeding activities by bifidobacteria. Trends Microbiol. 2018, 26, 339–350. [Google Scholar] [CrossRef]
- Pokusaeva, K.; Fitzgerald, G.F.; van Sinderen, D. Carbohydrate metabolism in bifidobacteria. Genes Nutr. 2011, 6, 285–306. [Google Scholar] [CrossRef]
- Moreira de Gouveia, M.I.; Bernalier-Donadille, A.; Jubelin, G. Enterobacteriaceae in the human gut: Dynamics and ecological roles in health and disease. Biology 2024, 13, 142. [Google Scholar] [CrossRef]
- Song, Y.; Wu, X.; Li, Z.; Ma, Q.Q.; Bao, R. Molecular mechanism of siderophore regulation by the Pseudomonas aeruginosa BfmRS two-component system in response to osmotic stress. Commun. Biol. 2024, 7, 295. [Google Scholar] [CrossRef]
- Shin, N.R.; Whon, T.W.; Bae, J.W. Proteobacteria: Microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015, 33, 496–503. [Google Scholar] [CrossRef]
- Derrien, M.; Collado, M.C.; Ben-Amor, K.; Salminen, S.; de Vos, W.M. The mucin degrader Akkermansia muciniphila is an abundant resident of the human intestinal tract. Appl. Environ. Microbiol. 2008, 74, 1646–1648. [Google Scholar] [CrossRef]
- Kostic, A.D.; Gevers, D.; Pedamallu, C.S.; Michaud, M.; Duke, F.; Earl, A.M.; Ojesina, A.I.; Jung, J.; Bass, A.J.; Tabernero, J.; et al. Genomic analysis identifies association of Fusobacterium with colorectal carcinoma. Genome Res. 2012, 22, 292–298. [Google Scholar] [CrossRef]
- Rubinstein, M.R.; Wang, X.; Liu, W.; Hao, Y.; Cai, G.; Han, Y.W. Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-cadherin/β-catenin signaling via its FadA adhesin. Cell Host Microbe 2013, 14, 195–206. [Google Scholar] [CrossRef]
- Jumas-Bilak, E.; Marchandin, H. The phylum Synergistetes. In The Prokaryotes; Rosenberg, E., DeLong, E.F., Lory, S., Stackebrandt, E., Thompson, F., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 931–954. [Google Scholar] [CrossRef]
- Obregon-Tito, A.J.; Tito, R.Y.; Metcalf, J.; Sankaranarayanan, K.; Clemente, J.C.; Ursell, L.K.; Xu, Z.Z.; Van Treuren, W.; Knight, R.; Gaffney, P.M.; et al. Subsistence strategies in traditional societies distinguish gut microbiomes. Nat. Commun. 2015, 6, 6505. [Google Scholar] [CrossRef]
- Schnorr, S.L.; Candela, M.; Rampelli, S.; Centanni, M.; Consolandi, C.; Basaglia, G.; Turroni, S.; Biagi, E.; Peano, C.; Severgnini, M.; et al. Gut microbiome of the Hadza hunter-gatherers. Nat. Commun. 2014, 5, 3654. [Google Scholar] [CrossRef]
- Wu, S.; Sun, C.; Li, Y.; Wang, T.; Jia, L.; Lai, S.; Yang, Y.; Luo, P.; Dai, D.; Yang, Y.Q.; et al. GMrepo: A database of curated and consistently annotated human gut metagenomes. Nucleic Acids Res. 2020, 48, D545–D553. [Google Scholar] [CrossRef]
- Bor, B.; Bedree, J.K.; Shi, W.; McLean, J.S.; He, X. Saccharibacteria (TM7) in the human oral microbiome. J. Dent. Res. 2019, 98, 500–509. [Google Scholar] [CrossRef]
- Hu, C.; Rzymski, P. Non-photosynthetic Melainabacteria (Cyanobacteria) in human gut: Characteristics and association with health. Life 2022, 12, 476. [Google Scholar] [CrossRef]
- Hansen, E.E.; Lozupone, C.A.; Rey, F.E.; Wu, M.; Guruge, J.L.; Narra, A.; Goodfellow, J.; Zaneveld, J.R.; McDonald, D.T.; Goodrich, J.A.; et al. Pan-genome of the dominant human gut-associated archaeon, Methanobrevibacter smithii, studied in twins. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. S1), 4599–4606. [Google Scholar] [CrossRef]
- Samuel, B.S.; Gordon, J.I. A humanized gnotobiotic mouse model of host-archaeal-bacterial mutualism. Proc. Natl. Acad. Sci. USA 2006, 103, 10011–10016. [Google Scholar] [CrossRef]
- Dridi, B.; Henry, M.; El Khéchine, A.; Raoult, D.; Drancourt, M. High prevalence of Methanobrevibacter smithii and Methanosphaera stadtmanae detected in the human gut using an improved DNA detection protocol. PLoS ONE 2009, 4, e7063. [Google Scholar] [CrossRef]
- Shkoporov, A.N.; Hill, C. Bacteriophages of the human gut: The “known unknown” of the microbiome. Cell Host Microbe 2019, 25, 195–209. [Google Scholar] [CrossRef]
- Mirzaei, M.K.; Maurice, C.F. Ménage à trois in the human gut: Interactions between host, bacteria and phages. Nat. Rev. Microbiol. 2017, 15, 397–408. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Valera, F.; Martin-Cuadrado, A.B.; Rodriguez-Brito, B.; Pasić, L.; Thingstad, T.F.; Rohwer, F.; Mira, A. Explaining microbial population genomics through phage predation. Nat. Rev. Microbiol. 2009, 7, 828–836. [Google Scholar] [CrossRef]
- Hallen-Adams, H.E.; Suhr, M.J. Fungi in the healthy human gastrointestinal tract. Virulence 2017, 8, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Underhill, D.M.; Iliev, I.D. The mycobiota: Interactions between commensal fungi and the host immune system. Nat. Rev. Immunol. 2014, 14, 405–416. [Google Scholar] [CrossRef] [PubMed]
- Matovelle, C.; Tejedor, M.T.; Monteagudo, L.V.; Beltrán, A.; Quílez, J. Prevalence and associated factors of Blastocystis sp. infection in patients with gastrointestinal symptoms in Spain: A case-control study. Trop. Med. Infect. Dis. 2022, 7, 226. [Google Scholar] [CrossRef]
- Deng, L.; Tan, K.S.W. From parasite to partner: Unravelling the multifaceted role of Blastocystis in human health and disease. Lancet Microbe 2025, 6, 101155. [Google Scholar] [CrossRef]
- Donaldson, G.P.; Lee, S.M.; Mazmanian, S.K. Gut biogeography of the bacterial microbiota. Nat. Rev. Microbiol. 2016, 14, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Savage, D.C. Microbial ecology of the gastrointestinal tract. Annu. Rev. Microbiol. 1977, 31, 107–133. [Google Scholar] [CrossRef]
- Jensen, B.A.H.; Heyndrickx, M.; Jonkers, D.; Mackie, A.; Millet, S.; Naghibi, M.; Pærregaard, S.I.; Pot, B.; Saulnier, D.; Sina, C.; et al. Small intestine vs. colon ecology and physiology: Why it matters in probiotic administration. Cell Rep. Med. 2023, 4, 101190. [Google Scholar] [CrossRef]
- Rowland, I.; Gibson, G.; Heinken, A.; Scott, K.; Swann, J.; Thiele, I.; Tuohy, K. Gut microbiota functions: Metabolism of nutrients and other food components. Eur. J. Nutr. 2018, 57, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Azzouz, L.L.; Sharma, S. Physiology, large intestine. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK507857/ (accessed on 24 September 2025).
- Derrien, M.; Vaughan, E.E.; Plugge, C.M.; de Vos, W.M. Akkermansia muciniphila gen. nov., sp. nov., a human intestinal mucin-degrading bacterium. Int. J. Syst. Evol. Microbiol. 2004, 54, 1469–1476. [Google Scholar] [CrossRef]
- Arpaia, N.; Campbell, C.; Fan, X.; Dikiy, S.; van der Veeken, J.; deRoos, P.; Liu, H.; Cross, J.R.; Pfeffer, K.; Coffer, P.J.; et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 2013, 504, 451–455. [Google Scholar] [CrossRef]
- Furusawa, Y.; Obata, Y.; Fukuda, S.; Endo, T.A.; Nakato, G.; Takahashi, D.; Nakanishi, Y.; Uetake, C.; Kato, K.; Kato, T.; et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 2013, 504, 446–450. [Google Scholar] [CrossRef]
- LeBlanc, J.G.; Milani, C.; de Giori, G.S.; Sesma, F.; van Sinderen, D.; Ventura, M. Bacteria as vitamin suppliers to their host: A gut microbiota perspective. Curr. Opin. Biotechnol. 2013, 24, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Magnúsdóttir, S.; Ravcheev, D.; de Crécy-Lagard, V.; Thiele, I. Systematic genome assessment of B-vitamin biosynthesis suggests co-operation among gut microbes. Front. Genet. 2015, 6, 148. [Google Scholar] [CrossRef]
- Barone, M.; D’Amico, F.; Brigidi, P.; Turroni, S. Gut microbiome–micronutrient interaction: The key to controlling the bioavailability of minerals and vitamins? BioFactors 2022, 48, 307–314. [Google Scholar] [CrossRef]
- Ridlon, J.M.; Kang, D.J.; Hylemon, P.B. Bile salt biotransformations by human intestinal bacteria. J. Lipid Res. 2006, 47, 241–259. [Google Scholar] [CrossRef]
- Wahlström, A.; Sayin, S.I.; Marschall, H.U.; Bäckhed, F. Intestinal crosstalk between bile acids and microbiota and its impact on host metabolism. Cell Metab. 2016, 24, 41–50. [Google Scholar] [CrossRef]
- Haghikia, A.; Li, X.S.; Liman, T.G.; Bledau, N.; Schmidt, D.; Zimmermann, F.; Kränkel, N.; Widera, C.; Sonnenschein, K.; Haghikia, A.; et al. Gut microbiota-dependent trimethylamine N-oxide predicts risk of cardiovascular events in patients with stroke and is related to proinflammatory monocytes. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 2225–2235. [Google Scholar] [CrossRef]
- Wang, Z.; Klipfell, E.; Bennett, B.J.; Koeth, R.; Levison, B.S.; Dugar, B.; Feldstein, A.E.; Britt, E.B.; Fu, X.; Chung, Y.M.; et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011, 472, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Clarke, J.K. On the bacterial factor in the ætiology of dental caries. Br. J. Exp. Pathol. 1924, 5, 141–147. [Google Scholar]
- Scardovi, V.; Crociani, F. Bifidobacterium catenulatum, Bifidobacterium dentium, and Bifidobacterium angulatum: Three new species and their deoxyribonucleic acid homology relationships. Int. J. Syst. Evol. Microbiol. 1974, 24, 6–20. [Google Scholar]
- Xu, J.; Bjursell, M.K.; Himrod, J.; Deng, S.; Carmichael, L.K.; Chiang, H.C.; Hooper, L.V.; Gordon, J.I. A genomic view of the human–Bacteroides thetaiotaomicron symbiosis. Science 2003, 299, 2074–2076. [Google Scholar] [CrossRef] [PubMed]
- Rosero, J.A.; Killer, J.; Sechovcová, H.; Mrázek, J.; Benada, O.; Fliegerová, K.; Havlík, J.; Kopečný, J. Reclassification of Eubacterium rectale (Hauduroy et al. 1937) Prévot 1938 in a new genus Agathobacter gen. nov. as Agathobacter rectalis comb. nov., and description of Agathobacter ruminis sp. nov., isolated from the rumen contents of sheep and cows. Int. J. Syst. Evol. Microbiol. 2016, 66, 768–773. [Google Scholar] [CrossRef]
- Louis, P.; Flint, H.J. Diversity, metabolism and microbial ecology of butyrate-producing bacteria from the human large intestine. FEMS Microbiol. Lett. 2009, 294, 1–8. [Google Scholar] [CrossRef]
- Miller, T.L.; Wolin, M.J.; Conway de Macario, E.; Macario, A.J. Isolation of Methanobrevibacter smithii from human feces. Appl. Environ. Microbiol. 1982, 43, 227–232. [Google Scholar] [CrossRef]
- Metchnikoff, E. Étude sur la flore intestinale. IV. Le Bacillus sporogenes. Ann. Inst. Pasteur 1908, 22, 942–946. [Google Scholar]
- Tian, X.; Wu, Y.; Duan, C.; Zhou, X.; Li, Y.; Zheng, J.; Lai, W.; Zhang, S.; Cao, L.; Zhong, S. Tryptophan was metabolized into beneficial metabolites against coronary heart disease or prevented from producing harmful metabolites by the in vitro drug screening model based on Clostridium sporogenes. Front. Microbiol. 2022, 13, 1013973. [Google Scholar] [CrossRef]
- Hall, I.C.; O’Toole, E. Intestinal flora in new-born infants with a description of a new pathogenic anaerobe, Bacillus difficilis. Am. J. Dis. Child. 1935, 49, 390–402. [Google Scholar] [CrossRef]
- Harrison, M.A.; Kaur, H.; Wren, B.W.; Dawson, L.F. Production of p-cresol by decarboxylation of p-HPA by all five lineages of Clostridioides difficile provides a growth advantage. Front. Cell. Infect. Microbiol. 2021, 11, 757599. [Google Scholar] [CrossRef]
- Tao, X.; Li, Y.; Huang, H.; Chen, Y.; Liu, P.; Li, X. Desulfovibrio vulgaris Hildenborough prefers lactate over hydrogen as electron donor. Ann. Microbiol. 2014, 64, 451–457. [Google Scholar] [CrossRef]
- Baron, E.J.; Summanen, P.; Downes, J.; Roberts, M.C.; Wexler, H.; Finegold, S.M. Bilophila wadsworthia, gen. nov. and sp. nov., a unique gram-negative anaerobic rod recovered from appendicitis specimens and human faeces. J. Gen. Microbiol. 1989, 135, 3405–3411. [Google Scholar] [CrossRef] [PubMed]
- Daniel, S.L.; Ridlon, J.M. Clostridium scindens: History and current outlook for a keystone species in the mammalian gut involved in bile acid and steroid metabolism. FEMS Microbiol. Rev. 2025, 49, fuaf016. [Google Scholar] [CrossRef] [PubMed]
- Darby, T.M.; Jones, R.M. Beneficial influences of Lactobacillus plantarum on human health and disease. In The Microbiota in Gastrointestinal Pathophysiology; Academic Press: Cambridge, MA, USA, 2017; pp. 109–117. [Google Scholar]
- Lim, J.Y.; Yoon, J.; Hovde, C.J. A brief overview of Escherichia coli O157:H7 and its plasmid O157. J. Microbiol. Biotechnol. 2010, 20, 5–14. [Google Scholar] [CrossRef]
- Meganathan, R. Biosynthesis of menaquinone (vitamin K2) and ubiquinone (coenzyme Q): A perspective on enzymatic mechanisms. Vitam. Horm. 2001, 61, 173–218. [Google Scholar] [CrossRef]
- Yang, Y.M.; Zhang, M.Y.; Wu, Y.Y.; Zhang, L.; Zhang, Y.X. Survival and morphological changes of Clostridium butyricum spores co-exposed to antibiotics and simulated gastrointestinal fluids: Implications for antibiotic stewardship. Microorganisms 2025, 13, 1347. [Google Scholar] [CrossRef] [PubMed]
- Walls, W.G.; Moody, J.D.; McDaniel, E.C.; Villanueva, M.; Shepard, E.M.; Broderick, W.E.; Broderick, J.B. The B12-independent glycerol dehydratase activating enzyme from Clostridium butyricum cleaves SAM to produce 5′-deoxyadenosine and not 5′-deoxy-5′-(methylthio)adenosine. J. Inorg. Biochem. 2022, 227, 111662. [Google Scholar] [CrossRef]
- Chmiel, J.A.; Carr, C.; Stuivenberg, G.A.; Venema, R.; Chanyi, R.M.; Al, K.F.; Giguere, D.; Say, H.; Akouris, P.P.; Domínguez Romero, S.A.; et al. New perspectives on an old grouping: The genomic and phenotypic variability of Oxalobacter formigenes and the implications for calcium oxalate stone prevention. Front. Microbiol. 2022, 13, 1011102. [Google Scholar] [CrossRef]
- Rogosa, M. The genus Veillonella I: General cultural, ecological, and biochemical considerations. J. Bacteriol. 1964, 87, 162–170. [Google Scholar] [CrossRef]
- Dobranowski, P.A.; Stintzi, A. Resistant starch, microbiome, and precision modulation. Gut Microbes 2021, 13, 1926842. [Google Scholar] [CrossRef]
- Moffett, J.R.; Puthillathu, N.; Vengilote, R.; Jaworski, D.M.; Namboodiri, A.M. Acetate revisited: A key biomolecule at the nexus of metabolism, epigenetics and oncogenesis—Part 1: Acetyl-CoA, acetogenesis and acyl-CoA short-chain synthetases. Front. Physiol. 2020, 11, 580167. [Google Scholar] [CrossRef]
- De Vadder, F.; Kovatcheva-Datchary, P.; Goncalves, D.; Vinera, J.; Zitoun, C.; Duchampt, A.; Bäckhed, F.; Mithieux, G. Microbiota-generated metabolites promote metabolic benefits via gut–brain neural circuits. Cell 2014, 156, 84–96. [Google Scholar] [CrossRef]
- Bartlett, A.; Kleiner, M. Dietary protein and the intestinal microbiota: An understudied relationship. iScience 2022, 25, 105313. [Google Scholar] [CrossRef]
- Kushkevych, I.; Dordević, D.; Vítězová, M. Possible synergy effect of hydrogen sulfide and acetate produced by sulfate-reducing bacteria on inflammatory bowel disease development. J. Adv. Res. 2020, 27, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, F.; Thiele, I. Microbial metabolism marvels: A comprehensive review of microbial drug transformation capabilities. Gut Microbes 2024, 16, 2387400. [Google Scholar] [CrossRef] [PubMed]
- Dodd, D.; Spitzer, M.H.; Van Treuren, W.; Merrill, B.D.; Hryckowian, A.J.; Higginbottom, S.K.; Le, A.; Cowan, T.M.; Nolan, G.P.; Fischbach, M.A.; et al. A gut bacterial pathway metabolizes aromatic amino acids into nine circulating metabolites. Nature 2017, 551, 648–652. [Google Scholar] [CrossRef] [PubMed]
- Agus, A.; Planchais, J.; Sokol, H. Gut microbiota regulation of tryptophan metabolism in health and disease. Cell Host Microbe 2018, 23, 716–724. [Google Scholar] [CrossRef]
- Andrei, P.I.; Pierik, A.J.; Zauner, S.; Andrei-Selmer, L.C.; Selmer, T. Subunit composition of the glycyl radical enzyme p-hydroxyphenylacetate decarboxylase: A small subunit, HpdC, is essential for catalytic activity. Eur. J. Biochem. 2004, 271, 2225–2230. [Google Scholar] [CrossRef]
- Peck, S.C.; Denger, K.; Burrichter, A.; Irwin, S.M.; Balskus, E.P.; Schleheck, D. A glycyl radical enzyme enables hydrogen sulfide production by the human intestinal bacterium Bilophila wadsworthia. Proc. Natl. Acad. Sci. USA 2019, 116, 3171–3176. [Google Scholar] [CrossRef]
- Begley, M.; Hill, C.; Gahan, C.G. Bile salt hydrolase activity in probiotics. Appl. Environ. Microbiol. 2006, 72, 1729–1738. [Google Scholar] [CrossRef]
- Olivos-Caicedo, K.Y.; Fernandez-Materan, F.V.; Daniel, S.L.; Anantharaman, K.; Ridlon, J.M.; Alves, J.M.P. Pangenome analysis of Clostridium scindens: A collection of diverse bile acid- and steroid-metabolizing commensal gut bacterial strains. Microorgani sms 2025, 13, 857. [Google Scholar] [CrossRef]
- Gao, Y.; Lin, J.; Ye, C.; Guo, S.; Jiang, C. Microbial transformations of bile acids and their receptors in the regulation of metabolic dysfunction-associated steatotic liver disease. Liver Res. 2023, 7, 165–176. [Google Scholar] [CrossRef]
- Plovier, H.; Everard, A.; Druart, C.; Depommier, C.; Van Hul, M.; Geurts, L.; Chilloux, J.; Ottman, N.; Duparc, T.; Lichtenstein, L.; et al. A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat. Med. 2017, 23, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Pompei, A.; Cordisco, L.; Amaretti, A.; Zanoni, S.; Matteuzzi, D.; Rossi, M. Folate production by bifidobacteria as a potential probiotic property. Appl. Environ. Microbiol. 2007, 73, 179–185. [Google Scholar] [CrossRef]
- Lopez, H.W.; Leenhardt, F.; Coudray, C.; Remesy, C. Minerals and phytic acid interactions: Is it a real problem for human nutrition? Int. J. Food Sci. Technol. 2002, 37, 727–739. [Google Scholar] [CrossRef]
- Koch, C.D.; Gladwin, M.T.; Freeman, B.A.; Lundberg, J.O.; Weitzberg, E.; Morris, A. Enterosalivary nitrate metabolism and the microbiome: Intersection of microbial metabolism, nitric oxide and diet in cardiac and pulmonary vascular health. Free Radic. Biol. Med. 2017, 105, 48–67. [Google Scholar] [CrossRef] [PubMed]
- Forceville, X. Seleno-enzymes and seleno-compounds: The two faces of selenium. Crit. Care 2006, 10, 180. [Google Scholar] [CrossRef] [PubMed][Green Version]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tong, J.; Chang, B.; Wang, B.; Zhang, D.; Wang, B. Effects of alcohol on intestinal epithelial barrier permeability and expression of tight junction-associated proteins. Mol. Med. Rep. 2014, 9, 2352–2356. [Google Scholar] [CrossRef]
- Chassaing, B.; Koren, O.; Goodrich, J.K.; Poole, A.C.; Srinivasan, S.; Ley, R.E.; Gewirtz, A.T. Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature 2015, 519, 92–96. [Google Scholar] [CrossRef]
- Suez, J.; Korem, T.; Zeevi, D.; Zilberman-Schapira, G.; Thaiss, C.A.; Maza, O.; Israeli, D.; Zmora, N.; Gilad, S.; Weinberger, A.; et al. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature 2014, 514, 181–186. [Google Scholar] [CrossRef]
- Dethlefsen, L.; Huse, S.; Sogin, M.L.; Relman, D.A. The pervasive effects of an antibiotic on the human gut microbiota, as revealed by deep 16S rRNA sequencing. PLoS Biol. 2008, 6, e280. [Google Scholar] [CrossRef]
- Jackson, M.A.; Goodrich, J.K.; Maxan, M.E.; Freedberg, D.E.; Abrams, J.A.; Poole, A.C.; Sutter, J.L.; Welter, D.; Ley, R.E.; Bell, J.T.; et al. Proton pump inhibitors alter the composition of the gut microbiota. Gut 2016, 65, 749–756. [Google Scholar] [CrossRef]
- Dominguez-Bello, M.G.; Costello, E.K.; Contreras, M.; Magris, M.; Hidalgo, G.; Fierer, N.; Knight, R. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc. Natl. Acad. Sci. USA 2010, 107, 11971–11975. [Google Scholar] [CrossRef]
- Azad, M.B.; Konya, T.; Maughan, H.; Guttman, D.S.; Field, C.J.; Sears, M.R.; Becker, A.B.; Scott, J.A.; Kozyrskyj, A.L. Infant gut microbiota and the hygiene hypothesis of allergic disease: Impact of household pets and siblings on microbiota composition and diversity. Allergy Asthma Clin. Immunol. 2013, 9, 15. [Google Scholar] [CrossRef]
- Arrieta, M.C.; Stiemsma, L.T.; Amenyogbe, N.; Brown, E.M.; Finlay, B. The intestinal microbiome in early life: Health and disease. Front. Immunol. 2014, 5, 427. [Google Scholar] [CrossRef]
- O’Toole, P.W.; Jeffery, I.B. Gut microbiota and aging. Science 2015, 350, 1214–1215. [Google Scholar] [CrossRef]
- Leung, C.; Rivera, L.; Furness, J.B.; Angus, P.W. The role of the gut microbiota in NAFLD. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 412–425. [Google Scholar] [CrossRef]
- Goodrich, J.K.; Waters, J.L.; Poole, A.C.; Sutter, J.L.; Koren, O.; Blekhman, R.; Beaumont, M.; Van Treuren, W.; Knight, R.; Bell, J.T.; et al. Human genetics shape the gut microbiome. Cell 2014, 159, 789–799. [Google Scholar] [CrossRef]
- Moura, F.A.; de Andrade, K.Q.; Dos Santos, J.C.F.; Araújo, O.R.P.; Goulart, M.O.F. Antioxidant therapy for treatment of inflammatory bowel disease: Does it work? Redox Biol. 2015, 6, 617–639. [Google Scholar] [CrossRef]
- Maslowski, K.M.; Vieira, A.T.; Ng, A.; Kranich, J.; Sierro, F.; Yu, D.; Schilter, H.C.; Rolph, M.S.; Mackay, F.; Artis, D.; et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 2009, 461, 1282–1286. [Google Scholar] [CrossRef]
- Everard, A.; Belzer, C.; Geurts, L.; Ouwerkerk, J.P.; Druart, C.; Bindels, L.B.; Guiot, Y.; Derrien, M.; Muccioli, G.G.; Delzenne, N.M.; et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl. Acad. Sci. USA 2013, 110, 9066–9071. [Google Scholar] [CrossRef]
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Poggi, M.; Knauf, C.; Bastelica, D.; Neyrinck, A.M.; Fava, F.; Tuohy, K.M.; Chabo, C.; et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007, 56, 1761–1772. [Google Scholar] [CrossRef] [PubMed]
- Machiels, K.; Joossens, M.; Sabino, J.; De Preter, V.; Arijs, I.; Eeckhaut, V.; Ballet, V.; Claes, K.; Van Immerseel, F.; Verbeke, K.; et al. A decrease of the butyrate-producing species Roseburia hominis and Faecalibacterium prausnitzii defines dysbiosis in patients with ulcerative colitis. Gut 2014, 63, 1275–1283. [Google Scholar] [CrossRef] [PubMed]
- Vandeputte, D.; Kathagen, G.; D’hoe, K.; Vieira-Silva, S.; Valles-Colomer, M.; Sabino, J.; Wang, J.; Tito, R.Y.; De Commer, L.; Darzi, Y.; et al. Quantitative microbiome profiling links gut community variation to microbial load. Nature 2017, 551, 507–511. [Google Scholar] [CrossRef] [PubMed]
- Albenberg, L.G.; Wu, G.D. Diet and the intestinal microbiome: Associations, functions, and implications for health and disease. Gastroenterology 2014, 146, 1564–1572. [Google Scholar] [CrossRef]
- Carbonero, F.; Benefiel, A.C.; Alizadeh-Ghamsari, A.H.; Gaskins, H.R. Microbial pathways in colonic sulfur metabolism and links with health and disease. Front. Physiol. 2012, 3, 448. [Google Scholar] [CrossRef] [PubMed]
- Beaumont, M.; Andriamihaja, M.; Lan, A.; Khodorova, N.; Audebert, M.; Blouin, J.M.; Grauso, M.; Lancha, L.; Benetti, P.H.; Benamouzig, R.; et al. Detrimental effects for colonocytes of an increased exposure to luminal hydrogen sulfide: The adaptive response. Free Radic. Biol. Med. 2016, 93, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Huang, Y.; Huang, M.; Wang, M.; Ming, Y.; Chen, W.; Chen, Y.; Tang, Z.; Jia, B. From nitrate to NO: Potential effects of nitrate-reducing bacteria on systemic health and disease. Eur. J. Med. Res. 2023, 28, 425. [Google Scholar] [CrossRef]
- Cai, J.; Su, W.; Chen, X.; Zheng, H. Advances in the study of selenium and human intestinal bacteria. Front. Nutr. 2022, 9, 1059358. [Google Scholar] [CrossRef]
- Bernstein, H.; Bernstein, C.; Payne, C.M.; Dvorakova, K.; Garewal, H. Bile acids as carcinogens in human gastrointestinal cancers. Mutat. Res. Rev. Mutat. Res. 2005, 589, 47–65. [Google Scholar] [CrossRef]
- O’Keefe, S.J. Diet, microorganisms and their metabolites, and colon cancer. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 691–706. [Google Scholar] [CrossRef]
- Schwarcz, R.; Stone, T.W. The kynurenine pathway and the brain: Challenges, controversies and promises. Neuropharmacology 2017, 112, 237–247. [Google Scholar] [CrossRef]
- Knight, R.; Vrbanac, A.; Taylor, B.C.; Aksenov, A.; Callewaert, C.; Debelius, J.; Gonzalez, A.; Kosciolek, T.; McCall, L.I.; McDonald, D.; et al. Best practices for analysing microbiomes. Nat. Rev. Microbiol. 2018, 16, 410–422. [Google Scholar] [CrossRef] [PubMed]
- Tourlousse, D.M.; Narita, K.; Miura, T.; Sakamoto, M.; Ohashi, A.; Shiina, K.; Matsuda, M.; Miura, D.; Shimamura, M.; Ohyama, Y.; et al. Validation and standardization of DNA extraction and library construction methods for metagenomics-based human fecal microbiome measurements. Microbiome 2021, 9, 95. [Google Scholar] [CrossRef]
- Bäckhed, F.; Ding, H.; Wang, T.; Hooper, L.V.; Koh, G.Y.; Nagy, A.; Semenkovich, C.F.; Gordon, J.I. The gut microbiota as an environmental factor that regulates fat storage. Proc. Natl. Acad. Sci. USA 2004, 101, 15718–15723. [Google Scholar] [CrossRef]
- Round, J.L.; Mazmanian, S.K. The gut microbiota shapes intestinal immune responses during health and disease. Nat. Rev. Immunol. 2009, 9, 313–323. [Google Scholar] [CrossRef]
- Ridaura, V.K.; Faith, J.J.; Rey, F.E.; Cheng, J.; Duncan, A.E.; Kau, A.L.; Griffin, N.W.; Lombard, V.; Henrissat, B.; Bain, J.R.; et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science 2013, 341, 1241214. [Google Scholar] [CrossRef]
- Stämmler, F.; Gläsner, J.; Hiergeist, A.; Holler, E.; Weber, D.; Oefner, P.J.; Gessner, A.; Spang, R. Adjusting microbiome profiles for differences in microbial load by spike-in bacteria. Microbiome 2016, 4, 28. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y. Bioinformatic and statistical analysis of microbiome data. Methods Mol. Biol. 2023, 2629, 183–229. [Google Scholar] [CrossRef]
- Galloway-Peña, J.; Hanson, B. Tools for analysis of the microbiome. Dig. Dis. Sci. 2020, 65, 674–685. [Google Scholar] [CrossRef] [PubMed]
- Hansen, S.J.Z.; Tang, P.; Kiefer, A.; Galles, K.; Wong, C.; Morovic, W. Droplet digital PCR is an improved alternative method for high-quality enumeration of viable probiotic strains. Front. Microbiol. 2020, 10, 3025. [Google Scholar] [CrossRef]
- Miquel, S.; Martín, R.; Rossi, O.; Bermúdez-Humarán, L.G.; Chatel, J.M.; Sokol, H.; Thomas, M.; Wells, J.M.; Langella, P. Faecalibacterium prausnitzii and human intestinal health. Curr. Opin. Microbiol. 2013, 16, 255–261. [Google Scholar] [CrossRef]
- Almeida, A.; Nayfach, S.; Boland, M.; Strozzi, F.; Beracochea, M.; Shi, Z.J.; Pollard, K.S.; Sakharova, E.; Parks, D.H.; Hugenholtz, P.; et al. A unified catalog of 204,938 reference genomes from the human gut microbiome. Nat. Biotechnol. 2021, 39, 105–114. [Google Scholar] [CrossRef]
- Frank, J.A.; Pan, Y.; Tooming-Klunderud, A.; Eijsink, V.G.H.; McHardy, A.C.; Nederbragt, A.J.; Pope, P.B. Improved metagenome assemblies and taxonomic binning using long-read circular consensus sequence data. Sci. Rep. 2016, 6, 25373. [Google Scholar] [CrossRef] [PubMed]
- Douglas, G.M.; Maffei, V.J.; Zaneveld, J.R.; Yurgel, S.N.; Brown, J.R.; Taylor, C.M.; Huttenhower, C.; Langille, M.G.I. PICRUSt2 for prediction of metagenome functions. Nat. Biotechnol. 2020, 38, 685–688. [Google Scholar] [CrossRef]
- Wright, R.J.; Langille, M.G. PICRUSt2-SC: An update to the reference database used for functional prediction within PICRUSt2. Bioinformatics 2025, 41, btaf269. [Google Scholar] [CrossRef]
- Franzosa, E.A.; Morgan, X.C.; Segata, N.; Waldron, L.; Reyes, J.; Earl, A.M.; Giannoukos, G.; Boylan, M.R.; Ciulla, D.; Gevers, D.; et al. Relating the metatranscriptome and metagenome of the human gut. Proc. Natl. Acad. Sci. USA 2014, 111, E2329–E2338. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, W.; Ning, Z.; Mayne, J.; Mack, D.; Stintzi, A.; Tian, R.; Figeys, D. Deep metaproteomics approach for the study of human microbiomes. Anal. Chem. 2017, 89, 9407–9415. [Google Scholar] [CrossRef]
- Raja, G.; Gupta, H.; Gebru, Y.A.; Youn, G.S.; Choi, Y.R.; Kim, H.S.; Yoon, S.J.; Kim, D.J.; Kim, T.J.; Suk, K.T. Recent advances of microbiome-associated metabolomics profiling in liver disease: Principles, mechanisms, and applications. Int. J. Mol. Sci. 2021, 22, 1160. [Google Scholar] [CrossRef]
- Lund, P.J.; Gates, L.A.; Leboeuf, M.; Smith, S.A.; Chau, L.; Lopes, M.; Friedman, E.S.; Saiman, Y.; Kim, M.S.; Shoffler, C.A.; et al. Stable isotope tracing in vivo reveals a metabolic bridge linking the microbiota to host histone acetylation. Cell Rep. 2022, 41, 111809. [Google Scholar] [CrossRef]
- Xiao, X.; Zhou, Y.; Li, X.; Jin, J.; Durham, J.; Ye, Z.; Wang, Y.; Hennig, B.; Deng, P. 13C-stable isotope resolved metabolomics uncovers dynamic biochemical landscape of gut microbiome–host organ communications in mice. Microbiome 2024, 12, 90. [Google Scholar] [CrossRef]
- Rouskas, K.; Guela, M.; Pantoura, M.; Pagkalos, I.; Hassapidou, M.; Lalama, E.; Pfeiffer, A.F.H.; Decorte, E.; Cornelissen, V.; Wilson-Barnes, S.; et al. The influence of an AI-driven personalized nutrition program on the human gut microbiome and its health implications. Nutrients 2025, 17, 1260. [Google Scholar] [CrossRef] [PubMed]
- Noecker, C.; Eng, A.; Srinivasan, S.; Theriot, C.M.; Young, V.B.; Jansson, J.K.; Fredricks, D.N.; Borenstein, E. Metabolic and ecological interactions in microbial communities: Combining statistical and mechanistic modeling to understand function. Front. Microbiol. 2016, 7, 632. [Google Scholar]
- Eicher, T.; Kinnebrew, G.; Patt, A.; Spencer, K.; Ying, K.; Ma, Q.; Machiraju, R.; Mathé, A.E.A. Metabolomics and multi-omics integration: A survey of computational methods and resources. Metabolites 2020, 10, 202. [Google Scholar] [CrossRef] [PubMed]
- BCC Research. Microbiome Therapeutics Market. Available online: https://www.bccresearch.com/market-research/biotechnology/microbiome-therapeutics-market.html#:~:text=Report%20Highlights,54.8%25%20from%202022%20through%202027 (accessed on 24 September 2025).
- Grand View Research. Nutritional Supplements Market. Available online: https://www.grandviewresearch.com/industry-analysis/nutritional-supplements-market (accessed on 24 September 2025).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).