Molecular Networks Underlying Wheat Resistance and Susceptibility to Pyrenophora tritici-repentis
Abstract
1. Introduction
2. Materials and Methods
2.1. Expression and Trait Data
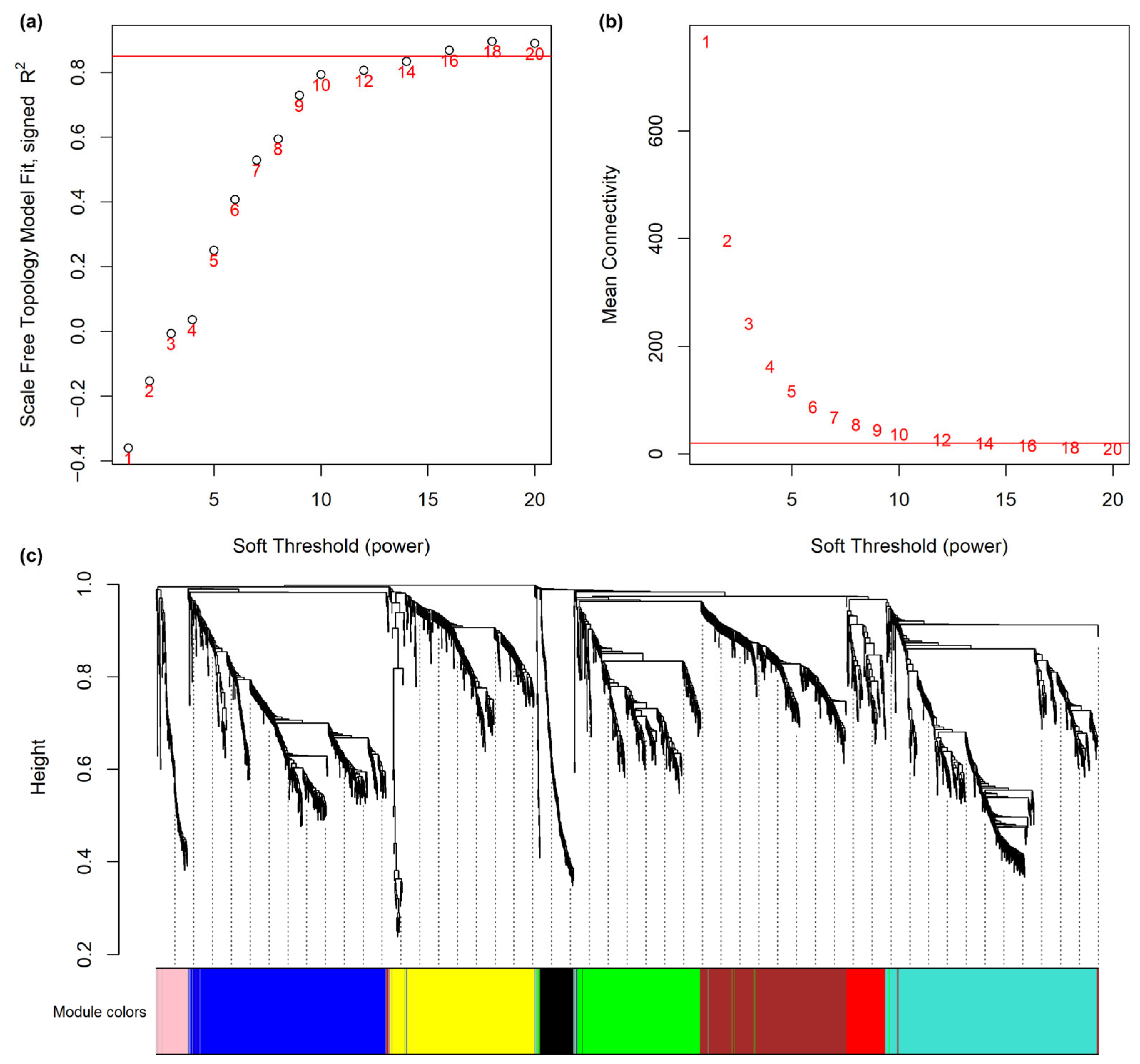
2.2. Construction of WGCNA
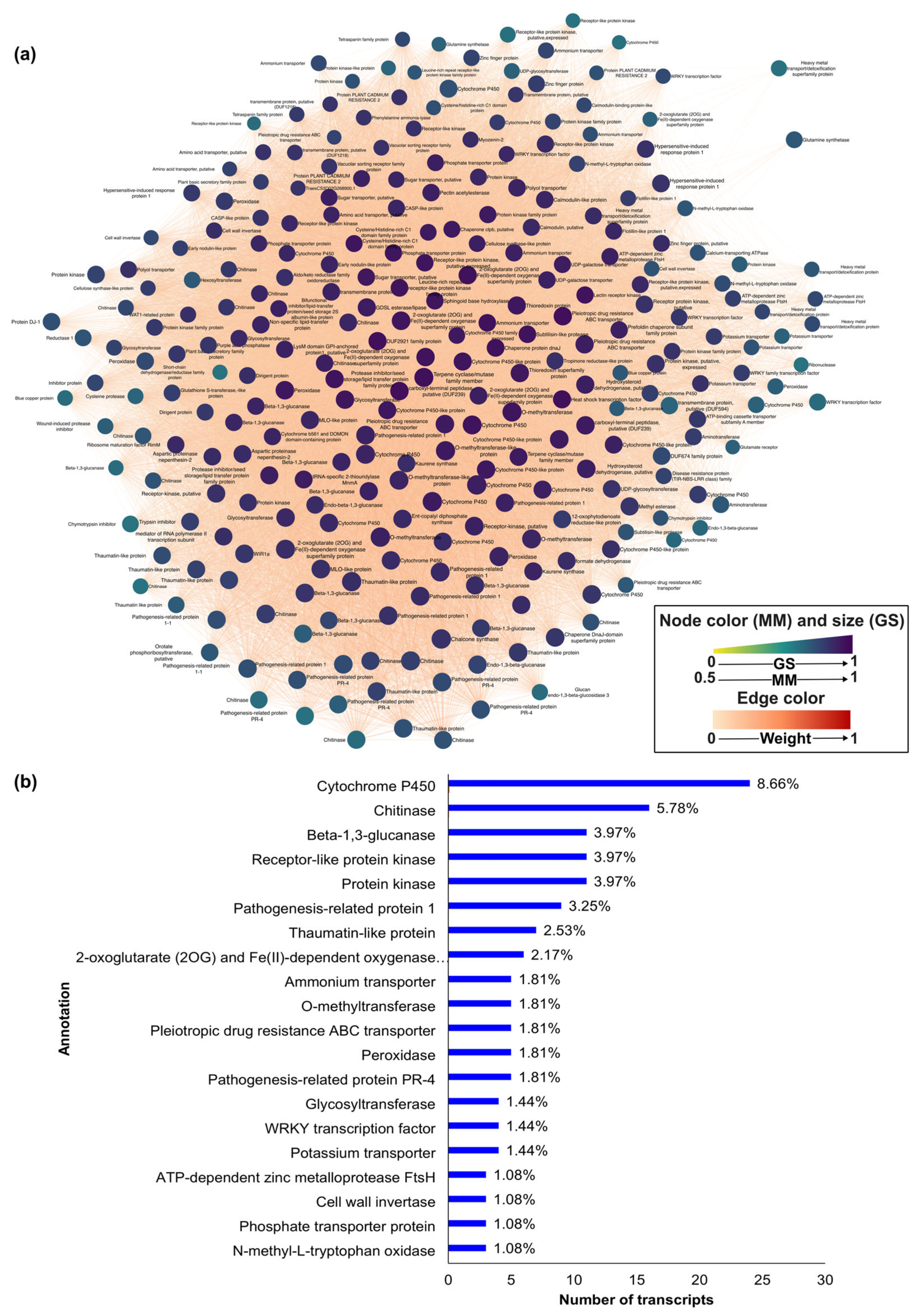
2.3. Intra-Modular Analysis and Identification of Hub Genes
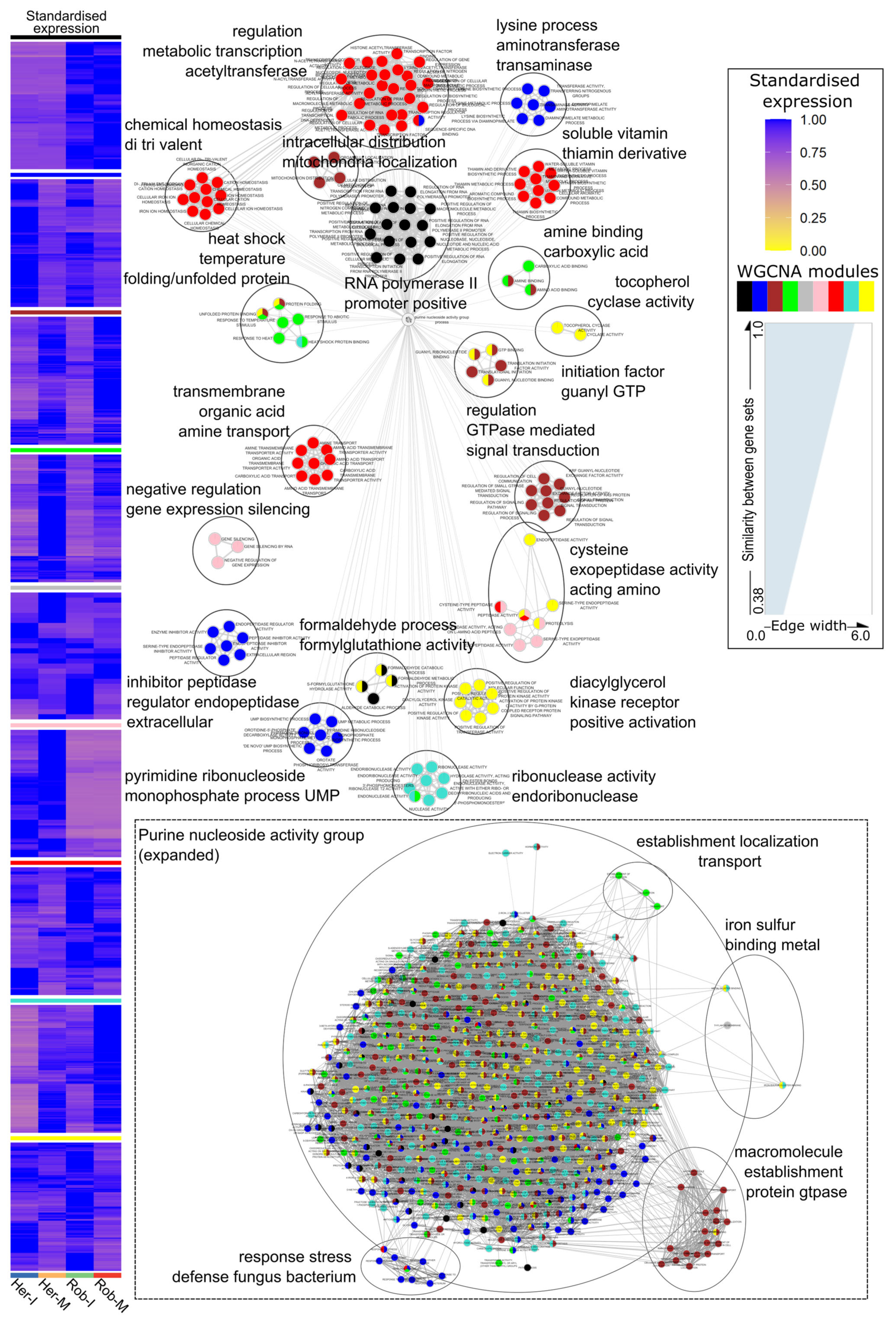
2.4. Functional Annotation and Enrichment Analysis
3. Results
3.1. Global Co-Expression Network
3.2. Module–Trait Relationships Reveal Common Responses Triggered by Ptr Infection in Resistant and Susceptible Genotypes
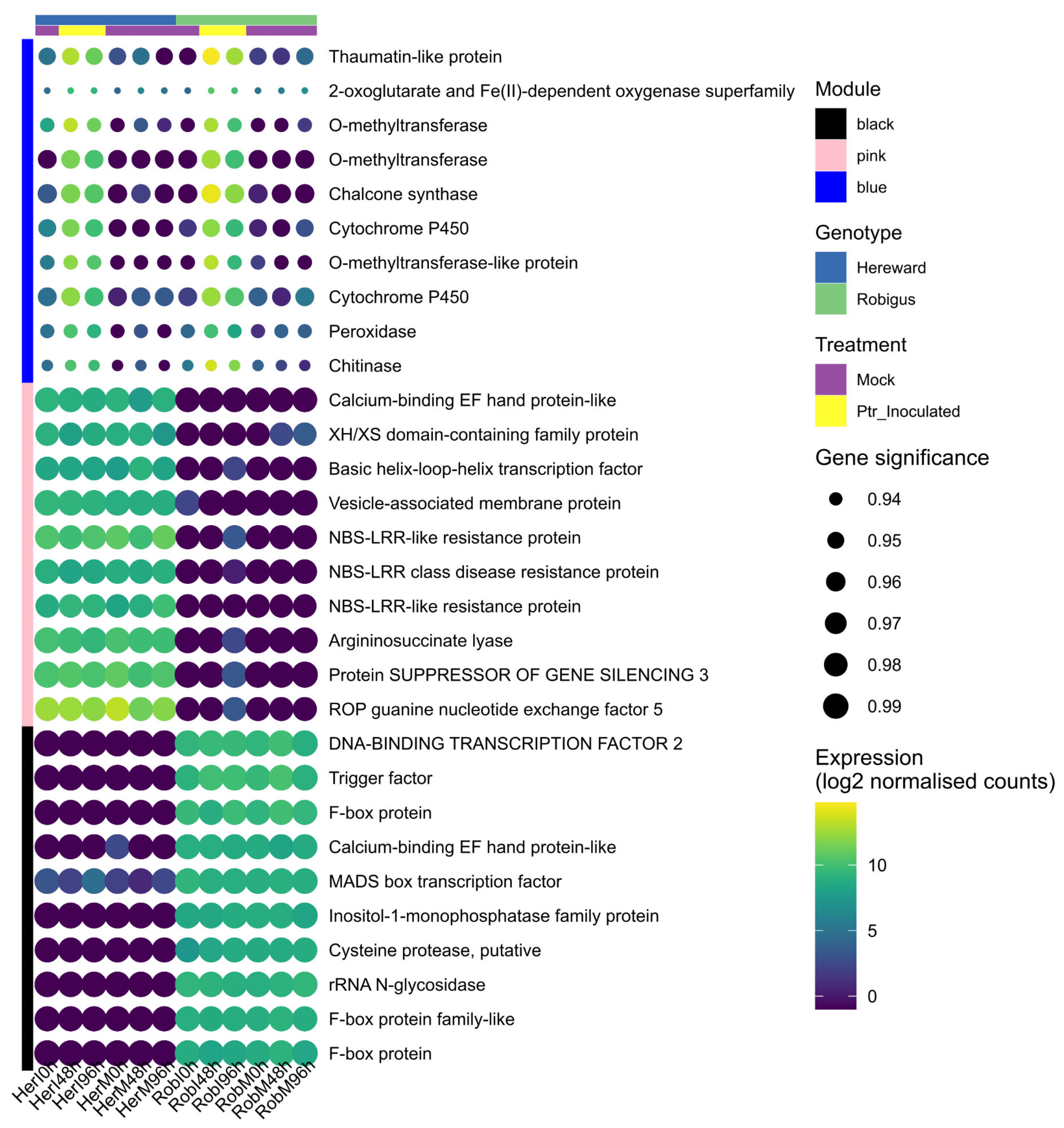
3.3. Intramodular Analysis and Network Analysis of Hub Genes
4. Discussion
4.1. The Blue Module: Pyrenophora Tritici-Repentis Is Likely to Suppress Basal and Effector-Triggered Immunity of Wheat
4.2. The Blue Module: Pyrenophora Tritici-Repentis Influences Nitrogen Metabolism
4.3. The Black Module: Defining the Sources Partial Resistance to Pyrenophora Tritici-Repentis in Robigus
4.4. The Pink Module: Changes Associated with Susceptibility in Hereward
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Ptr | Pyrenophora tritici-repentis |
| hpi | Hours post inoculation |
| Rob | Robigus |
| Her | Hereward |
| corr | Correlation |
| ME | Module eigengene |
| GS | Gene significance |
| MM | Module membership |
| FDR | False discovery rate |
References
- Ciuffetti, L.M.; Manning, V.A.; Pandelova, I.; Faris, J.D.; Friesen, T.L.; Strelkov, S.E.; Weber, G.L.; Goodwin, S.B.; Wolpert, T.J.; Figueroa, M. Pyrenophora tritici-repentis: A Plant Pathogenic Fungus with Global Impact. Genom. Plant-Assoc. Fungi Monocot Pathog. 2014, 1–39. [Google Scholar] [CrossRef]
- Mironenko, N.; Krämer, I.; Ordon, F.; Mikhailova, L.; Kopahnke, D.; Timopheeva, E. Intraspecific Genetic Diversity of Pyrenophora tritici-repentis (Died.) Drechs. (Drechslera tritici-repentis [Died.] Shoem.) Detected by Random Amplified Polymorphic DNA Assays. Arch. Phytopathol. Plant Prot. 2007, 40, 431–440. [Google Scholar] [CrossRef]
- Sautua, F.J.; Carmona, M.A. Detection and Characterization of QoI Resistance in Pyrenophora tritici-repentis Populations Causing Tan Spot of Wheat in Argentina. Plant Pathol. 2021, 70, 2125–2136. [Google Scholar] [CrossRef]
- Singh, R.P.; Singh, P.K.; Rutkoski, J.; Hodson, D.P.; He, X.; Jørgensen, L.N.; Hovmøller, M.S.; Huerta-Espino, J. Disease Impact on Wheat Yield Potential and Prospects of Genetic Control. Annu. Rev. Phytopathol. 2016, 54, 303–322. [Google Scholar] [CrossRef]
- Lamari, L.; Bernier, C.C. Toxin of Pyrenophora Tritici-Repentis: Host-Specificity, Significance in Disease, and Inheritance of Host Reaction. Phytopathology 1989, 79, 740. [Google Scholar] [CrossRef]
- Faris, J.D.; Anderson, J.A.; Francl, L.J.; Jordahl, J.G. Chromosomal Location of a Gene Conditioning Insensitivity in Wheat to a Necrosis-Inducing Culture Filtrate from Pyrenophora Tritici-Repentis. Phytopathology 1996, 86, 459–463. [Google Scholar] [CrossRef]
- Effertz, R.J.; Meinhardt, S.W.; Anderson, J.A.; Jordahl, J.G.; Francl, L.J. Identification of a Chlorosis-Inducing Toxin from Pyrenophora tritici-repentis and the Chromosomal Location of an Insensitivity Locus in Wheat. Phytopathology 2007, 92, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Friesen, T.; Faris, J. Molecular Mapping of Resistance to Pyrenophora tritici-repentis Race 5 and Sensitivity to Ptr ToxB in Wheat. Theor. Appl. Genet. 2004, 109, 464–471. [Google Scholar] [CrossRef]
- Faris, J.D.; Liu, Z.; Xu, S.S. Genetics of Tan Spot Resistance in Wheat. Theor. Appl. Genet. 2013, 126, 2197–2217. [Google Scholar] [CrossRef]
- Lamari, L.; Sayoud, R.; Boulif, M.; Bernier, C.C. Identification of a New Race in Pyrenophora Tritici-Repentis: Implications for the Current Pathotype Classification System. Can. J. Plant Pathol. 1995, 17, 312–318. [Google Scholar] [CrossRef]
- Ali, S.; Francl, L.J. A New Race of Pyrenophora tritici-repentis from Brazil. Plant Dis. 2002, 86, 1050. [Google Scholar] [CrossRef]
- Lamari, L.; Strelkov, S.E.; Yahyaoui, A.; Orabi, J.; Smith, R.B. The Identification of Two New Races of Pyrenophora tritici-repentis from the Host Center of Diversity Confirms a One-to-One Relationship in Tan Spot of Wheat. Phytopathology 2003, 93, 391–396. [Google Scholar] [CrossRef]
- McDonald, M.C.; Ahren, D.; Simpfendorfer, S.; Milgate, A.; Solomon, P.S. The Discovery of the Virulence Gene ToxA in the Wheat and Barley Pathogen Bipolaris sorokiniana. Mol. Plant Pathol. 2018, 19, 432–439. [Google Scholar] [CrossRef]
- Friesen, T.L.; Stukenbrock, E.H.; Liu, Z.; Meinhardt, S.; Ling, H.; Faris, J.D.; Rasmussen, J.B.; Solomon, P.S.; McDonald, B.A.; Oliver, R.P. Emergence of a New Disease as a Result of Interspecific Virulence Gene Transfer. Nat. Genet. 2006, 38, 953–956. [Google Scholar] [CrossRef]
- Oliver, R.P.; Friesen, T.L.; Faris, J.D.; Solomon, P.S. Stagonospora Nodorum: From Pathology to Genomics and Host Resistance. Annu. Rev. Phytopathol. 2011, 50, 23–43. [Google Scholar] [CrossRef] [PubMed]
- Manning, V.A.; Ciuffetti, L.M. Localization of Ptr ToxA Produced by Pyrenophora tritici-repentis Reveals Protein Import into Wheat Mesophyll Cells. Plant Cell 2005, 17, 3203–3212. [Google Scholar] [CrossRef] [PubMed]
- Manning, V.A.; Hardison, L.K.; Ciufetti, L.M. Ptr ToxA Interacts with a Chloroplast-Localized Protein. Mol. Plant-Microbe Interact. 2007, 20, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Manning, V.A.; Hamilton, S.M.; Karplus, P.A.; Ciuffetti, L.M. The Arg-Gly-Asp-Containing, Solvent-Exposed Loop of Ptr ToxA Is Required for Internalization. Mol. Plant-Microbe Interact. 2008, 21, 315–325. [Google Scholar] [CrossRef]
- Manning, V.A.; Andrie, R.M.; Trippe, A.F.; Ciuffetti, L.M. Ptr ToxA Requires Multiple Motifs for Complete Activity. Mol. Plant-Microbe Interact. 2004, 17, 491–501. [Google Scholar] [CrossRef][Green Version]
- Manning, V.A.; Chu, A.L.; Steeves, J.E.; Wolpert, T.J.; Ciuffetti, L.M. A Host-Selective Toxin of Pyrenophora Tritici- Repentis, Ptr ToxA, Induces Photosystem Changes and Reactive Oxygen Species Accumulation in Sensitive Wheat. Mol. Plant-Microbe Interact. 2009, 22, 665–676. [Google Scholar] [CrossRef] [PubMed]
- Pandelova, I.; Betts, M.F.; Manning, V.A.; Wilhelm, L.J.; Mockler, T.C.; Ciuffetti, L.M. Analysis of Transcriptome Changes Induced by Ptr ToxA in Wheat Provides Insights into the Mechanisms of Plant Susceptibility. Mol. Plant 2009, 2, 1067–1083. [Google Scholar] [CrossRef] [PubMed]
- Pandelova, I.; Figueroa, M.; Wilhelm, L.J.; Manning, V.A.; Mankaney, A.N.; Mockler, T.C.; Ciuffetti, L.M. Host-Selective Toxins of Pyrenophora tritici-repentis Induce Common Responses Associated with Host Susceptibility. PLoS ONE 2012, 7, e40240. [Google Scholar] [CrossRef] [PubMed]
- Andersen, E.J.; Nepal, M.P.; Ali, S. Necrotrophic Fungus Pyrenophora tritici-repentis Triggers Expression of Multiple Resistance Components in Resistant and Susceptible Wheat Cultivars. Plant Pathol. J. 2021, 37, 99. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, L.C.; Santana, F.M.; Beckmann, M.; Mur, L.A.J. Using MAGIC against Tan Spot Disease: How Multiparent Advanced Generation Intercross Wheat Lines Respond to Pyrenophora tritici-repentis Infection. Phytopathology 2020, 110, S2.94–S2.95. [Google Scholar] [CrossRef]
- Ferreira, L.; Santana, F.; Scagliusi, S.; Beckmann, M.; Mur, L. Omic Characterisation of Multi-Component Defences against the Necrotrophic Pathogen Pyrenophora tritici-repentis in Wheat. Plant Biol. 2025, 27, 347–361. [Google Scholar] [CrossRef]
- Mackay, I.J.; Bansept-Basler, P.; Barber, T.; Bentley, A.R.; Cockram, J.; Gosman, N.; Greenland, A.J.; Horsnell, R.; Howells, R.; O’Sullivan, D.M.; et al. An Eight-Parent Multiparent Advanced Generation Inter-Cross Population for Winter-Sown Wheat: Creation, Properties, and Validation. G3 Genes Genomes Genet. 2014, 4, 1603–1610. [Google Scholar] [CrossRef]
- Yan, J.; Risacher, S.L.; Shen, L.; Saykin, A.J. Network Approaches to Systems Biology Analysis of Complex Disease: Integrative Methods for Multi-Omics Data. Brief. Bioinform. 2018, 19, 1370–1381. [Google Scholar] [CrossRef]
- van Dam, S.; Võsa, U.; van der Graaf, A.; Franke, L.; de Magalhães, J.P. Gene Co-Expression Analysis for Functional Classification and Gene–Disease Predictions. Brief. Bioinform. 2018, 19, 575–592. [Google Scholar] [CrossRef]
- Pandaranayaka, E.P.; Frenkel, O.; Elad, Y.; Prusky, D.; Harel, A. Network Analysis Exposes Core Functions in Major Lifestyles of Fungal and Oomycete Plant Pathogens. BMC Genomics 2019, 20, 1020. [Google Scholar] [CrossRef]
- Cheng, C.-H.; Shen, B.-N.; Shang, Q.-W.; Liu, L.-Y.D.; Peng, K.-C.; Chen, Y.-H.; Chen, F.-F.; Hu, S.-F.; Wang, Y.-T.; Wang, H.-C.; et al. Gene-to-Gene Network Analysis of the Mediation of Plant Innate Immunity by the Eliciting Plant Response-Like 1 (Epl1) Elicitor of Trichoderma Formosa. Mol. Plant-Microbe Interact. 2018, 31, 683–691. [Google Scholar] [CrossRef]
- Hu, Q.; Tan, L.; Gu, S.; Xiao, Y.; Xiong, X.; Zeng, W.; Feng, K.; Wei, Z.; Deng, Y. Network Analysis Infers the Wilt Pathogen Invasion Associated with Non-Detrimental Bacteria. NPJ Biofilms Microbiomes 2020, 6, 8. [Google Scholar] [CrossRef]
- Zhang, B.; Horvath, S. A General Framework for Weighted Gene Co-Expression Network Analysis. Stat. Appl. Genet. Mol. Biol. 2005, 4. [Google Scholar] [CrossRef]
- Li, Y.; Pearl, S.A.; Jackson, S.A. Gene Networks in Plant Biology: Approaches in Reconstruction and Analysis. Trends Plant Sci. 2015, 20, 664–675. [Google Scholar] [CrossRef]
- Langfelder, P.; Horvath, S. WGCNA: An R Package for Weighted Correlation Network Analysis. BMC Bioinform. 2008, 9, 559. [Google Scholar] [CrossRef]
- Zhang, H.; Fu, Y.; Guo, H.; Zhang, L.; Wang, C.; Song, W.; Yan, Z.; Wang, Y.; Ji, W. Transcriptome and Proteome-Based Network Analysis Reveals a Model of Gene Activation in Wheat Resistance to Stripe Rust. Int. J. Mol. Sci. 2019, 20, 1106. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A Software Environment for Integrated Models of Biomolecular Interaction Networks. Genome Res. 2003, 13, 2498. [Google Scholar] [CrossRef] [PubMed]
- Appels, R.; Eversole, K.; Feuillet, C.; Keller, B.; Rogers, J.; Stein, N.; Pozniak, C.J.; Stein, N.; Choulet, F.; Distelfeld, A.; et al. Shifting the Limits in Wheat Research and Breeding Using a Fully Annotated Reference Genome. Science 2018, 361, eaar7191. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-González, R.H.; Borrill, P.; Lang, D.; Harrington, S.A.; Brinton, J.; Venturini, L.; Davey, M.; Jacobs, J.; Van Ex, F.; Pasha, A.; et al. The Transcriptional Landscape of Polyploid Wheat. Science 2018, 361, eaar6089. [Google Scholar] [CrossRef]
- Maere, S.; Heymans, K.; Kuiper, M. BiNGO: A Cytoscape Plugin to Assess Overrepresentation of Gene Ontology Categories in Biological Networks. Bioinformatics 2005, 21, 3448–3449. [Google Scholar] [CrossRef] [PubMed]
- Merico, D.; Isserlin, R.; Stueker, O.; Emili, A.; Bader, G.D. Enrichment Map: A Network-Based Method for Gene-Set Enrichment Visualization and Interpretation. PLoS ONE 2010, 5, e13984. [Google Scholar] [CrossRef] [PubMed]
- Fenton, A.; Antonovics, J.; Brockhurst, M.A. Inverse-Gene-for-Gene Infection Genetics and Coevolutionary Dynamics. Am. Nat. 2009, 174, E230–E242. [Google Scholar] [CrossRef] [PubMed]
- Faris, J.D.; Zhang, Z.; Lu, H.; Lu, S.; Reddy, L.; Cloutier, S.; Fellers, J.P.; Meinhardt, S.W.; Rasmussen, J.B.; Xu, S.S.; et al. A Unique Wheat Disease Resistance-like Gene Governs Effector-Triggered Susceptibility to Necrotrophic Pathogens. Proc. Natl. Acad. Sci. USA 2010, 107, 13544–13549. [Google Scholar] [CrossRef]
- Jones, J.D.G.; Dangl, J.L. The Plant Immune System. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef]
- Nadimpalli, R.; Yalpani, N.; Johal, G.S.; Simmons, C.R. Prohibitins, Stomatins, and Plant Disease Response Genes Compose a Protein Superfamily That Controls Cell Proliferation, Ion Channel Regulation, and Death. J. Biol. Chem. 2000, 275, 29579–29586. [Google Scholar] [CrossRef]
- Rostoks, N.; Schmierer, D.; Kudrna, D.; Kleinhofs, A. Barley Putative Hypersensitive Induced Reaction Genes: Genetic Mapping, Sequence Analyses and Differential Expression in Disease Lesion Mimic Mutants. Theor. Appl. Genet. 2003, 107, 1094–1101. [Google Scholar] [CrossRef]
- Zhou, L.; Cheung, M.Y.; Zhang, Q.; Lei, C.L.; Zhang, S.H.; Sun, S.S.M.; Lam, H.M. A Novel Simple Extracellular Leucine-Rich Repeat (ELRR) Domain Protein from Rice (OsLRR1) Enters the Endosomal Pathway and Interacts with the Hypersensitive-Induced Reaction Protein 1 (OsHIR1). Plant Cell Environ. 2009, 32, 1804–1820. [Google Scholar] [CrossRef]
- Zhou, L.; Cheung, M.Y.; Li, M.W.; Fu, Y.; Sun, Z.; Sun, S.M.; Lam, H.M. Rice Hypersensitive Induced Reaction Protein 1 (OsHIR1) Associates with Plasma Membrane and Triggers Hypersensitive Cell Death. BMC Plant Biol. 2010, 10, 290. [Google Scholar] [CrossRef]
- Jarosch, B.; Kogel, K.H.; Schaffrath, U. The Ambivalence of the Barley Mlo Locus: Mutations Conferring Resistance Against Powdery Mildew (Blumeria graminis f. sp. hordei) Enhance Susceptibility to the Rice Blast Fungus Magnaporthe grisea. Mol. Plant-Microbe Interact. 2007, 12, 508–514. [Google Scholar] [CrossRef]
- Douchkov, D.; Johrde, A.; Nowara, D.; Himmelbach, A.; Lueck, S.; Niks, R.; Schweizer, P. Convergent Evidence for a Role of WIR1 Proteins during the Interaction of Barley with the Powdery Mildew Fungus Blumeria graminis. J. Plant Physiol. 2011, 168, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Tufan, H.A.; Mcgrann, G.R.D.; Maccormack, R.; Boyd, L.A. TaWIR1 Contributes to Post-Penetration Resistance to Magnaporthe Oryzae, but Not Blumeria graminis f. Sp. Tritici, in Wheat. Mol. Plant Pathol. 2012, 13, 653–665. [Google Scholar] [CrossRef]
- Lu, S.; Faris, J.D.; Sherwood, R.; Friesen, T.L.; Edwards, M.C. A Dimeric PR-1-Type Pathogenesis-Related Protein Interacts with ToxA and Potentially Mediates ToxA-Induced Necrosis in Sensitive Wheat. Mol. Plant Pathol. 2014, 15, 650–663. [Google Scholar] [CrossRef]
- Gao, L.; Wang, S.; Li, X.Y.; Wei, X.J.; Zhang, Y.J.; Wang, H.Y.; Liu, D.Q. Expression and Functional Analysis of a Pathogenesis-Related Protein 1 Gene, TcLr19PR1, Involved in Wheat Resistance Against Leaf Rust Fungus. Plant Mol. Biol. Report. 2015, 33, 797–805. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, F.; Liang, F.; Zhang, Y.; Ma, L.; Wang, H.; Liu, D. Functional Analysis of a Pathogenesis-Related Thaumatin-like Protein Gene TaLr35PR5 from Wheat Induced by Leaf Rust Fungus. BMC Plant Biol. 2018, 18, 76. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Sun, Y.; Song, N.; Zhao, M.; Liu, R.; Feng, H.; Wang, X.; Kang, Z. Puccinia striiformis f. sp. tritici MicroRNA-like RNA 1 (Pst-MilR1), an Important Pathogenicity Factor of Pst, Impairs Wheat Resistance to Pst by Suppressing the Wheat Pathogenesis-Related 2 Gene. New Phytol. 2017, 215, 338–350. [Google Scholar] [CrossRef]
- Sanaz Ramezanpour, S.; Mohammadi, M.; Navabpour, S.; Soltanloo, H.; Kia, S.; Kalateh Arabi, M. Study on Expression Pattern of Chalcone Synthase And-1,3-Glucanase under Septoria Tritici Treatment in Wheat by Quantitative Real Time PCR. J. Agric. Environ. Sci. 2012, 12, 1431–1436. [Google Scholar] [CrossRef]
- Xu, J.; Wang, X.Y.; Guo, W.Z. The Cytochrome P450 Superfamily: Key Players in Plant Development and Defense. J. Integr. Agric. 2015, 14, 1673–1686. [Google Scholar] [CrossRef]
- Nomura, T.; Ishihara, A.; Iwamura, H.; Endo, T.R. Molecular Characterization of Benzoxazinone-Deficient Mutation in Diploid Wheat. Phytochemistry 2007, 68, 1008–1016. [Google Scholar] [CrossRef]
- Pandian, B.A.; Sathishraj, R.; Djanaguiraman, M.; Prasad, P.V.V.; Jugulam, M. Role of Cytochrome P450 Enzymes in Plant Stress Response. Antioxidants 2020, 9, 454. [Google Scholar] [CrossRef]
- Saito, K.; Kobayashi, M.; Gong, Z.; Tanaka, Y.; Yamazaki, M. Direct Evidence for Anthocyanidin Synthase as a 2-Oxoglutarate-Dependent Oxygenase: Molecular Cloning and Functional Expression of CDNA from a Red Forma of Perilla Frutescens. Plant J. 1999, 17, 181–189. [Google Scholar] [CrossRef]
- Mitchell, A.J.; Weng, J.K. Unleashing the Synthetic Power of Plant Oxygenases: From Mechanism to Application. Plant Physiol. 2019, 179, 813. [Google Scholar] [CrossRef] [PubMed]
- Hahlbrock, K.; Grisebach, H. Enzymic Controls in the Biosynthesis of Lignin and Flavonoids. Ann. Rev. Plant Physiol. 1979, 30, 105–130. [Google Scholar] [CrossRef]
- Chen, Z.; Zheng, Z.; Huang, J.; Lai, Z.; Fan, B. Biosynthesis of Salicylic Acid in Plants. Plant Signal Behav. 2009, 4, 493–496. [Google Scholar] [CrossRef] [PubMed]
- Ortuño, A.; Díaz, L.; Alvarez, N.; Porras, I.; García-Lidón, A.; Del Río, J.A. Comparative Study of Flavonoid and Scoparone Accumulation in Different Citrus Species and Their Susceptibility to Penicillium digitatum. Food Chem. 2011, 125, 232–239. [Google Scholar] [CrossRef]
- Marschner, H. General Introduction to the Mineral Nutrition of Plants. In Inorganic Plant Nutrition; Springer: Berlin/Heidelberg, Germany, 1983; pp. 5–60. [Google Scholar] [CrossRef]
- Mur, L.A.J.; Simpson, C.; Kumari, A.; Gupta, A.K.; Gupta, K.J. Moving Nitrogen to the Centre of Plant Defence against Pathogens. Ann. Bot. 2017, 119, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wang, M.; Mur, L.A.J.; Shen, Q.; Guo, S. Unravelling the Roles of Nitrogen Nutrition in Plant Disease Defences. Int. J. Mol. Sci. 2020, 21, 572. [Google Scholar] [CrossRef]
- Devadas, R.; Simpfendorfer, S.; Backhouse, D.; Lamb, D.W. Effect of Stripe Rust on the Yield Response of Wheat to Nitrogen. Crop J. 2014, 2, 201–206. [Google Scholar] [CrossRef]
- Brennan, R.F. The Role of Manganese and Nitrogen Nutrition in the Susceptibility of Wheat Plants to Take-All in Western Australia. Fertil. Res. 1992, 31, 35–41. [Google Scholar] [CrossRef]
- Huber, D.M.; Tsai, C.Y. Influence of the Form of Nitrogen on Ammonium, Amino Acids and N-assimelating Enzyme Activity in Maize Genotypes 1. J. Plant Nutr. 2008, 18, 747–763. [Google Scholar] [CrossRef]
- Mead, O.; Thynne, E.; Winterberg, B.; Solomon, P.S. Characterising the Role of GABA and Its Metabolism in the Wheat Pathogen Stagonospora Nodorum. PLoS ONE 2013, 8, e78368. [Google Scholar] [CrossRef]
- Gupta, K.J.; Brotman, Y.; Segu, S.; Zeier, T.; Zeier, J.; Persijn, S.T.; Cristescu, S.M.; Harren, F.J.M.; Bauwe, H.; Fernie, A.R.; et al. The Form of Nitrogen Nutrition Affects Resistance against Pseudomonas syringae Pv. phaseolicola in Tobacco. J. Exp. Bot. 2013, 64, 553–568. [Google Scholar] [CrossRef]
- Zhang, L.; Tan, Q.; Lee, R.; Trethewy, A.; Lee, Y.H.; Tegeder, M. Altered Xylem-Phloem Transfer of Amino Acids Affects Metabolism and Leads to Increased Seed Yield and Oil Content in Arabidopsis. Plant Cell 2010, 22, 3603–3620. [Google Scholar] [CrossRef]
- Perchlik, M.; Tegeder, M. Leaf Amino Acid Supply Affects Photosynthetic and Plant Nitrogen Use Efficiency under Nitrogen Stress. Plant Physiol. 2018, 178, 174–188. [Google Scholar] [CrossRef]
- Perchlik, M.; Tegeder, M. Improving Plant Nitrogen Use Efficiency through Alteration of Amino Acid Transport Processes. Plant Physiol. 2017, 175, 235–247. [Google Scholar] [CrossRef]
- MacIntosh, G.C.; Castandet, B. Organellar and Secretory Ribonucleases: Major Players in Plant RNA Homeostasis1[OPEN]. Plant Physiol. 2020, 183, 1438–1452. [Google Scholar] [CrossRef]
- Olmedo, G.; Guzmán, P. Processing Precursors with RNase III in Plants. Plant Sci. 2008, 175, 741–746. [Google Scholar] [CrossRef]
- Gan, J.; Shaw, G.; Tropea, J.E.; Waugh, D.S.; Court, D.L.; Ji, X. A Stepwise Model for Double-Stranded RNA Processing by Ribonuclease III. Mol. Microbiol. 2008, 67, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.J.; Kim, J.B.; Seo, Y.W.; Kim, D.Y. F-Box Genes in the Wheat Genome and Expression Profiling in Wheat at Different Developmental Stages. Genes 2020, 11, 1154. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wei, C.; Meng, Y.; Fan, R.; Zhao, W.; Wang, X.; Yu, X.; Laroche, A.; Kang, Z.; Liu, D. Identification and Expression Analysis of Some Wheat F-Box Subfamilies during Plant Development and Infection by Puccinia triticina. Plant Physiol. Biochem. 2020, 155, 535–548. [Google Scholar] [CrossRef]
- Stefanowicz, K.; Lannoo, N.; Van Damme, E.J.M. Plant F-Box Proteins—Judges between Life and Death. Crit. Rev. Plant Sci. 2015, 34, 523–552. [Google Scholar] [CrossRef]
- Law, J.A.; Vashisht, A.A.; Wohlschlegel, J.A.; Jacobsen, S.E. SHH1, a Homeodomain Protein Required for DNA Methylation, as Well as RDR2, RDM4, and Chromatin Remodeling Factors, Associate with RNA Polymerase IV. PLoS Genet. 2011, 7, e1002195. [Google Scholar] [CrossRef] [PubMed]
- Haag, J.R.; Brower-Toland, B.; Krieger, E.K.; Sidorenko, L.; Nicora, C.D.; Norbeck, A.D.; Irsigler, A.; LaRue, H.; Brzeski, J.; McGinnis, K.; et al. Functional Diversification of Maize RNA Polymerase IV and V Subtypes via Alternative Catalytic Subunits. Cell Rep. 2014, 9, 378–390. [Google Scholar] [CrossRef]
- Zhang, H.; Ma, Z.Y.; Zeng, L.; Tanaka, K.; Zhang, C.J.; Ma, J.; Bai, G.; Wang, P.; Zhang, S.W.; Liu, Z.W.; et al. DTF1 Is a Core Component of RNA-Directed DNA Methylation and May Assist in the Recruitment of Pol IV. Proc. Natl. Acad. Sci. USA 2013, 110, 8290–8295. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, X.; Luo, J.; Lv, S.; Liu, R.; Du, X.; Jia, B.; Yuan, F.; Zhang, H.; Du, J. Recognition of H3K9me1 by Maize RNA-Directed DNA Methylation Factor SHH2. J. Integr. Plant Biol. 2021, 63, 1091–1096. [Google Scholar] [CrossRef]
- Menna, A.; Dora, S.; Sancho-Andrés, G.; Kashyap, A.; Meena, M.K.; Sklodowski, K.; Gasperini, D.; Coll, N.S.; Sánchez-Rodríguez, C. A Primary Cell Wall Cellulose-Dependent Defense Mechanism against Vascular Pathogens Revealed by Time-Resolved Dual Transcriptomics. BMC Biol. 2021, 19, 161. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, L.; Fu, Y.; Cheung, M.Y.; Wong, F.L.; Phang, T.H.; Sun, Z.; Lam, H.M. Expression of an Apoplast-Localized BURP-Domain Protein from Soybean (GmRD22) Enhances Tolerance towards Abiotic Stress. Plant Cell Environ. 2012, 35, 1932–1947. [Google Scholar] [CrossRef]
- Ding, X.; Hou, X.; Xie, K.; Xiong, L. Genome-Wide Identification of BURP Domain-Containing Genes in Rice Reveals a Gene Family with Diverse Structures and Responses to Abiotic Stresses. Planta 2009, 230, 149–163. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Li, Y.; Yan, Y.; Wang, K.; Gao, Y.; Hu, Y. Genome-Scale Identification of Soybean BURP Domain-Containing Genes and Their Expression under Stress Treatments. BMC Plant Biol. 2010, 10, 197. [Google Scholar] [CrossRef]
- Nourbakhsh, A.; Collakova, E.; Gillaspy, G.E. Characterization of the Inositol Monophosphatase Gene Family in Arabidopsis. Front. Plant Sci. 2015, 5, 725. [Google Scholar] [CrossRef]
- Sharma, N.; Chaudhary, C.; Khurana, P. Wheat Myo-Inositol Phosphate Synthase Influences Plant Growth and Stress Responses via Ethylene Mediated Signaling. Sci. Rep. 2020, 10, 10766. [Google Scholar] [CrossRef] [PubMed]
- Levine, E.E.; Frank Loewus, B.A.; Kelly, S.; Neufeldt, E.F. Metabolism of myo-inositol in plants: Conversion to pectin, hemicellulose, D-xylose, and sugar acids. Proc. Natl. Acad. Sci. USA 1962, 48, 421. [Google Scholar] [CrossRef]
- Lu, Y.; Yao, J. Chloroplasts at the Crossroad of Photosynthesis, Pathogen Infection and Plant Defense. Int. J. Mol. Sci. 2018, 19, 3900. [Google Scholar] [CrossRef]
- Yang, F.; Xiao, K.; Pan, H.; Liu, J. Chloroplast: The Emerging Battlefield in Plant–Microbe Interactions. Front. Plant Sci. 2021, 12, 218. [Google Scholar] [CrossRef]
- Morales, R.; Charon, M.H.; Kachalova, G.; Serre, L.; Medina, M.; Gómez-Moreno, C.; Frey, M. A Redox-Dependent Interaction between Two Electron-Transfer Partners Involved in Photosynthesis. EMBO Rep. 2000, 1, 271. [Google Scholar] [CrossRef] [PubMed]
- Goss, T.; Hanke, G. The End of the Line: Can Ferredoxin and Ferredoxin NADP(H) Oxidoreductase Determine the Fate of Photosynthetic Electrons? Curr. Protein Pept. Sci. 2014, 15, 385. [Google Scholar] [CrossRef]
- Jeon, Y.; Ahn, C.S.; Jung, H.J.; Kang, H.; Park, G.T.; Choi, Y.; Hwang, J.; Pai, H.S. DER Containing Two Consecutive GTP-Binding Domains Plays an Essential Role in Chloroplast Ribosomal RNA Processing and Ribosome Biogenesis in Higher Plants. J. Exp. Bot. 2014, 65, 117–130. [Google Scholar] [CrossRef][Green Version]
- Fukunaga, R.; Doudna, J.A. DsRNA with 5′ Overhangs Contributes to Endogenous and Antiviral RNA Silencing Pathways in Plants. EMBO J. 2009, 28, 545–555. [Google Scholar] [CrossRef]
- Mourrain, P.; Béclin, C.; Elmayan, T.; Feuerbach, F.; Godon, C.; Morel, J.B.; Jouette, D.; Lacombe, A.M.; Nikic, S.; Picault, N.; et al. Arabidopsis SGS2 and SGS3 Genes Are Required for Posttranscriptional Gene Silencing and Natural Virus Resistance. Cell 2000, 101, 533–542. [Google Scholar] [CrossRef]
- Cho, Y.W.; Hong, T.; Hong, S.H.; Guo, H.; Yu, H.; Kim, D.; Guszczynski, T.; Dressler, G.R.; Copeland, T.D.; Kalkum, M.; et al. PTIP Associates with MLL3- and MLL4-Containing Histone H3 Lysine 4 Methyltransferase Complex. J. Biol. Chem. 2007, 282, 20395–20406. [Google Scholar] [CrossRef] [PubMed]
- Sterner, D.E.; Berger, S.L. Acetylation of Histones and Transcription-Related Factors. Microbiol. Mol. Biol. Rev. 2000, 64, 435. [Google Scholar] [CrossRef]
- Kim, J.H.; Baek, S.H. Emerging Roles of Desumoylating Enzymes. Biochim. Et Biophys. Acta (BBA)—Mol. Basis Dis. 2009, 1792, 155–162. [Google Scholar] [CrossRef]
- Gray, W.M.; Hellmann, H.; Dharmasiri, S.; Estelle, M. Role of the Arabidopsis RING-H2 Protein RBX1 in RUB Modification and SCF Function. Plant Cell 2002, 14, 2137. [Google Scholar] [CrossRef]
- Nitika; Porter, C.M.; Truman, A.W.; Truttmann, M.C. Post-Translational Modifications of Hsp70 Family Proteins: Expanding the Chaperone Code. J. Biol. Chem. 2020, 295, 10689–10708. [Google Scholar] [CrossRef] [PubMed]
- Backe, S.J.; Sager, R.A.; Woodford, M.R.; Makedon, A.M.; Mollapour, M. Post-Translational Modifications of Hsp90 and Translating the Chaperone Code. J. Biol. Chem. 2020, 295, 11099–11117. [Google Scholar] [CrossRef]
- Sharma, M.; Fuertes, D.; Perez-Gil, J.; Lois, L.M. SUMOylation in Phytopathogen Interactions: Balancing Invasion and Resistance. Front. Cell Dev. Biol. 2021, 9, 2189. [Google Scholar] [CrossRef]
- Ehrlich, E.S.; Wang, T.; Luo, K.; Xiao, Z.; Niewiadomska, A.M.; Martinez, T.; Xu, W.; Neckers, L.; Yu, X.F. Regulation of Hsp90 Client Proteins by a Cullin5-RING E3 Ubiquitin Ligase. Proc. Natl. Acad. Sci. USA 2009, 106, 20330–20335. [Google Scholar] [CrossRef]
- Yang, Z.; Fu, Y. ROP/RAC GTPase Signaling. Curr. Opin. Plant Biol. 2007, 10, 490–494. [Google Scholar] [CrossRef] [PubMed]
- Nagawa, S.; Xu, T.; Yang, Z. RHO GTPase in Plants: Conservation and Invention of Regulators and Effectors. Small GTPases 2010, 1, 78. [Google Scholar] [CrossRef] [PubMed]
- Berken, A.; Thomas, C.; Wittinghofer, A. A New Family of RhoGEFs Activates the Rop Molecular Switch in Plants. Nature 2005, 436, 1176–1180. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.-m.; Hazak, O.; Cheung, A.Y.; Yalovsky, S. RAC/ROP GTPases and Auxin Signaling. Plant Cell 2011, 23, 1208–1218. [Google Scholar] [CrossRef]
- Yalovsky, S.; Bloch, D.; Sorek, N.; Kost, B. Regulation of Membrane Trafficking, Cytoskeleton Dynamics, and Cell Polarity by ROP/RAC GTPases. Plant Physiol. 2008, 147, 1527–1543. [Google Scholar] [CrossRef] [PubMed]




| Sample | Genotype | Treatment | hpi | Trait Data | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Rob | RobM | RobI | Her | HerM | HerI | HerI_RobI | ||||
| RIT0R1 | Rob | Untreated | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 |
| RIT0R2 | Rob | Untreated | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 |
| RIT0R3 | Rob | Untreated | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 |
| RMT0R1 | Rob | Untreated | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 |
| RMT0R2 | Rob | Untreated | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 |
| RMT0R3 | Rob | Untreated | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 |
| HIT0R1 | Her | Untreated | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 |
| HIT0R2 | Her | Untreated | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 |
| HIT0R3 | Her | Untreated | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 |
| HMT0R1 | Her | Untreated | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 |
| HMT0R2 | Her | Untreated | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 |
| HMT0R3 | Her | Untreated | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 |
| RMT48R1 | Rob | Mock | 48 | 1 | 1 | 0 | 0 | 0 | 0 | 0 |
| RMT48R2 | Rob | Mock | 48 | 1 | 1 | 0 | 0 | 0 | 0 | 0 |
| RMT48R3 | Rob | Mock | 48 | 1 | 1 | 0 | 0 | 0 | 0 | 0 |
| HMT48R1 | Her | Mock | 48 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| HMT48R2 | Her | Mock | 48 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| HMT48R3 | Her | Mock | 48 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| RIT48R1 | Rob | Inoculated | 48 | 1 | 0 | 1 | 0 | 0 | 0 | 1 |
| RIT48R2 | Rob | Inoculated | 48 | 1 | 0 | 1 | 0 | 0 | 0 | 1 |
| RIT48R3 | Rob | Inoculated | 48 | 1 | 0 | 1 | 0 | 0 | 0 | 1 |
| HIT48R1 | Her | Inoculated | 48 | 0 | 0 | 0 | 1 | 0 | 1 | 1 |
| HIT48R2 | Her | Inoculated | 48 | 0 | 0 | 0 | 1 | 0 | 1 | 1 |
| HIT48R3 | Her | Inoculated | 48 | 0 | 0 | 0 | 1 | 0 | 1 | 1 |
| RMT96R1 | Rob | Mock | 96 | 1 | 1 | 0 | 0 | 0 | 0 | 0 |
| RMT96R2 | Rob | Mock | 96 | 1 | 1 | 0 | 0 | 0 | 0 | 0 |
| RMT96R3 | Rob | Mock | 96 | 1 | 1 | 0 | 0 | 0 | 0 | 0 |
| HMT96R1 | Her | Mock | 96 | 0 | 0 | 0 | 1 | 1 | 0 | 0 |
| HMT96R2 | Her | Mock | 96 | 0 | 0 | 0 | 1 | 1 | 0 | 0 |
| HMT96R3 | Her | Mock | 96 | 0 | 0 | 0 | 1 | 1 | 0 | 0 |
| RIT96R1 | Rob | Inoculated | 96 | 1 | 0 | 1 | 0 | 0 | 0 | 1 |
| RIT96R2 | Rob | Inoculated | 96 | 1 | 0 | 1 | 0 | 0 | 0 | 1 |
| RIT96R3 | Rob | Inoculated | 96 | 1 | 0 | 1 | 0 | 0 | 0 | 1 |
| HIT96R1 | Her | Inoculated | 96 | 0 | 0 | 0 | 1 | 0 | 1 | 1 |
| HIT96R2 | Her | Inoculated | 96 | 0 | 0 | 0 | 1 | 0 | 1 | 1 |
| HIT96R3 | Her | Inoculated | 96 | 0 | 0 | 0 | 1 | 0 | 1 | 1 |
| Module | GO Term Name | Term ID | FDR | Num. hubs |
|---|---|---|---|---|
| blue | intrinsic component of plasma membrane | GO:0031226 | 6.30 × 10−11 | 13 |
| anchored component of plasma membrane | GO:0046658 | 9.82 × 10−7 | 8 | |
| extracellular region | GO:0005576 | 2.92 × 10−6 | 31 | |
| anchored component of membrane | GO:0031225 | 9.08 × 10−6 | 8 | |
| endoplasmic reticulum lumen | GO:0005788 | 9.19 × 10−6 | 5 | |
| integral component of plasma membrane | GO:0005887 | 1.07 × 10−4 | 5 | |
| intrinsic component of membrane | GO:0031224 | 2.43 × 10−3 | 104 | |
| plasma membrane | GO:0005886 | 3.26 × 10−3 | 19 | |
| plant-type vacuole membrane | GO:0009705 | 1.61 × 10−2 | 3 | |
| plant-type vacuole | GO:0000325 | 3.89 × 10−2 | 3 | |
| integral component of membrane | GO:0016021 | 3.89 × 10−2 | 97 | |
| extracellular space | GO:0005615 | 4.44 × 10−2 | 5 | |
| black | plastoglobule | GO:0010287 | 2.88 × 10−2 | 2 |
| plastid thylakoid | GO:0031976 | 3.43 × 10−2 | 3 | |
| chloroplast stroma | GO:0009570 | 3.43 × 10−2 | 3 | |
| chloroplast thylakoid | GO:0009534 | 3.43 × 10−2 | 3 | |
| plastid stroma | GO:0009532 | 3.43 × 10−2 | 3 | |
| chloroplast thylakoid membrane protein complex | GO:0098807 | 4.00 × 10−2 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira, L.C.; Santana, F.M.; Mur, L.A.J. Molecular Networks Underlying Wheat Resistance and Susceptibility to Pyrenophora tritici-repentis. Microbiol. Res. 2025, 16, 242. https://doi.org/10.3390/microbiolres16110242
Ferreira LC, Santana FM, Mur LAJ. Molecular Networks Underlying Wheat Resistance and Susceptibility to Pyrenophora tritici-repentis. Microbiology Research. 2025; 16(11):242. https://doi.org/10.3390/microbiolres16110242
Chicago/Turabian StyleFerreira, Larissa Carvalho, Flavio Martins Santana, and Luis A. J. Mur. 2025. "Molecular Networks Underlying Wheat Resistance and Susceptibility to Pyrenophora tritici-repentis" Microbiology Research 16, no. 11: 242. https://doi.org/10.3390/microbiolres16110242
APA StyleFerreira, L. C., Santana, F. M., & Mur, L. A. J. (2025). Molecular Networks Underlying Wheat Resistance and Susceptibility to Pyrenophora tritici-repentis. Microbiology Research, 16(11), 242. https://doi.org/10.3390/microbiolres16110242







